Abstract
Cortical reorganization of function due to the growth of an adjacent brain tumor has clearly been demonstrated in a number of surgically proven cases. Such cases demonstrate the unmistakable implications for the neurosurgical treatment of brain tumors, as the cortical function may not reside where one may initially suspect based solely on the anatomical magnetic resonance imaging (MRI). Consequently, preoperative localization of eloquent areas adjacent to a brain tumor is necessary, as this may demonstrate unexpected organization, which may affect the neurosurgical approach to the lesion. However, in interpreting functional MRI studies, the interpreting physician must be cognizant of artifacts, which may limit the accuracy of functional MRI in the setting of brain tumors.
Keywords: brain tumors, cortical reorganization, fMRI, neurosurgery
The human brain is constantly changing. This is seen over long time scales, such as the development of the brain of a growing child or the involutional changes in the elderly. In addition, the brain undergoes fundamental changes on a much shorter time scale. For example, every acquired memory is reflected as a physical change in the brain.1,2 With the advent of advanced neuroimaging techniques, the changes in the brain due to acquisition of new competencies can be appreciated in both structural and functional studies.3
Attempts by the brain to overcome injury and to reacquire capabilities lost due the anatomical damage by having other parts of the brain take over (or try to take over) the lost functions can be termed cortical reorganization. Successful cortical reorganization in response to various insults has been described in a number of clinical scenarios. For example, in early Alzheimer’s disease, the brain is able to initially overcome decreased functionality in parts of the brain, for example, the inferior parietal cortex in the study by Lind et al4 or the hippocampus in the study by Trivedi et al5, by recruiting other parts of the brain (eg, the temporal lobe as in the study by Bondi et al6). These compensatory changes, which are efficient in the initial stages of the disease, can be seen on neuroimaging studies.4–6 Similarly, partial recovery is well documented in patients with stokes.7 However, the ability of the brain to undergo reorganization is limited and is soon exhausted. In the Alzheimer’s disease example, the progression of neurodegeneration is relentless and eventually overcomes the inadequate compensatory capability of the brain. Similarly, it is unusual to completely recover from devastating cortical infarcts. A further confounding factor is that the ability of the brain to recover from various insults decreases with age.
Currently, the understanding of cortical plasticity is in its infancy. It is unknown which factors influence recovery of neurological function of successful cortical reorganization. Perhaps more importantly, the factors that accentuate cortical plasticity to allow for more effective recovery from neurological damage remains completely obscured from the view of neuroscientists and clinicians. Clearly, the ability to enhance the recuperative powers of the human central nervous system would be a major boon for patients suffering from neurological damage such as strokes or spinal cord injuries.
CORTICAL REORGANIZATION DUE TO BRAIN TUMOR GROWTH
The possibility of cortical reorganization of brain function in response to the growth of a tumor is clearly important when considering therapeutic options for the treatment of brain tumors. This is perhaps most important when considering neurosurgical intervention. Generally, brain functions can be localized to specific neuroanatomical locations. However, the involvement of a neuroanatomical location by a tumor, which leads to cortical reorganization and the displacement of this function to another location, can lead to the expansion of neurosurgical options. In other words, the displacement of neurological function away for the area of the tumor opens up the possibility of a more complete resection of the tumor without the incurrence of iatrogenic damage. In order to appreciate such changes, preoperative functional imaging is essential. Specifically, the results of preoperative functional imaging that demonstrates the displacement of neurological function away from the expected area can convert a case wherein a neurosurgeon declines an operation to the successful resection of a tumor. Therefore, reorganization is an important phenomenon to consider in treatment planning of neurosurgical candidates.
As a tumor invades part of the brain and affects the underlying functions, other parts of the brain attempt to compensate for the functional deficit through cortical reorganization, or plasticity.8,9 Although the exact mechanism for reorganization is not known, it is likely that many factors, such as the type and extent of injury and the affected function, influence intrahemispheric or interhemispheric compensations.10 Evidence suggests that not only can a tumor lead to neuronal hyperactivity in the immediate vicinity, but also tumor growth may elicit reorganization and transfer of functional control to the contralateral hemisphere, as shown to occur in strokes.8,10–13
Two examples that highlight reorganization are as follows: A 34-year-old, right-handed man was first diagnosed with an astrocytoma involving the left inferior frontal cortex 7 years before the current admission.14 A stereotactic biopsy at that time demonstrated a grade II/IV astrocytoma. As the patient was right handed, there was a 95% to 98% chance that the lesion involved Broca’s area. Therefore, surgical resection was not performed for fear of causing mutism in a young patient with a long expected life span. Instead, the patient was treated with fractionated external radiation therapy and chemotherapy. He was followed with serial magnetic resonance imaging (MRI) scans that demonstrated slow progression of the tumor. The current admission was prompted by the presence of uncal herniation with compression of the brainstem and mild right-sided weakness and dysarthria. A functional MRI (fMRI) scan with 2 language paradigms revealed that Wernicke’s area was in its expected location in the left hemisphere, while Broca’s area localized to the right hemisphere (Fig. 1). During the operation, the patient did not tolerate conscious sedation and therefore intraoperative mapping was not performed. The patient’s dysarthria and right hemiparesis resolved after resection of the temporal component of the tumor. Pathologic examination confirmed a grade III/IV astrocytoma.
FIGURE 1.
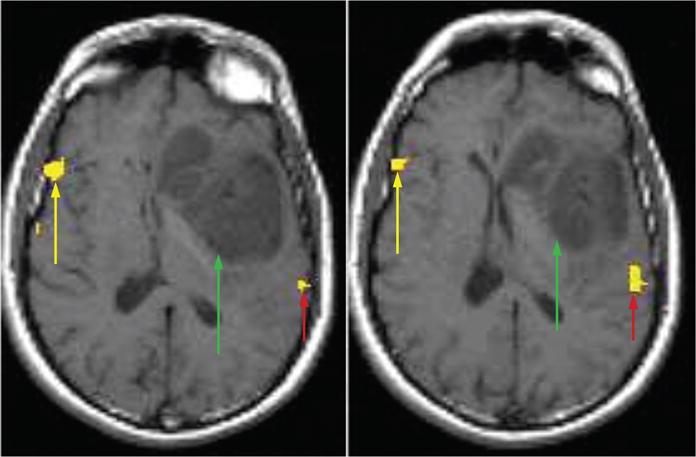
fMRI scan with language paradigms coregistered to axial T1-weighted images in a 34-year-old man with a left inferior frontal glioma. The 2 axial slices localize Broca area to the right hemisphere (yellow arrows), Wernicke area to the left hemisphere (red arrows), and show their relationship to the tumor location (green arrows). The localization of Broca area to the right hemisphere suggests cortical reorganization. Axial slices taken from a figure in J Comput Assist Tomogr 2002; 26:941–943.
The second example is of a 62-year-old right-handed man who had a left temporoparietal lesion involving the expected location of Wernicke’s area.15 An fMRI scan with multiple language paradigms revealed that Broca’s area localized to its expected location in the left hemisphere, while Wernicke’s area unexpectedly localized to the right hemisphere (Fig. 2). During the operation, the patient tolerated conscious sedation and underwent intraoperative mapping for language localization. Intraoperative mapping confirmed fMRI localization of Broca’s area. For Wernicke’s area, the patient’s language function remained intact during stimulation of the expected location in the left hemisphere. Total resection of the enhancing portion of the tumor, including the expected Wernicke’s area, followed and the patient suffered no postoperative language dysfunction. Had fMRI not been performed and cortical reorganization was not considered, the patient would not have undergone optimal treatment for his lesion. Together, these cases highlight cortical reorganization and how preoperative fMRI aids in treatment planning for neurosurgical candidates.
FIGURE 2.
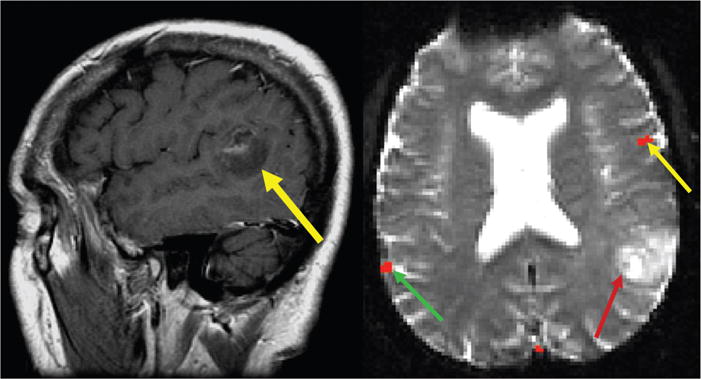
A, Postcontrast sagittal image from a 62-year-old right-handed man with a mostly nonenhancing temporoparietal neoplasm involving the expected location of Wernicke area (yellow arrow). B, Axial fMRI results show Broca area localizing to the left hemisphere (yellow arrow) and Wernicke area localizing to the right hemisphere (green arrow) opposite of the tumor (red arrow). Intraoperative mapping and surgical resection confirmed fMRI results. Adapted from AJNR Am J Neuroradiol 2004; 25:130–133.
MOTOR CORTEX AND THE SUPPLEMENTARY MOTOR AREA
The supplementary motor area (SMA), located in the superior frontal gyrus, plays a vital role in motor planning and organization and is split into 2 functional parts.16,17 The part related to motor planning is the caudal SMA proper and its function is intimately related to motor control. SMA localization is more difficult than the primary motor cortex (M1) on routine MRI examinations, as there is not a clear anatomical delineation. Nevertheless, iatrogenic damage can lead to temporary paresis.18 The SMA has 2 main characteristics that allow for it to be helpful in motor mapping using fMRI. First, the hemodynamic response of the SMA occurs before the motor cortex during a motor paradigm. Second, despite not having a clear anatomical delineation from the motor cortex, the SMA is located a distance away from the precentral gyrus, which allows for it to be used in the study of motor function when a tumor infiltrates the motor cortex.
Because of the characteristics of the SMA, our group hypothesized that when a high-grade tumor invades in the motor cortex, the ipsilateral SMA takes on some of the function of the motor cortex. Evidence to support this hypothesis exists as the volume of activation in the SMA ipsilateral to a tumor increases. As a tumor causes a decrease in the volume of activation in the motor cortex, a greater area of the SMA activates in response to a motor paradigm.19 In addition, the hemodynamic response function of the SMA ipsilateral to a tumor shifts temporally and looks more like the response function of a normal motor cortex. Figure 3 shows an example of a patient with a glioblastoma multiforme (GBM) in the left motor cortex performing a bilateral finger tapping fMRI paradigm. The peak in the hemodynamic response function curve occurs 1.5 seconds earlier in the SMA than the motor cortex in the normal, contralateral hemisphere, while in the tumor hemisphere, the SMA peak has temporally shifted and is only 0.5 seconds sooner than in the motor cortex.19 Thus, the hemodynamic response function ipsilateral to the tumor assumes a configuration closely resembling the configuration of the hemodynamic response function in the primary motor cortex. The conclusions we can draw from this are that the SMA appears to assume at least some of the function of the motor cortex when infiltrated by a tumor and that hemodynamic response function analysis may offer a sensitive alternative to volume of activation measurements in our understanding of plasticity.
FIGURE 3.
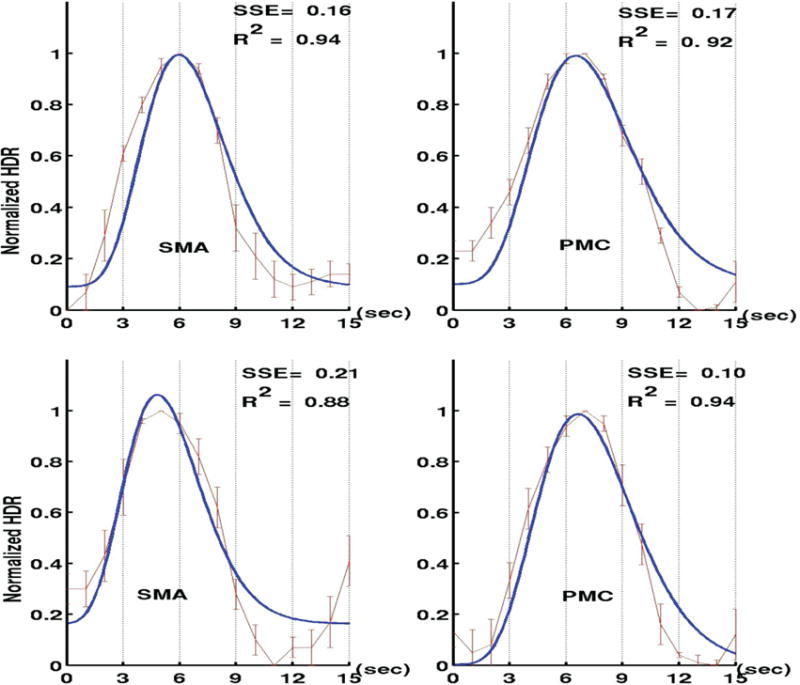
A patient with a GBM in the left motor cortex performing a bilateral finger tapping paradigm. The top graphs (A) are the response curves of finger tapping on the right hand (which are mapped to the tumor-affected hemisphere) and the bottom graphs (B) are the response curves of finger tapping on the left hand (which are mapped to the normal, contralateral hemisphere). In A, we see that that the peak in the hemodynamic response function curve occurs 0.5 seconds sooner in the SMA than the motor cortex, while in B, the SMA peak 1.5 seconds sooner than in the motor cortex. The shift in the SMA peak in the tumor-affected hemisphere suggests that the SMA may be taking on some motor functions from the motor cortex. Used and adapted with permission from Med Sci Monit 2009; 15: MT55–MT62.
EVIDENCE FOR CORTICAL REORGANIZATION FROM OTHER MODALITIES
Direct Cortical, Intraoperative Stimulation
Cortical reorganization of language function described in using the blood oxygenation level-dependent (BOLD) fMRI method has been confirmed using other methods, including a landmark study published in the New England Journal of Medicine, by Sanai et al.20 These authors studied language function in 250 consecutive patients with gliomas using direct cortical stimulation. They reported that the cortical maps generated with intraoperative language data showed surprising variability in language localization within the dominant hemisphere. Specifically, the authors averred that sites associated with speech function were variably located along the cortex and extended well beyond the classic anatomical boundaries of Broca area and Wernicke areas. This finding suggested that language function in these patients was translocated to other areas of the brain not classically associated with language function.
Transcranial Magnetic Stimulation (TMS)
A recent study using repetitive navigated transcranial magnetic stimulation (rTMS) confirmed unusual language organization in patients with brain tumors in the inferior left frontal lobe, implying cortical reorganization.21 In this study, 15 patients with lesions of left-sided language-eloquent brain areas and 50 healthy, right-handed volunteers underwent bilateral rTMS language mapping via an object-naming task. Left-sided language function was confirmed by direct, intraoperative cortical stimulation in all patients. The rTMS-induced language errors were categorized into 6 different error types. The error ratio (induced errors/number of stimulations) was determined for each brain region on both hemispheres. A hemispheric dominance ratio was then defined for each region as the quotient of the error ratio (left/right) of the corresponding area of both hemispheres (ratio >1 = left dominant; ratio <1 = right dominant). Patients with language-eloquent lesions showed a statistically significantly lower ratio than healthy participants, which indicates a higher participation of the right hemisphere in language function. Hence, this study demonstrated a shift of language function to the nondominant hemisphere in patients with brain tumors in the dominant hemisphere using TMS.
CORTICAL REORGANIZATION BENEFITS RETENTION OF LANGUAGE FUNCTION IN BRAIN TUMOR PATIENTS
The case studies outlined above have shed light on the possibility that the brain’s language network can reorganize as a result of tumor invasion, recruiting Broca’s homologue in the right hemisphere after damage to Broca’s area proper in the typically dominant left hemisphere. A recent study from our group22 sought both to identify whether the unexpected right-frontal fMRI activation seen in such case studies exists as a group-level trend in patients with left-frontal tumors and also examine the possible compensatory nature of this activation. The authors conducted a retrospective analysis of 159 brain tumor patients who had undergone presurgical fMRI language mapping. Patients with left-frontal tumors were hypothesized to be more likely to show right- or codominant fMRI language activation than patients who had tumors elsewhere in the brain (H1). Patients with left-frontal tumors who were identified as right- or codominant for language were expected to possess more intact language function as measured by the Boston Naming Test (H2). All patients underwent presurgical fMRI for purposes of identifying language-related regions of cortex to be avoided during surgical resection. A trained neuropsychologist administered the Boston Naming Test before the scan and at least 1 block-design language paradigm during fMRI. Each patient’s language laterality was determined by the neuroradiologist. Left-handed or ambidextrous patients and those with technically suboptimal scans were excluded. To test H1, patients were split into 2 groups: (1) tumors in the left-frontal lobe (n = 81), and (2) tumors elsewhere in the brain (n = 78). A 2-tailed. z-test comparing the distribution of hemispheric dominance of the left-frontal group and the overall distribution was significant, with more right- or codominant patients in the left frontal group than would be expected (z = 2.16, P = 0.0308). To test H2, patients with left-frontal tumors were split into (1) left language dominant (n = 21) and (2) right- or colanguage dominant (n = 8). A 2-tailed independent-samples t test using each group’s Boston Naming scores found that the mean was significantly higher (t[23] = −2.3, P =.031) for the right- or codominant group (M = 56.38; SD = 2.72) group than for the left-dominant (M = 48.76; SD = 14.49), suggesting that the atypical was not merely an artifact of the ratio-dependent nature of the fMRI laterality index. These results suggest that the functional language network may exhibit a greater degree of plasticity than previously known. Importantly, they also suggest that the right hemisphere may be able to compensate for damage to putative language cortex.
In another recent study from our group,23 we evaluated which anatomical areas were associated with fMRI evidence for cortical reorganization. Prior studies have shown that some patients with left-hemispheric brain tumors have an increased propensity for developing right-sided language support. However, the precise trigger for establishing codominant language function in brain tumor patients remains unknown. We analyzed the magnetic resonance (MR) scans of patients with left-hemispheric tumors and either codominant (n = 35) or left-hemisphere dominant (n = 35) language function on fMRI to investigate anatomical factors influencing hemispheric language dominance. Of 11 neuroanatomical areas evaluated for tumor involvement, the basal ganglia was significantly correlated with codominant language function (P < 0.001). Perhaps more significantly, among patients whose tumors invaded the basal ganglia, those with language codominance performed significantly better on the Boston Naming Test, a clinical measure of aphasia, than their left-lateralized counterparts (56.5 vs 36.5, P =.025). Although further studies are needed to elucidate the role of the basal ganglia in establishing codominance, our results suggest that reactive codominance may afford a behavioral advantage to patients with left-hemispheric tumors.
PSEUDO-REORGANIZATION
However, in interpreting fMRI examinations in patient with brain tumors, it is essential to appreciate the possibility of false-negative activations due to various factors that result in pseudo-reorganization rather than true reorganization. Acknowledgment of this distinct possibility is crucial in interpreting fMRI examination so as not to lull the neurosurgeon into a false sense of security that the brain she/he is contemplating on resecting is void of function.
False-negative activation is due to limitations of BOLD fMRI as a test. A common source of false-negative results in fMRI is susceptibility artifacts. fMRI uses T2* gradient echo (GE) sequences that lack the refocusing pulse of routine spin echo (SE) sequences. Although the sequences used in fMRI are sensitive enough to detect the subtle susceptibility differences between oxyhemoglobin (HbO2) and deoxyhemoglobin (dHb), one consequence is susceptibility artifacts that cause local field inhomogeneities are accentuated as well. Susceptibility artifacts occur when tissues or objects with large differences in magnetic susceptibility are in close proximity to each other and are a problem because they cause signal distortions that can mask fMRI signals from truly active sites.10 The large susceptibility differences cause distortions in the local magnetic field and therefore interfere with image acquisition. The effect of susceptibility artifacts on fMRI results depends on the neurological surgery history of the patient. In those without prior surgery, artifacts localize to areas of abrupt magnetic susceptibility changes such as air–tissue interfaces and near cavities or moving tissues.24,25 Examples of air–tissue interfaces leading to fMRI signal dropout are the anterior temporal lobe and orbitofrontal cortex due to their localization near the ear canal and sinuses, respectively.24,25 In those with prior surgery, titanium plates, metallic staples, hemorrhage and blood products, and residual surgical metal make artifacts worse.10 Prior surgery causes decreased volumes of fMRI activation on the side of the brain ipsilateral to the tumor. The most likely reason for the decrease is the prominent signal intensity dropout on the T2* images due to the susceptibility artifacts resulting from the previous operation.24,25 It is also essential to appreciate that the presence of abnormal neovasculature and the resultant neurovascular uncoupling (NVU) can also lead to a muting of the BOLD effect and false-negative findings that result in pseudo-reorganization.26–35 The basis for the BOLD signal is neurovascular coupling (NVC), wherein vascular changes are tied to neuronal activity. With neuronal activity, there is oxygen extraction from the site of activity followed by an increase in cerebral blood flow (CBF) that delivers oxygenated blood and diamagnetic HbO2. The increase in CBF overshoots the oxygen demand from neuronal activity delivering an influx of HbO2 that is greater than what was lost due to oxygen extraction as well as diluting dHb. The increase in HbO2 relative to dHb causes an increase in BOLD signal strength. This coupling of vascular changes and neuronal activity is compromised in tumors, especially high-grade tumors such as GBM.26–35
In GBMs, vessels are tortuous and disorganized, highly permeable, and have abnormalities in their endothelial walls.36 Functionally, tumor vessels respond abnormally to both pharmacological challenges, such as papaverine injections and induced hypertension, and physiological challenges, such as hypercapnia and hypocapnia.37–39 Taken together, these abnormal vessels have a decreased or absent ability to increase blood flow in response to neuronal activity, which leads to a muting of the BOLD response. A major problem caused by NVU on fMRI results is neither false negatives nor truly eloquent cortical areas appear as neither active nor contributing to a specific task, especially as studies have shown the presence of functional neurons within tumor beds.40,41 However, being mindful of the effects of tumors and NVU allow for more accurate interpretation of fMRI results and allows for proper planning and use of other techniques to verify functional cortical centers, such as with intra-operative direct cortical stimulation.
CONCLUSION
Cortical reorganization of function due to the growth of an adjacent brain tumor has clearly been demonstrated in a number of surgically proven cases. Such cases demonstrate the unmistakable implications for the neurosurgical treatment of brain tumors, as the cortical function may not reside where one may initially suspect based solely on the anatomical MRI. Consequently, preoperative localization of eloquent areas adjacent to a brain tumor is necessary, as this may demonstrate unexpected organization, which may affect the neurosurgical approach to the lesion. However, in interpreting fMRI studies, the interpreting physician must be cognizant of artifacts, which may limit the accuracy of fMRI in the setting of brain tumors.
References
- 1.Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758. doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Temple E, Deutsch GK, Poldrack RA, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci U S A. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lind J, Persson J, Ingvar M, et al. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006;129(pt 5):1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi MA, Schmitz TW, Ries ML, et al. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer’s disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondi MW, Houston WS, Eyler LT, et al. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auriat AM, Neva JL, Peters S, et al. A Review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front Neurol. 2015;6:226. doi: 10.3389/fneur.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peck KK, Holodny AI. fMRI clinical applications. In: Reiser MF, Semmler W, Hricak H, editors. Magnetic Resonance Tomography. Berlin: Springer Verlag; 2007. pp. 1308–1331. [Google Scholar]
- 9.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7:732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 10.Bogomolny DL, Petrovich NM, Hou BL, et al. Functional MRI in the brain tumor patient. Top Magn Reson Imaging. 2004;15:325–335. doi: 10.1097/00002142-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 12.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 13.Roberts TPL, Tran Q, Ferrari P, et al. Increased somatosensory neuromagnetic fields ipsilateral to lesions in neurosurgical patients. NeuroReport. 2002;13:699–702. doi: 10.1097/00001756-200204160-00032. [DOI] [PubMed] [Google Scholar]
- 14.Holodny AI, Schulder M, Ybasco A, et al. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr. 2002;26:941–943. doi: 10.1097/00004728-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Petrovich NM, Holodny AI, Brennan CW, et al. Isolated translocation of Wernicke’s areato the right hemisphere in a 62 year old man with a temporoparietal glioma. AJNR Am J Neuroradiol. 2004;25:130–133. [PMC free article] [PubMed] [Google Scholar]
- 16.Peck KK, Bradbury MS, Hou BL, et al. The role of the supplementary motor area (SMA) in the execution of primary motor activities in brain tumor patients: functional MRI detection of time-resolved differences in the hemodynamic response. Med Sci Monit. 2009;15:MT55–MT62. [PubMed] [Google Scholar]
- 17.Peck KK, Bradbury M, Psaty EL, et al. Joint activation of the supplementary motor area and presupplementary motor area during simultaneous motor and language functional MRI. Neuroreport. 2009;20:487–491. doi: 10.1097/WNR.0b013e3283297d71. [DOI] [PubMed] [Google Scholar]
- 18.Brennan NP. Preparing the patient for the fMRI Study and optimization of paradigm selection and delivery. In: Holodny AI, editor. Functional Neuroimaging: A Clinical Approach. New York: Informa Healthcare USA, Inc; 2008. pp. 13–21. [Google Scholar]
- 19.Peck KK, Holodny AI, Hou HL, et al. Reorganization of the cortical control of movement due to brain tumor growth: evidence from the hemodynamic response function of the supplementary motor area. Proc Intl Soc Mag Reson Med. 2005;13:569. [Google Scholar]
- 20.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 21.Krieg SM, Sollmann N, Hauck T, et al. Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS One. 2013;8:e75403. doi: 10.1371/journal.pone.0075403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jost E, Christiansen MH, Brennan N, Holodny A. Behavioral advantage in confrontation naming performance in brain tumor patients with left-frontal tumors. Conference Proceedings of the Society for the Neurobiology of Language. 2014;6:86. [Google Scholar]
- 23.Shaw K, Brennan NM, Woo K, et al. Infiltration of the basal ganglia by brain tumors is associated with the development of co-dominant language function on fMRI [manuscript submitted] doi: 10.1016/j.bandl.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peck KK, Bradbury M, Petrovich N, et al. Presurgical evaluation of language using functional magnetic resonance imaging in brain tumor patients with previous surgery. Neurosurgery. 2009;64:644–652. doi: 10.1227/01.NEU.0000339122.01957.0A. [DOI] [PubMed] [Google Scholar]
- 25.Kim MJ, Holodny AI, Hou BL, et al. The effect of prior surgery on blood oxygen level-dependent functional MR imaging in the preoperative assessment of brain tumors. AJNR Am J Neuroradiol. 2005;26:1980–1985. [PMC free article] [PubMed] [Google Scholar]
- 26.Holodny AI, Schulder M, Wen-Ching L, et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 1999;20:609–612. [PMC free article] [PubMed] [Google Scholar]
- 27.Holodny AI, Schulder M, Wen-Ching L, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 2000;21:1415–1422. [PMC free article] [PubMed] [Google Scholar]
- 28.Hou BL, Bradbury M, Peck KK, et al. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. NeuroImage. 2006;32:489–497. doi: 10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- 29.Chen CM, Hou BL, Holodny AI. Effect of age and tumor grade on BOLD functional MR imaging in preoperative assessment of patients with glioma. Radiology. 2008;248:971–978. doi: 10.1148/radiol.2483071280. [DOI] [PubMed] [Google Scholar]
- 30.Ulmer JL, Krouwer HG, Mueller WM, et al. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular coupling. AJNR Am J Neuroradiol. 2003;24:213–217. [PMC free article] [PubMed] [Google Scholar]
- 31.Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudodominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569–581. doi: 10.1227/01.neu.0000134384.94749.b2. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal S, Sair HI, Yahyavi-Firouz-Abadi N, et al. Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.25012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Pillai JJ, Zacà D. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol Cancer Res Treat. 2012;11:361–374. doi: 10.7785/tcrt.2012.500284. [DOI] [PubMed] [Google Scholar]
- 34.Zaca D, Hua J, Pillai JJ. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J Clin Oncol. 2011;2:289–298. doi: 10.5306/wjco.v2.i7.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai JJ, Zacá D. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J Clin Oncol. 2011;2:397–403. doi: 10.5306/wjco.v2.i12.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 37.Huber P. Functional tests in angiography of brain tumors. Neuroradiology. 1970;1:132–141. [Google Scholar]
- 38.Bradac GB, Simon RS, Heidieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence on different CO2 tensions. Neuroradiology. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- 39.Pronin IN, Holodny AI, Kornienko VN, et al. The use of hyperventilation in contrast-enhanced MR of brain tumors. AJNR. 1997;18:1705–1708. [PMC free article] [PubMed] [Google Scholar]
- 40.Ojemann J, Miller J, Silbergeld D. Preserved function in brain invaded by tumor. Neurosurgery. 1996;39:253–259. doi: 10.1097/00006123-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Skirboll SS, Ojemann GA, Berger MS, et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678–685. [PubMed] [Google Scholar]


