Abstract
Biliverdin reductase A (BVR) and Akt isozymes have overlapping pleiotropic functions in the insulin/PI3K/MAPK pathway. Human BVR (hBVR) also reduces the hemeoxygenase activity product biliverdin to bilirubin and is directly activated by insulin receptor kinase (IRK). Akt isoenzymes (Akt1–3) are downstream of IRK and are activated by phosphatidylinositol-dependent kinase 1 (PDK1) phosphorylating T308 before S473 autophosphorylation. Akt (RxRxxSF) and PDK1 (RFxFPxFS) binding motifs are present in hBVR. Phosphorylation of glycogen synthase kinase 3 (GSK3) isoforms α/β by Akts inhibits their activity; nonphosphorylated GSK3β inhibits activation of various genes. We examined the role of hBVR in PDK1/Akt1/GSK3 signaling and Akt1 in hBVR phosphorylation. hBVR activates phosphorylation of Akt1 at S473 independent of hBVR’s kinase competency. hBVR and Akt1 coimmunoprecipitated, and in-cell Förster resonance energy transfer (FRET) and glutathione S-transferase pulldown analyses identified Akt1 pleckstrin homology domain as the interactive domain. hBVR activates phosphorylation of Akt1 at S473 independent of hBVR’s kinase competency. Site-directed mutagenesis, mass spectrometry, and kinetic analyses identified S230 in hBVR 225RNRYLSF sequence as the Akt1 target. Underlined amino acids are the essential residues of the signaling motifs. In cells, hBVR-activated Akt1 increased both GSK3α/β and forkhead box of the O class transcription class 3 (FoxO3) phosphorylation and inhibited total GSK3 activity; depletion of hBVR released inhibition and stimulated glucose uptake. Immunoprecipitation analysis showed that PDK1 and hBVR interact through hBVR’s PDK1 binding 161RFGFPAFS motif and formation of the PDK1/hBVR/Akt1 complex. sihBVR blocked complex formation. Findings identify hBVR as a previously unknown coactivator of Akt1 and as a key mediator of Akt1/GSK3 pathway, as well as define a key role for hBVR in Akt1 activation by PDK1.—Miralem, T., Lerner-Marmarosh, N., Gibbs, P. E. M., Jenkins, J. L., Heimiller, C., Maines, M. D. Interaction of human biliverdin reductase with Akt/protein kinase B and phosphatidylinositol-dependent kinase 1 regulates glycogen synthase kinase 3 activity: a novel mechanism of Akt activation.
Keywords: activation of hBVR, Akt/hBVR/GSK3 axis, bilirubin, heme oxygenase 1
Biliverdin reductase A (BVR) is an evolutionarily conserved, soluble protein found in all mammalian cell types analyzed to date, albeit at different levels. The reductase is an adenine nucleotide (ATP, NADPH, NADH) binding protein with a unique dual cofactor/pH-dependence activity profile (1, 2). BVR is indispensable for reduction of biliverdin to bilirubin, the product of heme oxidation by the heme oxygenase isozymes (HO-1 and HO-2) (3). Bilirubin is an effective intracellular antiinflammatory agent, antioxidant, and quencher of reactive oxygen species (ROS) (4–7).
The human form of the reductase (hBVR) is a phosphorylated 296 aa residue protein and is the product of the BLVRA gene. The BVRB protein bears little sequence or structural similarity to BVRA, and it has a broader range of substrate specificity (8, 9). This study is focused on the BVRA protein, and hBVR is therefore synonymous with BVRA. hBVR has been extensively studied in the context of its function in the insulin/IGF-1/PI3K/MAPK signaling network (reviewed in refs. 9–13). The protein is present in all tissue, with the highest abundance in kidney. hBVR is directly activated by insulin-stimulated insulin receptor kinase (IRK) (14) that involves interaction of its phosphotyrosine-binding motif, Y198MKM, with the NPXY motif of IRK. Proteins that contain YMXM, including IRS1 and -2, and PI3K, when tyrosine phosphorylated by IRK, become the recognition platform and docking site for src-homology domain containing proteins and assembly of multiprotein signaling complexes. Recently an hBVR fragment was found to activate insulin/IGF-1 signaling by its ability to interact with the intracellular kinase domain of the insulin receptor and to stimulate glucose uptake (15).
Three isoforms of the S/T kinase, Akt/PKB (1, 2, 3 or α, β, γ, respectively), have been characterized (16, 17). Although Akt1 and -2 share similar downstream targets and have primary structural similarities, this does not extend to the roles they play in biologic systems and the outputs of their activities (18, 19). Indeed, the 2 forms have been reported to have opposing roles in cell proliferation (20, 21). Akt1, but not Akt2, is required for cell proliferation (20). Akt3 is the most prominent form in brain and testis (22). While this isoform is necessary for postnatal development, it does not serve a function in glucose homeostasis (23). Akt isozymes and the stress-response genes that include HO-1 are downstream in the insulin/IGF-1/PI3K/MAPK signaling network. The kinases are also downstream effectors of the signaling network, and they regulate a number of functions in the cell, including phosphorylation of glycogen synthase kinase (GSK3) α and β isoforms, kinases that prominently feature in regulation of glucose metabolism. The activated Akts phosphorylate the serine-threonine kinase GSK3 isoforms, which are expressed in most cell types. The 2 isoforms of GSK3 have a high degree of amino acid identity (24, 25) and function in a similar manner. Unlike other kinases, phosphorylation of the isoforms leads to inhibition of their signaling activity (24, 25). Activation of GSK3β has been shown to inhibit the antioxidant response element of the HO-1 promoter and NRF2 (26–28). Activation of the Akt/GSK3 pathway and hBVR have opposing effects on the oxidative stress response of HO-1 (26–29). Akt isozymes are also the main regulators of the forkhead box of the O class (FoxO) transcription factors (FoxO-1, -3, -4, and -6), the key regulators of cell survival that promote resistance to oxidative stress and ROS response and that control cell cycle progression and apoptosis (30–32). FoxO proteins are phosphorylated at 3 conserved residues (T32, S253, and S315) by Akt kinases; phosphorylated T32 and S253 form the binding site for 14-3-3 protein and cytoplasmic sequestration of FoxO, and termination of its transcriptional activity (32). FoxO1 and FoxO3 are close homologs of the Caenorhabditis elegans Daf-16 protein, which was shown to mediate insulin signaling downstream of Akt (33).
The Akt kinases have in common a threonine in the activation loop of the catalytic domain, the phosphorylation of which is the first step in kinase activation. The multistep process of activation of Akt kinases is initiated in response to extracellular stimuli with translocation of the kinases to the cell membrane, followed by phosphorylation by phosphatidylinositol-dependent kinase 1 (PDK1) of T308 in Akt1 (T309 in Akt2) in the activation loop (34, 35); phosphorylation of a second site, S473 (Akt1, S474 in Akt2), leads to maximal activity (34, 36). The mechanism of serine phosphorylation is not fully elucidated; autophosphorylation is likely involved in the process, and the involvement of other kinases and indirect contribution of PDK1 has also been suggested (37). A recent summation of mechanisms of Akt activation has implicated several different kinases as targeting the S473 site, including mammalian target of rapamycin (mTOR) and DNA-PK (reviewed in ref. 38). A threonine (T450) in the turn motif of the C-terminal regulatory domain is also a phosphorylation target; modification of this residue, however, is not required for full activity. Akt tyrosine phosphorylation also has been reported, although at present its functional significance is not fully known (39). The structure, regulation, and biologic functions of the Akt enzymes have been recently reviewed (40, 41).
hBVR and Akt are functionally linked (42, 43). Additional notable criteria suggested to us the likelihood of a direct regulatory link between the enzymes. To elaborate, to a great extent, Akt and hBVR are activated by similar stimuli that include ROS, growth factors (IGF-1, -2), insulin, and hypoxia (17, 29, 44, 45). The regulatory effects of Akt and hBVR directly and indirectly merge at various levels, such as regulation of VEGF, HO-1, NRF2, BTB and CNC homology 1, basic leucine zipper transcription factor 1 (BACH1), and transcription factors for the stress response c-Fos and c-Jun (42, 46–53). In turn, HO-1 is an effector in regulation of eNOS, iNOS, and the heme-dependent transcription factors NRF2 and BACH1 (48, 50, 52, 53). Also, at the structural level, Akt isoforms and hBVR share certain similarities in their protein–protein interaction domain. The N-terminal pleckstrin homology (PH) domain of the Akt isoforms (aa1–113) (54, 55) and the C-terminal half of hBVR, which folds as a large 6-stranded β sheet (56, 57), are the protein–protein interaction domains (58). The 225RNRYLSF sequence, which is identical to the canonical Akt interaction motif RxRxxSF motif, is present in the β sheet; the motif is present in all Akt substrates, in nonsubstrate binding proteins, and in Akt coactivator proteins such as members of the proto-oncogene TCL1 family (TCL1, MTCP1, TCL1b) (59, 60). Underlined amino acids are the essential residues of the signaling motifs. In addition, the consensus PDK1 binding motif, RFGFP165AFS, which corresponds to the C-Box, FXFPXF, motif identified by Jacobs et al. (61), is split between hBVR H8 and H9 (56, 57), with the proline residue positioned at the junction and accessible to binding and recognition of lipids and hydrophobic ligands.
In addition, hBVR and Akt isoforms, particularly Akt1, play parallel roles in the cell. Akt1-regulated activities include cellular metabolism, gene expression, cell proliferation, and survival. hBVR also promotes cell proliferation and survival/antiapoptotic, primarily as a function of its activity in the heme metabolism pathway and its role in ERK1/2 activation by MEK1/2 and by PKCδ (58, 62).
Because of the above noted overlap of hBVR and Akt1 at various levels of structure, function, and regulation, we questioned whether a direct regulatory interaction links hBVR and Akt; if so, we wondered there was a reciprocal interaction among the proteins. Because of more extensive similarities between hBVR and Akt1, the study was focused on Akt1 isoform. We found that hBVR directly interacts with and is a coactivator of Akt1 and is a component of the PDK1/Akt complex. Even though hBVR S230 was shown to be a phosphate acceptor site and potentially an Akt target, before this study, the identity of the kinase for transfer of a phosphate group was not confirmed. We chose GSK3α/β phosphorylation as a prototype function for evaluation of the link between hBVR and Akt1. Finding a reciprocal link between hBVR and GSK3 activity suggests the likelihood that a number of Akt-regulated processes in insulin/IGF-1-responsive cells are influenced by hBVR.
MATERIALS AND METHODS
Materials
Recombinant N-terminally his6-tagged full-length Akt1 expressed in insect cells and activated by PDK1 was obtained from EMD Millipore (Billerica, MA, USA). [γ32P]-ATP was purchased from PerkinElmer (Waltham, MA, USA). hBVR peptides were synthesized in both myristoylated and native forms by EZBiolab (Westfield, IN, USA). hBVR antibodies were produced in our laboratory (63); all other antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Aktide and Crosstide substrates were obtained from R&D Systems (Minneapolis, MN, USA) and Biomol (Enzo Life Sciences) (Farmingdale, NY, USA), respectively. siRNA against hBVR was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). IGF-1 was purchased from Calbiochem (San Diego, CA, USA) and TNF-α from Shenandoah Biotechnology (Warwick, PA, USA). The pcDNA-HA-GSK3β plasmid was obtained from Addgene (Cambridge, MA, USA).
Plasmids
Cloning of wild-type (wt) hBVR into pcDNA3 and pGEX-4T2 has been described elsewhere (14); the HA epitope tag sequence was introduced into the pcDNA3 clones during PCR amplification of the open reading frame. C-box (162FGFPAF-VGAPAV) and kinase dead G17-A mutations were introduced by site-directed mutagenesis; these mutations have been described previously (62, 64).
The open reading frames of Akt1 and Akt2 were amplified by PCR and cloned into pcDNA (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA), pEGFP-C1 (Clontech Laboratories, Mountain View, CA, USA), pDsRed2-C1 (Clontech Laboratories), and/or pET-28a (Novagen, Madison, WI, USA) by standard methods. The PH domains of the Akts were obtained by amplification of aa 1–250 of each isoform and subsequent cloning into the pEGFP-C1, pDsRed2-C1, and pET-28a vectors. Likewise, plasmids encoding truncated Akt1 (initiating at M118) and Akt2 (start at M120), and therefore lacking the PH domain encoded by aa1–113 (55), were assembled in pEGFP-C1 using appropriate PCR products. The open reading frame for PDK1 was amplified from a clone (pDONR223-PDPK1, Addgene plasmid 23801; a gift from W. Hahn and D. Root, Harvard Medical School, Boston, MA, USA), and was cloned into pcDNA3. All constructs were verified by DNA sequencing.
Protein expression in Escherichia coli
hBVR, expressed as a glutathione S-transferase (GST) fusion protein or with an N-terminal his6 tag, was expressed in E. coli BL-21 (Thermo Fisher Scientific Life Sciences) cultured at 30°C after induction by isopropylthiophenylgalactoside, and purified as described previously from cell lysates by batch affinity binding to glutathione (GSH) agarose or nickel–nitrilotriacetic acid (Ni-NTA) agarose, as appropriate (65). GST protein was prepared using identical procedures from cells transformed with the empty pGEX-4T2 (GE Healthcare, Waukesha, WI, USA) vector. The proteins were stored in elution buffer (50 mM Tris pH 8.0, 60 mM NaCl, 5 mM GSH containing 10% glycerol). His6-tagged Akts were expressed from pET-Akt plasmids; protein expression was induced with isopropylthiophenylgalactoside. The proteins were purified by affinity chromatography with Ni-NTA agarose (Thermo Fisher Scientific Life Sciences), eluted with 250 mM imidazole, and finally dialyzed to remove the imidazole. Folding of the proteins was examined using PDK1 to phosphorylate the protein while it was still bound to the Ni-NTA agarose, followed by measurement of Akt kinase activity.
Kinase activity
Akt kinase activity was measured in 50 mM HEPES, pH 7.4, 20 mM MgCl2, 0.5 mM EGTA, 2 mM DTT, 100 µM ATP (5 nmol, labeled with 10 µCi [γ32P]-ATP), with 30 µM peptide substrate; the substrate was either Crosstide (GRPRTSSFAEGKK) or Aktide (ARKRERTYSFGHHA). Reaction products were assayed on P81 filters by scintillation counting or by gel electrophoresis and autoradiography (14). The specific activity of Akt1 under these conditions is ∼550 pmol [32P] incorporated per microgram. GSK-3 (α and β) kinase assays were performed as described elsewhere (66) using 25 µM glycogen synthase peptide 2 as substrate. Products were analyzed as for Akt. The PDK1 assay was performed in 50 mM Tris-HCl, pH 7.5, 20 mM Mg(OAc)2, 5 mg/ml bovine serum albumin (BSA), 0.5% 2-mercaptoethanol, and 100 µM ATP (with 10 μCi of [γ32P]-ATP as above). Reaction products were visualized by gel electrophoresis and autoradiography.
Cell culture and transfection
Cultures of human embryonic kidney cells (HEK293) were maintained in DMEM with 10% fetal bovine serum (FBS). Cells were transfected with plasmids using Fugene-6 (Promega, Madison, WI, USA). Transfected cells were serum starved in DMEM containing 0.1% FBS for up to 24 h before treatment with 40 ng/ml IGF-1 (62). Variations from this approach are described in the figure captions. Preparation of cell lysates, immunoprecipitation, and Western blot tests were performed as described previously (58, 67). GST pull-down experiments with GST-hBVR for cells transfected with full-length or truncated pEGFP-Akt fusion plasmids were performed as described previously (68).
FoxO3 phosphorylation
Cells were transfected with either pcDNA-HA-hBVR or sihBVR. After 24 h, cells were treated with 40 ng/ml IGF-1 for 0, 1, or 4 h. Cell lysates were analyzed by Western blot test using antibodies to phospho-FoxO3, followed by anti-FoxO3A. Signals were quantified by densitometry, and the phospho-FoxO signal was normalized using that for FoxO3A.
In-cell Förster resonance energy transfer
The analysis was performed essentially as described by Periasamy et al. (69, 70). HEK293 cells were seeded into glass-bottomed dishes (MatTek, Ashland, MA, USA) and cotransfected with pEGFP-Akt fusion constructs (full-length or truncated) and pDsRed2-hBVR; 24 h later, cells were serum starved (0.1% FBS) overnight. Förster resonance energy transfer (FRET) images were taken on an Olympus (Tokyo, Japan) FV1000 laser scanning confocal microscope in the presence or absence of 40 ng/ml IGF-1, which was added during image collection. A 488 nm laser was used to excite EGFP, while a 559 nm laser was used to excite DsRed2. Photomultiplier tubes were used to record fluorescence emission. Sensitized emission FRET was performed by obtaining the following images: 1) donor-only dish imaged with donor only excitation (both channels were recorded); and 2) acceptor-only dish imaged with donor-only excitation (emissions were recorded; then the same dish was excited using the 559 nm laser to record acceptor excitation with acceptor emission). Cells from the above 2 steps were used to remove background from the unprocessed FRET image (DSBT and ASBT). Double-labeled cells were then excited with donor-only excitation, and both channels were recorded. A final image was taken by exciting the double-labeled cells with acceptor excitation (559 nm). The 7 images obtained were then added to the FV1000 workstation updated FRET module to obtain FRET efficiencies and Förster distances.
Affinity binding of GST-hBVR by Akt in vitro
His6-tagged Akts were expressed in E. coli, and 0 to 10 µg was bound to Ni-NTA agarose (∼10 µl packed resin) in 50 mM Tris-HCl, pH 7.5 60 mM NaCl. GST-hBVR (10 µg) in the same buffer was added and was allowed to bind at 4°C overnight. After extensive washing in the same buffer, the beads were suspended in 2× SDS gel loading buffer and analyzed by gel electrophoresis and Western blot test using anti-GST antibodies. A standard curve of GST-hBVR was included on the blot. The blot was stripped before probing with Akt antibodies. To test whether Akt as isolated from E. coli was correctly folded, the protein was immobilized on Ni-NTA agarose as before, then washed twice with the buffer described above for PDK1 kinase assay. PDK1 and unlabeled ATP were then added to phosphorylate the immobilized protein. After washing and equilibrating the immobilized Akt1 with its kinase buffer, Aktide and [γ32P]-ATP were added; incorporation of [32P] was measured using P81 filters. The Ni-NTA agarose beads with bound Akt were then washed and suspended in SDS gel loading buffer. An aliquot was subjected to electrophoresis and Western blot testing, and probed sequentially with anti-phospho-Akt T308 and anti-Akt2 antibodies.
Glucose uptake
Glucose uptake was assessed using 2-deoxy 1-[3H] glucose as before (14). Cells in 24-well plates were grown to near confluence, and starved in calcium and magnesium supplemented PBS (Thermo Fisher Scientific Life Sciences) containing 1 mg/ml BSA for 2 h, followed by treatment with 100 nM insulin or 40 ng/ml IGF-1 for 15 min. Deoxyglucose (0.2 mM, containing 1μCi/ml [3H]-deoxyglucose) was added for various lengths of times. Cells were washed 3 times with cold PBS and solubilized in 150 μl 50 mM NaOH. Radioactivity was measured and normalized to protein concentration. Assessments were the average of triplicate samples for each condition.
Preparation of protein for mass spectrometry
His6-hBVR was incubated with Akt1 and unlabeled ATP using the reaction conditions described above but without [γ32P]-ATP. Triplicate 2 µg aliquots of the reaction product were resolved by gel electrophoresis and digested with one of trypsin, chymotrypsin, or Arg-C protease. Mass spectrometry was performed essentially as described previously (67).
Statistical analysis
All experiments were performed at least 3 times. Statistical analysis by 1-way ANOVA and nonlinear regression were performed by Prism software (GraphPad Software, La Jolla, CA, USA).
RESULTS
hBVR binds and stimulates Akt1 kinase activity in the cell
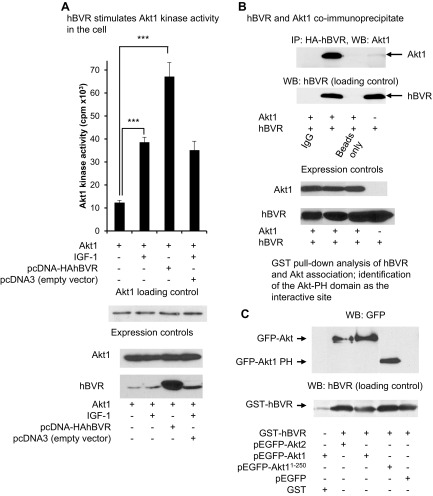
Because of the overlap between hBVR and Akt1 at various levels such as of structure, function, and regulation, we questioned whether there is a reciprocal interaction among the proteins. In order to test this, we examined their interaction in cells. Akt is activated in the cell by extracellular stimuli, including insulin and IGF-1, which both act through PI3K. We compared the activity of Akt in cells treated with IGF-1 with those transfected with hBVR expression plasmid. As shown in Fig. 1A, the kinase activity of Akt1 in the cell increased in response to treatment with 40 ng/ml IGF-1. The same induction was seen with overexpressed hBVR; overexpression of BVR was verified by Western blot analysis of the cell lysates. Cells transfected with empty vector and treated with IGF-1 showed no increase in Akt activity compared to similarly treated, untransfected cells. Western blot indicated that there was no increase in Akt levels in either transfected or IGF-1-treated cells. It should be noted that transfection with the hBVR expression plasmid leads to an increase in hBVR protein of 5- to 8-fold (Fig. 1A).
Figure 1.
A) hBVR binds and stimulates Akt1 kinase activity in cell. Cells were transfected with pcDNA-Akt1 with or without pcDNA-HA-hBVR or pcDNA3 vector. Some cells, as indicated, were treated with 40 ng/ml IGF-1. Immunoprecipitates were prepared using anti-Akt1 antibody, and Akt1 kinase activity was measured using Crosstide as substrate. Western blot of immunoprecipitated protein was probed with anti-Akt1. Expression of hBVR and Akt1 was verified by Western blot test. ***P < 0.001. B) hBVR and Akt1 coimmunoprecipitate. HEK cells overexpressing HA-hBVR either alone or cotransfected with pcDNA-Akt1 were treated with 40 ng/ml IGF-1 for 15 min; cell lysates were subjected to immunoprecipitation using anti-HA antibody. Immunoprecipitates were analyzed by Western blot analysis with anti-Akt1 antibody followed by anti-hBVR. Expression of hBVR and Akt1 was verified by Western blot test. C) hBVR and Akt association is mediated by Akt PH domain. GST-BVR bound to GSH agarose was added to lysates of cells that had been transfected with indicated EGFP fusion plasmids. Proteins bound by immobilized BVR were assayed by Western blot test, which was probed with anti-GFP antibodies. GST was included as additional control.
In this series of studies, we examined whether hBVR and Akt proteins bind. Coimmunoprecipitation was performed using antibody to the influenza hemagglutinin epitope (HA) on lysates of cells cotransfected with expression plasmids for Akt1 and HA-hBVR, and stimulated with IGF-1 (Fig. 1B). The presence of Akt1 in the immunoprecipitate indicated association of proteins in the cell. The immunoprecipitates from cells transfected with plasmid pcDNA-HA-hBVR contained detectable hBVR, while no signal was detected in lysates of empty vector transfected cells. The overexpression of both hBVR and Akt1 was verified by Western blot analysis; specificity of the interaction was demonstrated by the absence of either protein in mock immunoprecipitations using either control IgG or no antibody.
To confirm the association of hBVR and Akt in cells, GST-BVR bound to GSH agarose was added to lysates from cells transfected with the indicated pEGFP-Akt fusion plasmids or with empty pEFGP-C1 vector. The GSH agarose was collected by centrifugation, and the bound proteins were analyzed by Western blot test (Fig. 1C). To avoid complications due to the potential loss of epitopes in truncated proteins, the blot was probed with antibody to green fluorescent protein (GFP). GFP alone did not bind to BVR, whereas the full-length Akt1 and Akt2 fusions and a fusion that included only aa 1–250 of Akt1 (Akt11–250) were both bound by BVR. There was no association of EGFP-Akt1 with immobilized GST. These observations confirm those of Fig. 1B and moreover indicate that BVR binds to the N-terminal half of Akt1 that contains the PH domain.
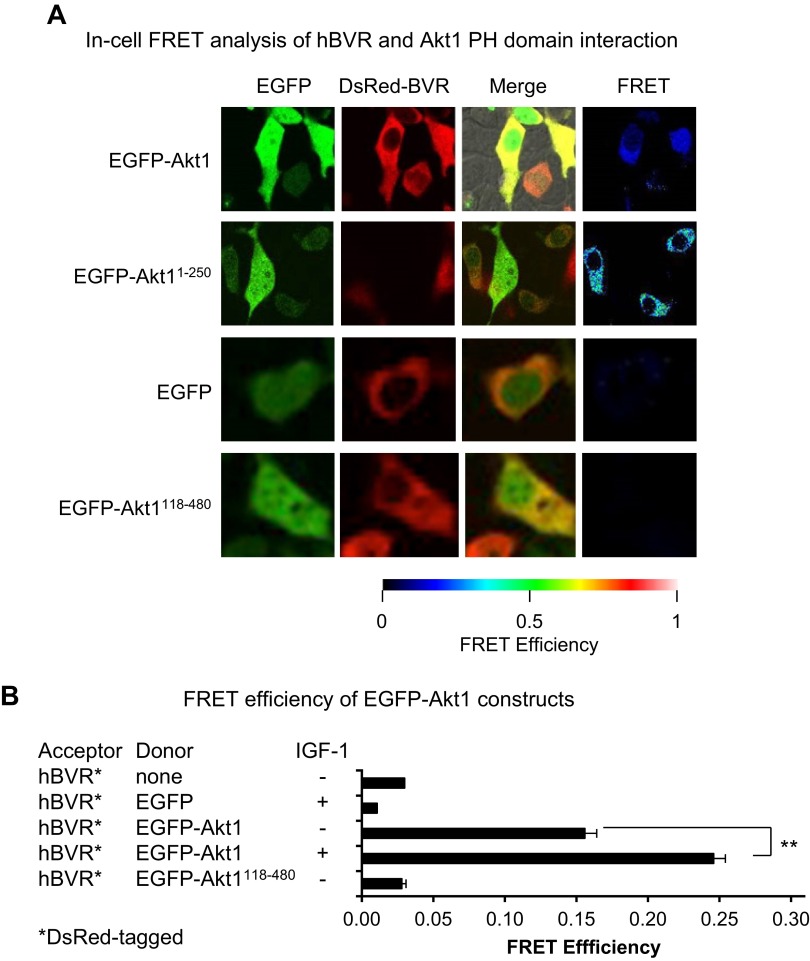
To examine more closely the association of Akt and hBVR in the cell, and to circumvent problems that might arise during preparation of lysates, we used a combination of confocal microscopy and FRET analysis (Figs. 2 and 3). All cells in these experiments were transfected with a plasmid encoding the FRET acceptor, pDsRed-hBVR. A series of cotransfected FRET donors (pEGFP-Akt1 and 2, both wt and mutant) was examined, as indicated in Figs. 2 and 3. Cells were serum starved for 16 h before treatment with IGF-1 for 15 min. Cells expressing both transfected plasmids, as judged by the presence of yellow color in merged images for the EGFP and DsRed2 signals, were subsequently examined for FRET; FRET efficiency and Förster distances were measured. Cells that expressed only the DsRed-hBVR acceptor species and cells cotransfected with the acceptor and empty pEGFP vector were used as negative FRET controls. Increased binding of 2 proteins results in a reduced Förster distance and increased FRET efficiency. A subset of doubly transfected cells with some apparent signal localization was detected in all cotransfections, including that using empty pEGFP vector. Because the latter showed no evidence of FRET, the EGFP moiety of the other constructs is not the source of interaction. In cells cotransfected with pDsRed-hBVR and pEGFP-Akt1, FRET efficiency and Förster resonance distances were measured before and after treatment with IGF-1–FRET efficiency for the protein interaction is depicted as a false-color image. The FRET efficiency was significantly increased upon IGF-1 treatment (Fig. 2B), representing a reduction in Förster distance (Table 1) and indicating that Akt1 and BVR become closely associated in the cell in response to the stimulus. Despite an apparent colocalization suggested by microscopy (Fig. 2), EGFP-Akt1118–480 failed to give a FRET signal in IGF-1-treated cells.
Figure 2.
A) In-cell FRET analysis of hBVR and Akt1 interaction. Cells cotransfected with pDsRed2-hBVR and indicated pEGFP-Akt1 constructs, wt or truncation mutants, were examined by confocal microscopy after stimulation with 40 ng/ml IGF-1. Empty pEGFP vector was used as control. FRET was detected from multiple images as described in Materials and Methods; FRET images shown depict same cells as shown for confocal microscopy. B) FRET efficiency was calculated for at least 3 microscope fields for each condition. Values for no donor were calculated for cells that had been transfected with pDsRed2-hBVR only. **P < 0.01.
Figure 3.
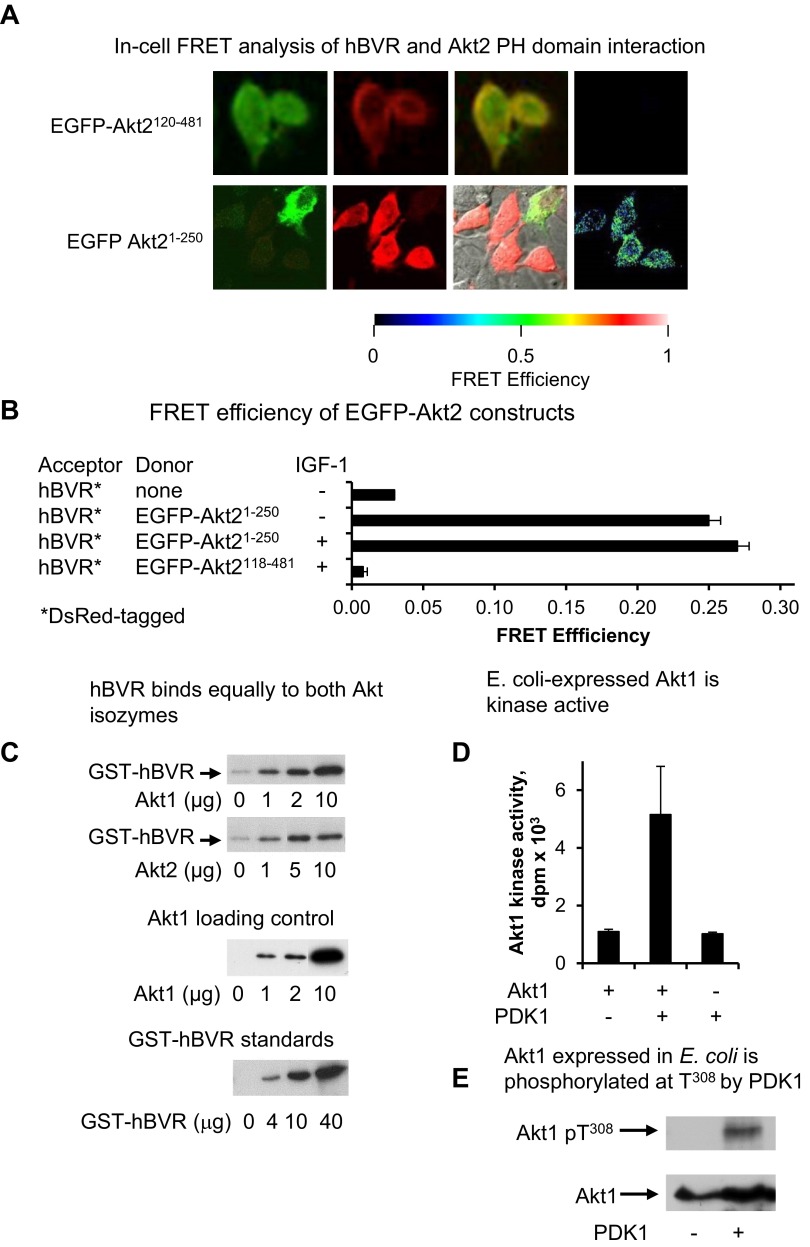
A) In-cell FRET analysis of hBVR and Akt2 PH domain interaction. Cells cotransfected with pEGFP-Akt2 constructs and pDsRed2-hBVR were examined by FRET as in Fig. 2A. B) FRET efficiency was calculated as in Fig. 2B; efficiency for Akt21–250 construct was measured before and after IGF-1 treatment. C) hBVR does not display preferential affinity for Akt enzymes. His6-tagged Akts, expressed in E. coli and bound to Ni-NTA agarose, were incubated with GST-BVR. After washing, proteins bound on beads were analyzed by gel electrophoresis and Western blot test using anti-GST antibodies. A series of loading controls for GST-BVR was also included on blot to allow approximate quantification of GST-hBVR bound to Akt. The blot was subsequently stripped and probed with anti-Akt1 antibodies to verify input Akt1 levels. D) E. coli–expressed Akt1 is kinase active. his6-tagged Akt1 bound to Ni-NTA agarose was equilibrated in PDK1 kinase buffer and treated with PDK1. PDK1 was then washed from agarose beads, immobilized protein was equilibrated in Akt kinase buffer, and kinase activity was measured with Aktide substrate. E) Akt1 expressed in E. coli is phosphorylated at T308 by PDK1. PDK1 kinase reaction products from panel D were examined for T308 phosphorylation by Western blot test.
TABLE 1.
Förster distance calculated from FRET analysis of cells transfected with EGFP-Akt donor and DsRed-hBVR acceptor constructs
| Donor | FRET efficiency | Förster distance |
|---|---|---|
| Akt1 | 15.6 ± 3% | 5.36 ± 0.14 nm |
| Akt1 + IGF-1 | 24.6 ± 5% | 4.76 ± 0.18 nm |
| Akt21–250 | 25.0 ± 2% | 4.77 ± 0.12 nm |
As noted in Fig. 1C, the binding site in Akt1 for hBVR is likely to be located in aa 1–250 of Akt1. The pEGFP-Akt11–250 and pEGFP-Akt21–250 constructs, which encompass the Akt PH domain, were used. A strong FRET response was observed in cells transfected with these constructs; in contrast to the full-length Akt1 construct, the FRET efficiency was the same in untreated and IGF-1-treated cells (Fig. 3A, B). Although the T308 site is phosphorylated upon IGF-1 stimulation, it is not present in the constructs used in Fig. 3A, B, and the absence of change in FRET indicates that binding of hBVR and Akt is not mediated by T308 phosphorylation. The Förster distance between DsRed and EGFP was virtually identical in the IGF-1-treated cells expressing full-length Akt1 or the PH-domain-containing protein (Table 1), confirming that the mode of binding of each construct to hBVR is the same. In contrast, the PH domain of full-length Akt in untreated cells is likely to be less accessible for binding to hBVR than it is in the truncated protein, resulting in the observed weaker FRET response.
To compare Akt1 and Akt2 binding to hBVR, an in vitro experiment was performed using Akt1 and Akt2 proteins that had been expressed in E. coli. Increasing amounts of the his6-tagged proteins were bound on Ni-NTA agarose and incubated with GST-hBVR. Binding was assayed by Western blot test, which was probed with anti-GST antibody; the gel included a series of standards for GST-BVR to estimate the efficiency of binding. GST-hBVR binding was approximately proportional to the immobilized Akt for each sample (Fig. 3C), and no significant difference in binding to Akt1 or Akt2 was detected. The immobilized Akt was examined for the presence of correctly folded protein by phosphorylation with PDK1 using unlabeled ATP followed by determining its kinase activity. There was at least a 5-fold increase in Akt1 kinase activity after PDK1 treatment, while PDK1 itself showed minimal activity in the Akt kinase assay (Fig. 3D). Spent beads from the kinase assay were then examined by Western blot test (Fig. 3E). Akt1 treated with PDK1 was phosphorylated at T308, whereas the untreated protein was not, as expected for protein produced in E. coli, suggesting a proper folding of the immobilized Akt.
Taken together, the data in Figs. 1, 2, and 3 suggest that the mechanism of hBVR binding and activation of Akts involves the Akt PH domain.
Mechanism of activation of Akt1 and Akt2 by hBVR
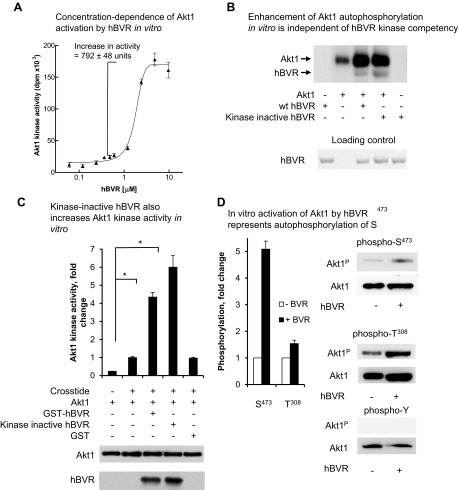
To examine further the hBVR and Akt interaction, several in vitro experiments were performed that focused on Akt1. In the first series of in vitro experiments, we explored whether Akt1 and hBVR are coactivators using his6-tagged Akt1 (EMD Millipore; activated in insect cells with PDK1) and measuring incorporation of [32P] into an Akt-specific peptide substrate. The activation of Akt1 by GST-tagged hBVR was shown to be dose dependent (Fig. 4A), with a pronounced sigmoid dose response. In light of this observation, 3 µM GST-hBVR was used in each subsequent in vitro assay unless otherwise indicated. We have previously demonstrated that the G17-A mutation in the ATP-binding site rendered hBVR kinase incompetent (64). Data in Fig. 4B indicate that addition of the wt or mutant hBVR to an Akt1 autophosphorylation reaction led to a 2.5-fold increase in incorporation of phosphate into Akt1; the extent of incorporation was identical whether wt or kinase-incompetent hBVR was added. This observation holds irrespective of the preparation used—whether tagged with GST or his6, or whether the tag had been removed with thrombin. The increased phosphorylation of hBVR in the presence of Akt1 is consistent with hBVR being a substrate for Akt. Autophosphorylation of hBVR is unlikely to be relevant in the experiment because kinase-incompetent hBVR was equally well phosphorylated in the presence of Akt.
Figure 4.
hBVR activates Akt1 in vitro. A) Concentration dependence of Akt1 activation by hBVR in vitro. Phosphorylation of Crosstide substrate by his6-tagged recombinant Akt1 was measured in presence of increasing concentrations of GST-tagged hBVR. Data were fitted to sigmoid curve using nonlinear regression. B) Enhancement of Akt1 autophosphorylation in vitro is independent of hBVR kinase competency. Autophosphorylation of Akt1 was measured in presence or absence of 2 µg wt or kinase dead (G17 > A) hBVR. Kinase reaction products were separated by SDS-PAGE and detected by autoradiography; loading of hBVR was detected by Ponceau S staining. C) Kinase-inactive hBVR also increases Akt1 kinase activity in vitro. Akt1 activity was measured as in (A) using Crosstide as substrate and wt GST-hBVR, G17 > A kinase inactive mutant, or GST alone. Akt1 and hBVR input is indicated by Western blots. *P < 0.05. D) In vitro activation of Akt1 by hBVR represents autophosphorylation of S473. Akt1 was incubated under conditions favoring Akt kinase activity in presence or absence of hBVR, as described in (C). T308, S473, and tyrosine phosphorylated products were detected by Western blot test by sequentially probing membranes with antibody against specific phosphorylation products, followed by anti-Akt1. Densitometry of T308, S473, and Akt1 blots was used to correct antiphosphopeptide signals for differences in loading.
Quantification of [32P] incorporation and densitometric analysis of the blot were used to determine the proportion of Akt molecules that were autophosphorylated. The data indicated that in the reactions with Akt1 alone, 36% of Akt1 molecules are autophosphorylated if no more than one phosphate is added to each protein molecule. This increases to 84% in the samples with added hBVR. This observation was extended to the Akt1 kinase activity toward a peptide substrate (Fig. 4C), where both wt and kinase-inactive hBVR stimulated Akt1 activity. Kinase reactions containing GST-hBVR in addition to the peptide substrate showed an approximately 5-fold increase in [32P] incorporation compared to reactions lacking hBVR. It is not clear why the kinase-dead hBVR is a somewhat more effective activator of Akts because virtually identical amounts of hBVR protein were included in the reaction, as shown by the Western blot test. The fact that kinase-incompetent BVR was fully supportive of Akt1 stimulation argues strongly against the possibility of Akt1 being a substrate for hBVR’s kinase activity. No increase in Akt activity was observed if GST, prepared using the same procedure as used for GST-hBVR, was added to the kinase reaction, confirming that the stimulation was dependent only on hBVR. Western blot analyses of the reactions confirmed equal input of Akt1 in each reaction.
In order to test which sites of Akt1 are phosphorylated in the presence of hBVR, a cold (no [32P]-ATP added) Akt1 kinase reaction was performed in vitro in the presence or absence of hBVR and examined by Western blot test. It was noted that there was little increase in phosphorylation at T308 in the samples with hBVR compared to untreated controls (Fig. 4D), as would be expected for an enzyme that had been activated by PDK1 phosphorylation at this site. On the other hand, phosphorylation at S473 was increased 5-fold, as indicated by densitometric analysis of the blots. No phosphorylation at tyrosine was detected; this is not surprising, as Akt1 is not a tyrosine kinase, and the reaction conditions do not favor hBVR’s tyrosine kinase activity.
Identification of hBVR phosphorylation site
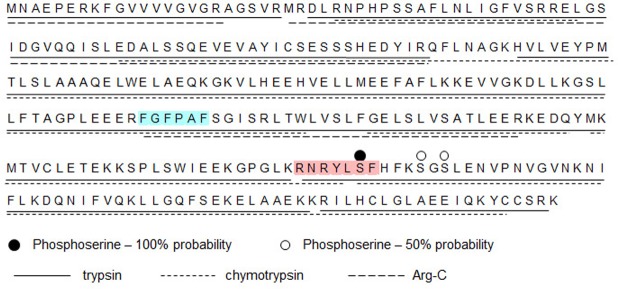
RxRxxSF has been established as the site of interaction for Akt in its targets (71). We questioned whether the RNRYLSF sequence in hBVR, which is identical to the consensus, is the target site of interaction with Akt. For the current analyses, his6-tagged hBVR prepared from E. coli was incubated in vitro in a standard Akt1 kinase assay using only unlabeled ATP. A control reaction with [32P]-ATP confirmed that phosphorylated hBVR was present in the reaction. The unlabeled hBVR reaction product was purified by gel electrophoresis; as previously, 3 independent digests (trypsin, chymotrypsin, and Arg-C) were analyzed by mass spectrometry (67). Peptide coverage of the protein exceeded 99%, with only the C-terminal lysine not appearing in the digestion products (Fig. 5). A tryptic peptide that includes S230 was shown to contain phosphoserine (Table 2). A second site in hBVR, either S235 or S237, was phosphorylated at low frequency (2 of 83 occurrences), but which of the 2 residues was modified could not be identified unequivocally. We note that this second site is close to S230 in both the primary and secondary structures (57, 63).
Figure 5.
Sequence coverage of phosphorylated hBVR by proteolysis and mass spectrometry. Products of kinase reaction that included Akt1 and his6-hBVR (purified from E. coli) were resolved by gel electrophoresis. Three identical samples, each containing 2 µg of protein, were digested with trypsin, chymotrypsin, or Arg-C protease. Products were identified by mass spectrometry. Extent of sequence coverage by each digest is indicated by underlining; serine residues identified as phosphorylation sites are indicated by dots above sequence. Red shading indicates Akt binding/phosphorylation site as predicted by computational analysis, and blue shading indicates RFxFPxFS PDK1 binding motif.
TABLE 2.
Identification of S230 as high-probability target in hBVR by Akt1
| Sequence | n | Site | Phosphorylated | Unmodified |
|---|---|---|---|---|
| YLPSFHFK | 4 | S230 | 4 | |
| NRYLSFHFK | 10 | 11 | ||
| YLSFHFKSGSLENVPNVGVNK | 1 | |||
| PSGSLENVPNVGVNK or | 2 | S235/S237 | 2 | |
| SGPSLENVPNVGVNK | ||||
| SGSLENVPNVGVNK | 76 | |||
| SGSLENVPNVGVNKNIFLK | 4 | 81 | ||
| YLSFHFKSGSLENVPNVGVNK | 1 |
Phosphorylated peptides and their unmodified counterparts were detected in tryptic digest. It was not possible in this analysis to unequivocally assign phosphoserine in peptide containing S235 and S237.
Sequences including hBVR S230 site are substrates/inhibitors of Akt
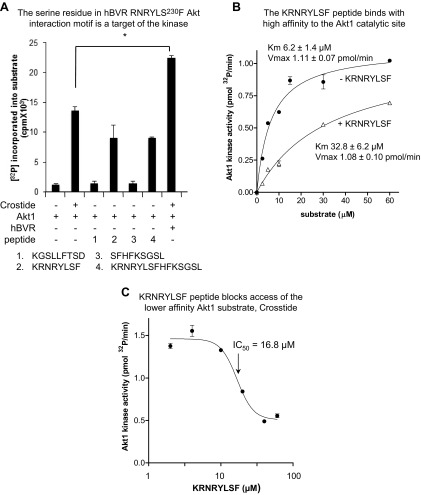
We further investigated the role of the RNRYLSF motif in the interaction between Akt1 and hBVR using several hBVR-derived peptides that include variations of the motif (Fig. 6A). It was observed that peptides containing this motif were substrates for Akt1 (i.e., 224KRNRYLSF, 224KRNRYLSFHFKSGSL), although they were utilized somewhat less efficiently than an equimolar concentration of commercial substrate. Two peptides that contained only a partial sequence of the RNRYLSF motif or that were derived from a sequence in hBVR of similar composition (230SFHFKSGSL, 147KGSLLFTSD) were not substrates.
Figure 6.
A) Serine residue in hBVR RNRYLS230F Akt interaction motif is target of kinase. Akt1 was assayed in vitro in presence of Crosstide as substrate or peptides (each at 20 µM) derived from hBVR, as described in Materials and Methods. Effect of adding 1 µg hBVR to reaction mixture with Crosstide was also examined. B) KRNRYLSF peptide binds with high affinity to Akt1 catalytic site. Akt was assayed in presence or absence of 20 µM KRNRYLSF while varying Crosstide concentration. Data were fitted to Michaelis-Menten equation by nonlinear regression. C) KRNRYLSF peptide blocks access of lower-affinity Akt1 substrate Crosstide. Akt1 was assayed in vitro in presence of 30 µM Crosstide as substrate and increasing amounts of KRNRYLSF.
In light of these findings, the relative affinities of the Crosstide substrate and KRNRYLSF for the Akt1 active site were examined. Comparison of the kinetics of phosphorylation of Crosstide in the presence and absence of a fixed concentration of KRNRYLSF (Fig. 6B) indicated that the hBVR-derived peptide caused a 5-fold increase in apparent Km for Crosstide (6.2 ± 1.4 µM with no peptide, 32.8 ± 6.2 µM with peptide), while there was no change in Vmax (1.11 ± 0.07 pmol/min, 1.08 ± 0.10 pmol/min, respectively). The hBVR peptide is therefore acting as a high-affinity substrate for Akt1 that, by binding tightly in the active site, prevents access of Crosstide substrate to the site. Effectively, the slow off-rate of KRNRYLSF makes it both a substrate and a competitive inhibitor of Akt1.
We undertook a further kinetic analysis of Akt1 with the KRNRYLSF peptide, varying its concentration in the presence of a fixed concentration of Crosstide. The peptide led to a concentration-dependent decrease in phosphorylation of Crosstide substrate by Akt1 (Fig. 6C). The plateau at high KRNRYLSF concentration we interpret as being due to phosphorylation of the hBVR peptide. From the nonlinear regression analysis, an apparent IC50 (drug concentration causing 50% inhibition) of 16.8 µM was estimated. While the BVR S230 peptides show a high affinity for the active site of Akt1, it is also noted that hBVR also binds to the PH domain of Akt, which is expected to stabilize Akt in its active conformation.
hBVR/Akt/PDK1 form a complex involving hBVR’s consensus PDK1 binding sequence
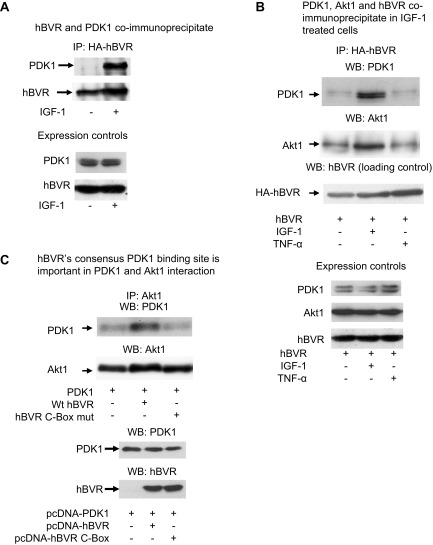
We have shown previously that hBVR is a scaffold protein (11, 58, 64). It is therefore possible that hBVR interacts with Akt binding partners such as PDK1, the established kinase for phosphorylation of T308 (Akt2, T309). This possibility was tested in the experiments shown in Fig. 7. Cells cotransfected with pcDNA-HA-hBVR and pcDNA-PDK1 were treated with IGF-1, cell lysates were immunoprecipitated with anti-HA antibody, and the precipitates were analyzed by Western blot test. It is apparent that activation of PDK1 in response to IGF-1 resulted in its binding to hBVR (Fig. 7A). Western blot analysis of whole cell lysates indicated that there was no difference in expression of hBVR and PDK1 in the treated and untreated cells.
Figure 7.
A) Coimmunoprecipitation of PDK1 and hBVR. Cells were cotransfected with PDK1 expression plasmid and pcDNA-HA-hBVR. Lysates were prepared from cells with or without stimulation with 40 ng/ml IGF-1 and immunoprecipitated with anti-HA antibody. Immunoprecipitates were analyzed by Western blot test; blot was sequentially probed with antibodies to PDK1 and hBVR. Expression of PDK1 and hBVR in lysates was verified by Western blot test. B) PDK1, Akt1, and hBVR coimmunoprecipitate in IGF-1 stimulated cells. Lysates prepared from cells cotransfected with pcDNA-HA-hBVR and pcDNA-Akt1 and stimulated with 40 ng/ml IGF-1 or 50 ng/ml TNF-α were immunoprecipitated with anti-HA antibodies. Immunoprecipitates were analyzed by Western blot test, which was sequentially probed with antibodies to PDK1, Akt1, and hBVR. Overexpression of Akt1 and HA-hBVR, and presence of PDK1 were verified by Western blot test of cell lysates. C) hBVR consensus PDK1 binding site is important in PDK1 and Akt1 interaction. Cells were cotransfected with PDK1 expression plasmid with or without pcDNA-HA-hBVR or pcDNA-HA-hBVR C-Box (RFGFPAFS to RVGAPAVS) mutant. Immunoprecipitates obtained with Akt1 antibody were analyzed for PDK1 as in (B); equality of loading was confirmed by probing blot with anti-Akt1 antibody. Overexpression of proteins was verified by Western blot test of cell lysates.
In a second experiment, cells were cotransfected with the same hBVR and PDK1 plasmids, then stimulated with 2 different extracellular activators, IGF-1 and TNF-α (40 ng/ml or 50 ng/ml, respectively), which activate different sets of pathways after binding to their specific receptors. It should also be noted that TNF-α activates hBVR by a mechanism that involves S-T phosphorylation (64). Cell lysates were immunoprecipitated with anti-HA antibodies, then analyzed by a Western blot test that was sequentially probed with antibodies against PDK1, Akt1, and hBVR. In the absence of specific stimulation by IGF-1, there was a basal level of PDK1 and Akt1 bound by hBVR in the immunoprecipitate; these levels were unaltered in cells treated with TNF-α (Fig. 7B). However, in IGF-1-activated cells, there were significant increases in binding of PDK1 and Akt1 to immunoprecipitated hBVR. PDK1 as seen in this immunoprecipitate was apparently resolved as 2 bands. This may reflect the presence of splicing variants of PDK1 in HEK cells. Again, analysis of cell lysates indicated that the increased PDK1 in the immunoprecipitate was not merely due to the presence of elevated PDK1 expression.
The sequence 161RFGFPAFS was identical to the consensus PDK1 binding motif, and we examined whether this motif mediates hBVR-PDK1 interaction. Cells were transfected with pcDNA-PDK1 alone or were cotransfected with wt pcDNA-HA-hBVR or pcDNA-HA-hBVR C-box mutant (RFGFPAFS changed to RVGAPAVS). Cell lysates were examined by a Western blot test of immunoprecipitates obtained with anti-Akt1 antibody, which was probed sequentially with antibodies against PDK1 and Akt1. Association of PDK1 with Akt1 was markedly increased in cells transfected with wt hBVR but not in cells expressing the mutant protein (Fig. 7C). This is unlikely to be due to the slight variation in total Akt1 in the lysate from wt hBVR-transfected cells. The experiment leads to the conclusion that the hydrophobic motif in hBVR is the primary site for interaction with PDK1.
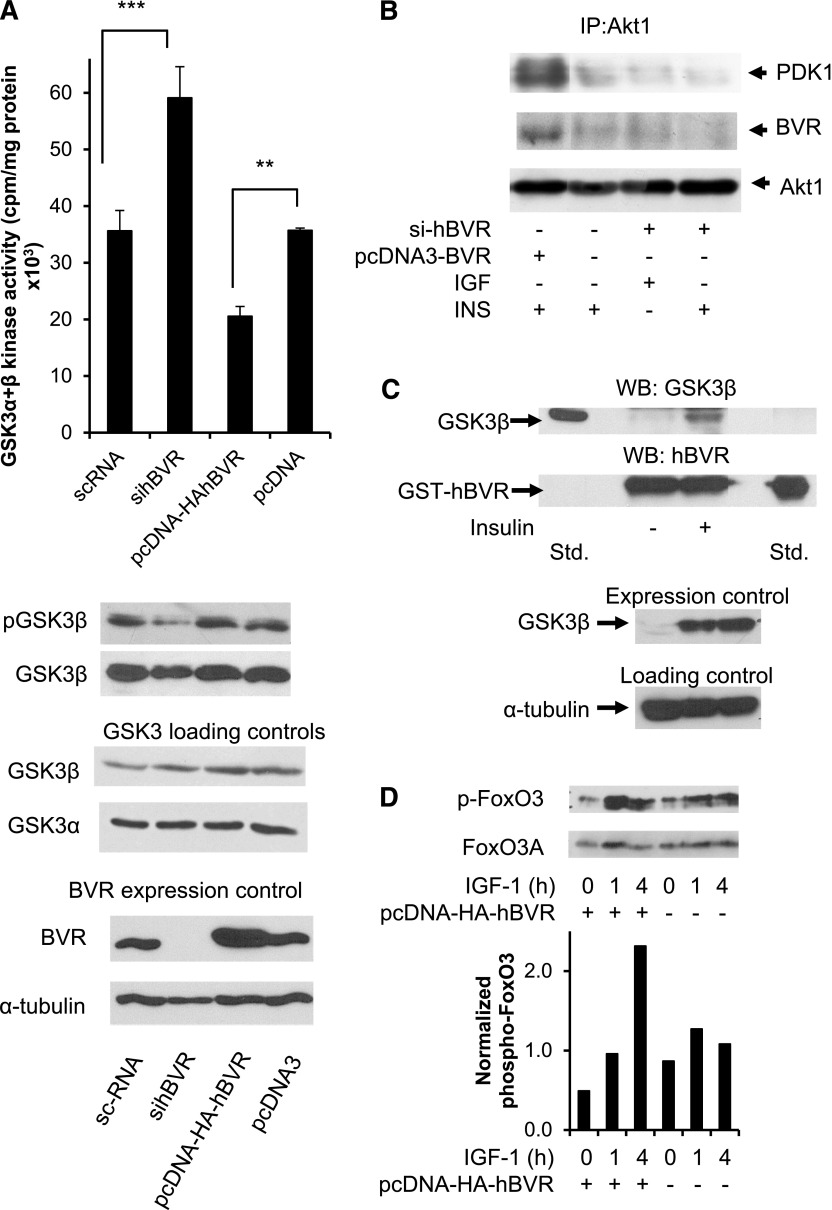
The consequence of hBVR activating Akt in the cell was examined in the context of the Akt substrate GSK3. Unlike many other kinases, phosphorylation of GSK by Akt leads to its inhibition. Our hypothesis was that if hBVR activates Akt1/2, then overexpression or ablation of hBVR should, respectively, inhibit or activate GSK3. The data in Fig. 8A were obtained from cells in which hBVR was overexpressed from plasmid or was down-regulated by siRNA to hBVR. Overexpression or down-regulation of hBVR was confirmed in the accompanying Western blot tests; it is apparent that there was no change in the expression of either GSK3α or GSK3β. Cell lysates were examined for total GSK3 kinase activity using a specific substrate. Elevated hBVR expression led to a reduction in GSK3 kinase activity compared to that seen in cells treated with the empty pcDNA3 vector, whereas ablation of hBVR in the cell by treatment with siRNA led to an enhanced activity compared to that seen with a control duplex RNA. There was no discernable effect arising from the different transfection regimens used for siRNA and plasmids. Moreover, the activity of GSK3β was reflected in the phosphorylation status of S9. Activation of GSK by siRNA treatment and inhibition by elevated hBVR were as expected based on modulation of Akt1 activity by hBVR. Formation of the hBVR/PDK1/Akt1 complex was examined in these cell lysates. Complex formation was observed in cells treated with insulin, and this was enhanced in cells transfected with pcDNA-HA-hBVR (Fig. 8B). In contrast, cells treated with sihBVR showed minimal complex formation on addition of either insulin or IGF-1. The data emphasize the importance of hBVR in Akt activation by PDK1.
Figure 8.
A) Presence of hBVR decreases GSK3α/β activity in cell. Cells were transfected with either pcDNA-HA-hBVR or with siBVR. Lysates prepared from cells were assayed for GSK3α/β activity using peptide substrate from glycogen synthase, as described in text. **P < 0.01, ***P < 0.001. Western blots of lysates were probed with antibodies to phospho-GSK3β, GSK3α, GSK3β, hBVR, and anti–tubulin. B) sihBVR treatment prevents formation of hBVR/PDK1/Akt1 complex. Immunoprecipitates were analyzed by Western blot test, which was sequentially probed with PDK1 and Akt1 antibodies. Observed pattern of PDK1 is consistent with presence of splice variants that are recognized by antibody. C) GST pulldown shows association of GSK3β from insulin-treated cells with hBVR. Cells were transfected with pcDNA-GSK3β and subsequently treated with insulin or left untreated. Lysates were analyzed by GST pulldown using GST-hBVR, followed by Western blot analysis. Blots were probed with antibodies to GSK3β followed by anti-hBVR. Overexpression of GSK3β was verified by Western blot test. D) Phosphorylation of FoxO3 in response to IGF-1 is mediated by hBVR. Cells were transfected with pcDNA-HA-hBVR or with empty vector. After 24 h, transfected cells were treated with 40 ng/ml IGF-1 for 1 or 4 h or were left untreated. Phosphorylated FoxO3C and FoxO3A were detected by Western blot test, which was then probed with antibody to FoxO3A. Densitometry was used to normalize phospho-FoxO3 signals.
To examine further the consequences of inactivation of GSK3 by BVR-stimulated Akt, a GST pulldown experiment was carried out using lysates from cells transfected with pcDNA-GSK3β. No GSK3β was detected in the lysate prepared from cells that had not been treated with insulin (Fig. 8C), whereas association between GST-BVR and GSK3β was seen after insulin treatment. It is likely that BVR associates only with the phosphorylated, inactive form of GSK3β. A second phosphorylation target of Akt is the transcription factor FoxO3; the end result of this phosphorylation is inactivation of FoxO3 and its sequestration in the cytoplasm (32). Cells were transfected with pcDNA-HA-hBVR or empty vector, starved, and then treated with IGF-1 for up to 4 h. Cell lysates were analyzed by Western blot test using anti-phospho-FoxO antibodies followed by anti-FoxO3A antibody. Overexpression of hBVR resulted in elevated phosphorylation of FoxO3 in response to IGF-1 treatment above the levels observed in untransfected cells (Fig. 8D).
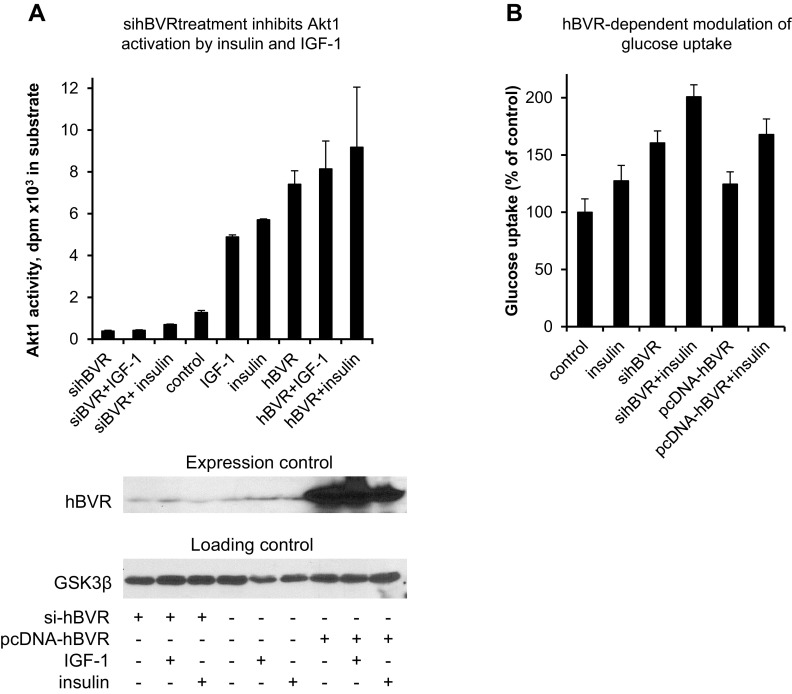
Because BVR expression levels correlated with phosphorylation of GSK3 and FoxO3, the activity of Akt was examined in cells transfected with either sihBVR or pcDNA-HA-hBVR, then treated with insulin or IGF-1. Akt1 was immunoprecipitated from cell lysate for determination of kinase activity. hBVR, insulin, or IGF-1, singly or in combination, increased the activity (Fig. 9A), while sihBVR treatment inhibited basal Akt1 activity and prevented activation in response to either insulin or IGF-1. The data indicate that the hBVR-dependent modulation of GSK3 and FoxO3 is likely to be mediated through hBVR’s activation of Akt.
Figure 9.
sihBVR inhibits Akt1 activation and stimulates glucose uptake. A) sihBVR treatment inhibits Akt1 activation by insulin and IGF-1. Cells were transfected with siRNA against hBVR or with pcDNA-HA-hBVR, and treated with IGF-1 or insulin. Akt1 was immunoprecipitated from cell lysate and assayed for kinase activity with Aktide substrate. Expression levels of hBVR were verified by Western blot test, with GSK3β serving as loading control. B) sihBVR treatment stimulates glucose uptake. Cells were transfected with siRNA against hBVR or with pcDNA-HA-hBVR, and assayed for glucose uptake in presence or absence of insulin, as described in Materials and Methods.
In a related experiment, the effect of raising or lowering the cellular hBVR level on insulin-dependent glucose uptake was examined. Overexpression of hBVR had little effect (Fig. 9B), whereas ablation of hBVR with siRNA resulted in increased glucose uptake, as was observed in an earlier study (14). The role of hBVR in glucose uptake is complex because it appears to affect pathways that stimulate glucose uptake as well as those that are inhibitory, such as IRS-1.
DISCUSSION
The primary and secondary structural features hBVR result in its playing multiple functions in the insulin/IGF-1/PI3K/MAPK signaling network, while Akts mediate a number of downstream events of the same pathway. Because of similarities in regulation and effector functions of hBVR and Akt1, as well as the presence of a predicted interaction site and convergence on their ultimate target of activation, HO-1, we hypothesize a regulatory link between hBVR and Akt1. Our findings identify hBVR as a coactivator and substrate for Akt, and as a binding partner for PDK1-Akt activation. Data point to hBVR functioning as an interactive protein, rather than a kinase, in the activation of Akt1. hBVR is an IRK-interactive protein; in addition to Y198 in the YMKM motif, 2 other tyrosines, Y228 in the YLSF motif and Y291 in the C-terminus of the protein, are directly phosphorylated by IRK (14). Although hBVR has tyrosine kinase activity, its targets do not include the noted tyrosine residues (14). Phosphorylated hBVR predictably would be a platform for recruitment of PI3K, PDK1, and Akt enzymes. The suggested mechanism is consistent with the finding that kinase competency of hBVR has no bearing on activation of Akts by PDK1. Taken together, these data indicate formation of a complex involving PDK1, hBVR, and Akts that is likely a significant contributor to activation of Akt in the IGF-1/insulin signaling cascade. It is noteworthy that the hBVR fragment that activates IRK intracellular kinase domain contains Y291 (15).
Finding that hBVR markedly increased activity of T308-activated/phosphorylated Akt1 in vitro suggests hBVR involvement in S473 phosphorylation. Because kinase competency of hBVR is not relevant to Akt1 phosphorylation, protein–protein interaction is the only other mechanism that can be considered responsible for the increased S473 phosphorylation in the presence of hBVR. The question is, by what mechanism? As noted above, phosphorylation of 2 key residues, T308 in the activation loop of Akt1 and S473 in its C-terminal hydrophobic motif, are required for maximal activation. T308 is the known target of PDK1 (34). Autophosphorylation is one of the mechanisms that have been suggested to be involved in phosphorylation of S473 (40, 41). Two possibilities are considered to explain the increased S473 phosphorylation: first, protein–protein interaction causes conformational changes in Akt1 to stimulate autophosphorylation of S473; and/or second, the interaction facilitates accessibility of the serine residue to other kinases. In the cell, these possibilities are not mutually exclusive. However, the finding that in vitro in the presence of hBVR and absence of any other kinase there was an impressive (4- to 5-fold) enhancement of Akt1 kinase activity (Fig. 4C) is supportive of the first possibility for in vitro activity. It is noted that further stimulation of already activated Akt is not unprecedented (59).
Akt-hBVR interaction involves specific structural features of the proteins. Data shown in Fig. 6A, obtained using hBVR-based peptides with a core sequence of RXRXXSF, corresponding to the canonical consensus Akt-binding motif, indicate that 225RNRYLSF sequence of hBVR is an Akt-interactive site. At this point, the possibility of the presence of additional Akt1 interactive sites in the hBVR sequence cannot be ruled out. Furthermore, FRET analysis of hBVR interaction with full-length Akt1 and the isolated PH domain are supportive of protein–protein interaction in the cell. It is noted that the N-terminal 250 residues of Akt1, which are upstream of T308 and S473, include the PH domain (aa 1–113). Therefore, the finding that FRET analysis was not influenced by the presence or absence of IGF-1 was an expected event if the PH domain were the site of interaction with hBVR. Moreover, data obtained with Akt2 indicate that this domain of Akts is the hBVR interactive site, independent of the isozyme.
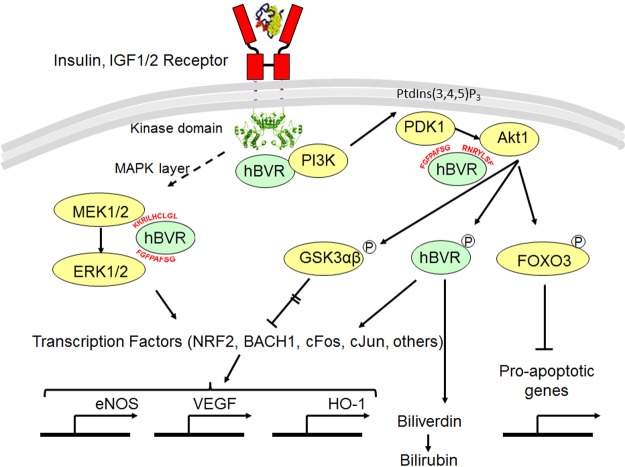
The functions of hBVR in the insulin signaling pathway are shown in Fig. 10. Activation of IRK and of the MAPK arm of the signaling pathway have been described elsewhere (14, 62), as has the IRK-BVR stimulation of PI3K, leading to the synthesis of phosphatidyl inositol 3,4,5-triphosphate (PtdIns(3,4,5)P3) (43, 72). PtdIns(3,4,5)P3 recruits the PH domain proteins PDK1 and Akt1 to the membrane. Findings of experiments that analyzed interactions of PDK1/Akt1/hBVR suggest a second process by which hBVR modulates Akt1 activity in the cell. Specifically, in addition to the direct binding of hBVR and Akt, the in-cell data shown in Fig. 7B indicate formation of a complex with Akt and PDK1. By analogy with previous observation of a MEK1/ERK2/hBVR complex (62), it is likely that hBVR facilitates interaction of Akt with PDK1 and that the hBVR hydrophobic motif 161RFGFPAFS is involved in binding and coimmunoprecipitation of PDK1 and hBVR (Fig. 7C). The consensus motif for high-affinity PDK1 binding site RxxFPxFS is also present in PKCζ and PKCδ (73). It is noteworthy that the hBVR hydrophobic motif was also identified by the Swiss-Prot motif search as a high probability PDK1 binding site, although, as with Akt1 and -2, the possibility of additional protein–protein interaction sites cannot be ruled out. This study only examined the PDK1-hBVR interaction in the context of Akt1 activation. A second upstream activator of Akt is mTOR. It is reasonable to suggest formation of a complex of mTOR/hBVR/Akt, based on a study that found a functional BVR/mTOR interaction (74).
Figure 10.
Roles of hBVR in activating IRK/PI3K/Akt pathway. BVR binds directly to IRK, where it functions as platform for interaction with IRS substrates (14). Activation of MAPK pathway (MAPK layer) is multistep process that leads to activation of MEK1/2. Interaction of hBVR with MEK1/2 and ERK1/2 to enhance activation of MEK has been defined elsewhere (62). Activation of ERK1/2 results in activation of as many as 50 transcription factors, including those shown (92). IRK and BVR together activate PI3K (43, 72), source for PtdIns(3,4,5)P3, which interacts with PH domain of Akt and recruits inactive protein to membrane, where it undergoes conformational change and is phosphorylated at T308 by 3-phosphoinositide-dependent kinase PDK1 (34, 35). Specific sequences in hBVR that mediate interactions with proteins in signaling pathways are indicated. Akts phosphorylate GSK3α/β, resulting in its inactivation; inactive protein in turn cannot phosphorylate and inactivate transcription factors, enabling expression of genes including those encoding eNOS, VEGF, and HO-1 (42, 46–48). hBVR is also instrumental in activating HO-1 (47, 93); in its capacity as reductase, it also converts biliverdin to bilirubin (1). Akt phosphorylation of FoxO3 results in its sequestration in cytoplasm and thereby prevents transcription of proapoptotic genes (32).
Activation of Akt leads to transcription of genes whose products are involved in cytoprotection (Fig. 10). A second mechanism of cytoprotection by Akt is via its effect on the transcription factor FoxO3, which regulates the expression of proapototic genes (31). Phosphorylation of FoxO3 by Akt inactivates the transcription factor and promotes its export to the cytoplasm, where it is bound by the 14-3-3 proteins; the interaction masks the FoxO3 nuclear localization signal and is dependent on prior phosphorylation of FoxO3 by Akt (30, 75).
We did not investigate any aspect of Akt3; therefore, whether hBVR and Akt3 interact remains to be elucidated. However, specificity of Akt isoforms for a coactivator protein has been reported. For instance, all members of the proto-oncogene T-cell leukemia/lymphoma-1 (TCL1) family bind to and activate Akt1 and -2, whereas Akt3 is activated only by TCL1 (76). TCL oncogenes are small proteins, and their expression is limited to a specific subset of T and B cells (77). In contrast, hBVR expression is not restricted to any cell type, and it is expressed in all mammalian cells types and tissues, although at different levels. Therefore, hBVR may also modulate Akt3 kinase activity.
GSK3 isoforms (GSK3α/β) are multitasking kinases (78) that mediate a wide range of cellular functions (79). Akt1 activation will predictably influence the function of its downstream targets, which are several (41). The observed influence of hBVR on the Akt1/GSK3 pathway is consistent with the possibility of other cellular functions that are targets of Akt1 activity being affected by hBVR too. One such outcome could be Akt-mediated activation of modulators of the immune response and inflammation, such as the transcription factor NF-κB (80). This factor is a key regulator of the immune response and a promoter of inflammation (81, 82). NF-κB has a major input in tumorigenesis, particularly in those cancers that arise in response to inflammation (reviewed in refs. 83–85). Previous studies have demonstrated that hBVR mediates activation of NF-κB in cells treated with TNF-α (58, 86). In addition, a finding that directly links the contribution of hBVR to the Akt-dependent immune response and inflammation is the data shown in Fig. 8A, revealing a reciprocal relationship between hBVR and GSK3 activities. GSK3β has been implicated in activation of NF-κB (87, 88). The observation that hBVR associated with phosphorylated GSK3β raises a number of intriguing possibilities. These include hBVR binding mediating inactivation of GSK3, acting as a nuclear export transporter of GSK3, or being a means of sequestering GSK3 in the cytoplasm. The end result, however, would be the same: reduced GSK3-dependent phosphorylation of transcription factors and concomitant gene activation. It is also possible that by binding hBVR, phosphorylated GSK3 could dampen hBVR-dependent activation of its binding partners and thereby provide a feedback mechanism to prevent runaway activation.
With respect to activation of hBVR by Akt, it is likely that this is directly relevant to cellular defense mechanisms and complements Akt functions. The reductase’s activity is dependent on its phosphorylation status; the enzyme is the sole source of bilirubin production in physiologic conditions. Bilirubin is a most effective intracellular antioxidant and free radical quencher (4, 89). Additionally, hBVR is a transcription factor for the stress-responsive genes that regulate HO-1, the generator of biliverdin, the bilirubin precursor (47, 90, 91). Therefore, the link between transactivation of hBVR and Akt1 directly influences their roles in promoting cell survival and attenuation of cell death.
Acknowledgments
This work was supported by the U.S. National Institutes of Health, National Institute of Environmental Health Sciences (Grant ES004066) and funding from the University of Rochester Environmental Health Sciences Center (P30ES001247). The authors are particularly indebted to L. Callahan (Director of the Confocal and Conventional Microscopy Resource, University of Rochester) for her guidance and assistance with the in-cell FRET measurements. The authors also thank the University of Rochester Clinical and Translational Science Institute for purchase of the incubator chamber and Binder incubator, which facilitated these studies. Thanks are also due to S. Ghaemmeghami, K. Welle, and J. Hryhorenko (University of Rochester Proteomics Core Facility) for the mass spectrometry analyses.
Glossary
- BACH1
BTB and CNC homology 1, basic leucine zipper transcription factor 1
- BSA
bovine serum albumin
- BVR
biliverdin reductase A
- FBS
fetal bovine serum
- FoxO
forkhead box of the O class
- FRET
Förster resonance energy transfer
- GFP
green fluorescent protein
- GSH
glutathione
- GSK3
glycogen synthase kinase 3
- GST
glutathione S-transferase
- HA
influenza hemagglutinin epitope
- hBVR
human biliverdin reductase A
- HEK
human embryonic kidney
- HO
heme oxygenase
- IRK
insulin receptor kinase
- mTOR
mammalian target of rapamycin
- Ni-NTA
nickel–nitrilotriacetic acid
- PDK1
phosphatidylinositol-dependent kinase 1
- PH
pleckstrin homology
- PtdIns(3,4,5)P3
phosphatidyl inositol 3,4,5-triphosphate
- ROS
reactive oxygen species
- TCL
T-cell leukemia/lymphoma
- wt
wild-type
REFERENCES
- 1.Kutty R. K., Maines M. D. (1981) Purification and characterization of biliverdin reductase from rat liver. J. Biol. Chem. 256, 3956–3962 [PubMed] [Google Scholar]
- 2.Maines M. D., Trakshel G. M. (1993) Purification and characterization of human biliverdin reductase. Arch. Biochem. Biophys. 300, 320–326 [DOI] [PubMed] [Google Scholar]
- 3.Maines M. D., Trakshel G. M., Kutty R. K. (1986) Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 261, 411–419 [PubMed] [Google Scholar]
- 4.Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235, 1043–1046 [DOI] [PubMed] [Google Scholar]
- 5.Sedlak T. W., Snyder S. H. (2009) Cycling the wagons for biliverdin reductase. J. Biol. Chem. 284, le11; author reply le12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 80, 1895–1903 [DOI] [PubMed] [Google Scholar]
- 7.Vítek L. (2012) The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front. Pharmacol. 3, 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maines M. D., Gibbs P. E. M. (2013) Biliverdin reductase: its multiple functions in cell signaling and role in cytoprotection. In Handbook of Porphyrin Science (Ferreira G., Kadish K. M., Smith K. M., Guilard R., eds.), Vol. 26, pp. 380–441, World Scientific, Singapore: [Google Scholar]
- 9.O’Brien L., Hosick P. A., John K., Stec D. E., Hinds T. D. Jr (2015) Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 26, 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapitulnik J., Maines M. D. (2009) Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 30, 129–137 [DOI] [PubMed] [Google Scholar]
- 11.Gibbs P. E., Tudor C., Maines M. D. (2012) Biliverdin reductase: more than a namesake—the reductase, its peptide fragments, and biliverdin regulate activity of the three classes of protein kinase C. Front. Pharmacol. 3, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegiel B., Otterbein L. E. (2012) Go green: the anti-inflammatory effects of biliverdin reductase. Front. Pharmacol. 3, 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone E., Di Domenico F., Mancuso C., Butterfield D. A. (2014) The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: it’s time for reconciliation. Neurobiol. Dis. 62, 144–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerner-Marmarosh N., Shen J., Torno M. D., Kravets A., Hu Z., Maines M. D. (2005) Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 102, 7109–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs P. E., Lerner-Marmarosh N., Poulin A., Farah E., Maines M. D. (2014) Human biliverdin reductase–based peptides activate and inhibit glucose uptake through direct interaction with the kinase domain of insulin receptor. FASEB J. 28, 2478–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones P. F., Jakubowicz T., Pitossi F. J., Maurer F., Hemmings B. A. (1991) Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 88, 4171–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S. R., Brunet A., Greenberg M. E. (1999) Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 18.Yang Z. Z., Tschopp O., Baudry A., Dümmler B., Hynx D., Hemmings B. A. (2004) Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 32, 350–354 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez E., McGraw T. E. (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8, 2502–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Héron-Milhavet L., Franckhauser C., Rana V., Berthenet C., Fisher D., Hemmings B. A., Fernandez A., Lamb N. J. (2006) Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol. Cell. Biol. 26, 8267–8280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon R. L., Marcotte R., Hennessy B. T., Woodgett J. R., Mills G. B., Muller W. J. (2009) Akt1 and Akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 69, 5057–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodbeck D., Cron P., Hemmings B. A. (1999) A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J. Biol. Chem. 274, 9133–9136 [DOI] [PubMed] [Google Scholar]
- 23.Tschopp O., Yang Z. Z., Brodbeck D., Dummler B. A., Hemmings-Mieszczak M., Watanabe T., Michaelis T., Frahm J., Hemmings B. A. (2005) Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132, 2943–2954 [DOI] [PubMed] [Google Scholar]
- 24.Ali A., Hoeflich K. P., Woodgett J. R. (2001) Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 101, 2527–2540 [DOI] [PubMed] [Google Scholar]
- 25.Rayasam G. V., Tulasi V. K., Sodhi R., Davis J. A., Ray A. (2009) Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 156, 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar M., Rojo A. I., Velasco D., de Sagarra R. M., Cuadrado A. (2006) Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 281, 14841–14851 [DOI] [PubMed] [Google Scholar]
- 27.Khan A., Jamwal S., Bijjem K. R., Prakash A., Kumar P. (2015) Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid–induced neurotoxicity in rats. Neuroscience 287, 66–77 [DOI] [PubMed] [Google Scholar]
- 28.Shinohara M., Ybanez M. D., Win S., Than T. A., Jain S., Gaarde W. A., Han D., Kaplowitz N. (2010) Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J. Biol. Chem. 285, 8244–8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miralem T., Hu Z., Torno M. D., Lelli K. M., Maines M. D. (2005) Small interference RNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J. Biol. Chem. 280, 17084–17092 [DOI] [PubMed] [Google Scholar]
- 30.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 31.Dansen T. B., Burgering B. M. (2008) Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 18, 421–429 [DOI] [PubMed] [Google Scholar]
- 32.Singh A., Ye M., Bucur O., Zhu S., Tanya Santos M., Rabinovitz I., Wei W., Gao D., Hahn W. C., Khosravi-Far R. (2010) Protein phosphatase 2A reactivates FOXO3a through a dynamic interplay with 14-3-3 and AKT. Mol. Biol. Cell 21, 1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., Tissenbaum H. A., Ruvkun G. (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 [DOI] [PubMed] [Google Scholar]
- 34.Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G. F., Holmes A. B., Gaffney P. R., Reese C. B., McCormick F., Tempst P., Coadwell J., Hawkins P. T. (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279, 710–714 [DOI] [PubMed] [Google Scholar]
- 35.Najafov A., Shpiro N., Alessi D. R. (2012) Akt is efficiently activated by PIF-pocket- and PtdIns(3,4,5)P3-dependent mechanisms leading to resistance to PDK1 inhibitors. Biochem. J. 448, 285–295 [DOI] [PubMed] [Google Scholar]
- 36.Stokoe D., Stephens L. R., Copeland T., Gaffney P. R., Reese C. B., Painter G. F., Holmes A. B., McCormick F., Hawkins P. T. (1997) Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 [DOI] [PubMed] [Google Scholar]
- 37.Hanada M., Feng J., Hemmings B. A. (2004) Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim. Biophys. Acta 1697, 3–16 [DOI] [PubMed] [Google Scholar]
- 38.Hemmings B. A., Restuccia D. F. (2012) PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 4, a011189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen R., Kim O., Yang J., Sato K., Eisenmann K. M., McCarthy J., Chen H., Qiu Y. (2001) Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 276, 31858–31862 [DOI] [PubMed] [Google Scholar]
- 40.Kumar C. C., Madison V. (2005) AKT crystal structure and AKT-specific inhibitors. Oncogene 24, 7493–7501 [DOI] [PubMed] [Google Scholar]
- 41.Toker A., Marmiroli S. (2014) Signaling specificity in the Akt pathway in biology and disease. Adv. Biol. Regul. 55, 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pachori A. S., Smith A., McDonald P., Zhang L., Dzau V. J., Melo L. G. (2007) Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J. Mol. Cell. Cardiol. 43, 580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegiel B., Baty C. J., Gallo D., Csizmadia E., Scott J. R., Akhavan A., Chin B. Y., Kaczmarek E., Alam J., Bach F. H., Zuckerbraun B. S., Otterbein L. E. (2009) Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 284, 21369–21378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salim M., Brown-Kipphut B. A., Maines M. D. (2001) Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J. Biol. Chem. 276, 10929–10934 [DOI] [PubMed] [Google Scholar]
- 45.Gibbs P. E., Miralem T., Maines M. D. (2010) Characterization of the human biliverdin reductase gene structure and regulatory elements: promoter activity is enhanced by hypoxia and suppressed by TNF-alpha-activated NF-kappaB. FASEB J. 24, 3239–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Józkowicz A., Huk I., Nigisch A., Weigel G., Dietrich W., Motterlini R., Dulak J. (2003) Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid. Redox Signal. 5, 155–162 [DOI] [PubMed] [Google Scholar]
- 47.Kravets A., Hu Z., Miralem T., Torno M. D., Maines M. D. (2004) Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J. Biol. Chem. 279, 19916–19923 [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Chen Y., Sternberg P., Cai J. (2008) Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest. Ophthalmol. Vis. Sci. 49, 1671–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitamura T., Asai N., Enomoto A., Maeda K., Kato T., Ishida M., Jiang P., Watanabe T., Usukura J., Kondo T., Costantini F., Murohara T., Takahashi M. (2008) Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat. Cell Biol. 10, 329–337 [DOI] [PubMed] [Google Scholar]
- 50.Bitar M. S., Al-Mulla F. (2011) A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 301, E1119–E1129 [DOI] [PubMed] [Google Scholar]
- 51.Florczyk U., Jazwa A., Maleszewska M., Mendel M., Szade K., Kozakowska M., Grochot-Przeczek A., Viscardi M., Czauderna S., Bukowska-Strakova K., Kotlinowski J., Jozkowicz A., Loboda A., Dulak J. (2014) Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow–derived proangiogenic cells and hind limb ischemia. Antioxid. Redox Signal. 20, 1693–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes J. D., Chowdhry S., Dinkova-Kostova A. T., Sutherland C. (2015) Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 43, 611–620 [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann K., Baldinger J., Mayerhofer B., Atanasov A. G., Dirsch V. M., Heiss E. H. (2015) Activated AMPK boosts the Nrf2/HO-1 signaling axis—A role for the unfolded protein response. Free Radic. Biol. Med. 88(Pt B), 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coffer P. J., Jin J., Woodgett J. R. (1998) Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agamasu C., Ghanam R. H., Saad J. S. (2015) Structural and biophysical characterization of the interactions between calmodulin and the pleckstrin homology domain of Akt. J. Biol. Chem. 290, 27403–27413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kikuchi A., Park S. Y., Miyatake H., Sun D., Sato M., Yoshida T., Shiro Y. (2001) Crystal structure of rat biliverdin reductase. Nat. Struct. Biol. 8, 221–225 [DOI] [PubMed] [Google Scholar]
- 57.Whitby F. G., Phillips J. D., Hill C. P., McCoubrey W., Maines M. D. (2002) Crystal structure of a biliverdin IXalpha reductase enzyme–cofactor complex. J. Mol. Biol. 319, 1199–1210 [DOI] [PubMed] [Google Scholar]
- 58.Gibbs P. E., Miralem T., Lerner-Marmarosh N., Tudor C., Maines M. D. (2012) Formation of ternary complex of human biliverdin reductase-protein kinase Cδ-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-κB, and inducible nitric-oxidase synthase (iNOS). J. Biol. Chem. 287, 1066–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laine J., Künstle G., Obata T., Sha M., Noguchi M. (2000) The protooncogene TCL1 is an Akt kinase coactivator. Mol. Cell 6, 395–407 [DOI] [PubMed] [Google Scholar]
- 60.Brazil D. P., Park J., Hemmings B. A. (2002) PKB binding proteins. Getting in on the Akt. Cell 111, 293–303 [DOI] [PubMed] [Google Scholar]
- 61.Jacobs D., Glossip D., Xing H., Muslin A. J., Kornfeld K. (1999) Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 13, 163–175 [PMC free article] [PubMed] [Google Scholar]
- 62.Lerner-Marmarosh N., Miralem T., Gibbs P. E., Maines M. D. (2008) Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc. Natl. Acad. Sci. USA 105, 6870–6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maines, M. D., Polevoda, B. V., Huang, T. J., and McCoubrey, W. K., Jr. (1996) Human biliverdin IXalpha reductase is a zinc-metalloprotein. Characterization of purified and Escherichia coli expressed enzymes. Eur. J. Biochem. 235, 372–381 [DOI] [PubMed]
- 64.Lerner-Marmarosh N., Miralem T., Gibbs P. E., Maines M. D. (2007) Regulation of TNF-alpha-activated PKC-zeta signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB J. 21, 3949–3962 [DOI] [PubMed] [Google Scholar]
- 65.Maines M. D., Miralem T., Lerner-Marmarosh N., Shen J., Gibbs P. E. (2007) Human biliverdin reductase, a previously unknown activator of protein kinase C betaII. J. Biol. Chem. 282, 8110–8122 [DOI] [PubMed] [Google Scholar]
- 66.Doble B. W., Patel S., Wood G. A., Kockeritz L. K., Woodgett J. R. (2007) Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 12, 957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miralem T., Lerner-Marmarosh N., Gibbs P. E., Tudor C., Hagen F. K., Maines M. D. (2012) The human biliverdin reductase–based peptide fragments and biliverdin regulate protein kinase Cδ activity: the peptides are inhibitors or substrate for the protein kinase C. J. Biol. Chem. 287, 24698–24712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miralem T., Gibbs P. E., Revert F., Saus J., Maines M. D. (2010) Human biliverdin reductase suppresses Goodpasture antigen-binding protein (GPBP) kinase activity: the reductase regulates tumor necrosis factor-alpha-NF-kappaB-dependent GPBP expression. J. Biol. Chem. 285, 12551–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Periasamy A., Day R. N. (1998) Fret imaging of pit-1 protein interactions in living cells. J. Biomed. Opt. 3, 154–160 [DOI] [PubMed] [Google Scholar]
- 70.Periasamy A., Wallrabe H., Chen Y., Barroso M. (2008) Chapter 22: quantitation of protein–protein interactions: confocal FRET microscopy. Methods Cell Biol. 89, 569–598 [DOI] [PubMed] [Google Scholar]
- 71.Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 72.Maines M. D. (2007) Biliverdin reductase: PKC interaction at the cross-talk of MAPK and PI3K signaling pathways. Antioxid. Redox Signal. 9, 2187–2195 [DOI] [PubMed] [Google Scholar]
- 73.Parekh D. B., Ziegler W., Parker P. J. (2000) Multiple pathways control protein kinase C phosphorylation. EMBO J. 19, 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bisht K., Wegiel B., Tampe J., Neubauer O., Wagner K. H., Otterbein L. E., Bulmer A. C. (2014) Biliverdin modulates the expression of C5aR in response to endotoxin in part via mTOR signaling. Biochem. Biophys. Res. Commun. 449, 94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunet A., Kanai F., Stehn J., Xu J., Sarbassova D., Frangioni J. V., Dalal S. N., DeCaprio J. A., Greenberg M. E., Yaffe M. B. (2002) 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 156, 817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laine J., Künstle G., Obata T., Noguchi M. (2002) Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family. J. Biol. Chem. 277, 3743–3751 [DOI] [PubMed] [Google Scholar]
- 77.Virgilio L., Narducci M. G., Isobe M., Billips L. G., Cooper M. D., Croce C. M., Russo G. (1994) Identification of the TCL1 gene involved in T-cell malignancies. Proc. Natl. Acad. Sci. USA 91, 12530–12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doble B. W., Woodgett J. R. (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu C., Kim N. G., Gumbiner B. M. (2009) Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 8, 4032–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bai D., Ueno L., Vogt P. K. (2009) Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 125, 2863–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wijayanti N., Huber S., Samoylenko A., Kietzmann T., Immenschuh S. (2004) Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid. Redox Signal. 6, 802–810 [DOI] [PubMed] [Google Scholar]
- 82.Naidu S., Wijayanti N., Santoso S., Kietzmann T., Immenschuh S. (2008) An atypical NF-kappa B-regulated pathway mediates phorbol ester–dependent heme oxygenase-1 gene activation in monocytes. J. Immunol. 181, 4113–4123 [DOI] [PubMed] [Google Scholar]
- 83.Karin M. (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441, 431–436 [DOI] [PubMed] [Google Scholar]
- 84.Grivennikov S. I., Greten F. R., Karin M. (2010) Immunity, inflammation, and cancer. Cell 140, 883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben-Neriah Y., Karin M. (2011) Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 12, 715–723 [DOI] [PubMed] [Google Scholar]
- 86.Gibbs P. E., Maines M. D. (2007) Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase. Int. J. Cancer 121, 2567–2574 [DOI] [PubMed] [Google Scholar]
- 87.Hoeflich K. P., Luo J., Rubie E. A., Tsao M. S., Jin O., Woodgett J. R. (2000) Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406, 86–90 [DOI] [PubMed] [Google Scholar]
- 88.Zhang J. S., Herreros-Villanueva M., Koenig A., Deng Z., de Narvajas A. A., Gomez T. S., Meng X., Bujanda L., Ellenrieder V., Li X. K., Kaufmann S. H., Billadeau D. D. (2014) Differential activity of GSK-3 isoforms regulates NF-κB and TRAIL- or TNFα induced apoptosis in pancreatic cancer cells. Cell Death Dis. 5, e1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stocker R. (2004) Antioxidant activities of bile pigments. Antioxid. Redox Signal. 6, 841–849 [DOI] [PubMed] [Google Scholar]
- 90.Shibahara S., Müller R. M., Taguchi H. (1987) Transcriptional control of rat heme oxygenase by heat shock. J. Biol. Chem. 262, 12889–12892 [PubMed] [Google Scholar]
- 91.Keyse S. M., Tyrrell R. M. (1989) Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA 86, 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoon S., Seger R. (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24, 21–44 [DOI] [PubMed] [Google Scholar]
- 93.Tudor C., Lerner-Marmarosh N., Engelborghs Y., Gibbs P. E., Maines M. D. (2008) Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem. J. 413, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]