Abstract
Vitamin A bound to retinol binding protein 4 (RBP4) constitutes the major transport mode for retinoids in fasting circulation. Emerging evidence suggests that membrane protein, STRA6 (stimulated by retinoic acid 6), is the RBP4 receptor and vitamin A channel; however, the role of STRA6 in vitamin A homeostasis remains to be defined in vivo. We subjected Stra6-knockout mice to diets sufficient and insufficient for vitamin A and used heterozygous siblings as controls. We determined vitamin A levels of the eyes, brain, and testis, which highly express Stra6, as well as of tissues with low expression, such as lung and fat. We also studied the consequence of STRA6 deficiency on retinoid-dependent processes in tissues. Furthermore, we examined how STRA6 deficiency affected retinoid homeostasis of the aging mouse. The picture that emerged indicates a critical role for STRA6 in the transport of vitamin A across blood–tissue barriers in the eyes, brain, and testis. Concurrently, fat and lung rely on dietary vitamin A. In testis and brain, Stra6 expression was regulated by vitamin A. In controls, this regulation reduced vitamin A consumption when the dietary supply was limited, sequestering it for the eye. Thus, STRA6 is critical for vitamin A homeostasis and the adaption of this process to the fluctuating supply of the vitamin.—Kelly, M., Widjaja-Adhi, M. A. K., Palczewski, G., von Lintig, J. Transport of vitamin A across blood–tissue barriers is facilitated by STRA6.
Keywords: vision, retinoids, RBP4
Effective distribution of vitamin A [all-trans-retinol (ROL)] throughout the body maintains retinoid signaling in peripheral tissues and ensures photoreceptor function and survival in the eyes (1–3). In this process, dietary vitamin A is absorbed in the intestine, esterified, and transported, along with other dietary lipids, in triacylglycerol-rich chylomicrons. Tissues use this form of vitamin A in a lipoprotein lipase-dependent manner, with most vitamin A in this form ultimately being stored in the liver (4). The remainder in chylomicron remnants is deposited in the liver for storage in specialized stellate (Ito) cells (2). In the liver, ROL is loaded onto the serum retinol binding protein 4 (RBP4) and mobilized into the circulation (5). In fasting circulation, stored vitamin A bound to RBP4 (holo-RBP4) is the major transport mode of ROL and its levels are homeostatic in healthy, well-nourished individuals (2, 5). Once in the blood, holo-RBP4 escapes kidney glomeruli filtrations by forming a complex with 55-kDa transthyretin homotetramer at a 2:1 molar ratio (6).
Biochemical studies indicate the involvement of a cellular RBP4 receptor in vitamin A uptake from circulating holo-RBP4 (7–10). This receptor was identified as the stimulated by retinoic acid 6 (STRA6) gene product (11). Cell culture studies indicate that STRA6 protein facilitates the bidirectional flux of ROL between its blood carrier RBP4 and cellular retinol binding protein 1 (12, 13). Cellular accumulation of ROL is driven by conversion into all-trans-retinyl esters (REs) via lecithin retinol acyl transferase (LRAT) (12–15). LRAT is a microsomal, membrane-anchored enzyme that converts ROL into REs by transferring palmitate from lecithin to ROL (16, 17). Recent studies indicate that both Stra6 and Lrat expression are jointly governed via regulation by retinoid signaling in nonocular peripheral tissues (14).
Mutations in the STRA6 gene in humans result in the rare, yet fatal, Matthew Wood syndrome (MWS) (18, 19). MWS is characterized by severe bilateral microphthalmia, often in combination with pulmonary dysplasia, cardiac defects, and diaphragmatic hernia, among other anomalies and malformations (20). Symptoms of MWS are highly variable, even within the same family, and range in severity from isolated microphthalmia to complex microphthalmic syndromes (21). Similarly, mutations in the RBP4 gene can result in congenital eye malformations, including microphthalmia (22, 23). Again, large variations in the phenotypic manifestation of RBP4 mutations are reported, even within the same family (22).
Extensive variability of the phenotypic manifestations of RBP4 and STRA6 mutations has led to controversial discussions about vitamin A uptake homeostasis. Some researchers have suggested that greater availability of dietary vitamin A during development reduces the severity of birth defects in patients who are affected by RBP4 and STRA6 mutations (15, 22). Others have proposed that STRA6 is only required for vitamin A uptake in the eyes (24). In addition, a role of the STRA6 protein beyond its function as a RBP4 receptor has been proposed but remains controversial (25–27). Thus, the role of STRA6 in cellular vitamin A uptake homeostasis and its putative association with diseases requires clarification. In this study, we intended to clarify the role of STRA6 in adult mice.
Three laboratories, including ours, have established Stra6-knockout (Stra6−/−; KO) mouse strains. Stra6−/− mice are born at mendelian frequency when raised on vitamin A–rich diets (15, 28, 29). Stra6−/− mice display ocular phenotypes that are characteristic of a vitamin A–deficient retina, such as attenuated ERG responses, cone cell death, and shortening of photoreceptor outer segments (15, 28). Of note, Stra6−/− mice phenotypically resemble Rbp4-knockout (Rbp4−/−) mice, which also display these features (5, 15).
Because STRA6-deficient mice do not develop a human-like MWS phenotype, this mouse mutant presents a unique model to study the role of the RBP4 receptor throughout the mammalian life cycle. Thus, we maintained Stra6−/− mice and heterozygous siblings on diets with sufficient and deficient amounts of vitamin A. The diet lacking vitamin A forced the animals to rely on stored vitamin A, which circulates bound to RBP4. In addition, we intended to clarify the role of STRA6 in body vitamin A homeostasis during aging.
MATERIALS AND METHODS
Animal care and husbandry
Animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Statement for the Use of Animals in Ophthalmic and Vision Research (Association for Research in Vision and Ophthalmology, Rockville, MD, USA). The generation of Stra6−/− mice has been previously described (15). For experiments, male heterozygous Stra6 (Stra6+/−; Het) and homozygous KO mice were bred from heterozygous dams on regular vitamin A rich chow that contained ∼29,000 IU vitamin A/kg diet (Prolab RMH 3000; LabDiet, St. Louis, MO, USA) as previously described (15). After weaning, Het and KO mice were subjected to a vitamin A–sufficient (VAS) or vitamin A–deficient (VAD) experimental diet for 12 wk. Both diets were based on AIN93G formulation, but the VAD diet was without vitamin A supplementation (4000 IU vitamin A/kg diet). Individual diets were prepared by Research Diets (New Brunswick, NJ, USA). In addition, a group of KO (n = 7) and Het (n = 6) mice was maintained on standard chow that contained ∼29,000 IU vitamin A/kg diet (Prolab RMH 3000) for a time period of 12 mo. After dietary interventions, mice were anesthetized by intraperitoneal injection of a mixture that contained 15 mg ketamine, 3 mg xylazine, and sterile water or saline, with a dose of 0.2 ml/25 g of mouse. Blood was drawn directly from the heart by cardiac puncture. Cerebrospinal fluid (CSF) was extracted via cisterna magna puncture (30) with a glass pipette under deep anesthesia. CSF was observed under the microscope to confirm the absence of red blood cells. CSF that was contaminated with blood was discarded. Mice were then perfused with 20 ml PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.3) and killed by cervical dislocation for further tissue collection. Upon dissection, the choroid plexus (CP) was removed from the brain and discarded. Tissues were immediately used for analyses and/or snap frozen in liquid nitrogen and stored at −80°C until further use.
Optical coherence tomography image collection and analysis
A previously established method was used to quantify the optical coherence tomography (OCT) signal with respect to retinal thickness (15). With pupils fully dilated with 1% tropicamide, mice were anesthetized with an intraperitoneal injection of ketamine and xylazine. Whiskers were trimmed to avoid image artifacts. OCT images were acquired with linear B-scan mode by employing ultra high–resolution OCT. Photoreceptor thickness was calculated at points of constant distance from the center of the optic nerve as the thickness of the layer identified as photoreceptor inner/outer segments in number of pixels by the height of each pixel.
HPLC analysis of retinoids
Retinoids were extracted from 100 μl of serum, 1 whole eyecup, or a tissue homogenate in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4) that contained ∼50 mg of tissue. Hydroxylamine (200 μl, 2 M) was added to the eyecups before homogenization. Retinoid extraction was performed twice with a mixture that contained 200 μl methanol, 400 μl acetone, and 500 µl hexane. HPLC analysis was performed on a normal-phase Zobax Sil (5 μm, 4.6 × 150 mm) column. Chromatographic separation was achieved by isocratic flow of 10% ethyl acetate/90% hexane. For quantification of molar amounts of retinoids, HPLC has previously been scaled with synthesized standard compounds.
Western blots
For the STRA6 Western blot, 8% acrylamide SDS gels were hand cast. Samples were dissolved in RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8.0). To this, we added a 3× loading buffer (188 mM Tris, pH 6.8, 3% SDS, 30% glycerol, 0.01% bromophenol blue, 15% β-mercaptoethanol). Samples were not boiled before the experiment, as is typical for glycosylated membrane proteins. Tris-buffered saline-Tween 20 (TBS-T; 5 mM Tris pH 7.4, 150 mM NaCl, 0.1% Tween-20) was used as wash buffer. Membrane was blocked with 5% milk in TBS-T overnight. Incubation with STRA6 polyclonal antibody raised against the full length protein (Abnova, Taipei, Taiwan) was used at a dilution of 1:1000 and incubated overnight. This antibody was previously reported to identify mouse STRA6 (31). A goat anti-rabbit IgG was used as the secondary antibody (Promega, Madison, WI, USA) at a dilution of 1:10,000 and was incubated for 1 h. Antibodies were diluted in TBS-T.
For RBP4 Western blotting, 12% acrylamide SDS gels were hand cast. Loading buffer was added to the samples as described above. Samples were boiled before the experiment. Incubation with an anti-RBP4 polyclonal antibody raised against the full length protein (Dako, Carpinteria, CA, USA) was used at a dilution of 1:2000 and incubated for 1 h. A goat anti-rabbit IgG was used as the secondary antibody (Promega) at a dilution of 1:10,000 and was incubated for 1 h.
Quantitative RT-PCR
RNA was extracted from flash-frozen tissues by using Trizol reagent according to manufacturer instructions. Before quantitative PCR, cDNA was acquired by using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA). RT reaction was performed on an Eppendorf (Hamburg, Germany) thermocycler according to manufacturer instructions. Gene expression measurement via quantitative PCR was then performed by using an ABI Real Time PCR Instrument using TaqMan probes (Applied Biosystems) primer sets for Gapdh (Mm99999915_g1), Id4 (Mm00499701_m1), Sohlh1 (Mm01338424_g1), c-Kit (Mm00445212_m1), Stra8 (Mm00486473_m1), and Stra6 (Mm00486457_m1). TaqMan Fast Univ PCR Master Mix (2×) No AmpErase UNG (Applied Biosystems) was used according to manufacturer instructions. Of note, the probe set used for quantitative RT-PCR of STRA6 sequences does not distinguish between the full Stra6 transcript and the proposed Stra6 alternate transcripts that may derive from alternative splicing (32). Reactions were carried out by using 50 ng cDNA/ 10 μl reaction mixture. Expression levels are normalized to Gapdh expression by using a standard ΔΔCt method.
ELISA detection of RBP4
We developed an in-house indirect ELISA for accurate detection of RBP4 in blood, CSF, and liver samples. We can detect picomolar concentrations of RBP4 from 0.5 μl serum or CSF. Samples were diluted 1:100 in a bicarbonate buffer (pH 9.6) and incubated on a 96 well maxi-sorp plate (50 μl) to bind protein to the wells, followed by blocking with 5% bovine serum albumin in PBS (100 μl), incubation with rabbit anti-RBP4 antibody (Dako) diluted 1:500 (100 μl), and finally incubation with goat anti-rabbit IgG antibody (Promega) diluted 1:1000 (100 μl). Trimethylbenzidine (100 μl) was used for detection, and the reaction was stopped with 2N H2SO4 (100 μl). RBP4 was produced as described below to be used as standards.
We produced in Escherichia coli and purified recombinant RBP4 to use as standards for ELISA. RBP4 expression and purification from E. coli was accomplished as described previously (33). In brief, human RBP4 (hRBP4) cDNA was cloned into a pET3a expression vector and expressed in BL-21 DE3 cells. Bacterial cells were harvested and lysed by osmotic shock. Insoluble material was pelleted by centrifugation, washed, and solubilized in 7 M guanidine hydrochloride and 10 mM dithiothreitol. After overnight incubation, insoluble material was removed by ultracentrifugation. hRBP4 was slowly refolded in the presence of retinol. Holo-hRBP4 was dialyzed against 10 mM Tris/HCl buffer (pH 8.0) and loaded onto a DE53 anion exchange chromatography column. Holo-hRBP4 was eluted with linear gradient of NaCl (0–1 M) in 10 mM Tris/HCl buffer, pH 8.0). Collected fractions were examined by SDS-PAGE and UV-visible spectroscopy to ensure a proper protein/retinoid ratio. Fractions that contained at least 90% holo-hRBP4 were pooled together and concentrated.
Histology
We use the Visual Sciences Research Center core facility at Case Western Reserve University for the preparation of slides and for hematoxylin and eosin (H&E) staining. Tissues were fixed overnight in 10% formalin and embedded in paraffin blocks. Ten-micrometer slices were fixed onto glass slides. H&E sections were prepared by the Visual Sciences Research Center core.
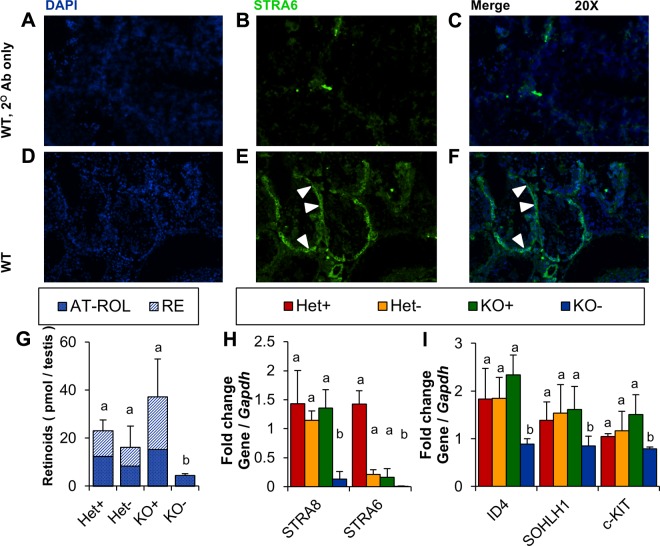
Testes and eyes were flash frozen immediately upon dissection and stored at −80°C until they were sliced with a cryomicrotome at a thickness of 5 μm. Slides were air dried briefly and fixed in 10% buffered formalin phosphate for 5 min. Slides were blocked for 1.5 h in a solution of 10% bovine serum albumin and 5% goat serum in PBS-Tween 20 (PBS-T; PBS plus 0.1% Tween-20). STRA6 antibody (Abnova) was diluted 1:250 in blocking buffer and applied overnight in a humidified chamber, followed by 3 × 15 min washes in PBS-T. Specificity for this antibody is shown in the Western blot in Fig. 1A. Slides were then incubated in goat anti-rabbit IgG (1:800; 488 nm; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1.5 h, immediately followed by an incubation in 1× DAPI for 15 min, and by 3 × 15 min washes in PBS-T. One drop ProLong Gold antifade reagent (Thermo Fisher Scientific Life Sciences. Waltham, MA, USA) was applied to each tissue section along with a coverslip. Slides were left in the dark overnight to set before fluorescence was detected with a confocal microscope (TCS SP5; Leica, Wetzlar, Germany).
Figure 1.
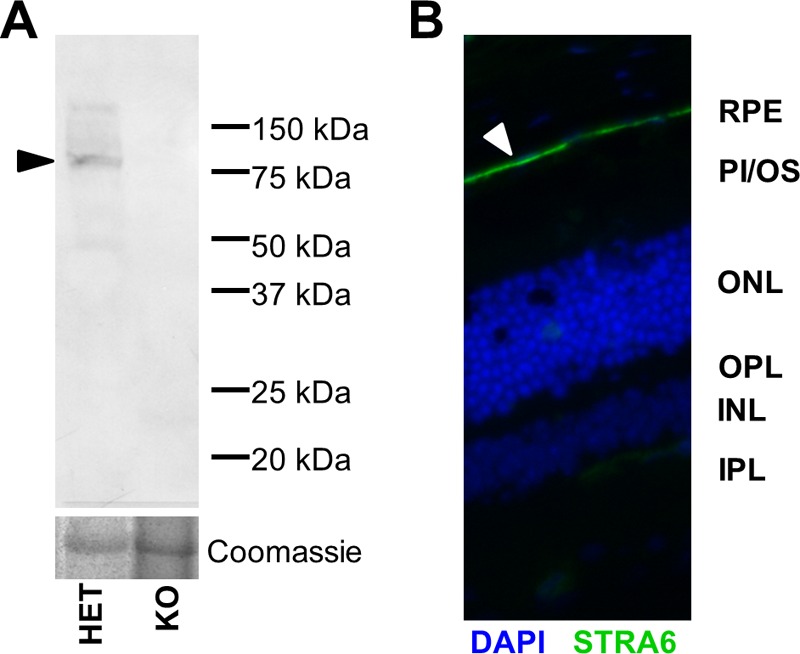
STRA6 is expressed as an 80-kDa membrane protein in the RPE. A) Western blot using a polyclonal antibody against STRA6, showing a single band of the antibody at 80 kDa in the RPE from Het but not KO mice. Coomassie stain was used as a loading control. (Arrowhead points to the STRA6 band.) B) Immunohistochemistry on a frozen section of an eye from a Het mouse using STRA6 antibody (green) showed strong staining (arrowhead) at the basolateral membrane of the RPE. DAPI (blue) was used as counterstain of nuclei of the cells in the image. (Arrowhead points to the STRA6.) INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PI/OS, photoreceptor inner/outer segments.
RESULTS
Study design
Studies in cell culture indicate that STRA6 mediates cellular vitamin A uptake. To test this hypothesis in live animals, we employed a Stra6−/− mouse model that we previously established (15). Because Stra6 mRNA splice variants exist and there is evidence that Stra6 is expressed from different promoters (32), we first confirmed a STRA6-null phenotype in our animals by employing a commercial polyclonal antibody. This antibody was generated against full-length human STRA6 and should detect different splice variants. In Western blots, a single band was stained in ocular protein extracts of Het (Stra6+/−) mice, which was absent in KO mice (Fig. 1A). The specificity of this antibody for STRA6 was further confirmed in immunohistochemistry where strong staining of the basolateral side of the retinal pigment epithelium (RPE) was observed (Fig. 1B). Similarly, the antibody specifically stained Sertoli cells in testes from Het mice (see “STRA6 is mandatory for vitamin A homeostasis of brain and testis” below) where STRA6 expression has been reported by others (24).
To reduce the influence from genetic background and gender, we employed male KO and Het siblings in our study. We bred and raised these mice on a chow diet with 29,000 IU/kg vitamin A in the form of RE. After weaning, we subjected these Het and KO mice to a VAD diet (Het− and KO−, respectively; Het/KO raised on VAD diet), which forced them to rely on stored vitamin A, which circulates as holo-RBP4. As a control, we raised an analogous cohort of Het and KO mice on a VAS diet that was supplemented with 4000 IU/kg vitamin A (Het+ and KO+, respectively; Het/KO raised on VAS diet). During the experimental period, we did not observe macroscopic phenotypic manifestations of VAD, such as growth retardation or weight or fur loss (Fig. 2A). At the end of the experimental period, we performed OCT analysis of the eyes.
Figure 2.
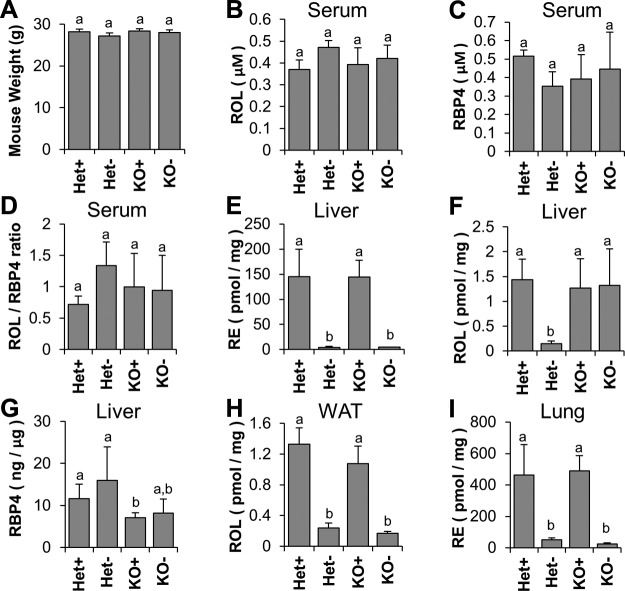
Effects of 12 wk of VAS and VAD dietary regimens on Het and KO mice. A) Mouse weights at end of study; B) HPLC measurements of serum ROL; C) ELISA measurement of serum RBP4; D) Serum ROL:RBP4 ratio; E) HPLC measurements of RE in liver; F) HPLC measurements of ROL in liver; G) ELISA measurements of RBP4 in liver; H) HPLC measurements of ROL in WAT; and I) HPLC measurements of ROL in lung. Letters shared between groups indicate no significant difference. Statistical significance was tested by applying a Student’s t test to each group compared with each other group. Data present values from n = 6 per genotype and dietary condition.
STRA6 knockout does not affect vitamin A homeostasis of blood, white adipose tissue, and lung
To investigate effects of STRA6 deficiency on circulating vitamin A levels, we measured retinoid and holo-RBP4 levels in serum of different dietary groups. HPLC analysis yielded no statistically significant differences in serum ROL levels (Fig. 2B). Moreover, ELISA determined that the serum RBP4 (Fig. 2C) and ROL/RBP4 ratio remained the same between different genotypes and dietary groups (Fig. 2D). HPLC analysis of hepatic retinoid content showed that mice that were subjected to VAD displayed ∼30-fold lower hepatic RE levels, which was mirrored by an accumulation of hepatic RBP4 (Fig. 2E–G). Of interest, a strong reduction in hepatic ROL levels was observed in Het−, but not KO− mice (Fig. 2F). In other body stores, such as white adipose tissue (WAT) and lung, Het− and KO− animals had largely depleted retinoids compared with Het+ and KO+, but there were no significant differences between genotypes (Fig. 2H, I).
STRA6 is critical for photoreceptor function and maintenance
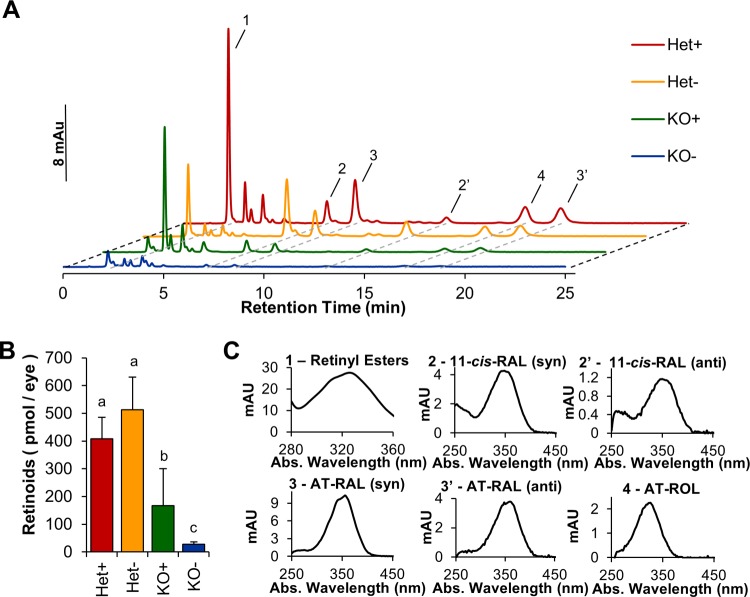
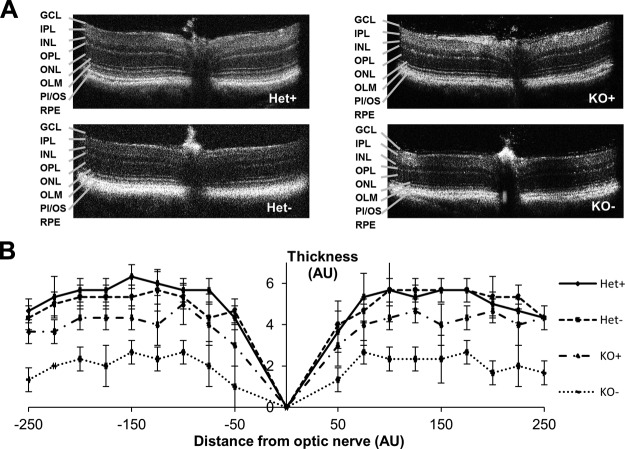
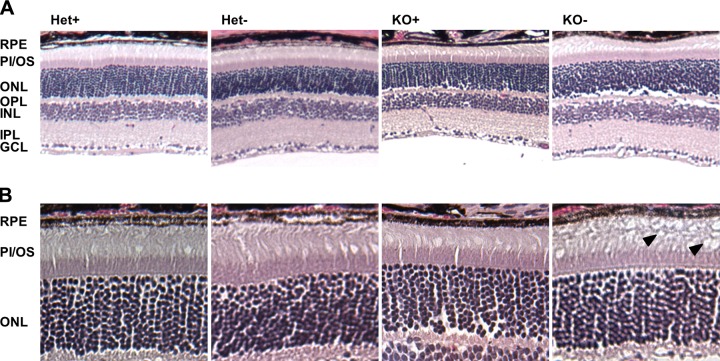
STRA6 is highly expressed in epithelia of blood–tissue barriers, such as the RPE of the retina, CP of the brain, and Sertoli cells of the testes (15, 24). HPLC analysis revealed that ocular retinoid levels were ∼3-fold lower in KO+ compared with Het+ controls (Fig. 3), and all retinoid metabolites of the visual cycle were detectable in the eyes of these mice (Supplemental Fig. S1). Under the VAD condition, ocular retinoids in KO mice were present near the lower limit of detection of our HPLC system (Fig. 3). In contrast, Het siblings were able to maintain ocular retinoid homeostasis (Fig. 3). When we analyzed OCT images acquired before euthanizing the mice, we observed that photoreceptor layer thickness reflected the retinoid status of the eyes. Thickness of the photoreceptor layer was reduced in KO− mice, and this reduction was seen throughout the retina (Fig. 4A, B). In contrast, Het− mice maintained normal photoreceptor layer thickness as measured by OCT (Fig. 4A, B). The critical requirement of STRA6 for photoreceptor maintenance also was confirmed in histologic, paraffin sections of postmortem eyes, which showed slight shortening in the photoreceptors in KO+ mice and deterioration of photoreceptors in KO− mice (Fig. 5). Thus, we concluded that STRA6 plays a pivotal role in maintaining ocular retinoid homeostasis even under conditions of continuous vitamin A supplementation. Vitamin A deprivation during adolescence induced retinoid deficiency of the eyes and thinning of photoreceptor outer segments in STRA6-deficient animals.
Figure 3.
Ocular vitamin A levels are affected by both genotype and diet. Comparison of ocular retinoid composition of Het and KO mice raised on VAS and VAD dietary regimens. A) Typical HPLC traces showing peaks for 1, REs; 2, 11-cis-retinal [11-cis-RAL (syn)]; 2′, 11-cis-RAL (anti); 3, all-trans-retinal (AT-RAL) (syn); 3′, AT-RAL (anti); 4, ROL. Red trace, Het+ mice; orange trace, Het− mice; green trace, KO+ mice; blue trace, KO− mice. B) Total ocular retinoids at the end of study of different genotypes and dietary regimens. C) Spectral characteristics of different retinoids detected in panel A. Letters shared between groups indicate no significant difference. Statistical significance was tested by applying a Student’s t test to each group compared with each other group. Data present values from n = 6 per genotype and dietary condition.
Figure 4.
Dietary vitamin A deprivation in KO mice results in thinning of the photoreceptor layer compared with Het mice raised on a VAS or VAD diet, or KO mice raised on a VAS diet. A) Typical OCT images of Het+, Het−, KO+, and KO− mice. B) Measurement of photoreceptor thickness from OCT images. AU, arbitrary units; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; PI/OS, photoreceptor inner/outer segments. Data present values from n = 6 per genotype and dietary condition.
Figure 5.
H&E-stained paraffin sections of retina from the eyes of Het and KO mice raised on a VAS or VAD diet; original magnification, ×10 (A), ×20 (B). Black arrows indicate deteriorated outer segments in KO− mice. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PI/OS, photoreceptor inner/outer segments.
STRA6 is mandatory for vitamin A homeostasis of brain and testis
STRA6 is also highly expressed in other epithelia of blood–tissue barriers, namely in Sertoli cells in the testis and the CP of the brain (11, 15, 24). Therefore, we analyzed retinoid levels in whole-brain homogenates, excluding the CP, via HPLC. As a result of the high lipid content of this organ, we subjected homogenates to saponification before analysis. We observed a significant reduction of ROL levels in the brains of KO+ mice, which became more pronounced in KO− mice. In addition, a significant reduction of ROL in the brain was observed in Het− mice compared with their isogenic Het+ siblings (Fig. 6A). This observation correlated with the regulation of the Stra6 mRNA expression in the brain. As expected for a gene responsive to retinoid signaling, Stra6 mRNA levels were significantly reduced in Het mice that were subjected to dietary deprivation of vitamin A (Fig. 6C). Of interest, we found no significant differences between groups with respect to RBP4 levels in CSF as measured by ELISA and using recombinant human RBP4 as standard (Fig. 6B, D). We performed quantitative RT-PCR analysis for Rbp4 expression in the brain. We used 400 ng cDNA from whole-brain homogenate and β-actin as a housekeeping gene and found very low levels of Rbp4 (30 ≥ Ct ≥34), which were consistent between groups.
Figure 6.
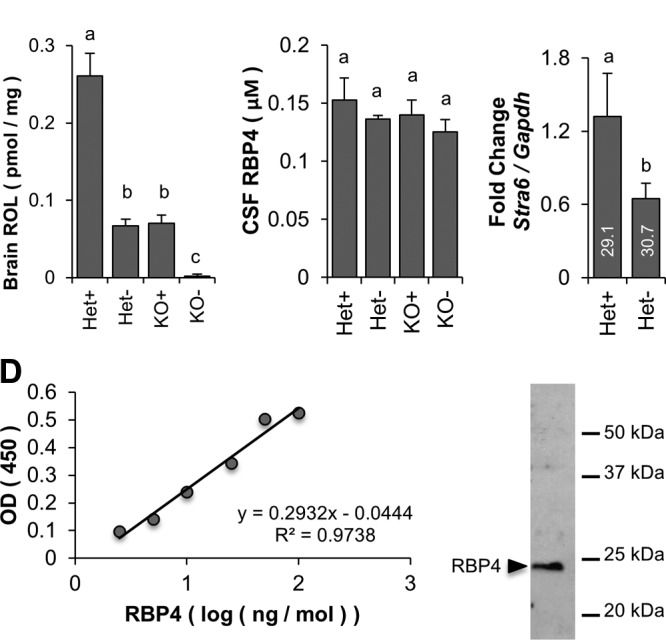
Vitamin A homeostasis of the brain is affected both by the Stra6 genotype and vitamin A content of the diet. A) HPLC measurements of ROL levels in whole-brain homogenates. B) ELISA measurement of RBP4 from CSF obtained from cisterna magna puncture. C) Quantitative RT-PCR measurement of Stra6 mRNA expression in Het mice subjected to feeding with VAD and VAS diet. Gapdh was used to normalize expression to a housekeeping gene. D) Standard curve for ELISA measurements with purified recombinant human RBP4. C) Immunoblot with purified recombinant human RBP4. Letters shared between groups indicate no significant difference. Statistical significance was tested by applying a Student’s t test to each group compared with each other group. Data present values from n = 6 per genotype and dietary condition.
Immunohistochemistry on paraffin sections of Het testes revealed that STRA6 was detectable in the Sertoli cell layer surrounding the testes (Fig. 7A–F). This epithelial cell layer provides nutrients, including vitamin A, to the testes and supports spermatogenesis (34). To assess whether STRA6 expression in Sertoli cells is required for vitamin A homeostasis in testes, we performed HPLC analyses with lipid extracts isolated from this organ. KO+ and Het+ mice displayed comparable levels of RE and ROL, which indicated that dietary vitamin A can maintain vitamin A homeostasis of the STRA6-deficient testes. This status changed when mice were subjected to vitamin A deprivation during adolescence. Retinoids were at the detection limit of the HPLC system in KO− mice. A slight but not significant reduction of retinoids was also observed in Het− testes (Fig. 7G). This correlated with a down-regulation of Stra6 mRNA levels in testes of Het− mice (Fig. 7H). Altered vitamin A homeostasis in the KO testes was mirrored in the expression of another vitamin A–responsive gene, the stimulated by retinoic acid gene product 8 (Stra8) (Fig 7H). This retinoic acid–inducible 45-kDa protein regulates meiotic initiation in spermatogenesis (35). Subsequent differentiation into spermatocytes is characterized by a finely coordinated expression of genes that drive spermatogenesis (36).
Figure 7.
Testicular vitamin A homeostasis and germ cell differentiation is affected by the Stra6 genotype. A–F) Immunohistochemistry on frozen sections of testes from Het mice stained with secondary antibody only (A–C) or a STRA6 and secondary antibody together (green; D–F). Sections were counterstained with DAPI (blue). White arrowheads indicate STRA6 protein expression in Sertoli cells of heterozygous mice. G) HPLC measurement of ROL and REs in lipophilic extracts of testes from Het and KO mice raised on VAS or VAD diets. H) Quantitative RT-PCR expression analysis of stimulated by retinoic acid genes in testes normalized to the Gapdh housekeeping gene. I) Quantitative RT-PCR analysis of expression of spermatogenesis marker genes in testes normalized to the housekeeping gene Gapdh. Letters shared between groups indicate no significant difference. Statistical significance was tested by applying a Student’s t test to each group compared with each other group. Data present values from n = 6 per genotype and dietary condition. AT-RAL, all-trans-retinal; c-KIT, tyrosine-protein kinase KIT; ID4, inhibitor of DNA binding 4; SOHLH1, spermatogenesis and oogenesis–specific basic helix-loop-helix 1; WT, wild-type. Original magnification, ×20.
To follow spermatogenesis in experimental animals, we selected marker genes for different stages in this process. Inhibitor of DNA binding 4 is a transcriptional repressor expressed specifically in spermatogonial stem cells (37). Tyrosine-protein kinase KIT is a member of the tyrosine receptor kinase family that is expressed in only differentiating and differentiated spermatogonia and is required for differentiation (38). Spermatogenesis and oogenesis–specific basic helix-loop-helix 1 is a transcriptional regulator of other genes necessary for spermatogonial differentiation, including tyrosine-protein kinase KIT, in progenitor and differentiating spermatogonia, but not in the spermatocyte (39). To determine the expression of these genes in our experimental groups, we isolated total RNA from testes and performed quantitative RT-PCR analyses with respective primer sets. In Het+ and KO+ mice, no difference was measured in the expression of these genes in testes. All 3 marker genes were significantly reduced in KO− mice. In contrast, testes from Het+ mice were able to maintain the expression of these genes devoted to spermatogenesis as a result of a functional Stra6 allele (Fig. 7I). Histologic sections of testes stained with H&E revealed no gross morphologic alteration of testes between genotypes and dietary groups (Supplemental Fig. S2). Furthermore, spermatocytes seemed to differentiate normally in DAPI-stained testicular sections in all groups under the applied conditions (Supplemental Fig. S2).
Vitamin A homeostasis is abrogated in aging, STRA6-null mice
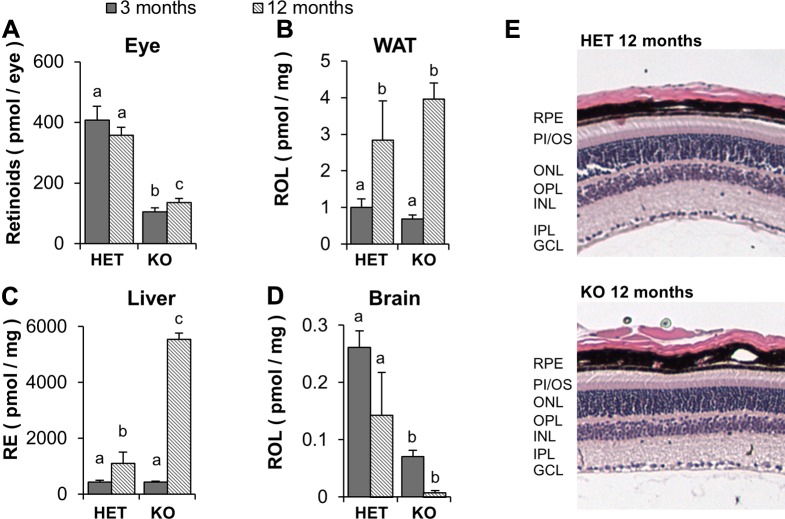
Our analyses indicated that STRA6 plays a pivotal role for vitamin A transport across blood–tissue barriers of the eyes, testis, and brain, in particular, under conditions of vitamin A deprivation. To examine consequences of STRA6 deficiency on global vitamin A homeostasis during aging, we maintained a cohort of KO and Het mice until these mice reached 12 mo of age on a chow diet that provided copious amounts of dietary vitamin A (29,000 IU/kg). We then analyzed retinoid levels in liver and peripheral tissues and compared them to 3-mo-old animals. These analyses revealed that liver stores for retinoids increased in aged mice. Liver RE levels in KO mice were 6-fold higher than those in age-matched Het siblings (Fig. 8). Conversely, KO mice displayed significantly lower levels of retinoids in peripheral tissues, such as the eye and the brain. In the eyes, total retinoid content was reduced ∼4-fold compared with Het siblings (Fig. 8A). Of note, all intermediates of the visual cycle were detected in KO mice, which indicated that the reduced retinoid content was not related to photoreceptor cell death. In fact, histologic sections of the eyes stained with H&E revealed photoreceptors are intact in aged KO mice (Fig. 8E). A similar phenomenon is evident in the brain. Analysis of retinoids of the brain in aged KO animals revealed a >10-fold reduction of retinoids compared with Het siblings. Of note, 12-mo-old KO mice displayed significantly lower retinoid levels in the brain than did 3-mo-old KO mice (Fig. 8D). Reduced retinoid levels in the brain of 12-mo-old mice were also observed in Het control mice (Fig. 8D). In contrast, retinoid content of WAT increased during aging and was comparable in KO and Het mice (Fig. 8B). Absence of an effect of STRA6 deficiency on retinoid content of WAT is consistent with the very low expression levels of the RBP4 receptor in this tissue (15, 26).
Figure 8.
The Stra6 genotype determines vitamin A homeostasis in the aging mouse. Het and KO mice were raised on a standard diet providing ample amounts of dietary vitamin A for 1 yr. A–D) HPLC analysis of ocular retinoids (A), retinoids (ROL and REs) in WAT (B), REs in liver (C), and ROL in brain (D). E) H&E-stained paraffin sections of the retina of the eyes of Het and KO mice. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PI/OS, photoreceptor inner/outer segments. Letters shared between groups indicate no significant difference. Statistical significance was tested by applying a Student’s t test to each group compared with each other group. Data present values from n = 6 per genotype and dietary condition. Original magnification, ×10.
DISCUSSION
STRA6 is highly expressed in epithelial barriers with tight junctions, including the RPE, the CP, the blood–brain barrier, and Sertoli cells of the testes (11, 15, 24, 40). These barrier epithelia have relatively small surface areas available for nutrient exchange. Thus, tissues separated from the blood stream by such barriers require a method for facilitating vitamin A import. In keeping with a critical role of STRA6 in vitamin A uptake, we found that the eyes of Het mice maintained retinoid homeostasis under VAD conditions, apparently as a result of the ability of STRA6 to take up circulating ROL from hepatic stores that are bound to RBP4. KO mice already showed significantly reduced retinoid levels on the VAS diet, which indicated that vitamin A from chylomicrons does not suffice to support ocular vitamin A homeostasis. This observation indicated that STRA6 is mandatory for maintenance of ocular retinoid homeostasis even when sufficient dietary vitamin A was available for a period of 12 mo. The dependence of the eyes on STRA6 became more evident when mice were subjected to VAD. KO combined with dietary VAD lead to an abrogation of ocular retinoid homeostasis and ultimately resulted in deterioration of photoreceptors. This finding clearly demonstrated that STRA6 is critical to acquire vitamin A from circulating holo-RPB4 and that vitamin A from chylomicron and/or passive diffusion of vitamin A from its RBP4 carrier cannot substitute for absence of STRA6 in the RPE.
Similarly, vitamin A levels were significantly reduced in the brains of KO+ mice and even more so in KO− mice, which were unable to acquire vitamin A from circulating holo-RBP4, the only available source. Brains from Het− mice displayed significantly higher levels of retinoids than did KO− mice. These Het− mice maintained neuronal vitamin A levels under vitamin A deprivation but at a lower level compared with their Het+ siblings. These results suggest that both dietary and stored vitamin A is used by the brain to maintain optimal levels in this organ.
In contrast to data from the eyes and brain, STRA6 in the Sertoli cells of the testes seems to serve as a back-up source of vitamin A rather than as a mandatory nutrient transport system. Here, dietary vitamin A was sufficient to maintain homeostasis in the absence of STRA6 as indicated by comparable retinoid levels in KO and Het mice. The difference in the brain might be explained by the larger surface-to-volume ratio of this organ, which allows retinoid uptake by a nonspecific mechanism. Testicular retinoid levels in KO−, but not Het−, mice significantly decrease compared with their VAS siblings after 12 wk. This finding indicates that STRA6 becomes critical in the testes when no dietary vitamin A is supplied. Consistent with this result, cessation of dietary vitamin A supply caused a reduction in spermatogenesis marker gene expression in KO− mice (41). Other studies investigating the consequences of VAD in the testes also found that spermatogenesis is dependent on vitamin A availability. Of note, effect of dietary vitamin A deprivation did manifest at a much later time point in the previous study (42). Thus, STRA6 seems to provide a fail-safe mechanism to protect spermatogenesis and ensure the production of progeny even when vitamin A is in short supply. Of interest, VAD in mice that lack LRAT, a protein that acts downstream of STRA6 in vitamin A uptake and promotes STRA6 function, inhibits meiotic initiation, which corroborates the importance of STRA6-mediated ROL transport for reproductive health of male mice (43). The observed reduction of vitamin A levels in KO− mice and the down-regulation of marker genes of spermatogenesis support a critical role for STRA6 in vitamin A homeostasis of germ cells in times of vitamin A deficiency. Of note, our Stra6−/− mice breed normally and viable pups seem to be born at a mendelian ratio when maintained on vitamin A–rich breeder chow (15). Investigations as to whether a VAD diet can affect the fertility of Stra6−/− mice might be within the scope of future work.
The slightly different responses of eyes, brain, and testes to vitamin A deprivation might be explained by the regulation of Stra6 gene expression. In the eyes, where vitamin A is absolutely essential for function and photoreceptor integrity, Stra6 is expressed at very high levels in the RPE in a retinoid-independent manner (14, 15). Thus, even when VAD is sustained for 12 wk, RPE can import enough retinoids from holo-RBP4 to maintain vision. This mechanism makes vision independent from dietary vitamin A fluctuation as long as the hepatic stores remain. Conversely, Stra6 expression is down-regulated in brain and testes under dietary deprivation, as shown in this study, thus explaining decreased testicular and neuronal retinoid levels of Het− mice. This down-regulation is likely caused by the retinoic acid responsiveness of Stra6 mRNA expression in these tissues (14, 15). A recent study confirmed this regulation at the promoter level (32).
Furthermore, we investigated the effects of our experimental conditions in tissues with low levels of Stra6 mRNA expression, such as WAT and lung, or with no expression at all, such as liver (15, 26). In these tissues, we observed no differences between genotypes with regard to RE stores when dietary vitamin A is removed. Vitamin A levels of these tissues dropped during VAD independent of the Stra6 genotype. This finding indicates STRA6 in WAT and lung does not significantly import vitamin A when dietary supply is cut off. This finding is consistent with our previous observation that STRA6 expression in lung and WAT drops from low to nearly undetectable levels in VAD mice (14). Thus, STRA6-depenent ROL uptake in these tissues may be used primarily for clearance of holo-RBP4 when excessive amounts of vitamin A are present in the circulation. In fact, pulmonary Stra6 mRNA expression is induced by retinoic acid (14, 44). In addition, it has been shown in rodents that retinoic acid increases pulmonary retinoids in a STRA6- and LRAT-dependent fashion (14, 44).
Our analyses in aging mice suggest that STRA6 is critical for proper vitamin A homeostasis not only in the eyes, but also throughout the body. Ocular retinoid levels were not significantly different between 3- and 12-mo-old mice of the same genotype; however, there was a significant difference between Het and KO mice. Low ocular retinoid levels of KO mice indicated that even long-term dietary vitamin A supplementation is not sufficient to establish normal vision in STRA6-deficient eyes. Histology confirmed that the lack of ocular retinoids in aged KO mice is not a result of a loss of photoreceptors. In both Het and KO mice, retinoid levels in the brain decreased with age, which suggested both lipoprotein lipase-dependent acquirement of vitamin A from chylomicrons and STRA6-dependent transport across the blood–brain barrier may become less efficient over time. This finding was surprising because aging is normally associated with a loss of tight junction integrity in the blood–brain barrier (45). Thus, an additional mechanism may contribute to the reduced retinoid levels in the brains of aged mice compared with those of younger animals. Of note, there was a significant decrease in brain retinoid levels between Het and KO mice, which demonstrated that, similar to the eyes, STRA6 is also critical for brain retinoid homeostasis.
Most strikingly, aged KO mice accumulated significantly more liver RE than did aged Het mice. This accumulation of the ester form of vitamin A, in its major storage organ, is intriguing and might reflect a decreased delivery of dietary vitamin A to the periphery in STRA6 deficiency. In addition, the second putative receptor for RBP4, denoted as RBPR2 (RBP4 receptor 2), is expressed in some non-STRA6 expressing tissues, including the liver, colon, spleen, and throughout the intestine (46); however, whereas little is known about its role in vitamin A homeostasis, RBPR2 may contribute to reuptake of vitamin A from circulating holo-RBP4 in this pathologic condition. Finally, we noticed in aged mice that compartments such as WAT have comparable levels of retinoids in aged KO and control mice, which suggests KO mice are not able to compensate for the hepatic retinoid overflow by diverting retinoids to other compartments.
Together, our studies revealed that STRA6 biology is an elegant example of nature’s engineering exploits to evolve complex regulatory networks. Our findings indicate that the role of RBP4 receptor in vitamin A homeostasis varies depending on tissue type and environmental condition. To this end, Stra6 is highly expressed in epithelia that constitute a blood–tissue barrier, especially in the RPE. In the eyes, which have the highest vitamin A requirement in the body, retinoid-independent Stra6 expression decouples ocular vitamin A supply from its dietary fluctuation. This regulation is critical for vision because photoreceptor function and survival depends on this nutrient.
In some nonocular tissues, such as testis and brain, Stra6 expression is higher than in most other tissues, but is regulated by retinoids. This regulation seemingly reduces ROL consumption of these tissues when its dietary supply is limited, which saves ROL to be used in the eye. Other peripheral tissues, such as lung and WAT, have a very low expression of STRA6 and seem to depend on dietary vitamin A, which they are able to store. In addition, these tissues may play a role in quickly removing vitamin A from the circulation when the body is flooded with retinoids (14).
The previously described coupling of STRA6-dependent vitamin A uptake with LRAT provides an additional layer of regulation and fine tuning in tissues with a high vitamin A demand (14). Thus, STRA6 acts as a biologic faucet, which allows for a higher flux of vitamin A into cells and tissues than is possible when this process is reliant on passive diffusion. The elaborated mechanisms of the opening and closing of this faucet ensures proper distribution of vitamin A to peripheral tissues and is regulated by demand and availability. It will be fascinating to discover further molecular details of this diet-responsive regulatory network in future studies.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) National Eye Institute (Grant EY020551); NIH Visual Sciences Training Program (Grant T32 EY007157; to M.K.); and histology was performed by the Visual Sciences Research Center core (Case Western Reserve University; NIH Grant P30-EY11373).
Glossary
- CP
choroid plexus
- CSF
cerebrospinal fluid
- H&E
hematoxylin and eosin
- Het
heterozygous Stra6 (Stra6+/−)
- Het+
Het raised on VAS diet
- Het−
Het raised on VAD diet
- KO
Stra6 knockout (Stra6−/−)
- KO+
KO raised on VAS diet
- KO−
KO raised on VAD diet
- LRAT
lecithin retinol acyl transferase
- MWS
Matthew Wood syndrome
- OCT
optical coherence tomography
- PBS-T
PBS-Tween 20
- RBP4
retinol binding protein 4
- RE
all-trans-retinyl ester
- ROL
all-trans-retinol
- RPE
retinal pigment epithelium
- STRA6
stimulated by retinoic acid 6
- TBS-T
Tris-buffered saline-Tween 20
- VAD
vitamin A–deficient
- VAS
vitamin A–sufficient
- WAT
white adipose tissue
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Napoli J. L. (2012) Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta 1821, 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Ambrosio D. N., Clugston R. D., Blaner W. S. (2011) Vitamin A metabolism: an update. Nutrients 3, 63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Lintig J. (2012) Metabolism of carotenoids and retinoids related to vision. J. Biol. Chem. 287, 1627–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomhoff R., Green M. H., Berg T., Norum K. R. (1990) Transport and storage of vitamin A. Science 250, 399–404 [DOI] [PubMed] [Google Scholar]
- 5.Quadro L., Blaner W. S., Salchow D. J., Vogel S., Piantedosi R., Gouras P., Freeman S., Cosma M. P., Colantuoni V., Gottesman M. E. (1999) Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 18, 4633–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Episkopou V., Maeda S., Nishiguchi S., Shimada K., Gaitanaris G. A., Gottesman M. E., Robertson E. J. (1993) Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc. Natl. Acad. Sci. USA 90, 2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bok D., Heller J. (1976) Transport of retinol from the blood to the retina: an autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein. Exp. Eye Res. 22, 395–402 [DOI] [PubMed] [Google Scholar]
- 8.Heller M., Bok D. (1976) A specific receptor for retinol binding protein as detected by the binding of human and bovine retinol binding protein to pigment epithelial cells. Am. J. Ophthalmol. 81, 93–97 [DOI] [PubMed] [Google Scholar]
- 9.Kelly M., von Lintig J. (2015) STRA6: role in cellular retinol uptake and efflux. Hepatobiliary Surg. Nutr. 4, 229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivaprasadarao A., Findlay J. B. (1988) The interaction of retinol-binding protein with its plasma-membrane receptor. Biochem. J. 255, 561–569 [PMC free article] [PubMed] [Google Scholar]
- 11.Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. (2007) A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi R., Yu J., Ter-Stepanian M., Zhong M., Cheng G., Yuan Q., Jin M., Travis G. H., Ong D., Sun H. (2011) Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem. Biol. 6, 1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isken A., Golczak M., Oberhauser V., Hunzelmann S., Driever W., Imanishi Y., Palczewski K., von Lintig J. (2008) RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amengual J., Golczak M., Palczewski K., von Lintig J. (2012) Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 287, 24216–24227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amengual J., Zhang N., Kemerer M., Maeda T., Palczewski K., Von Lintig J. (2014) STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum. Mol. Genet. 23, 5402–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz A., Winston A., Lim Y. H., Gilbert B. A., Rando R. R., Bok D. (1999) Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 274, 3834–3841 [DOI] [PubMed] [Google Scholar]
- 17.Ong D. E., Kakkad B., MacDonald P. N. (1987) Acyl-CoA-independent esterification of retinol bound to cellular retinol-binding protein (type II) by microsomes from rat small intestine. J. Biol. Chem. 262, 2729–2736 [PubMed] [Google Scholar]
- 18.Pasutto F., Sticht H., Hammersen G., Gillessen-Kaesbach G., Fitzpatrick D. R., Nürnberg G., Brasch F., Schirmer-Zimmermann H., Tolmie J. L., Chitayat D., Houge G., Fernández-Martínez L., Keating S., Mortier G., Hennekam R. C., von der Wense A., Slavotinek A., Meinecke P., Bitoun P., Becker C., Nürnberg P., Reis A., Rauch A. (2007) Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 80, 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golzio C., Martinovic-Bouriel J., Thomas S., Mougou-Zrelli S., Grattagliano-Bessieres B., Bonniere M., Delahaye S., Munnich A., Encha-Razavi F., Lyonnet S., Vekemans M., Attie-Bitach T., Etchevers H. C. (2007) Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am. J. Hum. Genet. 80, 1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chassaing N., Golzio C., Odent S., Lequeux L., Vigouroux A., Martinovic-Bouriel J., Tiziano F. D., Masini L., Piro F., Maragliano G., Delezoide A.-L., Attié-Bitach T., Manouvrier-Hanu S., Etchevers H. C., Calvas P. (2009) Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum. Mutat. 30, E673–E681 [DOI] [PubMed] [Google Scholar]
- 21.Casey J., Kawaguchi R., Morrissey M., Sun H., McGettigan P., Nielsen J. E., Conroy J., Regan R., Kenny E., Cormican P., Morris D. W., Tormey P., Chróinín M. N., Kennedy B. N., Lynch S., Green A., Ennis S. (2011) First implication of STRA6 mutations in isolated anophthalmia, microphthalmia, and coloboma: a new dimension to the STRA6 phenotype. Hum. Mutat. 32, 1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou C. M., Nelson C., Tarlé S. A., Pribila J. T., Bardakjian T., Woods S., Schneider A., Glaser T. (2015) Biochemical basis for dominant inheritance, variable penetrance, and maternal effects in RBP4 congenital eye disease. Cell 161, 634–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeliger M. W., Biesalski H. K., Wissinger B., Gollnick H., Gielen S., Frank J., Beck S., Zrenner E. (1999) Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest. Ophthalmol. Vis. Sci. 40, 3–11 [PubMed] [Google Scholar]
- 24.Berry D. C., Jacobs H., Marwarha G., Gely-Pernot A., O’Byrne S. M., DeSantis D., Klopfenstein M., Feret B., Dennefeld C., Blaner W. S., Croniger C. M., Mark M., Noy N., Ghyselinck N. B. (2013) The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J. Biol. Chem. 288, 24528–24539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry D. C., Jin H., Majumdar A., Noy N. (2011) Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. USA 108, 4340–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemany L., Kraus B. J., Norseen J., Saito T., Peroni O. D., Johnson R. L., Kahn B. B. (2014) Downregulation of STRA6 in adipocytes and adipose stromovascular fraction in obesity and effects of adipocyte-specific STRA6 knockdown in vivo. Mol. Cell. Biol. 34, 1170–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muenzner M., Tuvia N., Deutschmann C., Witte N., Tolkachov A., Valai A., Henze A., Sander L. E., Raila J., Schupp M. (2013) Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol. Cell. Biol. 33, 4068–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz A., Mark M., Jacobs H., Klopfenstein M., Hu J., Lloyd M., Habib S., Tosha C., Radu R. A., Ghyselinck N. B., Nusinowitz S., Bok D. (2012) Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest. Ophthalmol. Vis. Sci. 53, 3027–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terra R., Wang X., Hu Y., Charpentier T., Lamarre A., Zhong M., Sun H., Mao J., Qi S., Luo H., Wu J. (2013) To investigate the necessity of STRA6 upregulation in T cells during T cell immune responses. PLoS One 8, e82808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Duff K. (2008) A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J. Vis. Exp. 21, 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young P. A., Leonard S., Martin D. S., Findlay J. B. (2016) The effect of retinol binding protein on the proteome of C2C12 muscle cells. Diabetes Metab. Res. Rev. 32, 379–390 [DOI] [PubMed] [Google Scholar]
- 32.Laursen K. B., Kashyap V., Scandura J., Gudas L. J. (2015) An alternative retinoic acid-responsive Stra6 promoter regulated in response to retinol deficiency. J. Biol. Chem. 290, 4356–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golczak M., Maeda A., Bereta G., Maeda T., Kiser P. D., Hunzelmann S., von Lintig J., Blaner W. S., Palczewski K. (2008) Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 283, 9543–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogarth C. A., Griswold M. D. (2010) The key role of vitamin A in spermatogenesis. J. Clin. Invest. 120, 956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson E. L., Baltus A. E., Roepers-Gajadien H. L., Hassold T. J., de Rooij D. G., van Pelt A. M., Page D. C. (2008) Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 105, 14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S. R., Liu Y. X. (2015) Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 149, R159–R167 [DOI] [PubMed] [Google Scholar]
- 37.Oatley M. J., Kaucher A. V., Racicot K. E., Oatley J. M. (2011) Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol. Reprod. 85, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshinaga K., Nishikawa S., Ogawa M., Hayashi S., Kunisada T., Fujimoto T., Nishikawa S. (1991) Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113, 689–699 [DOI] [PubMed] [Google Scholar]
- 39.Suzuki H., Ahn H. W., Chu T., Bowden W., Gassei K., Orwig K., Rajkovic A. (2012) SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev. Biol. 361, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouillet P., Sapin V., Chazaud C., Messaddeq N., Décimo D., Dollé P., Chambon P. (1997) Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Dev. 63, 173–186 [DOI] [PubMed] [Google Scholar]
- 41.Clagett-Dame M., Knutson D. (2011) Vitamin A in reproduction and development. Nutrients 3, 385–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boucheron-Houston C., Canterel-Thouennon L., Lee T. L., Baxendale V., Nagrani S., Chan W. Y., Rennert O. M. (2013) Long-term vitamin A deficiency induces alteration of adult mouse spermatogenesis and spermatogonial differentiation: direct effect on spermatogonial gene expression and indirect effects via somatic cells. J. Nutr. Biochem. 24, 1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Palczewski K., Baehr W., Clagett-Dame M. (2011) Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol. Reprod. 84, 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu L., Ross A. C. (2010) Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J. Lipid Res. 51, 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elahy M., Jackaman C., Mamo J. C., Lam V., Dhaliwal S. S., Giles C., Nelson D., Takechi R. (2015) Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 12, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alapatt P., Guo F., Komanetsky S. M., Wang S., Cai J., Sargsyan A., Rodriguez Diaz E., Bacon B. T., Aryal P., Graham T. E. (2012) Liver retinol transporter and receptor for serum retinol binding protein (RBP4). J. Biol. Chem. 288, 1250–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]