Abstract
Background
Rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) patient populations face similar risks of chronic immunosuppression including corticosteroid use. We compared the receipt of preventive services between IBD and RA populations according to published quality metrics.
Methods
We defined a single-center cohort of patients with IBD or RA receiving specialty and primary care. Electronic health record abstraction assessed quality metrics, sociodemographics, comorbidity, and utilization. Comparisons used multivariate odds ratios and Student’s t-tests.
Results
218 RA and 190 IBD patients were included. In multivariate analysis, IBD patients were less likely to receive pneumococcal vaccination (OR=0.29, 95% CI: 0.11–0.85), while RA patients underwent glucocorticoid-induced osteoporosis screening more often (100% vs. 82.5%, p = 0.023).
Conclusions
Gastroenterologists can improve care quality for IBD patients by assuming greater responsibility for preventive care in IBD patients and/or collaborating with primary care and health systems to improve preventive care delivery.
Keywords: Inflammatory Bowel Disease, Rheumatoid Arthritis, Quality Care, Preventive Care, Vaccination
INTRODUCTION
Immunosuppressive therapy is the cornerstone of treatment for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA). [1,2] Advances in the treatment of IBD and RA with tumor necrosis factor (TNF) alpha inhibitors and immunomodulators, i.e., thiopurines and methotrexate, have improved rates of clinical remission, decreased corticosteroid use, and improved quality of life by producing higher rates of mucosal healing in IBD and decreasing joint damage in RA. [3,4] [5,6] They have also introduced new risks and preventive care needs.
Infections and bone loss are leading complications of treating IBD and RA with immunosuppressive agents such as corticosteroids. [7,8] Both populations are also at increased risk for lower respiratory tract infections. As such, all immunosuppressed patients are recommended to receive influenza and pneumococcal vaccination. [9,10] Despite these recommendations, RA and IBD patients are not always optimally vaccinated and may not receive other appropriate preventive care. [11–13]
To address this issue, the American Gastroenterological Association (AGA) and Crohn’s and Colitis Foundation of America (CCFA) have developed quality metrics. [14] The 2011 AGA ambulatory quality metrics for patients with IBD are as follows: (1) to identify disease location and phenotype, (2) to administer influenza and pneumococcal vaccinations, (3) to apply corticosteroid sparing therapy, (4) to screen for glucocorticoid-induced osteoporosis (GIO), (5) to perform and document tobacco cessation counseling, and (6) to evaluate for latent hepatitis B virus (HBV) (7) or tuberculosis (TB) infections prior to initiating anti-TNF therapy.
Similarly, vaccination, osteoporosis screening and other preventive care are key quality measures relating to chronic steroid use and immunosuppression in RA patients published by the American College of Rheumatology (ACR) in 2008. [1,15] The CCFA and the European Crohn’s and Colitis Organisation (ECCO) have called for more extensive quality metrics, e.g., Human papilloma virus vaccination. [16,17]We chose the AGA metrics which align with ACR quality metrics.
The aim of our study was to compare preventive care quality in IBD and RA patients receiving care within a single academic center, based upon the AGA and ACR ambulatory quality metrics. Recognizing that variation exists regarding gastroenterologist perspective on responsibility for such metrics, we examined receipt of care from any provider. We selected RA as our comparison population because they are also managed with immunosuppressants and have similar society-endorsed quality measures. We hypothesized that there would be clinically informative differences in receipt of preventive care between RA and IBD patients. We believe patients with RA are more likely to be screened for GIO, and those with IBD are more likely to evaluated for HBV infection, since these align with bone and liver health in their respective specialties. Moreover, examining differences could inform future health systems or provider improvement strategies in IBD populations.
MATERIALS AND METHODS
Cohort Study Population
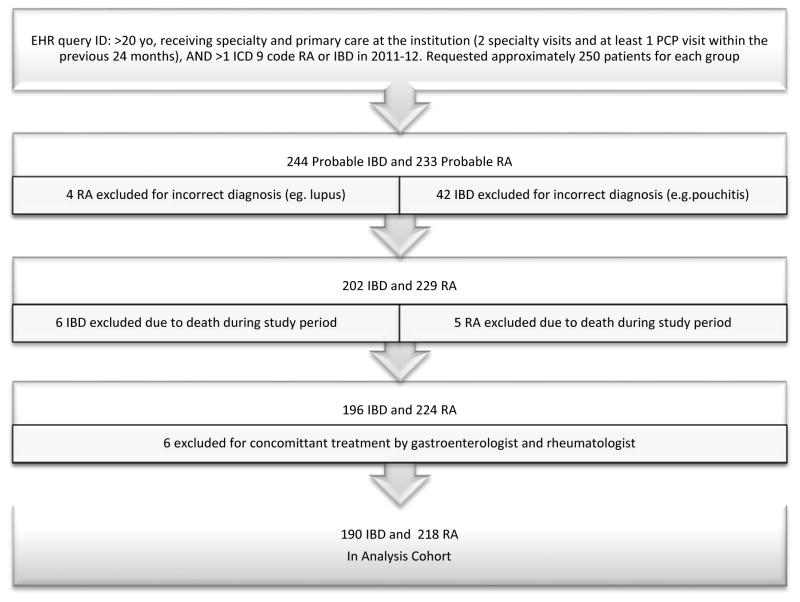
We performed a retrospective cohort study of IBD and RA patients receiving care at the University of Wisconsin Health System from 2011 to 2012, comparing the delivery of the specialty-specific ambulatory quality metrics to these populations (Table 1). An EPIC system (EPIC Corporation, Verona, WI, USA) electronic health record (EHR) query was performed to randomly identify approximately 500 patients meeting the following criteria: 1) age >20 years (ensuring patients had adult primary care providers (PCPs)), receipt of specialty and primary care at the institution and 2) >1 ICD-9 code for RA (714.0) [18] or IBD (555.xx & 556.xx ) [19] between January 1, 2011 and December 31, 2012. To examine quality measures specifically related to anti-TNF therapy (evaluation for latent HBV or TB), our goal was to have 50% of our sample comprised of patients on an anti-TNF therapy during the study period. We defined patients with at least two specialty clinic visits and at least one primary care visit as those with “regular care.” Patients who died prior to the study conclusion or who received care in both gastroenterology and rheumatology clinics were excluded (Figure 1).
Table 1.
Inflammatory Bowel Disease and Rheumatoid Arthritis Quality Metrics by Society
| Measure | IBD | RA | |
|---|---|---|---|
| 1 | Steroid sparing therapy | Approved by CMS and AGA in 2011 | Approved by CMS and ACR in 2008 |
| 2 | Rate of screening for tuberculosis (TB) prior to starting biologics | ||
| 3 | Rate of screening for osteoporosis if ≥10mg/d of prednisone ≥ 60 days | ||
| 4 | Rate of influenza vaccination | Recommended by U.S. Preventive Services Task Force & ACR 2008 | |
| 5 | Rate of pneumonia vaccination | ||
| 6 | Rate of screening for hepatitis B (HBV) prior to starting biologics | ||
| 7 | Counseling for smoking cessation if actively using tobacco |
CMS - Center for Medicare and Medicaid Services. AGA - American Gastroenterological Association. ACR - American College of Rheumatology
Figure 1.
Flowchart of RA and IBD patient study inclusion and exclusion.
The specialty clinics were comprised of 12 general rheumatology and 14 gastroenterology providers (including three dedicated IBD physicians; excluding hepatologists). The specialty clinics have multiple locations, some co-located with primary care, and both specialties provide care for a substantial proportion of the region’s IBD and RA patients. Influenza and pneumococcal vaccines were available at all specialty and primary care clinics. None of the providers were participating in quality-driven reimbursement programs before or during the study period.
EHR records included clinical notes, immunization, and orders. Sociodemographics, comorbidities, medications, and utilization of healthcare based on the annual number of PCP visits for each patient population were manually abstracted. We also computed a comorbidity score for each patient using the Charlson Comorbidity Index, a common tool to assess relative comorbidity risk in clinical research. [20]
Vaccination Quality Metrics
Influenza immunization was defined as the recommendation or administration of vaccine by a PCP or specialist once between January 1, 2011 and December 31, 2012. Pneumococcal immunization was defined as the recommendation or administration of the 23-valent polysaccharide vaccine by a PCP or specialist during the study period or within the previous 10 years. Documented patient refusal of influenza or pneumococcal immunization was scored as being recommended by the provider. Confirmation of immunization was evaluated using the Wisconsin Immunization Registry (WIR), a computerized internet database tracking immunization dates of Wisconsin children and adults since 2000, available within the EHR. [21]
Other Quality Metrics
Corticosteroid sparing therapy was evaluated if patients were prescribe corticosteroids (greater than 10mg/day for 60 or greater consecutive days) during the study period. Corticosteroid sparing medications included the following: disease modifying anti-rheumatic drugs. i.e., hydroxycholoroquine and leflunomide; thiopurines (azathioprine or 6-mercaptopurine); methotrexate; anti-TNF therapy (infliximab, adalimumab, certolizumab pegol, or etanercept); or non-TNF biologics for RA and IBD (natalizumab, abatacept, tocilizumab, tofacitinib, or rituximab). Our analysis did not include disease activity or medication efficacy during the study.
GIO screening was defined as recommending or ordering an assessment of bone mineral density by dual energy x-ray absorptiometry (DEXA) scan. This was evaluated in patients with a history of or current use of chronic corticosteroids. Chronic corticosteroid use was defined as the documented prescription of 10 mg daily or higher of prednisone for greater than 60 days. [14] Females screened for osteoporosis due to post-menopausal status alone were excluded.
All patients charts were reviewed for history or current tobacco use. All active tobacco users’ visit notes were evaluated for tobacco cessation counseling by primary care provider or specialist. Counseling for smoking cessation was defined as documented counseling or the prescription of pharmacological therapy.
Screening for latent TB and HBV was evaluated in patients on anti-TNF therapy during the study period, which included patients receiving induction or maintenance therapy. Records of patients who received long-term anti-TNF therapy were reviewed for documentation or evidence of evaluation of latent infection prior to starting anti-TNF therapy. TB screening was defined as documented check of either purified protein derivative (PPD) sensitivity or quantiferon-TB gold within six months of starting anti-TNF therapy. HBV screening was defined as a documented check of HBV surface antigen and HBV core antibody six months prior to receiving the first dose of anti-TNF therapy. If HBV or TB screening was not documented, it was considered not performed.
Two physician abstractors (FC and DC) reviewed the complete EHR using a standardized abstraction protocol. Data quality was evaluated by one of the abstractors (FC) by random re-abstractions of 10% of the charts.
Statistical Analyses
Demographic characteristics of patients were summarized using descriptive statistics. Differences between demographic groups were evaluated by chi-squared test for categorical values and Student’s t-test for continuous values. Unadjusted IBD and RA group outcomes were assessed using Student’s t-test.
A general estimating equation was used to model the probability of receiving influenza vaccine, pneumonia vaccine, or both, while controlling for correlation due to repeated measures within providers. [22] Beyond clinical group (IBD vs. RA), covariates of interest included age (continuous), gender, race (Caucasian, African American or other), number of PCP visits, number of specialty clinic visits, Charlson Comorbidity Index score, and ever being uninsured or enrolled in Medicaid. Covariate medications were ever treatment with biologic, immunosuppressant, or chronic corticosteroids (10 mg daily or higher of prednisone for greater than 60 days). All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
ETHICAL CONSIDERATIONS
This study was approved in 2013 by the University of Wisconsin-Madison Health Sciences Institutional Review Board with a waiver of informed consent.
RESULTS
Demographics
Between 2011 and 2012, 233 RA and 244 IBD patients receiving primary and specialty care at our institution were randomly selected from an EPIC EHR query, of which 218 and 190 met the final inclusion criteria (Figure 1). As expected, the RA cohort was older (mean age of 61 vs. 50 years, p < 0.0001) and predominantly female, (83% vs. 54%, p< 0.0001) (Table 2). RA patients had higher Charlson Comorbidity Index scores (1.7 vs. 0.6, p< 0.0001) and had more frequent visits with their PCPs and specialists compared with IBD patients. The RA cohort was also more likely to be on chronic immunosuppression compared to the IBD cohort (98% vs. 75%, p < 0.0001).
Table 2.
Rheumatoid Arthritis and Inflammatory Bowel Disease Cohort Descriptives
| RA (n=218) n, (%) |
IBD (n=190) n, (%) |
p value | |
|---|---|---|---|
| Age (mean +/− SD) | 60.5 +/− 13.9 | 49.8 +/− 16.0 | < 0.0001 |
| 20–34 | 9(4) | 43 (23) | |
| 35–49 | 35(16) | 52(27) | |
| 50–64 | 86(39) | 55(29) | |
| >65 | 88(40) | 40(21) | |
| Female | 181 (83) | 103 (54) | < 0.0001 |
| Caucasian | 197 (90) | 179 (94) | 0.15 |
| Crohn’s Disease | N/A | 115 (61) | |
| Ulcerative Colitis | 75 (39) | ||
| Tobacco Current User | 16 (7) | 13 (6) | 0.85 |
| PCP Specialty | |||
| Internal Medicine | 154 (70) | 122 (64) | |
| Family Medicine | 64 (29) | 67 (35) | |
| Medicaid | 14 (6) | 10 (5) | |
| Uninsured | 2 (1) | 4 (2) | |
| Charlson comorbidity index (mean, SD) | 1.7+/− 1.1 | 0.6 +/− (1.1) | < 0.0001 |
| Avg. PCP visits/year (mean, SD) | 1.9 +/−1.8 | 1.5 +/− 1.3 | 0.0085 |
| Avg. specialist visits/year (mean, SD) | 2.7 +/− 1.6 | 2.1 +/− 1.4 | < 0.0001 |
| Medications | |||
| Any immunosuppressant | 214 (98) | 142 (75) | < 0.0001 |
| Any Anti-TNF Therapy | 96 (44) | 77 (41) | 0.85 |
| TNF + Immunomodulator | 42 (19) | 30 (16) | 0.37 |
| Non-TNF biologic | 8(44) | 0 | n/a |
| Immunomodulator | 126 (58) | 81 (43) | 0.003 |
| Azathioprine/6MP | 4 (22) | 76 (40) | < 0.0001 |
| Methotrexate | 122 (56) | 5 (3) | < 0.0001 |
| Other DMARD* | 103 (47) | n/a | n/a |
| Mesalamine1 | 16 (7) | 97 (51) | < 0.0001 |
| Chronic Prednisone (>10mg for >60 days) | 76 (35) | 63(33) | 0.72 |
Other DMARD - hydroxycholorquine, leflunomide
Mesalamine - included sulfasalazine
Influenza and Pneumococcal vaccination
In unadjusted analysis, IBD patients were less likely to receive influenza (77% vs. 91%, p=0.0002) and pneumococcal vaccinations (57% vs. 80%, p < 0.0001) (Table 3). Documented refusal of immunization was low in both groups: 3% (n=5) among the IBD patients (three influenza and two pneumococcal) and 7% (n=15) of the RA group (ten influenza and five pneumococcal). Rheumatologists more often recommended or provided influenza (46% vs. 25%; p < 0.0001) and pneumococcal (38%.vs. 17%, p < 0.0001) immunization than did gastroenterologists. PCPs administered the majority of all vaccinations received by IBD patients (68% of influenza and 69% of pneumococcal) compared to approximately half of RA patients (48% of influenza and 52% of pneumococcal, p < 0.0001).
Table 3.
Receipt of Quality Metric Outcomes in RA and IBD Cohorts
| Quality Outcome | RA n, (%) |
IBD n, (%) |
p value |
|---|---|---|---|
| Steroid sparing therapy1 | 76 (100) | 63 (100) | NS |
| Influenza vaccination | 198 (91) | 147 (77) | 0.0002 |
| Specialist recommended | 102 (47) | 47 (25) | < 0.0001 |
| Pneumococcal vaccination | 175 (80) | 108 (57) | < 0.0001 |
| Specialist recommended | 83 (38) | 33 (17) | < 0.0001 |
| Glucocorticoid induced osteoporosis screening | 76/76 (100) | 52/63 (83) | 0.023 |
| Specialist recommended | 72 (95) | 29 (27) | < 0.0001 |
| Tobacco counseling | 12/16 (72) | 12/13 (92) | 0.23 |
| Specialist recommended | 7 (44) | 7 (54) | 0.60 |
| Screening for Infections prior to initiation of TNF inhibitor | |||
| Screening for latent TB* | 64/96 (72) | 49/77 (64) | 0.68 |
| Screening for latent Hepatitis B* | 19/96 (21) | 20/77 (26) | 0.34 |
Includes only eligible patients on chronic corticosteroids (>10mg for >60 days);
Includes only patients on a TNF inhibitor during study period.
In the fully controlled multivariate analysis of vaccinations (Table 4), IBD patients were less likely to receive pneumococcal vaccination (OR 0.30, 95% CI 0.11–0.85). Patients on azathioprine/ 6-MP (OR 2.16, 95% CI 1.08–4.30) or anti-TNF therapy (OR 2.49, 95% CI 1.20–5.19) were also more likely to have received the pneumococcal vaccine. More frequent primary care visits were associated with increased rates of influenza vaccination (OR 1.15, 95% CI 1.03–1.29). Patients cared for by family medicine physicians were less likely to be immunized compared to those seeing internal medicine physicians (OR 0.66, 95% CI 0.47–0.92).
Table 4.
Multivariate Predictors of Influenza and Pneumococcal Vaccination
| Influenza Vaccination | Pneumococcal Vaccination | Receipt of Both Influenza & Pneumococcal | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| RA (referent) | 1.00 | 1.00 | 1.00 | |||
| IBD | 0.53 | 0.23–1.24 | 0.30* | 0.11–0.85 | 0.35* | 0.14–.0.90 |
| Age | 1.01 | 0.99–1.02 | 1.04* | 1.02–1.06 | 1.03* | 1.01–1.05 |
| Male | 0.62 | 0.35–1.12 | 1.32 | 0.76–2.30 | 1.16 | 0.73–1.83 |
| Medicare | 2.74 | 0.39–18.93 | 5.29 | 0.93–30.17 | 3.29 | 0.62–17.42 |
| Caucasian | 3.54* | 1.51–8.29 | 0.92 | 0.29–2.92 | 1.38 | 0.46–4.09 |
| PCP type Family Medicine | 0.89 | 0.57–1.38 | 0.69* | 0.47–0.99 | 0.66* | 0.47–0.92 |
| PCP visits | 1.16* | 1.03–1.29 | 1.05 | 0.97–1.14 | 1.08 | 0.99–1.18 |
| Specialist visits | 0.94 | 0.84–1.05 | 1.01 | 0.90–1.13 | 0.99 | 0.91–1.09 |
| Charleston Comorbidity Index | 1.02 | 0.69–1.50 | 1.03 | 0.77–1.37 | 1.09 | 0.82–1.43 |
| TNF alpha Inhibitor | 1.46 | 0.82–2.58 | 2.05* | 1.44–2.93 | 1.86* | 1.33–2.58 |
| Azathioprine | 2.21 | 0.82–5.97 | 2.16* | 1.08–4.30 | 2.50* | 1.20–5.19 |
| Methotrexate | 1.09 | 0.45–2.64 | 0.51 | 0.22–1.18 | 0.73 | 0.34–1.53 |
| Mesalamine | 0.98 | 0.47–2.09 | 0.95 | 0.57–1.56 | 0.90 | 0.47–1.71 |
| Chronic Prednisone | 1.27 | 0.51–3.20 | 1.35 | 0.78–2.34 | 1.33 | 0.72–2.42 |
CI indicates confidence interval; OR, odds ratio; Chronic prednisone (>10mg for >60 days):
Indicates significant OR and 95% CI
Glucocorticoid-induced osteoporosis
Screening for GIO was more frequent for RA patients than for IBD patients (100% vs. 82.5%, p = 0.023). Rheumatologists were significantly more likely to screen for GIO compared to gastroenterologists (95% vs 27%, p < 0.0001). In the IBD cohort, 11 patients did not undergo screening (mean age 43, 45% female) and 52 underwent screening (mean age 55, 63% female). In the IBD cohort, older patients were more likely to be screened (mean age 55 vs 43, p <0.015). We found no significant difference in osteoporosis screening based on gender, comorbidity score, or number of provider visits.
Other Quality Metrics
All RA and IBD patients on chronic corticosteroids were on steroid sparing therapy. We found no difference between the two patient populations with regards to tobacco cessation counseling. However, the prevalence of current tobacco users in both groups was only 7% in both groups (16 RA and 13 IBD), limiting our ability to analyze this quality measure.
Anti-TNF therapy was received by 96 (44%) RA and 77 (41%) IBD patients during the study period. There was no significant differences in screening for latent TB (72% vs. 64% p = 0.68) or HBV (21% vs. 26% p = 0.34) between these groups. Furthermore, no patients were identified as having latent TB or HBV during the study period.
DISCUSSION
To our knowledge, this is the first study to compare the provision of quality care between RA and IBD patients receiving their regular primary and specialty care within a single multispecialty center. Our study population of IBD patients receiving primary care had higher rates of immunization (influenza 77%, pneumococcal 57%) than had been reported elsewhere (influenza 34% and pneumococcal 21%). [23] These rates were still suboptimal compared to RA patients at our institution. Screening IBD patients for GIO was also higher than a recent report (83% vs 32%), [23] but again lagged the RA cohort. Superior rates of screening for GIO by rheumatologists may reflect perceptions that bone health is part of their specialty. [24] Screening for latent infections prior to initiating anti-TNF therapy was inadequate in both cohorts, but similar to other published reports. [25] This may be due to suboptimal documentation, since patient verbal or historical paper chart reports of a negative PPD or HBV screening were not included. Notably, the RA quality metrics were published earlier than the IBD metrics, potentially influencing differences in receipt of preventive services between the cohorts.
Higher rates of immunization in the RA cohort may in part be related to their older age (40% of RA vs. 21% of IBD patients were > 65 years), given that the American College of Immunization Practice (ACIP) recommends that all adults ages >65 years receive pneumococcal vaccination. [26] The RA cohort also had more comorbidities and might have received vaccinations for other indications, e.g., heart disease or chronic obstructive pulmonary disease. [26] RA patients also had more provider visits, and thus more opportunities to be vaccinated. Nevertheless, even after controlling for age, gender, other comorbidities, and health care utilization, RA patients were more likely to receive vaccination than IBD patients, suggesting systematic differences in care and room for systems improvement in IBD patient care.
Ambiguity regarding who is responsible for providing immunization could partially explain suboptimal vaccination rates among immunosuppressed patients. A recent survey of gastroenterologists found that 64% of respondents felt that PCPs should be responsible for vaccinations. [27] A survey of rheumatologists showed similar attitudes with 57% of respondents perceiving PCPs as being responsible for vaccination. [28] To improve immunization rates, gastroenterologists and rheumatologists should assume a greater responsibility for recommending or providing appropriate immunization, as has been suggested by professional societies for both specialties. [1,29–31]
The Infectious Disease Society of America (IDSA) guidelines on vaccinating immunocompromised individuals recommend that specialists share responsibility with PCPs for ensuring that appropriate vaccinations are administered. [32] We posit that gastroenterologists should assume a greater responsibility for those younger than 65, since the majority of IBD patients are under 65 and lack other comorbidities that would require pneumococcal vaccination. [33] PCPs see a diversity of chronic disease patients and cannot be solely responsible for appropriately immunizing IBD patients. A typical primary care panel consists of about 2500 patients and one study estimated that it would require 21.7 hours daily to follow national clinical care guidelines for preventive service and chronic disease management. [34] Furthermore, PCP are often uncomfortable determining which vaccines patients should receive while receiving immunosuppressive therapies. [35]
Systematic improvements will be needed to improve immunization rates. One option is to recommend separate preventive care visits with a PCP, an advanced practice provider specializing in gastroenterology, a gastroenterology nurse or vaccine clinic, since in our study, more frequent PCP visits increased the likelihood of influenza vaccination. Quality improvement initiatives such as staff vaccination delegation protocols could also be implemented since they can improve immunization rates by sharing the responsibility across the entire health care team, i.e., medical assistants, nurses, and physicians. [36,37] In addition, EHR tools that alert patients or providers to preventive service eligibility dates may also improve care provided to IBD patients. [38]
Improving rates of GIO screening will also require that gastroenterologists assume a greater responsibility in preventive care delivery. The National Osteoporosis Foundation recommends screening in postmenopausal women age 65 and older and men at age 70 and older regardless of risk factors or those with risk factors, e.g., previous fracture, RA, or glucocorticoids. [39] As most IBD patients do not meet these screening criteria, PCPs should not be solely responsible for evaluating steroid exposure and the need for DEXA scans. Studies have found that BMD of the femur [40] or lumbar spine [41] can be measured from abdominal computed tomography (CT) scans. Since, IBD patients commonly undergo CT scans this could identify patients who should undergo a DEXA. Health system could implement changes so reports of BMD at the femur or lumbar spine are standard in the reports of in IBD patients undergoing abdominal CTs scan.
The AGA quality metrics only represent the minimum standards for improving the care quality of IBD patients. ECCO and CCFA previously called for more extensive quality metrics, e.g., cervical cancer screening, skin cancer screening, and etc. We believe gastroenterologists should consider adopting these additional quality metrics and sharing responsibility for these metrics with PCP and health systems.
Strengths of our study include measurement of quality metrics among IBD and RA patients who received their regular primary and specialty care within a single institution, use of a valid immunization registry which records receipt of vaccination across the state, and EHR use to document which health care professional recommended a given preventive service. However, there are some limitations to our study. Although preventive measures related to IBD have been published since 2009, [42] formal recommendations were not available until 2011. As prior studies have shown that adherence to informal practice guidelines can be low, we may have captured only the early adopters of the guidelines in the IBD cohort. [43] Follow-up studies examining evolving preventative practices in IBD clinics are therefore needed. Our high immunizations rates might not be generalizable to all institutions since local practice standards encourage immunization at any clinical contact, and a stated-wide immunization registry allowed confirmation of immunization. In addition, our patients almost certainly do not reflect the general RA or IBD populations as most had private insurance and were Caucasian. This may limit the generalizability of our findings. Furthermore, screening for GIO was not adjusted in a multi-variate analysis since all RA patients underwent screening. Thus, a predictive model could not be generated and the difference in screening may have been related to patient age. It is possible that rheumatologists might use more low dose corticosteroids and thus assume greater responsibility for osteoporosis screening. We also did not control for all specific individual comorbidities that might have qualified patients for influenza and pneumonia vaccination. Lastly, an inherent limitation of EHR and retrospective studies is that they require documentation of patient and provider actions. If recommendations meeting quality metrics were made by providers, but declined without documentation, or if vaccinations were received by patients outside of this health system, they would not be captured by our analysis.
In conclusion, although RA patients share several of the same risk factors for infection and bone loss as IBD patients, RA patients are meeting the goals for preventive care at higher rates at our institution even after controlling for multiple confounders. As gastroenterologists, we have ready opportunities to improve the quality of care for our IBD patients by sharing responsibility for provision of preventive services, particularly for those under 65.
Key issues.
Preventive services provided to our IBD cohort were higher than previously reported by other investigators, but lagged compared to RA patients.
The IBD cohort was less likely to receive appropriate influenza (77% vs. 91%, p = 0.0002) and pneumococcal vaccinations compared to the RA cohort (57% vs. 80%, p < 0.0001).
Primary care providers administered a greater proportion of vaccinations received by IBD patients than for RA (≥68% vs. ≤52% for RA patients, p < 0.0001).
RA patients more often underwent osteoporosis screening compared to IBD patients (100% vs. 82.5%, p = 0.02).
Gastroenterologists should assume greater responsibility for preventive care in IBD patients and/or collaborate with primary care and health systems to improve preventive care delivery.
Footnotes
Declaration of interest
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. CM Bartels received support from National Institutes of Health (NIH) National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) (K23 #AR062381) and also receives support from Independent Grants for Learning and Change (Pfizer). S Saha is a consultant with UCB Biosciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
* Of interest
** Of considerable interest
- 1.Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care & Research. 2012;64(5):625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dassopoulos T, Sultan S, Falck–Ytter YT, Inadomi JM, Hanauer SB. American Gastroenterological Association Institute Technical Review on the Use of Thiopurines, Methotrexate, and Anti–TNF-α Biologic Drugs for the Induction and Maintenance of Remission in Inflammatory Crohn’s Disease. Gastroenterology. 2013;145(6):1464–1478. doi: 10.1053/j.gastro.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. New England Journal of Medicine. 2010;362(15):1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 4.Panaccione R, Ghosh S, Middleton S, et al. Combination Therapy With Infliximab and Azathioprine Is Superior to Monotherapy With Either Agent in Ulcerative Colitis. Gastroenterology. 2014;146(2):392–400. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 5.Bejarano V, Quinn M, Conaghan PG, et al. Effect of the early use of the anti–tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Care & Research. 2008;59(10):1467–1474. doi: 10.1002/art.24106. [DOI] [PubMed] [Google Scholar]
- 6.Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. The Lancet. 2008;372(9636):375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 7.Kelly C, Hamilton J. What kills patients with rheumatoid arthritis? Rheumatology. 2007;46(2):183–184. doi: 10.1093/rheumatology/kel332. [DOI] [PubMed] [Google Scholar]
- 8.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. Journal of Crohn’s and Colitis. 2014;8(6):443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Coyne P, Hamilton J, Heycock C, Saravanan V, Coulson E, Kelly CA. Acute lower respiratory tract infections in patients with rheumatoid arthritis. The Journal of Rheumatology. 2007;34(9):1832–1836. [PubMed] [Google Scholar]
- 10**.Long MD, Martin C, Sandler RS, Kappelman MD. Increased Risk of Pneumonia Among Patients With Inflammatory Bowel Disease. Am J Gastroenterol. 2013;108(2):240–248. doi: 10.1038/ajg.2012.406. Patients with inflammatory bowel disease are at increased risk for pneumonia. Use of biologic medications, corticosteroids, and narcotics were independently associated with pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory Bowel Diseases. 2008;14(2):253–258. doi: 10.1002/ibd.20266. Patients with inflammatory bowel disease are less likely to receive routine preventive care services. [DOI] [PubMed] [Google Scholar]
- 12.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with Inflammatory Bowel Disease are at Risk for Vaccine-Preventable Illnesses. Am J Gastroenterol. 2006;101(8):1834–1840. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 13.Kremers HM, Bidaut-Russell M, Scott CG, Reinalda MS, Zinsmeister AR, Gabriel SE. Preventive medical services among patients with rheumatoid arthritis. The Journal of Rheumatology. 2003;30(9):1940–1947. [PubMed] [Google Scholar]
- 14.American Gastroenterological Association. [Accessed November 1, 2015];Adult inflammatory bowel disease Physician Performance Measure Set. 2011 http://www.gastro.org/practice/quality-initiatives/IBD_Measures.pdf.
- 15.American College or Rheumatology. [Accessed November 1, 2015];Rheumatoid Arthritis Physician Performance Measurement Set. http://www.rheumatology.org/practice/clinical/quality/NCQA_PCPI_ACR_Rheumatoid-Arthritis.pdf.
- 16.Melmed GY, Siegel CA, Spiegel BM, et al. Quality Indicators for Inflammatory Bowel Disease: Development of Process and Outcome Measures. Inflammatory Bowel Diseases. 2013;19(3):662–668. doi: 10.1097/mib.0b013e31828278a2. [DOI] [PubMed] [Google Scholar]
- 17.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. Journal of Crohn’s and Colitis. 2014;8(6):443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Barrett J, Liang MH, et al. Sensitivity and positive predictive value of medicare part B physician claims for rheumatologic diagnoses and procedures. Arthritis & Rheumatism. 1997;40(9):1594–1600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- 19.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflammatory Bowel Diseases. 2007;13(4):451–461. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]
- 20.Charlson MPP, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Wisconsin Immunization Registry. [Accessed November 1, 2015]; https://www.dhfswir.org/PR/portalHeader.do.
- 22.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. John Wiley & Son; Hoboken, N.J: 2004. [Google Scholar]
- 23.Feuerstein J, Lewandowski J, Martinez-Vazquez M, Leffler D, Cheifetz A. Documented Compliance with Inflammatory Bowel Disease Quality Measures Is Poor. Dig Dis Sci. 2014;60(2):339–344. doi: 10.1007/s10620-014-3385-y. [DOI] [PubMed] [Google Scholar]
- 24.Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care & Research. 2010;62(11):1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflammatory Bowel Diseases. 2012;18(6):1057–1063. doi: 10.1002/ibd.21824. [DOI] [PubMed] [Google Scholar]
- 26.Tomczyk SBN, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822. [PMC free article] [PubMed] [Google Scholar]
- 27**.Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: Deficiencies in gastroenterologists knowledge. Inflammatory Bowel Diseases. 2011;17(12):2536–2540. doi: 10.1002/ibd.21667. Shows most gastroenterologist feel primary care providers should be primaryly responsible for providing appropriate immunizations to patients with inflammatory bowel disease. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy EM, Azeez MA, Fitzpatrick FM, Donnelly S. Knowledge, Attitudes, and Clinical Practice of Rheumatologists in Vaccination of the At-Risk Rheumatology Patient Population. JCR: Journal of Clinical Rheumatology. 2012;18(5):237–241. doi: 10.1097/RHU.0b013e3182611547. [DOI] [PubMed] [Google Scholar]
- 29.van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Annals of the Rheumatic Diseases. 2011;70(3):414–422. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 30.Melmed GY. Immunizations and IBD: Whose responsibility is it? If I’m the prescribing doctor, shouldn’t it be mine? Inflammatory Bowel Diseases. 2012;18(1):41–42. doi: 10.1002/ibd.21666. [DOI] [PubMed] [Google Scholar]
- 31.Wasan SK, Baker SE, Skolnik PR, Farraye FA. A Practical Guide to Vaccinating the Inflammatory Bowel Disease Patient. Am J Gastroenterol. 2010;105(6):1231–1238. doi: 10.1038/ajg.2009.733. [DOI] [PubMed] [Google Scholar]
- 32**.Rubin LG, Levin MJ, Ljungman P, et al. IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clinical Infectious Diseases. 2013;58(3):309–318. doi: 10.1093/cid/cit816. Calls for specialist to share responsibility for assuring immunosuppressed patients receive appropriate immunizations. [DOI] [PubMed] [Google Scholar]
- 33.Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–9. [PubMed] [Google Scholar]
- 34**.Yarnall KSH, Østbye T, Krause KM, Pollak KI, Gradison M, Michener JL. Family Physicians as Team Leaders: “Time” to Share the Care. Preventing Chronic Disease. 2009;6(2):A59. Shows that primary care providers do not have sufficient time to be responsible for providing all preventive care in those with chronic illness. [PMC free article] [PubMed] [Google Scholar]
- 35.Selby L, Hoellein A, Wilson J. Are Primary Care Providers Uncomfortable Providing Routine Preventive Care for Inflammatory Bowel Disease Patients? Dig Dis Sci. 2011;56(3):819–824. doi: 10.1007/s10620-010-1329-8. [DOI] [PubMed] [Google Scholar]
- 36**.Parker S, Chambers White L, Spangler C, et al. A Quality Improvement Project Significantly Increased the Vaccination Rate for Immunosuppressed Patients with IBD. Inflammatory Bowel Diseases. 2013;19(9):1809–1814. doi: 10.1097/MIB.0b013e31828c8512. Shows quality improvement projects in specialty clinics can increase rates of immunization for IBD patients. [DOI] [PubMed] [Google Scholar]
- 37.Desai SP, Lu B, Szent-Gyorgyi LE, et al. Increasing pneumococcal vaccination for immunosuppressed patients: A cluster quality improvement trial. Arthritis & Rheumatism. 2013;65(1):39–47. doi: 10.1002/art.37716. [DOI] [PubMed] [Google Scholar]
- 38.Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Care & Research. 2009;61(11):1505–1510. doi: 10.1002/art.24873. [DOI] [PubMed] [Google Scholar]
- 39.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber NK, Fidler JL, Keaveny TM, et al. Validation of a CT-Derived Method for Osteoporosis Screening in IBD Patients Undergoing Contrast-Enhanced CT Enterography. The American journal of gastroenterology. 2014;109(3):401–408. doi: 10.1038/ajg.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Annals of Internal Medicine. 2013;158(8):588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moscandrew M, Mahadevan U, Kane S. General health maintenance in IBD. Inflammatory Bowel Diseases. 2009;15(9):1399–1409. doi: 10.1002/ibd.20944. [DOI] [PubMed] [Google Scholar]
- 43.Reddy SI, Friedman S, Telford JJ, Strate L, Ookubo R, Banks PA. Are Patients with Inflammatory Bowel Disease Receiving Optimal Care[quest] Am J Gastroenterol. 2005;100(6):1357–1361. doi: 10.1111/j.1572-0241.2005.40849.x. [DOI] [PubMed] [Google Scholar]