Abstract
Purpose
Pharmaceutical formulation and treatment process attributes, such as dose frequency and route of administration, can have an impact on quality of life, treatment adherence, and disease outcomes. The aim of this literature review was to examine studies on preferences for pharmaceutical treatment process attributes, focusing on research in diabetes, oncology, osteoporosis, and autoimmune disorders.
Methods
The literature search focused on identifying studies reporting preferences for attributes of the pharmaceutical treatment process. Studies were required to use formal quantitative preference assessment methods, such as utility valuation, conjoint analysis, or contingent valuation. Searches were conducted using Medline, EMBASE, Cochrane Library, Health Economic Evaluation Database, and National Health Service Economic Evaluation Database (January 1993–October 2013).
Results
A total of 42 studies met inclusion criteria: 19 diabetes, nine oncology, five osteoporosis, and nine autoimmune. Across these conditions, treatments associated with shorter treatment duration, less frequent administration, greater flexibility, and less invasive routes of administration were preferred over more burdensome or complex treatments. While efficacy and safety often had greater relative importance than treatment process, treatment process also had a quantifiable impact on preference. In some instances, particularly in diabetes and autoimmune disorders, treatment process attributes had greater relative importance than some or all efficacy and safety attributes. Some studies suggested that relative importance of treatment process depends on disease (eg, acute vs chronic) and patient (eg, injection experience) characteristics.
Conclusion
Despite heterogeneity in study methods and design, some general patterns of preference clearly emerged. Overall, the results of this review suggest that treatment process has a quantifiable impact on preference and willingness to pay for treatment, even in many situations where safety and efficacy were the primary concerns. Patient preferences for treatment process attributes can inform drug development decisions to better meet the needs of patients and deliver improved outcomes.
Keywords: preference, treatment process, pharmaceutical formulation, conjoint, utility, contingent valuation
Introduction
The effectiveness of pharmaceutical treatments depends not only on the chemical properties of the medication, but also on how medication is formulated and administered. Differences in treatment regimen and treatment process can have a profound effect on how patients experience pharmaceutical therapy. For example, while some medications are administered orally as tablets or capsules, others require intravenous (IV) administration in a hospital setting. Furthermore, treatment regimens can vary in terms of dose frequency and dose flexibility, including whether medications need to be taken with meals. These pharmaceutical formulation and treatment process attributes (subsequently referred to as “process attributes”) can impact patient adherence, and therefore indirectly affect the efficacy and safety of a medication.1–5 They can also have a direct effect on how patients experience treatment, which can impact health-related quality of life.
One way to examine and quantify the importance that patients place on the treatment process attributes is to use formal preference assessment methods, such as health state utility valuation and discrete choice experiments. These approaches permit quantitative comparison of the relative importance that patients place on a set of treatment attributes. While a substantial amount of research has documented the impact of efficacy and safety on patient preference for various medication options,6–9 less is known about the importance of treatment process attributes. Still, a smaller growing body of research has consistently highlighted the importance of how medications are taken.10–12 In addition, studies that include efficacy and/or safety attributes along with treatment process attributes can also quantify patients’ willingness to accept a risk of adverse events or reduced treatment benefit for the sake of improved comfort or convenience.
The aim of this literature review was to identify and examine published studies presenting preferences for pharmaceutical treatment process attributes. To facilitate synthesis of findings across studies, this review focused only on studies using formal preference assessment methodologies that provide a quantitative estimate of the value of treatment process attributes. Findings from these studies should have direct relevance to researchers working in drug development because results can provide insight into the value that patients place on treatment process attributes. Results may also aid clinicians in selecting treatments with attributes that have the potential to enhance treatment adherence.
Methods
Preference assessment methods
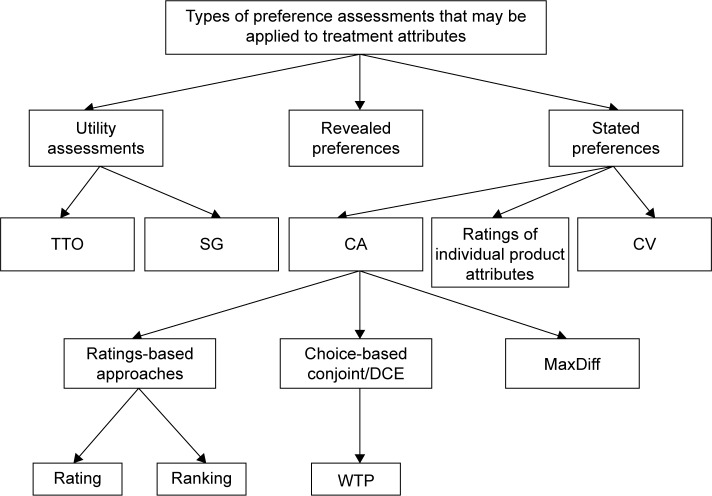
This review focused on studies that have used a range of methodologies to assess and quantify preference for process attributes. Preference assessment methods can be grouped into three broad categories (Figure 1). Stated preferences are derived from surveys or interviews with an experimental design such as conjoint or contingent valuation studies. Stated preference methods allow researchers to focus on specific attributes, control the way preferences are elicited, and assess preferences for hypothetical products.13–15 Results of these stated preference studies often allow researchers to compare the relative influence of multiple factors on patient preference. A second method commonly used in health care research is the health state utility assessment in which patients or members of the general public perform choice-based tasks to indicate their preferences for their own current health or descriptions of hypothetical health states (often called scenarios or vignettes).16–18 These methods yield utility values on a scale anchored to dead (0) and full health (1) that represent the strength of preferences for various health states, and may be used in cost-utility analyses. Utility studies most frequently focus on quantifying health status, symptoms, and treatment outcomes, but they have also been used to quantify preferences for treatment attributes and treatment processes.10,19 Revealed preferences are derived from actual observed market activities and real-world behavior.14
Figure 1.
Preference assessment methods.
Notes: This review focused on quantitative controlled studies examining preference for treatment process attributes. Preference assessment methods in the reviewed studies included both types of utility studies (TTO and SG), conjoint analysis (including DCE, conjoint with willingness to pay, and MaxDiff), and contingent valuation studies. MaxDiff is a form of conjoint analysis in which participants are asked to select attributes that are most and least important when making tradeoffs between treatments.32 Abbreviations: TTO, time trade-off; SG, standard gamble; CA, conjoint analysis; CV, contingent valuation; DCE, discrete-choice experiment; WTP, willingness to pay.
The current literature search was designed to identify stated preference studies and utility studies because these methods can provide a quantitative assessment of specific treatment process attributes. Although revealed preference data can provide an indication of trends across large samples, this methodology is not well suited for identifying preference among specific treatment process attributes. Consequently, the current literature search did not aim to identify revealed preference studies.
Literature search methods
Literature searches were conducted in the following databases: PubMed, EMBASE, Cochrane Library, Health Economic Evaluation Database, and National Health Service Economic Evaluation Database. The list of search terms was developed to identify articles that include the selected methods (ie, stated preference or utility assessment) and attributes related to treatment process. The following search terms (applied to article title and abstract) were intended to identify studies using the relevant preference methods: stated preference(s), time trade-off, TTO, time trade off, standard gamble, conjoint, contingent valuation, discrete choice, discrete-choice, willingness to pay, and willingness-to-pay. Treatment process search terms were intended to identify attributes related to route of administration, dose frequency, dose timing, dose size, convenience, and other process attributes. A full list of treatment process search terms is provided in the Supplementary material.
The search was limited to studies published in English between January 1, 1993 and October 16, 2013. Full-text primary articles were eligible for inclusion. Conference abstracts, editorials, and letters to the editor were excluded. Articles were considered for inclusion if they had both a preference methodology term and a process term. Articles were included if they evaluated preferences for one or more treatment attributes through utility, conjoint, contingent valuation, and/or discrete choice. Articles were excluded if they evaluated preferences for only efficacy and/or safety attributes (without assessment of preferences for treatment attributes, treatment processes, or treatment experience) or if they evaluated preferences through revealed preference rather than stated preference or utility methods. This review included studies examining treatment preferences from the patient perspective (either from patients themselves or nurses as patient proxies) and from general population participants.
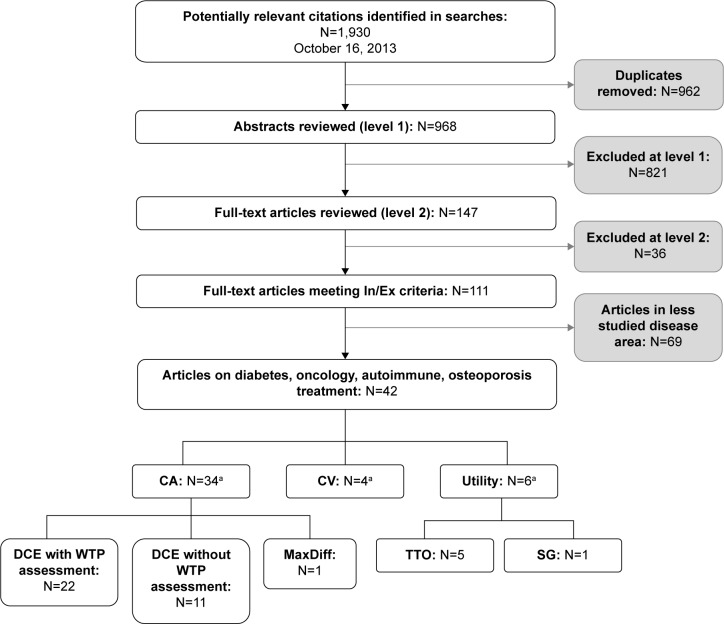
Abstracts of potential studies identified during the literature search (n=968) were screened and examined with regard to the inclusion/exclusion criteria. For any abstract that could not be confidently excluded, full-text articles were obtained and reviewed (n=147). A total of 111 articles met the criteria for inclusion (Figure 2). Four therapeutic areas were selected for detailed review (ie, diabetes, autoimmune disease, oncology, and osteoporosis) because these were areas with a substantial number of published articles, a range of disease severity, and a variety of treatment process attributes.
Figure 2.
Summary of literature search results.
Note: aSome articles presented more than one method of assessment.
Abbreviations: In, inclusion; Ex, exclusion; CA, conjoint analysis; CV, contingent valuation; DCE, discrete-choice experiment; WTP, willingness to pay; TTO, time trade-off; SG, standard gamble.
Data extraction methods
After articles were selected for inclusion, study characteristics were extracted and organized into table shells so that findings could be examined and summarized across studies. For each article, the following characteristics were captured in the data extraction tables: therapeutic area (diabetes, autoimmune disease, oncology, or osteoporosis), preference assessment method (conjoint, utility, contingent valuation, or multiple methods), respondent samples (patients, proxy, or general population), treatment process attribute results (route of administration, dose frequency, dose timing, dose size, treatment duration, and other), and comparison of treatment process attributes vs efficacy and safety.
As much as possible, an effort was made to present results consistently across studies, including preference for levels within each attribute and relative importance across attributes. However, the level of detail and presentation of results in the source articles varied greatly, and therefore, it was not always possible to extract the same quality or depth of information across studies.
Results
Included articles
A total of 42 studies met inclusion criteria in the following disease areas: 19 diabetes, nine oncology, five osteoporosis, and nine autoimmune. The most commonly used type of preference assessment method was conjoint analysis (n=34), which includes discrete choice experiments (DCEs) with willingness to pay assessment (n=22), DCEs without willingness to pay assessment (n=11), and one MaxDiff study. Other preference methods included utility assessments (n=6) and contingent valuation (n=4). These study methodological categories are not mutually exclusive. For example, there were studies that used both DCE and utility assessment methodology.48 Figure 1 lists three types of stated preference studies, two of which (conjoint analysis and contingent valuation) were identified in the current literature search. No stated preference studies examining ratings of individual product attributes outside the context of a larger treatment profile met the current inclusion criteria.
Most of the studies (n=33) were conducted in patient samples, although some were conducted with general population respondents (n=3) or nurses (n=1) serving as patient proxies. One study included both patient and general population respondents.34 Figure 2 summarizes article categorization, and Table 1 presents the clinical condition, preference assessment method, respondent sample, and results for each study.
Table 1.
Summary of studies included in the review
| Citation | Clinical condition | Preference assessment method | Respondent samples | Comparison of levels within treatment process attributes
|
Comparison of treatment process attributes to efficacy and safety
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Route of administration | Dose frequency | Dose timing | Dose size | Treatment duration | Other | Efficacy | Safety | ||||
| Diabetes studies | |||||||||||
| Aristides et al41 | Type 2 | DCE with WTP | Patients | E1* | − | + | |||||
| Bogelund et al20 | Type 2 | DCE with WTP | Patients | E1* | E* | E2* | + | ± | |||
| Boye et al33 | Type 2 | Utility (SG) | Patients | E* | NS2 | − | |||||
| Casciano et al31 | Type 1, Type 2 | DCE without WTP | Patients | E1, p = NR | X | ± | ± | ||||
| Chancellor et al26 | Type 1, Type 2 | Utility (TTO) | Patients | E3* | |||||||
| Evans et al34 | Type 1, Type 2 | Utility (TTO) | General population and patients | General population sample = E* Diabetes sample = E* |
General population sample: Basal only regimen = E2* Basal bolus regimen = E2* Diabetes sample: Basal only regimen = E2* Basal bolus regimen = NS |
||||||
| Guimaraes et al23 (results also reported in Guimaraes et al56,57) | Type 1, Type 2 | DCE with WTP | Patients | E1* E3* E4* |
+ | + | |||||
| Hauber et al52 | Type 2 | DCE with WTP | Patients | Inconclusive: confounded with dose size | Inconclusive: confounded with dose frequency | + | ± | ||||
| Jendle et al25 (results also reported in Jendle et al24) | Type 2 | DCE with WTP | Patients | E1* | E* | + | ± | ||||
| Lloyd et al36 | Type 1, Type 2 | DCE with WTP | Patients | E* | + | − | |||||
| Mohamed et al42 | Type 2 | DCE without WTP | Patients | Pills once a day: Swedish sample = NS German sample = NS Pills twice a day: Swedish sample = NS German sample = E* |
+ | ± | |||||
| Pinto et al58 | Type 1, Type 2 | Contingent valuation | Patients | NS3 | |||||||
| Polster et al48 | Type 2 | DCE without WTP Utility (TTO) | Patients | X | X | + | + | ||||
| Porzsolt et al49 | Type 1, Type 2 | DCE without WTP | Patients | E2, p = NR | + | + | |||||
| Sadri27 | Not specified | Contingent valuation | General population | E3* | |||||||
| Sadri et al28 | Type 1, Type 2 | Contingent valuation | Patients | E3* | |||||||
| Cancer treatment studies | |||||||||||
| Aristides et al45 | Advanced colorectal cancer | DCE with WTP | Proxy (oncology nurses) | NS | + | ||||||
| Bridges et al46 | Non-small-cell lung cancer | DCE without WTP | Patients | NSS | + | + | |||||
| Matzaet al19 | Cancer with bone metastases | Utility (TTO) | General population | E2* | E* | Renal monitoring blood test = E* | |||||
| Shafey et al50 | Relapsed follicular non-Hodgkins lymphoma | DCE without WTP | Patients | NS7 | NS | NS | + | + | |||
| Wong et al51 | Renal cell carcinoma | DCE without WTP | Patients | E5* | NS | NS | Taken with or without food = NS | + | + | ||
| Cancer supportive care studies | |||||||||||
| Langer et al47 | Solid tumors and anemia | DCE without WTP | Patients and providers (not discussed in current report) | Number of visits = U* | + | ||||||
| Ossa et al30 | Chemotherapy-related anemia | DCE with WTP | General population | E6* NS8 | E* | Administration in GP office or hospital = E* Administration in home or hospital = NS |
+ | + | |||
| Sung et al59 | Febrile neutropenia | DCE without WTP | Patients and parents of patients (not discussed in current report) | Inconclusive: confounded with inpatient/outpatient | Inconclusive: confounded with route of administration | ||||||
| Teuffel et al60 | Febrile neutropenia | Utility (TTO) Contingent valuation | Patients | NSS | E, p = NR | ||||||
| Autoimmune studies | |||||||||||
| Augustovski et al38 | Rheumatoid arthritis | DCE with WTP | Patients | ES NSI | Every 10 months vs every month = E* Every 10 months vs every week = E* Every 10 months vs every day = E* Every month vs every week = U |
± | ± | ||||
| Hauber et al35 | Plaque psoriasis | DCE with WTP | Patients | Inconclusive | E* | ||||||
| Hodgkins et al40 | Ulcerative colitis | DCE with WTP | Patients | Total sample = E* Canadian sample = E* UK sample = E* German sample = NS US sample = NS |
Total sample = E* Canadian sample = NS UK sample = E* German sample = NS US sample = E* |
+ | + | ||||
| Lichtenstein et al54 | Crohn’s disease | DCE without WTP | Patients | X | X | X | ± | ||||
| Ozdemir et al37 | Rheumatoid arthritis | DCE with WTP | Patients | Inconclusive | E* | ||||||
| Schaarschmidt et al53 | Psoriasis | DCE with WTP | Patients | X | X | X | Location of treatment = X | ± | − | ||
| Schaarschmidt et al44 | Psoriasis | DCE with WTP | Patients | X | X | X | Location of treatment = X | ||||
| Schmieder et al43 | Psoriasis | DCE with WTP | Patients | X | X | X | Location of treatment = X | ± | − | ||
| Shingler et al6 | Relapsing remitting MS | DCE without WTP | Patients | Ease of use = E* | |||||||
| Osteoporosis studies | |||||||||||
| Darba et al29 | Osteoporosis | DCE with WTP | Patients | U1* E2* |
At home with medical support vs admitted to hospital for administration = E* Self-administration at home vs administration at home with medical support = E* |
||||||
| de Bekker-Grob et al22 (results also reported in de Bekker-Grob et al21) | Osteoporosis | DCE with WTP | Patients | E1* | Oral = E* Injection = X |
E* | ± | + | |||
| Fraenkel et al39 | Osteoporosis | DCE with WTP | Patients | Inconclusive | Inconclusive | Inconclusive | − | − | |||
| Silverman et al32 | Osteoporosis | MaxDiff | Patients | E1, p = NR Inconclusive6 Inconclusive7 |
U, p = NR | + | + | ||||
Notes:
The preference is statistically significant. NS, the preference is not statistically significant, but there is a preference trend. p = NR, the statistical significance is not reported.
Route of administration: E, expected order of preference was found; U, unexpected order of preference was found; X, preference between levels of route of administration is not presented; blank, route of administration was not examined in this study; I, oral vs injection, 2, injection vs infusion, 3, inhaled vs injection, 4, oral vs inhaled, 5, oral vs infusion, 6, subcutaneous injection vs IV injection, 7, oral vs IV injections, and 8, IV injection vs cannula injection.
Dose frequency: E, expected order of preference was found indicating that less frequency dosing was preferred over more frequent dosing; U, unexpected order of preference was found; X, preference between levels of dose frequency is not presented; blank, dose frequency was not examined in this study.
Dose timing: E, expected order of preference was found; U, unexpected order of preference was found; X, preference between levels of dose timing not presented; blank, dose timing was not examined in this study; I, injecting immediately before meals vs injecting 30–45 minutes before meals, 2, more flexible timing (eg, any time of day) vs less flexible timing (eg, with meals).
Dose size: E, expected order of preference was found indicating that smaller dose size is preferred over a larger dose size; E, fewer tablets preferred over a greater number of tablets; U, unexpected order of preference found; X, preference between levels of dose size is not presented; blank, dose size was not examined in this study.
Treatment duration: E, expected order of preference was found indicating that shorter treatment duration was preferred over longer treatment duration; U, unexpected order of preference was found; X, preference between levels of treatment duration was not presented; blank, treatment duration was not examined in this study.
Other: E, expected order for the indicated attribute was found; U, unexpected order of preference for the indicated attribute was found; X, preference between levels of the indicated attribute was not presented; blank, other treatment process attributes were not examined in this study.
Efficacy: +, efficacy was more important than all treatment process attributes; −, treatment process attributes were more important than efficacy; ±, mixed results; blank, no comparison between treatment process attributes and efficacy.
Safety: +, safety was more important than all treatment process attributes; −, treatment process attributes were more important than safety; ±, mixed results; blank, no comparison between treatment process attributes and safety.
Abbreviations: DCE, discrete-choice experiment; WTP, willingness to pay; TTO, time trade-off; IV, intravenous; GP, general practitioner; MS, multiple sclerosis; SG, standard gamble.
Treatment process attributes
The most common treatment process attributes examined across the 42 studies were route of administration, dose frequency, dose timing, dose size, and treatment duration. The results for each of these attribute categories are described below and are summarized in Table 1. Results in Table 1 are grouped by therapeutic area rather than treatment process attribute to avoid redundancy, since many studies include more than one treatment process attribute. Results in this section are presented by treatment process attribute to highlight general patterns in preference for treatment process attributes. Statistical results across studies were often not directly comparable. For example, relative preference scores presented in different DCE studies were not necessarily on the same scale, and none of these yield numerical results that are directly comparable to health state utility studies. Therefore, to facilitate interpretation of results across studies, results in Table 1 are presented in terms of whether preferences for treatment process attributes followed expected or unexpected patterns.
Route of administration
Studies examining preferences among various routes of administration typically yielded findings in the expected direction, with easier or more convenient routes of administration preferred over more difficult routes of administration (Table 1). In multiple studies, respondents were found to prefer oral over injectable administration,20–25 inhaled medication over injections,23,26–28 and injections over infusions.19,29 Individual studies also reported a preference for oral over inhaled medication23 and IV injections over cannula injections.30
Examination of the results across studies highlights several potential factors that could mitigate or influence preference among routes of administration. For example, strength of preference for route of administration may be influenced by both disease status and current treatment.23,27,28,31 One study found that patients with diabetes were willing to pay significantly more for a preferred route of administration (inhaled insulin over injections) than general population respondents.27 Compared with insulin-naïve diabetes patients, insulin-treated patients were found to place less importance on route of administration31 and were willing to pay significantly less for inhaled insulin instead of insulin injections.28
The strength of preference for route of administration may also be influenced by other characteristics of the treatment itself, including treatment efficacy. Some studies suggest that patients are more willing to accept less convenient routes of administration when compensated by greater clinical benefit. Despite a preference for oral medications, patients with diabetes were willing to accept injectable medication if it was associated with improved glycated hemoglobin (HbA1c)20 or weight reduction.20,24,25 Preference for route of administration was also affected by treatment frequency21,22 and the location of treatment administration (eg, whether the treatment is administered at home or at a doctor’s office).32
Dose frequency
As expected, most studies examining dose frequency found that less frequent administration was preferred over more frequent administration.20,24,25,33–37 However, there were some instances when patients preferred more frequent dosing.32,38 For example, Augustovski et al38 reported that patients with rheumatoid arthritis preferred weekly treatment over monthly treatment and suggest that this may be to avoid having to remember or plan a less frequent treatment schedule.
Other studies suggest a possible interaction between dose frequency and route of administration. Patients may prefer more frequent dosing via a preferred route of administration over less frequent dosing with a less desirable route of administration.22,39
Finally, one study found that strength of preference for dose frequency could vary by geographic region.40 Patients in Canada and the United Kingdom had a statistically significant preference for fewer doses of oral medication per day, while no significant differences were found in Germany or the United States.
Dose timing
Respondents generally preferred flexible dose timing over dose timing linked to meals or other fixed times.20,34,41 One diabetes study found a potential interaction between dose timing and mode of administration.20 When dosing was less flexible (ie, linked to mealtimes), respondents were willing to pay more for oral over injectable medication than when dosing was more flexible (ie, not linked to mealtimes). Specifically, they were willing to pay €52 per month for tablets instead of injections when dosing was tied to mealtimes, but only €23 per month when doses could be administered at any time of day.
A study by Evans et al34 found that preferences for dose timing were affected by treatment regimen (basal-only vs basal-bolus) and disease status (patients with diabetes vs general population respondents). The preference for flexible basal insulin dosing was less pronounced when administered in a basal-bolus regimen where the timing of the bolus dose was fixed. In a basal-bolus regimen, preference for a once-daily time-flexible injection over a once-daily fixed time injection was significant for general population respondents, but not patients with diabetes.
Number of pills per dose
As expected, patients generally preferred treatment with fewer pills per dose.40,42 However, strength of preference for the number of pills at each dose may be influenced by geographic location. Mohamed et al42 found that in a sample of Swedish patients with Type 2 diabetes, the preference weights did not reveal a significant preference for the number of pills (one or two) for either the once a day or twice a day profiles. However, in the German sample, the preference weights indicate that patients preferred one pill in the morning and one pill in the evening over two pills at each administration.42 Hodgkins et al40 reported that patients in the US and UK were willing to pay significantly more each month for oral medication with a lower pill burden (one pill vs two pills and two pills vs three pills at each dose). However, among patients in Germany and Canada, there was no difference in willingness to pay for the preferred number of pills. In this study, respondents were told to “imagine that you are asked to pay the full cost each month in order to receive these new treatments”, regardless of whether they were typically required to pay for medication in their home country.
Treatment duration
Results of studies evaluating preference for treatment duration suggest that respondents generally prefer shorter treatment durations across disease areas.19,21,22,30 Two studies evaluating preference for duration of psoriasis treatment found that relative preference for treatment duration was influenced by respondent characteristics including comorbid depression43 and current treatment status.44 Schmieder et al43 found that the duration of treatment is relatively more important to patients with comorbid depression, but other comorbidities such as psoriatic arthritis, diabetes, and cardiovascular disease did not appear to influence the relative importance of treatment duration. Schaarschmidt et al44 found that patients who are currently receiving injectable treatment attached greater importance to treatment duration than patients treated with other treatment modalities.
Relative importance of treatment process compared with efficacy and safety
Results of the preference studies were also examined to compare the importance of treatment process relative to safety and efficacy. In most studies, treatment process attributes were relatively less important than safety and efficacy.23,30,32,40,43,45–51 However, in some instances, treatment process attributes had greater relative importance than some or all efficacy and safety attributes (Table 1).20,22,23,25,30–32,36,38–43,45–53
Disease area appears to be the primary factor influencing the importance of treatment process attributes relative to safety and efficacy. This difference in relative importance of treatment process is most obvious when comparing between results of autoimmune and cancer studies. At least one treatment process attribute was found to be relatively more important than safety or efficacy variables in four of the five autoimmune studies but in none of the six cancer studies that included treatment process and safety/efficacy attributes.
Sample characteristics such as disease status and treatment status may also influence the relative importance of treatment process in comparison with safety and efficacy attributes. Casciano et al31 compared subgroups of patients with Type 1 and Type 2 diabetes and found that route of administration was relatively more important than safety (side effects and risk of hypoglycemia) and efficacy attributes (maintenance of blood sugar levels) in the sample of patients with Type 2 diabetes, but not in the sample of patients with Type 1 diabetes. Hauber et al52 found that treatment experience influences the relative importance of daily dosing schedule (an attribute combining dose frequency and dose size) in relation to safety. This study included patients with a low current dosing burden (“light users” – patients taking fewer than five pills per day or taking medication only once a day or as needed) and patients with a high current dosing burden (“heavy users” – patients taking five or more pills per day or taking medications more than once a day). Among heavy users, dosing schedule was less important than safety attributes such as chance of stomach problems, frequency of hypoglycemia, and risk of congestive heart failure. Among light users, preference for daily dosing schedule was more important than stomach problems and risk of congestive heart failure, but less important than frequency of hypoglycemia. Schaarschmidt et al44 found that in a sample of patients with psoriasis, current treatment modality (topical therapy, phototherapy, tablets, injections, and infusions) led to differences in relative importance of magnitude and probability of benefit compared to delivery method, treatment frequency, and treatment duration.
Discussion
This review identified a substantial number of studies that quantitatively assessed preference for treatment process attributes. In many of these studies, it was found that treatment process was less important in determining preference than safety and efficacy. As listed in Table 1, this finding was reported across all four disease areas examined in this review, including oncology,45,47,51 diabetes,24,25,36,48 autoimmune disease,38,54 and osteoporosis.32
However, even when safety and efficacy attributes were more important, the treatment process often still had a quantifiable and potentially important impact on preference (Table 1).30,32,36,54 In conjoint studies, the impact of various aspects of the treatment process on preference was often quantified in relative importance scores representing the percentage of influence each attribute had on overall preference. In the studies reporting percentages, the impact of treatment process varied widely, accounting for 11.66%31–29.3%39 of treatment preference.
Furthermore, some studies reported that process attributes were equally or more important than safety and efficacy in determining treatment preference. Such results were found in samples of patients with diabetes,24,31 osteoporosis,39 and autoimmune disease,43,44,53 but not in samples of patients with cancer (Table 1). Perhaps the importance of treatment process attributes varies by disease condition and severity. For example, patients with cancer, often a terminal disease, were more concerned with safety and efficacy, while treatment process played less of a role in determining preference.
It is also likely that the importance of treatment process relative to treatment efficacy could depend on how outcomes are defined and quantified. Across the studies in this review, the definition of efficacy varied substantially. Given this heterogeneity, it is difficult to draw conclusions regarding the relative importance of treatment process compared to efficacy. When interpreting findings regarding relative preferences, it is important to remember that the way in which the concepts were operationalized (ie, through vignettes, attribute levels, etc) varies across studies, even among those employing the same methodology. The specific context of each study must be considered when interpreting the results, making cross-study comparisons difficult.
A wide range of studies documented preference among levels of treatment process attributes. As expected, these studies typically found that more convenient treatment processes tend to be preferred over more burdensome or more complex treatments (Table 1). For example, shorter durations of treatment administration were preferred over longer durations,19,22,30 and less frequent administration was preferred over more frequent administration.20,25,33–37 Fewer tablets at each administration were preferred over a greater number of tablets.40,52 Greater flexibility with regard to dose timing was preferred over less flexibility.20,34,41 Finally, less invasive routes of administration (eg, oral) were preferred over more invasive routes of administration (eg, injection and IV infusion).20,22,23,25
However, it should be noted that there were some exceptions to these patterns of preferences, with some studies failing to find significant differences in the expected direction.46,50,58 In addition, unexpected findings were occasionally reported, such as preferences for more frequent treatment doses (Table 1).32,38 Some studies suggest that there may be interactions among multiple treatment process attributes, such as dose frequency and dose timing,20,22,39 and these interactions among multiple treatment process issues could be causing some unexpected findings. Patients may consider each individual treatment process attribute in the larger treatment context of other process characteristics as well as safety and efficacy.
Several limitations of this literature review should be acknowledged. Although a broad literature search was conducted, the decision was eventually made to focus only on four disease areas. Therefore, this review should not be considered a comprehensive review of all published research on the topic. Other limitations stem from the content of the articles that were reviewed. For example, there was substantial variability among articles in terms of preference assessment methods, reported statistics, treatment attribute levels, respondent populations, and disease areas. This variability makes it difficult to compare findings and draw general conclusions. Adding to the difficulty of interpreting findings, levels of treatment process attributes often include multiple characteristics (eg, a blend of dose frequency and mode of administration), which confound the findings. Future studies on patient preference may address these limitations.
Despite inconsistencies in methodology, some general patterns of preference clearly emerged. Overall, the results of this review suggest that treatment process has a quantifiable impact on preference and willingness to pay for treatment, even in many situations where safety and efficacy were the primary concerns. Findings on specific treatment attributes could be used to inform the design of a target product profile for a molecule during early phases of drug development. The target product profile is a summary of drug development described in terms of labeling concepts and is intended to reflect treatment attributes that are believed to provide the greatest benefit and matter most to patients and prescribers.55 This profile is used to shape clinical studies supporting the development of a product and engage regulatory agencies in discussions of registration strategy. Patient preferences for treatment process attributes can serve as valuable input to the design of future studies that target innovative treatment approaches in order to better meet the needs of patients and deliver improved outcomes.
Supplementary materials
Search terms
The following search terms related to treatment process were applied to article titles and abstracts:
Route of administration: oral, pill, tablet, capsule, chewable, delayed-release, delayed release, sustained-release, sustained release, effervescent, granules, orodispersible, dissolvable, solution, suspension, parenteral, injection, subcutaneous, intramuscular, intravenous, intrathecal, depot, implant, infusion, transmucosal, buccal, nasal, ocular, transdermal, patch, microneedle, microporation, topical, cream, ointment, gel, spray, powder, rectal, vaginal, inhaled, inhaler, pump, intraperitoneal, mode of administration, delivery method, delivery system, drug administration route, drug administration routes, treatment modalities.
Dose frequency: dose, dosing.
Dose timing: food, meal.
Dose size: dosage.
Convenience: convenience, inconvenience.
Other process attributes: onset of action, dietary restriction, laboratory tests, monitoring, taste, sitting upright, treatment attributes, and process attributes.
Acknowledgments
Funding for this study was provided by Eli Lilly and Company, Indianapolis, IN, USA. The authors thank Katherine Kim for assistance with data extraction, Clarice P Hayes for assistance with study design, and Amara Tiebout for production assistance.
Footnotes
Disclosure
The study was funded by Eli Lilly and Company (Indianapolis, IN, USA). Four of the authors are employed by the sponsor: Joseph A Johnston, Sarah E Curtis, Henry A Havel, and Stephanie A Sweetana. Three of the authors are employed by Evidera, a company that received support from Eli Lilly and Company for time spent conducting this study: Katie D Stewart, Louis S Matza, and Heather L Gelhorn. A preliminary version of the abstract of this paper was presented at the ISOQOL 22nd Annual Conference, October 21–24, 2015 in Vancouver, BC, Canada, as a poster presentation with interim findings. The actual paper, however, has never been published. The authors report no other conflicts of interest in this work.
References
- 1.Hixson-Wallace JA, Dotson JB, Blakey SA. Effect of regimen complexity on patient satisfaction and compliance with warfarin therapy. Clin Appl Thromb Hemost. 2001;7(1):33–37. doi: 10.1177/107602960100700108. [DOI] [PubMed] [Google Scholar]
- 2.Morris LS, Schulz RM. Medication compliance: the patient’s perspective. Clin Ther. 1993;15(3):593–606. [PubMed] [Google Scholar]
- 3.Raue PJ, Schulberg HC, Heo M, Klimstra S, Bruce ML. Patients’ depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatr Serv. 2009;60(3):337–343. doi: 10.1176/appi.ps.60.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shikiar R, Rentz A, Barone J, Duncanson F, Katz E. Patient satisfaction with ofloxacin (F) and polymyxin B/neomycin/hydrocortisone (C) in the treatment of otitis externa: results from two randomized clinical trials. J Manage Care Med. 2002;6(3):24–27. [Google Scholar]
- 5.Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215. doi: 10.1111/j.1524-4733.2004.72252.x. [DOI] [PubMed] [Google Scholar]
- 6.Allan L, Hays H, Jensen NH, et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ. 2001;322(7295):1154–1158. doi: 10.1136/bmj.322.7295.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelhorn HL, Stringer SM, Brooks A, et al. Preferences for medication attributes among patients with type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2013;15(9):802–809. doi: 10.1111/dom.12091. [DOI] [PubMed] [Google Scholar]
- 8.Johnson FR, Ozdemir S, Mansfield C, et al. Crohn’s disease patients’ risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology. 2007;133(3):769–779. doi: 10.1053/j.gastro.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 9.Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients’ benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009;256(4):554–562. doi: 10.1007/s00415-009-0084-2. [DOI] [PubMed] [Google Scholar]
- 10.Brennan VK, Dixon S. Incorporating process utility into quality adjusted life years: a systematic review of empirical studies. Pharmacoeconomics. 2013;31(8):677–691. doi: 10.1007/s40273-013-0066-1. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson C, Shackley P. Does “process utility” exist? A case study of willingness to pay for laparoscopic cholecystectomy. Soc Sci Med. 1997;44(5):699–707. doi: 10.1016/s0277-9536(96)00215-8. [DOI] [PubMed] [Google Scholar]
- 12.Swan JS, Sainfort F, Lawrence WF, Kuruchittham V, Kongnakorn T, Heisey DM. Process utility for imaging in cerebrovascular disease. Acad Radiol. 2003;10(3):266–274. doi: 10.1016/s1076-6332(03)80100-9. [DOI] [PubMed] [Google Scholar]
- 13.Bridges JF. Stated preference methods in health care evaluation: an emerging methodological paradigm in health economics. Appl Health Econ Health Policy. 2003;2(4):213–224. [PubMed] [Google Scholar]
- 14.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowen D, Brazier J. Health utility measurement. In: Glied S, Smith P, editors. The Oxford Handbook of Health Economics. New York, NY: Oxford University Press; 2011. pp. 788–813. [Google Scholar]
- 17.Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5(1):1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 18.Torrance GW, Furlong W, Feeny D. Health utility estimation. Expert Rev Pharmacoecon Outcomes Res. 2002;2(2):99–108. doi: 10.1586/14737167.2.2.99. [DOI] [PubMed] [Google Scholar]
- 19.Matza LS, Cong Z, Chung K, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adherence. 2013;7:855–865. doi: 10.2147/PPA.S44947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogelund M, Vilsboll T, Faber J, Henriksen JE, Gjesing RP, Lammert M. Patient preferences for diabetes management among people with type 2 diabetes in Denmark – a discrete choice experiment. Curr Med Res Opin. 2011;27(11):2175–2183. doi: 10.1185/03007995.2011.625404. [DOI] [PubMed] [Google Scholar]
- 21.de Bekker-Grob EW, Essink-Bot ML, Meerding WJ, Koes BW, Steyerberg EW. Preferences of GPs and patients for preventive osteoporosis drug treatment: a discrete-choice experiment. Pharmacoeconomics. 2009;27(3):211–219. doi: 10.2165/00019053-200927030-00004. [DOI] [PubMed] [Google Scholar]
- 22.de Bekker-Grob EW, Essink-Bot ML, Meerding WJ, Pols HA, Koes BW, Steyerberg EW. Patients’ preferences for osteoporosis drug treatment: a discrete choice experiment. Osteoporos Int. 2008;19(7):1029–1037. doi: 10.1007/s00198-007-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimaraes C, Marra CA, Colley L, et al. A valuation of patients’ willingness-to-pay for insulin delivery in diabetes. Int J Technol Assess Health Care. 2009;25(3):359–366. doi: 10.1017/S0266462309990055. [DOI] [PubMed] [Google Scholar]
- 24.Jendle J, Torffvit O, Ridderstrale M, Ericsson A, Nilsen B, Bogelund M. Willingness to pay for diabetes drug therapy in type 2 diabetes patients: based on LEAD clinical programme results. J Med Econ. 2012;15(Suppl 2):1–5. doi: 10.3111/13696998.2012.703633. [DOI] [PubMed] [Google Scholar]
- 25.Jendle J, Torffvit O, Ridderstrale M, Lammert M, Ericsson A, Bogelund M. Willingness to pay for health improvements associated with anti-diabetes treatments for people with type 2 diabetes. Curr Med Res Opin. 2010;26(4):917–923. doi: 10.1185/03007991003657867. [DOI] [PubMed] [Google Scholar]
- 26.Chancellor J, Aballea S, Lawrence A, et al. Preferences of patients with diabetes mellitus for inhaled versus injectable insulin regimens. Pharmacoeconomics. 2008;26(3):217–234. doi: 10.2165/00019053-200826030-00005. [DOI] [PubMed] [Google Scholar]
- 27.Sadri H. Contingent valuation of inhaled insulin: a Canadian perspective. J Med Econ. 2007;10(4):475–487. [Google Scholar]
- 28.Sadri H, MacKeigan LD, Leiter LA, Einarson TR. Willingness to pay for inhaled insulin: a contingent valuation approach. Pharmacoeconomics. 2005;23(12):1215–1227. doi: 10.2165/00019053-200523120-00006. [DOI] [PubMed] [Google Scholar]
- 29.Darba J, Restovic G, Kaskens L, et al. Patient preferences for osteoporosis in Spain: a discrete choice experiment. Osteoporos Int. 2011;22(6):1947–1954. doi: 10.1007/s00198-010-1382-3. [DOI] [PubMed] [Google Scholar]
- 30.Ossa DF, Briggs A, McIntosh E, Cowell W, Littlewood T, Sculpher M. Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics. 2007;25(3):223–237. doi: 10.2165/00019053-200725030-00005. [DOI] [PubMed] [Google Scholar]
- 31.Casciano R, Malangone E, Ramachandran A, Gagliardino JJ. A quantitative assessment of patient barriers to insulin. Int J Clin Pract. 2011;65(4):408–414. doi: 10.1111/j.1742-1241.2010.02590.x. [DOI] [PubMed] [Google Scholar]
- 32.Silverman S, Calderon A, Kaw K, et al. Patient weighting of osteoporosis medication attributes across racial and ethnic groups: a study of osteoporosis medication preferences using conjoint analysis. Osteoporos Int. 2013;24(7):2067–2077. doi: 10.1007/s00198-012-2241-1. [DOI] [PubMed] [Google Scholar]
- 33.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 34.Evans M, Jensen HH, Bogelund M, Gundgaard J, Chubb B, Khunti K. Flexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off survey. J Med Econ. 2013;16(11):1357–1365. doi: 10.3111/13696998.2013.846262. [DOI] [PubMed] [Google Scholar]
- 35.Hauber AB, Gonzalez JM, Schenkel B, Lofland JH, Martin S. The value to patients of reducing lesion severity in plaque psoriasis. J Dermatolog Treat. 2011;22(5):266–275. doi: 10.3109/09546634.2011.588193. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd A, Nafees B, Barnett AH, et al. Willingness to pay for improvements in chronic long-acting insulin therapy in individuals with type 1 or type 2 diabetes mellitus. Clin Ther. 2011;33(9):1258–1267. doi: 10.1016/j.clinthera.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Ozdemir S, Johnson FR, Hauber AB. Hypothetical bias, cheap talk, and stated willingness to pay for health care. J Health Econ. 2009;28(4):894–901. doi: 10.1016/j.jhealeco.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Augustovski F, Beratarrechea A, Irazola V, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experiment. Value Health. 2013;16(2):385–393. doi: 10.1016/j.jval.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Fraenkel L, Gulanski B, Wittink D. Patient treatment preferences for osteoporosis. Arthritis Rheum. 2006;55(5):729–735. doi: 10.1002/art.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodgkins P, Swinburn P, Solomon D, Yen L, Dewilde S, Lloyd A. Patient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experiment. Patient. 2012;5(1):33–44. doi: 10.2165/11595390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Aristides M, Weston AR, FitzGerald P, Le Reun C, Maniadakis N. Patient preference and willingness-to-pay for Humalog Mix25 relative to Humulin 30/70: a multicountry application of a discrete choice experiment. Value Health. 2004;7(4):442–454. doi: 10.1111/j.1524-4733.2004.74007.x. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed AF, Zhang J, Johnson FR, et al. Avoidance of weight gain is important for oral type 2 diabetes treatments in Sweden and Germany: patient preferences. Diabetes Metab. 2013;39(5):397–403. doi: 10.1016/j.diabet.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Schmieder A, Schaarschmidt ML, Umar N, et al. Comorbidities significantly impact patients’ preferences for psoriasis treatments. J Am Acad Dermatol. 2012;67(3):363–372. doi: 10.1016/j.jaad.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Schaarschmidt ML, Umar N, Schmieder A, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27(2):187–198. doi: 10.1111/j.1468-3083.2011.04440.x. [DOI] [PubMed] [Google Scholar]
- 45.Aristides M, Chen J, Schulz M, Williamson E, Clarke S, Grant K. Conjoint analysis of a new chemotherapy: willingness to pay and preference for the features of raltitrexed versus standard therapy in advanced colorectal cancer. Pharmacoeconomics. 2002;20(11):775–784. doi: 10.2165/00019053-200220110-00006. [DOI] [PubMed] [Google Scholar]
- 46.Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224–231. doi: 10.1016/j.lungcan.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Langer CJ, Fastenau JM, Forlenza JB, et al. Effectiveness versus convenience: patient preferences for an erythropoietic agent to treat cancer-related anemia. Curr Med Res Opin. 2007;23(1):85–92. doi: 10.1185/030079906X162728. [DOI] [PubMed] [Google Scholar]
- 48.Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products – liraglutide and exenatide – for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–661. doi: 10.3111/13696998.2010.529377. [DOI] [PubMed] [Google Scholar]
- 49.Porzsolt F, Clouth J, Deutschmann M, Hippler HJ. Preferences of diabetes patients and physicians: a feasibility study to identify the key indicators for appraisal of health care values. Health Qual Life Outcomes. 2010;8:125. doi: 10.1186/1477-7525-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shafey M, Lupichuk SM, Do T, Owen C, Stewart DA. Preferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experiment. Bone Marrow Transplant. 2011;46(7):962–969. doi: 10.1038/bmt.2010.225. [DOI] [PubMed] [Google Scholar]
- 51.Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139–1148. doi: 10.3111/13696998.2012.708689. [DOI] [PubMed] [Google Scholar]
- 52.Hauber AB, Han S, Yang JC, et al. Effect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetes. Patient Prefer Adherence. 2013;7:937–949. doi: 10.2147/PPA.S43465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaarschmidt ML, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147(11):1285–1294. doi: 10.1001/archdermatol.2011.309. [DOI] [PubMed] [Google Scholar]
- 54.Lichtenstein GR, Waters HC, Kelly J, et al. Assessing drug treatment preferences of patients with Crohn’s disease: a conjoint analysis. Patient. 2010;3(2):113–123. [Google Scholar]
- 55.FDA Patient Preference Information – Submission, Review in PMAs, HDE Applications, and De Novo Requests, and Inclusion in Device Labeling Draft Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Silver Spring, MD: Center for Biologics Evaluation and Research (CBER); May, 2015. p. 26. [Google Scholar]
- 56.Guimaraes C, Marra CA, Colley L, et al. Socioeconomic differences in preferences and willingness-to-pay for insulin delivery systems in type 1 and type 2 diabetes. Diabetes Technol Ther. 2009;11(9):567–573. doi: 10.1089/dia.2009.0034. [DOI] [PubMed] [Google Scholar]
- 57.Guimaraes C, Marra CA, Gill S, et al. A discrete choice experiment evaluation of patients’ preferences for different risk, benefit, and delivery attributes of insulin therapy for diabetes management. Patient Prefer Adherence. 2010;4:433–440. doi: 10.2147/PPA.S14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinto SL, Holiday-Goodman M, Black CD, Lesch D. Identifying factors that affect patients’ willingness to pay for inhaled insulin. Res Social Adm Pharm. 2009;5(3):253–261. doi: 10.1016/j.sapharm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Sung L, Alibhai SM, Ethier MC, et al. Discrete choice experiment produced estimates of acceptable risks of therapeutic options in cancer patients with febrile neutropenia. J Clin Epidemiol. 2012;65(6):627–634. doi: 10.1016/j.jclinepi.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Teuffel O, Cheng S, Ethier MC, et al. Health-related quality of life anticipated with different management strategies for febrile neutropenia in adult cancer patients. Support Care Cancer. 2012;20(11):2755–2764. doi: 10.1007/s00520-012-1397-8. [DOI] [PubMed] [Google Scholar]
- 61.Shingler SL, Swinburn P, Ali S, Perard R, Lloyd AJ. A discrete choice experiment to determine patient preferences for injection devices in multiple sclerosis. J Med Econ. 2013;16(8):1036–1042. doi: 10.3111/13696998.2013.811079. [DOI] [PubMed] [Google Scholar]