Abstract
The bioactive sphingolipid sphingosine-1-phosphate (S1P) and the kinase that produces it have been implicated in inflammatory bowel diseases in mice and humans; however, little is known about the role of the 2 S1P-specific phosphohydrolase isoforms, SGPP1 and SGPP2, which catalyze dephosphorylation of S1P to sphingosine. To elucidate their functions, we generated specific knockout mice. Deletion of Sgpp2, which is mainly expressed in the gastrointestinal tract, significantly reduced dextran sodium sulfate (DSS)–induced colitis severity, whereas deletion of ubiquitously expressed Sgpp1 slightly worsened colitis. Moreover, Sgpp1 deletion enhanced expression of multifunctional proinflammatory cytokines, IL-6, TNF-α, and IL-1β, activation of the transcription factor signal transducer and activator of transcription 3, and immune cell infiltration into the colon. Conversely, Sgpp2-null mice failed to mount a DSS-induced systemic inflammatory response. Of interest, Sgpp2 deficiency suppressed DSS-induced intestinal epithelial cell apoptosis and improved mucosal barrier integrity. Furthermore, down-regulation of Sgpp2 attenuated LPS-induced paracellular permeability in cultured cells and enhanced expression of the adherens junction protein E-cadherin. Finally, in patients with ulcerative colitis, SGPP2 expression was elevated in colitis tissues relative to that in uninvolved tissues. These results indicate that induction of SGPP2 expression contributes to the pathogenesis of colitis by promoting disruption of the mucosal barrier function. SGPP2 may represent a novel therapeutic target in inflammatory bowel disease.—Huang, W.-C., Liang, J., Nagahashi, M., Avni, D., Yamada, A., Maceyka, M., Wolen, A. R., Kordula, T., Milstien, S., Takabe, K., Oravecz, T., Spiegel, S. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans.
Keywords: SGPP1, SGPP2, S1P, inflammatory bowel disease
Inflammatory bowel diseases (IBDs), such as ulcerative colitis (UC), are multifactorial inflammatory disorders of the gastrointestinal tract that affect several million people worldwide (1). Patients with IBD suffer from recurrent episodes of abdominal pain and severe diarrhea caused by chronic colonic inflammation. In addition to worsened quality of life, this refractory intestinal mucosal inflammation also increases the risk of developing colorectal cancer (2). The underlying cause of IBD involves injury of intestinal epithelial cells (IECs) with disruption of mucosal barrier function, and subsequent inflammation induced by bacterial invasion. The pathogenesis of IBD involves complex inter-relationships of various genetic and environmental factors, which make its treatment a great challenge (3, 4). Despite improved IBD therapy, including the use of anti-TNF and immunosuppressive drugs, not all patients respond, and many patients still experience insufficient improvement (5). A better understanding of the pathogenesis of IBD is needed to develop safer and more effective alternatives for long-term treatment of this disease.
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite that is present at high levels in the circulation and functions in innate and adaptive immunity (6). Previous preclinical studies have demonstrated that S1P and one of the kinases that produce it, sphingosine kinase 1 (SphK1), play important roles in colitis development in mice (7–9). Expression of SphK1 in the colon and circulating levels of S1P are increased by dextran sodium sulfate (DSS)–induced colitis in mice (7–9). Indeed, pharmacologic inhibition or genetic knockout of SphK1 attenuated experimental murine colitis (7, 9, 10). Of importance, expression of SphK1 is elevated in the colons of patients with UC (7, 11). Furthermore, expression of one of the S1P receptors, S1P receptor 1 (S1PR1), was greatly elevated in colonic mucosa during acute colitis in mice (8, 11). In agreement, FTY720/fingolimod, which is used for treatment of multiple sclerosis, and several other functional antagonists of S1PR1 as well as the competitive S1PR1 antagonist W146, which all attenuate S1P signaling via this receptor, reduce severity of colitis in several murine models (8, 12–16). It has also recently been shown that deletion of S1P lyase, which irreversibly degrades S1P, in gut epithelium enhanced colitis severity and inflammation after DSS treatment (11); however, S1P levels are regulated not only by synthesis and irreversible degradation but also by dephosphorylation. Intracellularly, S1P is dephosphorylated back to sphingosine by 2 specific, endoplasmic reticulum (ER) localized, S1P phosphatases (SGPP1 and SGPP2) (17). Extracellularly, S1P can also be degraded by 3 broad-specificity lipid phosphate phosphatases LPP1, LPP2, and LPP3, whose deletion blocked thymic egress of lymphocytes (18). Nevertheless, only a few studies have examined the functions of SGPP1 and SGPP2 in cultured cells. For example, overexpression of Sgpp1 decreased S1P, increased ceramide, and regulated apoptosis and ER-to-Golgi ceramide trafficking (19, 20). Conversely, silencing Sgpp1 increased S1P-induced resistance to apoptosis (21) and increased autophagy (22) and cell migration (23). This phosphatase also regulates resistance artery tone (24). In contrast, down-regulation of endothelial Sgpp2, an NF-κB–inducible gene, reduced TNF-α–induced IL-1β expression (25). Little is still known about the physiologic functions of these S1P phosphatases, although it was reported that deletion of Sgpp1 causes epidermal skin defects with abnormal keratinocyte differentiation (26). Because of the importance of S1P in colitis, and the predominant expression of Sgpp2 in the gastrointestinal tract, we sought to investigate the roles of these phosphatases in colitis.
MATERIALS AND METHODS
Mice generation
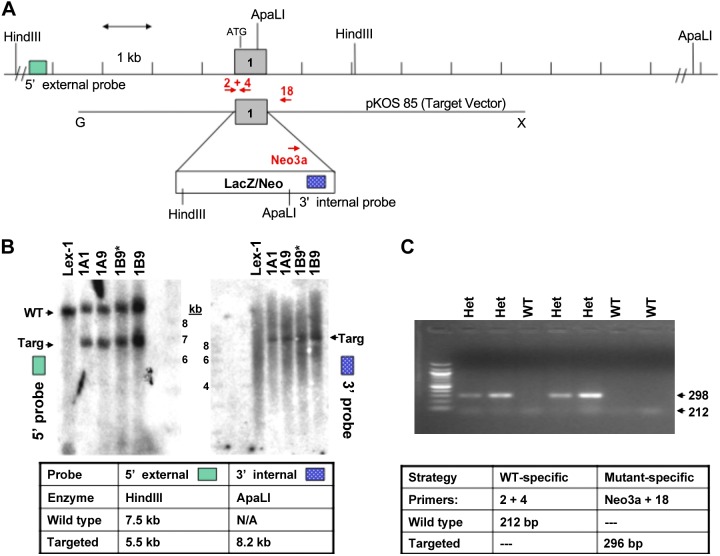
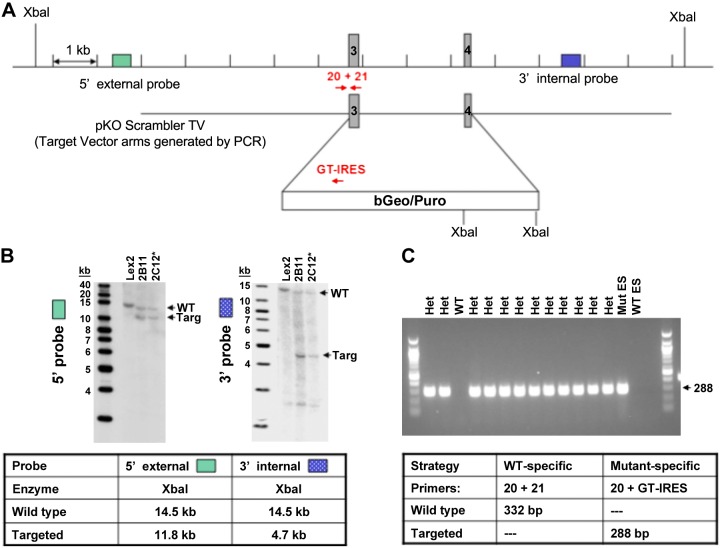
Sgpp1−/− and Sgpp2−/− mice were generated from embryonic stem cells by homologous recombination replacing exon 1 of Sgpp1 (Lexicon UTT074A; Genbank accession number AF247177) and exons 3 and 4 of Sgpp2 (Lexicon UTT074B; Genbank accession number NT039173) with LacZ/Neo or β-Geo/Puro selection cassettes, respectively (Figs. 1A and 2A) (Lexicon Pharmaceuticals, The Woodlands, TX, USA). Southern hybridization (Figs. 1B and 2B) and genomic PCR (Figs. 1C and 2C) analysis confirmed the targeted mutation in embryonic stem cells and mice, respectively. Mice on a C57/129sv background were maintained by heterozygous mating. Mice were genotyped 1–2 wk after birth by Southern hybridization and PCR of tail-snip DNA by using primers shown in Table 1. Mice (6–8 wk old) were used for all experiments, with wild-type (WT) littermates used as controls for gene-disrupted animals. All animal procedures were performed as approved by the Institutional Animal Care and Use Committee at Lexicon Pharmaceuticals or Virginia Commonwealth University.
Figure 1.
Generation of Sgpp1-knockout mice. A) The first exon of Sgpp1 was targeted for deletion by using a LacA/Neo selection cassette that contained an internal 3′ probe and ApaLI and HinDII restriction sites. B) Genomic DNA of parental (Lex-1) and transfected embryonic stem (ES) cells was analyzed by digestion with either (left) HinDIII or (right) ApaLI. When probed by Southern hybridization to a site 5′ of the insertion site, WT (Lex-1) generated only the expected fragment of 7.2 kb (WT), wheras ES that contained the insertion had this fragment as well as a shorter, 5.5 kb fragment (Targ) as a result of the inserted HinDIII site (left). Similarly, when genomic DNA was reprobed with a probe to an inserted sequence, a band was only found in the heterozygotes (Targ; right). C) Dual genomic PCR with the indicated primers demonstrated that only heterozygotes generated a fragment with insertion-specific primers. Het, heterozygote; Targ, target. *ES clone injected into embryo to generate knockout mice.
Figure 2.
Generation of Sgpp2-knockout mice. The third and fourth exons of Sgpp2 were targeted for deletion by using a β-Geo/Puro selection cassette that contained an internal pair of XbaI sites. A) Genomic DNA was analyzed by digestion with XbaI. B) When probed by Southern hybridization to a site 5′ of the insertion site but inside 2 flanking, genomic XbaI sites (left), parental embryonic stem (ES) cells (WT) generated the expected fragment of 14.5 kb, whereas heterozygotes had a shorter, 11.8 kb fragment as a result of the inserted XbaI sites. Similarly, when genomic DNA was reprobed with a probe 3′ to the inserted sequence but inside 2 flanking, genomic XbaI sites (right), parental ES cells (WT) again showed the 14.5 kb band, whereas the heterozygous, insertion-containing ES cells showed a shorter band as a result of the inserted XbaI sites. C) By using an insertion-specific probe, only heterozygous, insertion-containing animals generated a PCR fragment of the expected size, similar to that seen in the parental, insertion-containing ES (Mut ES) from which they were derived, whereas parental WT ES cells did not. Het, heterozygous; Targ, target. *ES clone injected into embryo to generate knockout mice.
TABLE 1.
Primer probes for Sgpp1 and Sgpp2 genotyping
| Type | Primers (5′–3′) |
|---|---|
| Southern probes for Sgpp1 deletion | GATTCCTCTCTTAATGGAGGC |
| GGGAATTGTCTGCTAGGTAC | |
| Neo2: CCTCAGAAGAACTCGTCAAG | |
| Neo5: GGCAGCGCGGCTATCGTG | |
| PCR genotyping for Sgpp1 deletion | ACTACCTGTTCTGCCTAGGC |
| GTTGTAGAAGACCTCCAGCT | |
| TCTAGAACTCTGACAGGACC | |
| Neo3a: GCAGCGCATCGCCTTCTATC | |
| Southern probes for Sgpp2 deletion | CTGTCATCAACCCAAACCCTAC |
| GCCATAAAAGCAAAAATCCAGG | |
| TTTCTTATCACCTTGGGTCACT | |
| CCAGCTCTATGCTCTGCAATTC | |
| PCR genotyping for Sgpp2 deletion | CCGTTGTGAGACTTGAAAAGAG |
| TCAGATTTGGGTGCTAAGTT | |
| GT-IRES: CCCTAGGAATGCTCGTCAAGA |
Induction and assessment of colitis
Acute colitis was induced with 5% DSS in drinking water for 5 d followed by 5 d of regular drinking water. The clinical course was followed daily by measurement of body weight and monitoring signs of rectal bleeding and diarrhea, which were scored as previously described (27). In brief, scoring for rectal bleeding was as follows: 0, no blood (Hemoccult; Beckman Coulter, Indianapolis, IN, USA); 2, positive Hemoccult; and 4, gross bleeding. Diarrhea scoring was as follows: 0, well-formed pellets; 2, pasty and semiformed stools that did not adhere to the anus; and 4, liquid stools that adhere to the anus. At d 10, mice were euthanized and colons were removed, photographed, and flushed with PBS. Portions of distal colon were either fixed with 10% neutral-buffered formalin for histologic analysis or snap-frozen with liquid nitrogen for Western blot analysis and quantitative PCR. Histologic assessments of colitis and severity scores were made in a double-blinded manner after hematoxylin and eosin staining as described (28). In brief, scoring for inflammation severity was as follows: 0, none; 1, mild; 2, moderate; and 3, severe. Scoring for inflammation extent was as follows: 0, none; 1, mucosa; 2, mucosa and submucosa; and 3, transmural. Crypt damage scoring was as follows: 0, none; 1, basal 1/3 damaged; 2, basal 2/3 damaged; 3, crypts lost; and 4, crypts and surface epithelium lost. Total colitis score was as follows: the sum of the 3 subscores.
S1P phosphatase assay
Colons were homogenized in buffer that contained 100 mM HEPES (pH 7.5), 10 mM EDTA, 1 mM DTT, and a cocktail of protease inhibitors. Lysates (2 mg protein) were incubated for 30 and 60 min at 37°C with 1.8 nmol of d17:1 S1P (Avanti Polar Lipids, Alabaster, AL, USA) as substrate for in vitro S1P phosphatase assay as described previously (29). Reactions were stopped by addition of 1 ml methanol and lipids were extracted after adding d20:1 sphingosine as internal standard. d17:1 sphingosine was quantified by liquid chromatography–electrospray ionization–tandem mass spectrometry (4000 QTrap; AB Sciex, Framingham, MA, USA) (8). Product formation was nearly linear with time ≤1 h and was proportional to amount of cell lysate (data not shown).
Quantification of sphingolipids
Lipids were extracted from serum and IECs and levels of sphingolipids were quantified by liquid chromatography–electrospray ionization–tandem mass spectrometry as described previously (8).
Complete blood count
Blood was withdrawn by cardiac puncture with a 25-gauge needle and immediately transferred into an EDTA tube. Complete blood counts were determined by Virginia Commonwealth University Health System Department of Pathology.
Western blotting
Frozen colon pieces were powdered under liquid nitrogen by mortar and pestle and lysis buffer was added that contained 50 mM Tris (pH 7.4), 100 mM NaCl, 0.5% NP40, 50 mM NaF, 1 mM DTT, 0.2 mM PMSF, and protease and phosphatase inhibitors cocktail (Sigma-Aldrich, St. Louis, MO, USA). Lysates were sonicated and centrifuged at 12,000 g at 4°C for 10 min. Supernatants were collected, protein concentration was determined, and aliquots were boiled in sample buffer for 5 min. Equal amounts of proteins were subjected to SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). Immunoblotting was performed with primary antibodies: p-signal transducer and activator of transcription 3 (STAT3; Tyr705), STAT3 (Cell Signaling Technology, Danvers, MA, USA); Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and glyceraldehyde 3-phosphate dehydrogenase as a loading control. Appropriate horseradish peroxidase–conjugated secondary antibodies were used (1:5000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), followed by enhanced chemiluminescence.
Immunohistochemistry
Paraffin-embedded slides were deparaffinized and antigen unmasking was carried out by microwave heating in citrate buffer for 20 min. Slides were incubated with 3% H2O2, washed, and then incubated with goat or horse serum (Dako, Carpinteria, CA, USA) for 30 min at room temperature. After washing with PBS, slides were incubated with primary antibodies. Biotinylated secondary antibodies were added and incubated at room temperature for 20 min. After 5 min with streptavidin–horseradish peroxidase, sections were stained with 3,3′-diaminobenzidine substrate and counterstained with hematoxylin. Apoptosis was determined by TUNEL assay using the ApopTag Peroxidase In Situ Apoptosis Detection Kit S7100 (EMD Millipore, Billerica, MA, USA). Slides were examined with a Zeiss Axioimager A1 and images were captured with an AxioCam MRc camera (Zeiss, Thornwood, NY, USA). The number of apoptotic cells was counted in 4–6 randomly selected fields at ×100 magnification.
Quantitative PCR
Total RNA was isolated by using Trizol (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA) and cDNA was synthesized by using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Amplification of cDNA was performed in triplicate with premixed primer probe sets and TaqMan master mix (Applied Biosystems) by using an ABI 7900HT. Gene expression levels were calculated by using the ΔΔCt method and were normalized to Gapdh expression.
ELISA
Supernatants from ex vivo colon cultures were used for ELISA determinations of TNF-α, IL-1β, IL-6, and IL-10 (BD Biosciences, San Jose, CA, USA) as described in Liang et al. (8).
Isolation of IECs
IECs were isolated essentially as previously described (30). In brief, dissected mouse colons were cut open longitudinally, washed, cut into small pieces, and incubated at 37°C for 30 min in 5 ml (per colon) of calcium- and magnesium-free HBSS (Thermo Fisher Scientific Life Sciences) that contained 10 mM DTT and 5 mM EDTA. Tubes were then vigorously shaken for 2 min to dislodge IECs. Cell suspensions were passed through a 100-μm cell strainer (BD Biosciences) and cells were pelleted at room temperature at 400 g for 5 min.
Colon epithelial barrier integrity analysis
To evaluate mucosal integrity, 300 μl FITC-dextran (2 mg/ml in saline; average MW 4400; Sigma-Aldrich) was administered orally 1 d after DSS treatment, and serum levels were measured 4 h later by fluorescence (31).
Paracellular tracer flux assay
Caco-2 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in EMEM that contained 20% fetal bovine serum. For the paracelllular tracer flux assay, Caco-2 cells (0.3 × 105 cells/insert) were seeded on 0.3 cm2 high-pore density polyethylene terephthalate membrane transwell inserts with 0.4-μm pores (Falcon; BD Biosciences), placed in 24-well plates, and transfected with siControl or SmartPool On-TargetPlus siSgpp2 (Dharmacon, Lafayette, CO, USA) using Lipofectamine RNAiMax (Thermo Fisher Scientific Life Sciences). Cell monolayers were treated with 1 μg/ml LPS for 24 h (32). FITC-dextran was then added to the apical compartment for 4 h (final concentration 6 μg/ml) (33). Paracellular flux was determined by measuring the fluorescence intensity in the basolateral compartment with a fluorometer (Victor ×4; PerkinElmer, Waltham, MA, USA) at excitation and emission wavelengths of 485 and 535 nm, respectively.
Immunofluorescence staining of E-cadherin
Caco-2 cells that were cultured on coverslips were transfected with siControl or siSgpp2 and then treated with vehicle or LPS (1 μg/ml) for 24 h, fixed in 3% paraformaldehyde for 10 min, quenched with 10 mM glycine in PBS for 10 min, and then incubated with E-cadherin antibody (1:100; ab1416; Abcam, Cambridge, MA, USA) for 20 min at room temperature. Cells were washed 3 times and incubated with secondary antibody conjugated with Alexa Fluor 555 (1:400) for 20 min at room temperature. Coverslips were washed and mounted on slides with 10 mM n-propylgallate in 100% glycerol. Images were collected with a Zeiss Axiovert fluorescence microscope at ×400 at the same microscope and camera settings.
SGPP2 gene expression in public databases
We used Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) data sets GSE22619 (34) and GSE11223 (35) to analyze the association of SGPP2 gene expression in inflamed sigmoid colons compared with uninflamed healthy individuals. All expression data was analyzed by using R core and Bioconductor (36). In the GSE22619 data set, microarray expression data is from sigmoid colon biopsies from 10 patients with UC and their healthy monozygotic twins without UC (discordant twin pairs). Expression profiles were measured by using Affymetrix Human Genome U133 Plus 2.0 platform (GPL570; http://www.affymetrix.com). We obtained raw CEL files from Gene Expression Omnibus and used the affy package (37) to analyze probe-level data. The MAS 5.0 absolute detection method was used to identify absent or low-intensity probe-sets. Of 54,675 probe sets included on the Human Genome U133 Plus 2.0 platform, we only considered the 19,053 that could be reliably detected in 50% of the GSE22619 samples. Probe set summaries were then generated by using the gcrma package. SGPP2 expression was examined by using 2 independent probe sets (238567_at; 244780_at), and statistical significance was determined by paired Student’s t test. The GSE11223 data set includes Agilent microarray data from analyses of inflamed colon epithelial biopsies of 57 patients with UC from different anatomic locations of the gastrointestinal tract and 27 uninflamed normal participants. These samples were run on the Agilent-012391 Whole Human Genome Oligo Microarray G4112A (GPL1708; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL1708), and SGPP2 expression levels in sigmoid colons were assayed by using probe A_23_P153971 and statistical significance was determined by unpaired Student’s t test. Microarray probes were mapped to genes by using the latest annotation data from the National Center for Biotechnology Information (Bethesda, MD, USA) (GPL570: ftp://ftp.ncbi.nlm.nih.gov/geo/platforms/GPLnnn/GPL570/annot/GPL570.annot.gz; GPL1708: ftp://ftp.ncbi.nlm.nih.gov/geo/platforms/GPL1nnn/GPL1708/annot/GPL1708.annot.gz).
Statistical analysis
Statistical analysis was performed by using unpaired 2-tailed Student’s t test for comparison of 2 groups and by 1-way ANOVA with Bonferroni post hoc comparison for experiments that consisted of ≥3 groups (Prism; GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered significant. Experiments were repeated at least 3 times with consistent results. For animal studies, measurements were blind with respect to group assignments.
RESULTS
Deletion of Sgpp2, but not Sgpp1, provides resistance to DSS-induced colitis
To knock out Sgpp1 and Sgpp2 expression in mice, gene-targeting vectors that replaced exon 1 of Sgpp1 (Fig. 1A) and exon 3 and 4 of Sgpp2 (Fig. 2A) with LacZ/Neo or β-Geo/Puro selection cassettes, respectively, were prepared and transfected into embryonic stem cells. Southern hybridization analysis confirmed the targeted mutation in embryonic stem cells (Figs. 1B and 2B) and was verified by PCR on genomic DNA from offspring (Figs. 1C and 2C). Although it has previously been reported that Sgpp1 deficiency in C57Bl/6 background results in neonatal lethality and skin abnormalities in newborn pups (26), no major phenotypes were noted in our Sgpp1-knockout mice on a C57/129sv mixed background. Moreover, heterozygote Sgpp1 and Sgpp2 mice were viable and fertile and their mating generated pups with genotype ratios with normal Mendelian frequencies (1:2:1), which indicated that neither SGPP1 nor SGPP2 are essential for neonatal viability. Deletion of Sgpp1 also did not influence expression of Sgpp2 and vice versa.
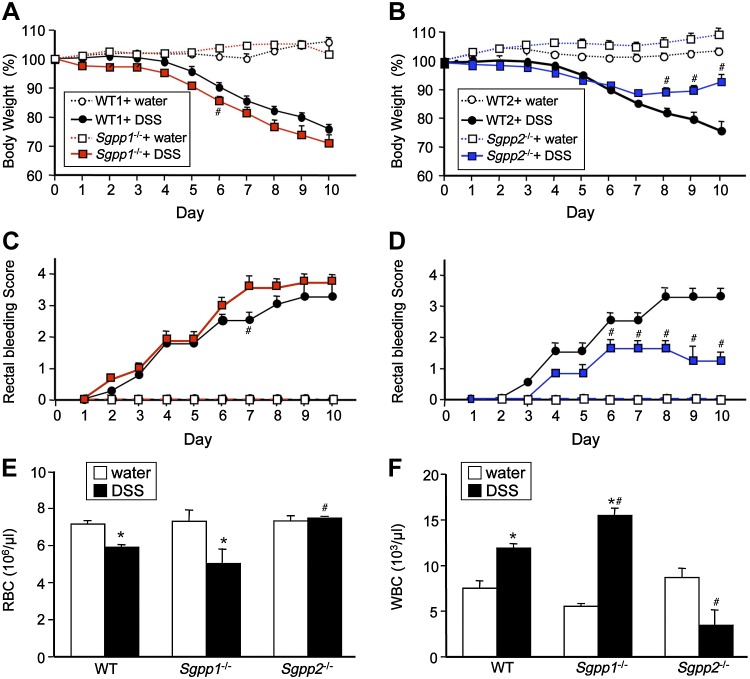
We were intrigued by the observation that, in contrast to Sgpp1, which is expressed in most mouse tissues, Sgpp2 is mainly expressed in the colon and stomach (26). Similarly, Sgpp2 was mainly expressed in stomach and large and small intestines, whereas Sgpp1 was ubiquitously expressed, as assessed by BioGPS (Scripps Research Institute, La Jolla, CA, USA). This led us to investigate the roles of these 2 isoenzymes in colitis induced by the luminal irritant, DSS, a murine model of intestinal inflammation. DSS was administered to Sgpp1−/− and Sgpp2−/− mice and their corresponding WT littermates in drinking water for 5 d followed by 5 d of plain water. As expected, DSS treatment of WT mice induced loss of body weight and decreased stool consistency with increased rectal bleeding scores (Fig. 3A–D). Although Sgpp1−/− mice showed slightly worse body weight loss, bloody stool, and diarrhea than their WT littermates (Fig. 3A, C and data not shown), surprisingly, these responses were greatly suppressed in Sgpp2−/− mice compared with WT mice (Fig. 3B, D). Moreover, DSS decreased red blood cells in the blood of WT mice and even more so in Sgpp1−/− mice, whereas circulating red blood cells of Sgpp2−/− mice were not significantly altered by DSS treatment (Fig. 3E). Similarly, after DSS treatment, there were also larger increases in circulating leukocytes in Sgpp1−/− mice than in WT mice. In sharp contrast to WT mice, circulating white blood cells were not increased in Sgpp2−/− mice after DSS treatment (Fig. 3F).
Figure 3.
DSS-induced colitis is greatly attenuated by deletion of Sgpp2. Acute colitis was induced in Sgpp1−/− and Sgpp2−/− mice and their corresponding WT littermates with 5% DSS in drinking water for 5 d followed by recovery for 5 d on normal drinking water. A–D) Changes in body weight (A, B) and blood stool scores (C, D) were determined; n = 10 mice per group. E, F) Circulating red blood cells (RBCs) (E) and white blood cells (WBCs) (F) were measured on d 10 in blood from Sgpp1−/− and Sgpp2−/− mice and their corresponding WT littermates that were treated without or with DSS. Data represent means ± sd. *P < 0.05 compared with water-treated mice. #P < 0.05 compared with WT + DSS group.
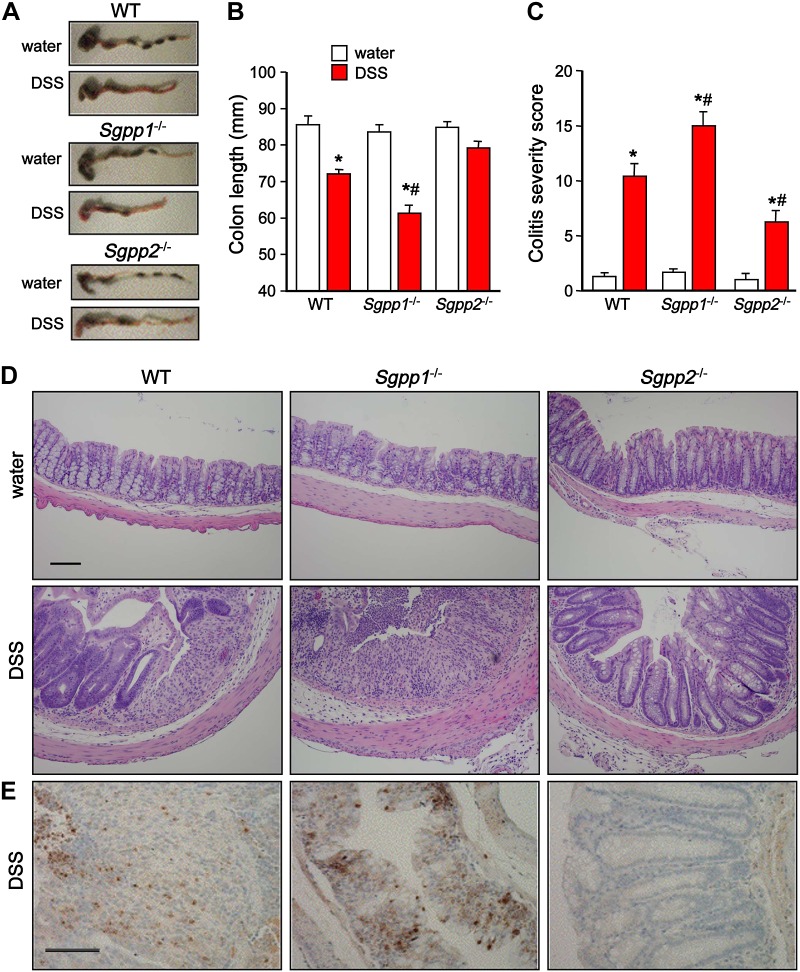
In agreement, whereas colon shortening in Sgpp1−/− mice was more severe compared with WT mice (Fig. 4A, B), DSS treatment did not cause significant colon shortening in Sgpp2−/− mice (Fig. 4A, B). Moreover, histopathologic analysis revealed severe epithelial erosion, areas of mucosal ulceration, and increased numbers of infiltrating mucosal and submucosal leukocytes and neutrophils in colons from DSS-treated WT or Sgpp1−/− mice, which is consistent with the pathologic assessment of colitis severity scores (Fig. 4C–E). In sharp contrast, colonic mucosa in Sgpp2−/− mice seemed relatively undamaged after DSS treatment, with minimal loss of crypt structures and epithelial cell denudation, smaller areas of ulceration, and reduced infiltration of inflammatory cells (Fig. 4C–E).
Figure 4.
Colitis severity is significantly reduced in Sgpp2−/− mice but increased in Sgpp1−/− mice. Acute colitis was induced with 5% DSS as described in Fig 3. A, B) Colon lengths were measured on day 10 in mice that were treated without or with DSS. Representative images (A) and quantification (B); n = 10 mice per group. C, D) Mucosal histology was examined by hematoxylin and eosin staining (D), and colitis severity scores (C) were determined in a double-blind manner. E) Neutrophils in colon sections were immunostained with antimyeloperoxidase. Data are means ± sd. Scale bar, 100 μm. *P < 0.05 compared with water control group. #P < 0.05 compared with WT + DSS group.
Inflammatory responses are mitigated in Sgpp2-null mice
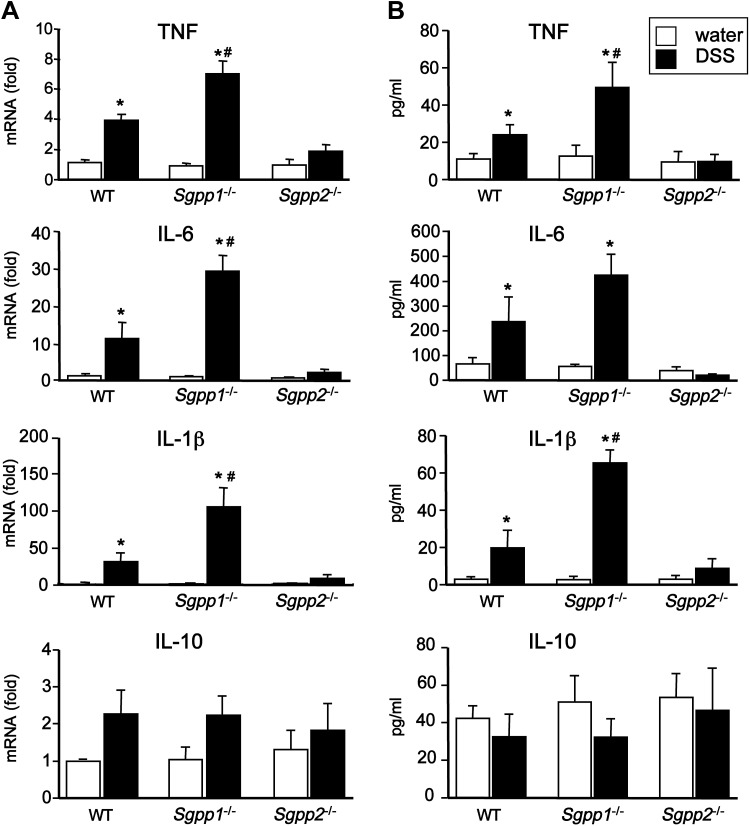
Given the importance of proinflammatory cytokines in the development of colitis (38), we next examined the effect of deletion of Sgpp1 and Sgpp2 on expression of cytokines in colon epithelium during acute colitis. Consistent with colitis severity, mRNA levels of the proinflammatory cytokines, IL-6, TNF-α, and IL-1β, were further increased in DSS-challenged Sgpp1−/− mice compared with WT mice, whereas, in contrast, their levels were not significantly elevated by DSS in colons of Sgpp2−/− mice (Fig. 5A). Similarly, release of these cytokines from colonic mucosa ex vivo was not elevated in Sgpp2−/− mice, whereas their release from colons was significantly increased by DSS treatment in WT mice and even more so in Sgpp1−/− mice (Fig. 5B). Nevertheless, DSS did not induce significant changes in expression or secretion of the anti-inflammatory cytokine IL-10 in WT mice or in any of the knockout mice (Fig. 5A, B).
Figure 5.
Opposite effects on Sgpp1 and Sgpp2 deletion on DSS-induced proinflammatory cytokines. Acute colitis was induced with 5% DSS in WT, Sgpp1−/−, and Sgpp2−/− mice. A) Cytokine mRNA levels in the colons was determined by quantitative PCR. B) Cytokines secreted from ex vivo colon cultures were measured by ELISA. *P < 0.05 compared with water control group. #P < 0.05 compared with WT + DSS group.
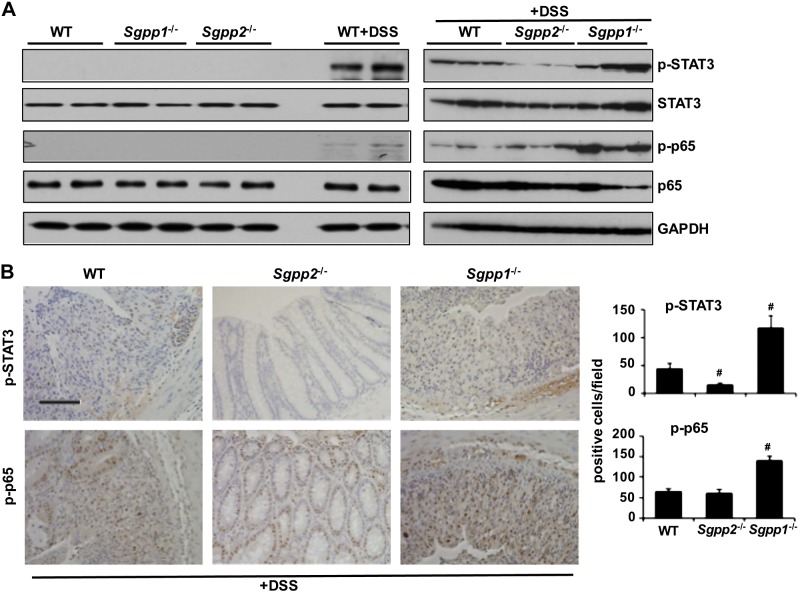
Transcription factors NF-κB and STAT3 have long been known to play critical roles in colitis (39). Although NF-κB was markedly stimulated during colitis development in Sgpp1-null mouse colon compared with WT mice, as shown by increased phosphorylation of its p65 subunit (Fig. 6A), no major differences were observed between WT and Sgpp2−/− mice. In agreement with the view that important functions of NF-κB–induced IL-6 in acute colitis are exerted via activation of STAT3 (40, 41), accumulation of phosphorylated STAT3 was clearly evident in the colonic mucosa of WT mice that were treated with DSS and considerably increased in Sgpp1−/− mice (Fig. 6A). In sharp contrast, STAT3 was not activated in colonic mucosa from DSS-challenged Sgpp2−/− mice (Fig. 6A). In agreement, immunohistochemical analysis revealed that p-STAT3 and p-p65 were evident in the nuclei of colonic epithelial cells of DSS-challenged WT mice and even enhanced in Sgpp1−/− but not in DSS-challenged Sgpp2−/− mice (Fig. 6B). Taken together, these data suggest that Sgpp2-null mice fail to mount a systemic inflammatory response and are protected against DSS-induced colitis.
Figure 6.
Sgpp2 deletion suppresses DSS-induced STAT3 activation. Acute colitis was induced with 5% DSS in WT, Sgpp1−/−, and Sgpp2−/− mice. A) Colonic lysates were analyzed by Western blotting with the indicated antibodies. B) Immunohistochemical analysis of p-STAT3 and p-p65. p-STAT3– and p-p65–positive cells were quantified in 6 randomly selected fields. #P < 0.05 compared with WT+DSS group. Scale bar, 100 μm.
S1P phosphatase activity is reduced in Sgpp1−/− and Sgpp2−/− colons
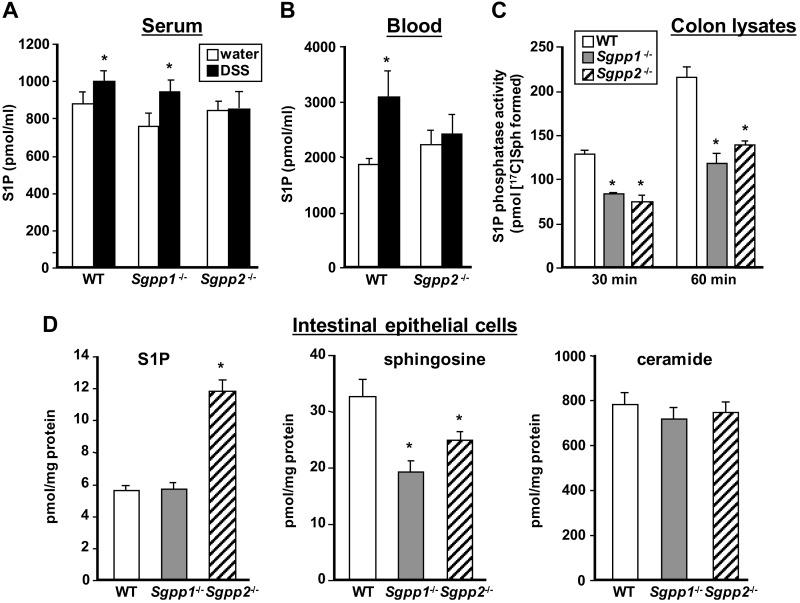
S1P has been implicated in the development of DSS-induced colitis, and it was suggested that increased blood S1P correlated with systemic inflammation and disease severity (7). Indeed, similar to previous studies (7, 8), we found that serum S1P levels in WT and Sgpp1−/− mice were increased during acute colitis (Fig. 7A); however, DSS challenge did not increase S1P levels in serum (Fig. 7A) or whole blood (Fig. 7B) of Sgpp2−/− mice, which corresponded with their reduced inflammatory responses. Nevertheless, S1P levels in the colon were not increased after DSS treatment nor were there detectable changes in colonic S1P levels in either of the Sgpp knockouts compared with WT mice (data not shown). Therefore, it was important to ensure that there is reduced S1P phosphatase activity in Sgpp-knockout colons. Indeed, degradation of S1P to sphingosine was greatly reduced in colonic extracts from Sgpp1- and Sgpp2-knockout mice compared with WT mice (Fig. 7C). Because expression of Sgpp1 and Sgpp2 was readily detectable in IECs that were isolated from WT mice by quantitative PCR but was below the level of detection in the respective knockout mice, we measured levels of sphingolipid metabolites in these cells. There were significantly higher levels of S1P in IECs from Sgpp2−/− colons compared with WT IECs, and sphingosine levels were correspondingly reduced (Fig. 7D). However, although there was a significantly lower level of sphingosine in IECs isolated from Sgpp1−/− mouse colons, no changes in S1P levels were observed. Moreover, there were no significant differences in ceramide levels in either of the knockout mouse IECs compared with WT mice (Fig. 7D).
Figure 7.
Effects of deletion of Sgpp1 and Sgpp2 on circulating S1P, colonic S1P phosphatase activity, and sphingolipid levels in IECs. WT, Sgpp1−/−, and Sgpp2−/− mice were treated with water or DSS as described in Fig. 3. A, B) S1P in serum (A) and whole blood (B) was measured by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS). C) Colonic extracts were prepared from WT, Sgpp1−/−, and Sgpp2−/− mice, and S1P phosphatase activity was determined at the indicated times. D) IECs were prepared from WT, Sgpp1−/−, and Sgpp2−/− mice. Levels of sphingosine, S1P, and ceramide were determined by LC-ESI-MS/MS. Data are expressed as picomoles per milligram protein. Data are means ± sd; n = 5 (A, B). *P < 0.05 compared to water-treated mice (A, B) or WT group (C, D).
Sgpp2 deficiency suppressed DSS-induced apoptosis and improved mucosal integrity
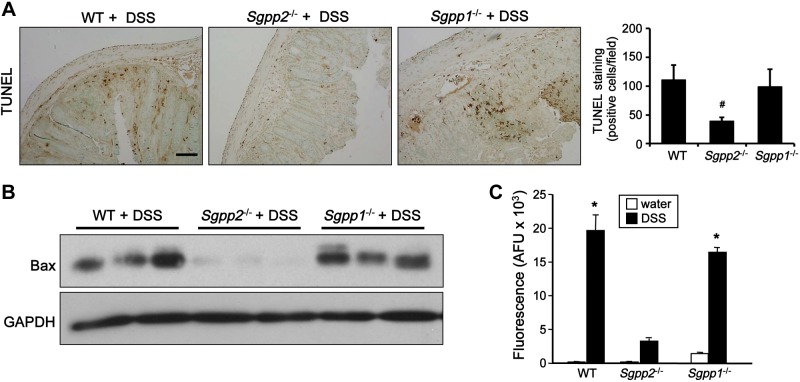
Our results suggest that Sgpp2 deficiency protects against DSS-induced intestinal damage; therefore, we wondered whether this is a result of effects on apoptosis of colonic epithelial cells, which leads to impairment of gut mucosal integrity, infiltration of bacteria into the gut mucosa, and subsequent recruitment of inflammatory cells. As shown by TUNEL staining, DSS markedly increased the number of apoptotic cells in the colonic mucosa of WT and Sgpp1−/− mice to a similar extent (Fig. 8A). In sharp contrast, apoptosis induced by DSS treatment was significantly suppressed by deletion of Sgpp2 (Fig. 8A). Similarly, in agreement with others (42, 43), we found that DSS-induced colitis also led to up-regulation of the proapoptotic protein Bax (Fig. 8B); however, DSS challenge did not increase Bax in mice devoid of Sgpp2 (Fig. 8B). Hence, induction of apoptosis correlated with the severity of colitis.
Figure 8.
Sgpp2 deletion protects against colonic apoptosis and impaired mucosal integrity induced by DSS. Acute colitis was induced in WT, Sgpp1−/−, and Sgpp2−/− mice with 5% DSS. A) Apoptotic cells in colons were examined by TUNEL staining, and positive cells were quantified in 6 randomly selected fields. B) Expression of proapoptotic Bax was determined in colon lysates by Western blotting. Blots were stripped and immunoblotted with anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody to demonstrate equal loading and transfer. C) WT, Sgpp1−/−, and Sgpp2−/− mice were treated with water or 5% DSS for 1 d. FITC-dextran was administered orally, and its serum level was measured 4 h later. Data are means ± sem. *P < 0.05 compared with water control group. #P < 0.05 compared with WT + DSS group.
To obtain mechanistic insight into the protective action of Sgpp2 deletion, we evaluated mucosal barrier function during induction of colitis by assessing the permeability of FITC-dextran. At 24 h after DSS treatment, FITC-dextran was administered orally, and 4 h later blood was drawn. Serum level of FITC-dextran in WT or Sgpp1−/− mice that were treated with DSS was significantly higher than that in nontreated mice, whereas only a small increase in permeability was observed in Sgpp2−/− mice that were treated with DSS (Fig. 8C). These results suggest that expression of Sgpp2 promotes disruption of the mucosal barrier during induction of colitis.
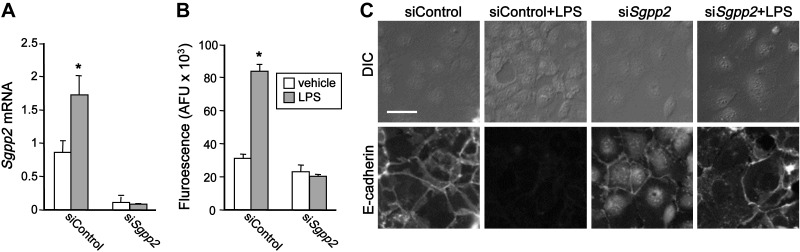
Down-regulation of Sgpp2 attenuated LPS-induced paracellular permeability in vitro and enhanced expression of E-cadherin
To further address the molecular mechanisms of the protective role of Sgpp2 deletion in DSS-induced changes to intestinal barrier function, we used Caco-2 cells, an in vitro model of intestinal epithelium. In agreement with previous studies (44, 45), treatment of a monolayer of Caco-2 cells with LPS, which is known to cause gut barrier dysfunction, greatly increased mucosal-to-serosal flux rates of FITC-dextran in the paracellular tracer flux assay (Fig. 9B). LPS also enhanced expression of Sgpp2 (Fig. 9A). Down-regulation of Sgpp2 completely eliminated LPS-induced flux increase (Fig. 9A, B). It has been shown that S1P enhances IEC barrier function by regulating expression of the adherens junction protein E-cadherin (46), which plays a central role in barrier regulation (47). Therefore, we next investigated alterations in the structures of the apical junctional complexes. Immunofluorescence staining of Caco-2 monolayers showed that E-cadherin was distributed along cell borders (Fig. 9C). In agreement with previous studies (44), LPS profoundly disrupted architecture of E-cadherin, decreased its expression, and reorganized its distribution (Fig. 9C). Down-regulation of Sgpp2 substantially prevented these LPS-induced alterations in E-cadherin (Fig. 9C). These results suggest that reducing expression of Sggp2 blocks LPS-induced barrier dysfunction.
Figure 9.
Deletion of Sgpp2 inhibits LPS-induced barrier dysfunction in Caco-2 cells. Caco-2 monolayers were transfected with siControl or siSgpp2 and then treated with vehicle or 1 µg/ml LPS for 24 h. A) Sgpp2 mRNA levels were determined by quantitative PCR and normalized to Gapdh. B) Permeability was assessed by measuring the paracellular flux of FITC-dextran added to the apical compartments. C) Caco-2 cells grown on cover slips were fixed and stained for E-cadherin with a specific antibody and corresponding secondary antibody conjugated with fluorescent probes, and images were acquired by confocal laser scanning microscopy. DIC, differential interference contrast. Data are arbitrary fluorescence units and are means ± sem. Scale bar, 50 μm. *P < 0.05 compared with vehicle.
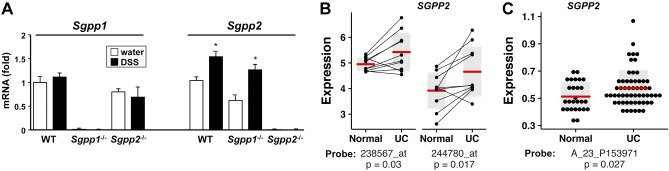
Sgpp2 expression is increased in DSS-induced colitis in mice and in sigmoid colon biopsies from patients with UC
Our results prompted us to examine the expression of Sgpp2 and Sgpp1 in colitis in mice. No significant changes were observed in Sgpp1 expression after DSS challenge; however, Sgpp2 mRNA was increased in colons from DSS-challenged mice (Fig. 10A). To begin to understand the link between SGPP2 and UC in humans, we analyzed SGPP2 expression in sigmoid colon biopsies from patients with UC and their healthy twins (discordant monozygotic twin pairs) in public domain microarray data (34). Analysis of these human twin pairs provides a unique ability to control for the contributions of genetic factors. Of interest, SGPP2 expression was significantly increased in sigmoid colons of patients with UC compared with their healthy twins (Fig. 10B). Because the twin study was focused on the sigmoid colon, which is generally affected in patients with UC, we next examined another set of microarray data from a larger cohort of patients (35). Increased SGPP2 expression was also observed in this microarray data from endoscopic mucosal biopsies taken at colonoscopy of patients with UC with histologically inflamed sigmoid colons compared with those from noninflamed healthy controls (Fig. 10C). These results suggest that SGPP2 expression is up-regulated in the sigmoid colons of patients with UC.
Figure 10.
Sgpp2 expression is increased after DSS treatment in mice and in sigmoid colon biopsies from patients with UC. A) Acute colitis was induced with 5% DSS in WT, Sgpp1−/−, and Sgpp2−/− mice. mRNA levels of Sgpp1 and Sgpp2 in colons were determined by quantitative PCR and were normalized to Gapdh. ∗P < 0.05 compared with water control group. B) Analysis of SGPP2 expression in Gene Expression Omnibus data set GSE22619 using 2 independent probe sets (238567_at; 244780_at) from colon mucosal tissue from 10 monozygotic twin pairs discordant for UC (34). C) Analysis of SGPP2 expression in Gene Expression Omnibus Data Set GSE11223202 using probe A_23_P153971 (35) of inflamed sigmoid colon biopsies of patients with UC (n = 57) compared with uninflamed biopsies from healthy individuals (n = 27). B, C) P values for specific probes are indicated. Horizontal bars indicate the group means, gray vertical bars = 1 sd from means.
DISCUSSION
In this report, we demonstrated the differential roles of the 2 isoforms of S1P phosphatases, SGPP1 and SGPP2, in acute colitis. Sgpp1-null mice had more severe colitis, with increased inflammatory cell infiltration and greater proinflammatory cytokine production, whereas DSS-induced colitis was greatly attenuated by deletion of Sgpp2. These findings suggest that although SGPP1 and SGPP2 are both localized to the ER (19, 21, 48), they have nonredundant roles and distinct physiologic functions that might be related to their unique expressions and/or functions in specific cell types. In this regard, only deletion of Sgpp2, and not deletion of Sgpp1, increased S1P levels in IECs, which is likely related to the high expression of Sgpp2 in the gastrointestinal tract (http://biogps.org/) (26). Our idea that S1P in different cell types has unique and complex actions during colitis is supported by previous studies (7–9). Using bone marrow transfers from SphK1−/− mice, it was demonstrated that hematopoietic-derived SphK1 regulates sphingolipid levels in circulation and systemic inflammation after DSS-induced colitis (7–9). Similarly, disruption of SphK2 in hematopoietic cells recapitulated the effects of global deletion of SphK2, which enhances SphK1 expression and colitis severity and that indicates that S1P in hematopoietic cells contributes to colitis severity, IL-6 production, and activation of STAT3 and NF-κB (7–9). Moreover, in agreement with the observation that S1P levels in circulation directly regulate circulating neutrophil levels (9), DSS-induced colitis increased infiltrating neutrophils in colons of WT and Sgpp1−/− mice with increased circulating S1P levels but not by deletion of Sgpp2 that prevents elevation of serum S1P during colitis.
Our findings raise the important question of how deletion of Sgpp2 significantly decreases colitis severity. Gut epithelium is critically involved in the maintenance of intestinal immune homeostasis, acting as a physical barrier to luminal bacteria and controlling immune cell infiltration (3). Impairment of intestinal epithelial integrity in humans has been suggested to be an important cause of the development of intestinal inflammation in patients with IBD (49). Experimental DSS-induced colitis in mice mimics this process by damaging the colon epithelium and interrupting barrier integrity to allow bacteria infiltration, which is detected by the innate immune system. This then orchestrates inflammatory responses, in part, by releasing cytokines and chemokines that recruit other cellular components, including adaptive immune cells. Of interest, we found that deletion of Sgpp2 suppressed DSS-induced epithelial cell apoptosis and, thus, preserved epithelial integrity and prevented bacterial infiltration into the mucosa. This, in turn, also prevented infiltration of immune cells and the expression of proinflammatory cytokines, such as TNF-α and IL-1β, which are known to further cause destruction of colonic epithelium and recruitment of additional immune cells. Hence, Sggp2 is needed to sustain this malicious feed-forward amplification loop that is involved in the development of DSS-induced colitis.
Consistent with a role for Sgpp2 in colitis, expression of Sgpp2, but not of Sgpp1, was increased after DSS treatment. Moreover, analysis of SGPP2 expression in endoscopic biopsies from 57 patients with UC and 27 control subjects revealed statistically significant increases in SGPP2 expression only in sigmoid colons from patients with UC. In agreement, analysis of data on discordant twin pairs revealed that SGPP2 was increased in sigmoid colon of the twin with UC compared with their healthy sibling. This is likely clinically relevant as the inflammation is generally most severe in the sigmoid and rectum and usually diminishes higher in the colon. Moreover, perforations that happen more often during the first episodes of colitis commonly occur in the sigmoid colon.
Induction of Sgpp2 mRNA in mice after DSS challenge and increased SGPP2 expression in human UC samples suggest that SGPP2 activity and reduction of S1P in IECs may enhance their apoptosis and impair intestinal barrier function as a significant modulator of UC. It is tempting to speculate that induction of Sgpp2 by DSS or SGPP2 in humans during colitis is driven, at least in part, by TNF-α, the major cytokine that initiates and perpetuates intestinal mucosal inflammation and damage, as it was shown that Sgpp2 expression is regulated by NF-κB (25). LPS and TNF-α can induce Sgpp2 expression probably via NF-κB as the Sgpp2 promoter has NF-κB motifs. This sequence of inflammatory events can lower the level of S1P specifically in IECs, which leads to disruption of S1P-maintaned barrier integrity. Modulation of SGPP2 expression, among other NF-κB–dependent genes, may contribute to the effectiveness of anti-TNF therapeutics that are currently used for IBD treatment. It is possible that targeting NF-κB signaling may block IEC apoptosis by preventing increases in SGPP2 expression leading to decreased S1P. Indeed, we and others have observed that S1P protects IECs from apoptosis by increasing expression of the adherens junction protein E-cadherin and enhancing intestinal epithelial barrier function (46, 47). Further delineation of the role of metabolism of S1P as well as the cellular targets of S1P and signaling will be invaluable in the understanding of the pathogenesis of IBD and might lead to development of new therapies.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Cancer Institute (NCI) Grant R01CA61774 and NIH National Institute of General Medical Sciences Grant R01GM043880 (to S.S.). This study was also supported, in part, by NIH NCI Grant R01CA160688 (to K.T.) and NIH Center for Clinical and Translational Research Grant UL1TR000058 (to A.R.W.). The authors acknowledge the Virginia Commonwealth University Lipidomics and Microscopy Cores, which are supported, in part, by funding from the NIH NCI Cancer Center Support Grant P30CA016059.
Glossary
- DSS
dextran sodium sulfate
- ER
endoplasmic reticulum
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- LPP
lipid phosphate phosphatase
- S1P
sphingosine-1-phosphate
- S1PR1
S1P receptor 1
- SphK1
sphingosine kinase 1
- STAT3
signal transducer and activator of transcription 3
- UC
ulcerative colitis
- WT
wild-type
REFERENCES
- 1.Molodecky N. A., Soon I. S., Rabi D. M., Ghali W. A., Ferris M., Chernoff G., Benchimol E. I., Panaccione R., Ghosh S., Barkema H. W., Kaplan G. G. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42, quiz e30 [DOI] [PubMed] [Google Scholar]
- 2.Von Roon A. C., Reese G., Teare J., Constantinides V., Darzi A. W., Tekkis P. P. (2007) The risk of cancer in patients with Crohn’s disease. Dis. Colon Rectum 50, 839–855 [DOI] [PubMed] [Google Scholar]
- 3.Xavier R. J., Podolsky D. K. (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 [DOI] [PubMed] [Google Scholar]
- 4.Khor B., Gardet A., Xavier R. J. (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen R. D. (2010) The pharmacoeconomics of biologic therapy for IBD. Nat. Rev. Gastroenterol. Hepatol. 7, 103–109 [DOI] [PubMed] [Google Scholar]
- 6.Maceyka M., Spiegel S. (2014) Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snider A. J., Kawamori T., Bradshaw S. G., Orr K. A., Gilkeson G. S., Hannun Y. A., Obeid L. M. (2009) A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 23, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang J., Nagahashi M., Kim E. Y., Harikumar K. B., Yamada A., Huang W. C., Hait N. C., Allegood J. C., Price M. M., Avni D., Takabe K., Kordula T., Milstien S., Spiegel S. (2013) Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snider A. J., Ali W. H., Sticca J. A., Coant N., Ghaleb A. M., Kawamori T., Yang V. W., Hannun Y. A., Obeid L. M. (2014) Distinct roles for hematopoietic and extra-hematopoietic sphingosine kinase-1 in inflammatory bowel disease. PLoS One 9, e113998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maines L. W., Fitzpatrick L. R., French K. J., Zhuang Y., Xia Z., Keller S. N., Upson J. J., Smith C. D. (2008) Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig. Dis. Sci. 53, 997–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degagné E., Pandurangan A., Bandhuvula P., Kumar A., Eltanawy A., Zhang M., Yoshinaga Y., Nefedov M., de Jong P. J., Fong L. G., Young S. G., Bittman R., Ahmedi Y., Saba J. D. (2014) Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Invest. 124, 5368–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deguchi Y., Andoh A., Yagi Y., Bamba S., Inatomi O., Tsujikawa T., Fujiyama Y. (2006) The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol. Rep. 16, 699–703 [PubMed] [Google Scholar]
- 13.Daniel C., Sartory N., Zahn N., Geisslinger G., Radeke H. H., Stein J. M. (2007) FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 178, 2458–2468 [DOI] [PubMed] [Google Scholar]
- 14.Daniel C., Sartory N. A., Zahn N., Schmidt R., Geisslinger G., Radeke H. H., Stein J. M. (2007) FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol. Immunol. 44, 3305–3316 [DOI] [PubMed] [Google Scholar]
- 15.Sanada Y., Mizushima T., Kai Y., Nishimura J., Hagiya H., Kurata H., Mizuno H., Uejima E., Ito T. (2011) Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One 6, e23933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J., Matsuda C., Kai Y., Nishida T., Nakajima K., Mizushima T., Kinoshita M., Yasue T., Sawa Y., Ito T. (2008) A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J. Pharmacol. Exp. Ther. 324, 276–283 [DOI] [PubMed] [Google Scholar]
- 17.Le Stunff H., Peterson C., Liu H., Milstien S., Spiegel S. (2002) Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta 1582, 8–17 [DOI] [PubMed] [Google Scholar]
- 18.Bréart B., Ramos-Perez W. D., Mendoza A., Salous A. K., Gobert M., Huang Y., Adams R. H., Lafaille J. J., Escalante-Alcalde D., Morris A. J., Schwab S. R. (2011) Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208, 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Stunff H., Galve-Roperh I., Peterson C., Milstien S., Spiegel S. (2002) Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J. Cell Biol. 158, 1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giussani P., Maceyka M., Le Stunff H., Mikami A., Lépine S., Wang E., Kelly S., Merrill A. H. Jr., Milstien S., Spiegel S. (2006) Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide. Mol. Cell. Biol. 26, 5055–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson K. R., Johnson K. Y., Becker K. P., Bielawski J., Mao C., Obeid L. M. (2003) Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J. Biol. Chem. 278, 34541–34547 [DOI] [PubMed] [Google Scholar]
- 22.Lépine S., Allegood J. C., Park M., Dent P., Milstien S., Spiegel S. (2011) Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ. 18, 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Stunff H., Mikami A., Giussani P., Hobson J. P., Jolly P. S., Milstien S., Spiegel S. (2004) Role of sphingosine-1-phosphate phosphatase 1 in epidermal growth factor-induced chemotaxis. J. Biol. Chem. 279, 34290–34297 [DOI] [PubMed] [Google Scholar]
- 24.Peter B. F., Lidington D., Harada A., Bolz H. J., Vogel L., Heximer S., Spiegel S., Pohl U., Bolz S. S. (2008) Role of sphingosine-1-phosphate phosphohydrolase 1 in the regulation of resistance artery tone. Circ. Res. 103, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mechtcheriakova D., Wlachos A., Sobanov J., Kopp T., Reuschel R., Bornancin F., Cai R., Zemann B., Urtz N., Stingl G., Zlabinger G., Woisetschläger M., Baumruker T., Billich A. (2007) Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell. Signal. 19, 748–760 [DOI] [PubMed] [Google Scholar]
- 26.Allende M. L., Sipe L. M., Tuymetova G., Wilson-Henjum K. L., Chen W., Proia R. L. (2013) Sphingosine-1-phosphate phosphatase 1 regulates keratinocyte differentiation and epidermal homeostasis. J. Biol. Chem. 288, 18381–18391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegmund B., Lehr H. A., Fantuzzi G., Dinarello C. A. (2001) IL-1 beta-converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl. Acad. Sci. USA 98, 13249–13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams K. L., Fuller C. R., Dieleman L. A., DaCosta C. M., Haldeman K. M., Sartor R. B., Lund P. K. (2001) Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120, 925–937 [DOI] [PubMed] [Google Scholar]
- 29.Maceyka M., Milstien S., Spiegel S. (2007) Measurement of mammalian sphingosine-1-phosphate phosphohydrolase activity in vitro and in vivo. Methods Enzymol. 434, 243–256 [DOI] [PubMed] [Google Scholar]
- 30.Weigmann B., Tubbe I., Seidel D., Nicolaev A., Becker C., Neurath M. F. (2007) Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 [DOI] [PubMed] [Google Scholar]
- 31.Kabashima K., Saji T., Murata T., Nagamachi M., Matsuoka T., Segi E., Tsuboi K., Sugimoto Y., Kobayashi T., Miyachi Y., Ichikawa A., Narumiya S. (2002) The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest. 109, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu L. C., Flynn A. N., Turner J. R., Buret A. G. (2005) SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: a novel cellular rescue mechanism? FASEB J. 19, 1822–1835 [DOI] [PubMed] [Google Scholar]
- 33.Akbari P., Braber S., Gremmels H., Koelink P. J., Verheijden K. A., Garssen J., Fink-Gremmels J. (2014) Deoxynivalenol: a trigger for intestinal integrity breakdown. FASEB J. 28, 2414–2429 [DOI] [PubMed] [Google Scholar]
- 34.Lepage P., Häsler R., Spehlmann M. E., Rehman A., Zvirbliene A., Begun A., Ott S., Kupcinskas L., Doré J., Raedler A., Schreiber S. (2011) Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 141, 227–236 [DOI] [PubMed] [Google Scholar]
- 35.Noble C. L., Abbas A. R., Cornelius J., Lees C. W., Ho G. T., Toy K., Modrusan Z., Pal N., Zhong F., Chalasani S., Clark H., Arnott I. D., Penman I. D., Satsangi J., Diehl L. (2008) Regional variation in gene expression in the healthy colon is dysregulated in ulcerative colitis. Gut 57, 1398–1405 [DOI] [PubMed] [Google Scholar]
- 36.Huber W., Carey V. J., Gentleman R., Anders S., Carlson M., Carvalho B. S., Bravo H. C., Davis S., Gatto L., Girke T., Gottardo R., Hahne F., Hansen K. D., Irizarry R. A., Lawrence M., Love M. I., MacDonald J., Obenchain V., Oleś A. K., Pagès H., Reyes A., Shannon P., Smyth G. K., Tenenbaum D., Waldron L., Morgan M. (2015) Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautier L., Cope L., Bolstad B. M., Irizarry R. A. (2004) affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20, 307–315 [DOI] [PubMed] [Google Scholar]
- 38.Neurath M. F. (2014) Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 [DOI] [PubMed] [Google Scholar]
- 39.Karin M. (2009) NF-kB as a critical link between inflammation and cancer. In Cold Spring Harbor Perspectives in Biology (Staudt L. M., Karin M., eds.), pp. 1–14, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bollrath J., Phesse T. J., von Burstin V. A., Putoczki T., Bennecke M., Bateman T., Nebelsiek T., Lundgren-May T., Canli O., Schwitalla S., Matthews V., Schmid R. M., Kirchner T., Arkan M. C., Ernst M., Greten F. R. (2009) gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15, 91–102 [DOI] [PubMed] [Google Scholar]
- 41.Grivennikov S., Karin E., Terzic J., Mucida D., Yu G. Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., Karin M. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vetuschi A., Latella G., Sferra R., Caprilli R., Gaudio E. (2002) Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig. Dis. Sci. 47, 1447–1457 [DOI] [PubMed] [Google Scholar]
- 43.Halpert G., Eitan T., Voronov E., Apte R. N., Rath-Wolfson L., Albeck M., Kalechman Y., Sredni B. (2014) Multifunctional activity of a small tellurium redox immunomodulator compound, AS101, on dextran sodium sulfate-induced murine colitis. J. Biol. Chem. 289, 17215–17227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang T., Wang L., Sun R., Chen H., Zhang H., Yu Y., Wang Y., Wang G., Yu Y., Xie K. (2016) Hydrogen-rich medium ameliorates lipopolysaccharide-induced barrier dysfunction via Rhoa-Mdia1 signaling in CACO-2 cells. Shock 45, 228–237 [DOI] [PubMed] [Google Scholar]
- 45.Guo S., Al-Sadi R., Said H. M., Ma T. Y. (2013) Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 182, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenspon J., Li R., Xiao L., Rao J. N., Sun R., Strauch E. D., Shea-Donohue T., Wang J. Y., Turner D. J. (2011) Sphingosine-1-phosphate regulates the expression of adherens junction protein E-cadherin and enhances intestinal epithelial cell barrier function. Dig. Dis. Sci. 56, 1342–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider M. R., Dahlhoff M., Horst D., Hirschi B., Trülzsch K., Müller-Höcker J., Vogelmann R., Allgäuer M., Gerhard M., Steininger S., Wolf E., Kolligs F. T. (2010) A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One 5, e14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa C., Kihara A., Gokoh M., Igarashi Y. (2003) Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. J. Biol. Chem. 278, 1268–1272 [DOI] [PubMed] [Google Scholar]
- 49.Laukoetter M. G., Nava P., Nusrat A. (2008) Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 14, 401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]