Abstract
Background
As targeted therapy becomes increasingly important, diagnostic techniques for identifying targeted biomarkers have also become an emerging issue. The study aims to evaluate the cost-effectiveness of treating patients as guided by epidermal growth factor receptor (EGFR) mutation status compared with a no-testing strategy that is the current clinical practice in South Korea.
Methods
A cost-utility analysis was conducted to compare an EGFR mutation testing strategy with a no-testing strategy from the Korean healthcare payer’s perspective. The study population consisted of patients with stage 3b and 4 lung adenocarcinoma. A decision tree model was employed to select the appropriate treatment regimen according to the results of EGFR mutation testing and a Markov model was constructed to simulate disease progression of advanced non-small cell lung cancer. The length of a Markov cycle was one month, and the time horizon was five years (60 cycles).
Results
In the base case analysis, the testing strategy was a dominant option. Quality-adjusted life-years gained (QALYs) were 0.556 and 0.635, and total costs were $23,952 USD and $23,334 USD in the no-testing and testing strategy respectively. The sensitivity analyses showed overall robust results. The incremental cost-effectiveness ratios (ICERs) increased when the number of patients to be treated with erlotinib increased, due to the high cost of erlotinib.
Conclusion
Treating advanced adenocarcinoma based on EGFR mutation status has beneficial effects and saves the cost compared to no testing strategy in South Korea. However, the cost-effectiveness of EGFR mutation testing was heavily affected by the cost-effectiveness of the targeted therapy.
Introduction
Targeted therapy is becoming increasingly emphasized for cancer patients. If a targeted therapy is used to treat patients without the corresponding biomarker, it may result in not only increased burdens of disease, but also unfavorable therapeutic consequences, as targeted therapy is associated with shorter overall survival and progression-free survival (PFS) than standard chemotherapy in treating mutation-negative patients [1].
Therefore, techniques identifying mutation biomarkers are an important issue in the use of targeted therapy. In vitro diagnostics (IVD) is a technique that can detect diseases or infection in laboratories using commercialized devices. A laboratory-developed test (LDT) is an IVD that is designed, manufactured, and used within a single laboratory, and follows the protocol of that individual laboratory for testing [2]. Companion diagnostics (CDx) is characterized as an IVD providing information for the safe and effective use of targeted therapy [3]. Accordingly, an assessment of CDx is performed by evaluating diagnostic accuracy and clinical utility, which is defined as the extent of improved clinical outcome of a targeted therapy.
Lung cancer is one of the most common causes of death in the world [4]. Diagnostic techniques for epidermal growth factor receptor (EGFR) mutations are important in treatment of advanced non-small cell lung cancer (NSCLC) thanks to the availability of tyrosine-kinase inhibitors (TKIs). EGFR mutations in NSCLC are more common in adenocarcinomas, females, never-smokers and ethnic Asians [5]. The prevalence of EGFR mutations is known to be 20–40% in Asian populations and 5–20% in Caucasians [5]. The National Comprehensive Cancer Network (NCCN) Guidelines recommends EGFR mutation testing in cases of non-squamous cell carcinoma [6].
Traditionally, platinum-based chemotherapy such as cisplatin or carboplatin has been used as the first-line therapy in stage 3b and 4 NSCLC. The combination of cisplatin plus pemetrexed is more effective in patients with non-squamous histology than cisplatin plus gemcitabine, whereas the latter is more effective in patients with squamous cell histology [6–8]. However, if patients test positive for an EGFR mutation, monotherapy with a TKI is recommended [6,7].
In Korea, TKIs tend to be used as a second-line therapy, generally without testing for EGFR mutations. This treatment pattern may be due to the high cost of TKIs and additional testing. Unfortunately, this therapeutic practice may lead to a lost opportunity to use TKIs earlier in the treatment of EGFR mutation-positive patients. Moreover, although genetic testing technology is being actively developed in South Korea, there is still a lack of awareness regarding companion diagnostics of the approval authorities. Therefore, it is necessary to provide evidence of cost-effectiveness in order to make the best use of the new technology and to diffuse the concept of CDx.
In the past, studies of cost-effectiveness related to this issue were limited; studies now are more likely to be published. Previous studies were conducted with different conditions: gefitinib as a first-line therapy [5, 9], erlotinib as a first-line therapy [10,11], erlotinib as a second-line therapy [12,13], or an evaluation of the cost-effectiveness among test techniques with erlotinib as a first-line therapy [14]. The cost-effectiveness of testing strategies, compared to the no-testing strategy, is still unclear because the results vary across studies due to differences in models, epidemiological data, clinical efficacy data or healthcare costs. For example, the prevalence applied in the studies varied by ethnicity, ranging from 12.8% to 32%. Chemotherapy regimens used were different from one study to another such as cisplatin plus docetaxel [5], carboplatin plus docetaxel [11], carboplatin plus paclitaxel [9] and so forth. Also, a study [5] did consider second-line treatment in the model whereas others considered first-line therapy only.
Our interest was in the cost-effectiveness of TKI treatment and CDx in real-world settings. Thus, we aimed to assess the cost-effectiveness of targeted therapy based on EGFR mutation status compared with the current clinical practice in South Korean healthcare after application of all the relevant clinical data. The study is expected to provide important evidence for the treatment of NSCLC patients to healthcare policy makers and genetic assay developers, as well as patients, healthcare providers, and insurers.
Materials and Methods
Study Population
The study population consisted of patients with advanced (stage 3b and 4) lung adenocarcinoma, because the prevalence of EGFR mutations in adenocarcinoma patients is high, and chemotherapy is indicated as the main therapy in stage 3b or 4 NSCLC. In this model, EGFR mutation testing was performed to detect the presence of EGFR mutations before using erlotinib as the first-line treatment. Erlotinib of TKIs was our main interest because it was marketed more recently (in 2008) than gefitinib in Korea. Afatinib was not considered because of lack of data for the analysis, which was sold after 2014. Furthermore, any cost-effectiveness study for erlotinib has not included the OPTIMAL trial [15] for the analysis, which investigated clinical outcomes for erlotinib and EGFR testing in Asians.
Strategies Compared
We compared an EGFR mutation testing strategy with a no-testing strategy. The EGFR mutation testing strategy (‘testing strategy’) was defined as treatment guided by the result of EGFR mutation testing in advanced NSCLC patients: erlotinib for EGFR mutation-positive patients and conventional chemotherapy for wild type patients. In contrast, a strategy with no EGFR mutation testing (‘no-testing strategy’) was defined as patient treatment according to the current clinical practice: conventional chemotherapy for all patients regardless of EGFR mutation status.
Model Structure and Methodology
The model consisted of two components: a decision model and a Markov model. A decision tree model was used to select an appropriate treatment regimen according to test result in the testing strategy, and a Markov model was used to simulate NSCLC progression in both the testing and no-testing strategies. To calculate the proportions of each decision tree branch, we estimated the positive and negative predictive values of EGFR mutation testing based on test sensitivity and specificity, prevalence of EGFR mutations in patients with stage 3b and 4 lung adenocarcinoma, and the proportion of unknown test results which are the cases where EGFR mutation status cannot be determined due to inadequate tumor tissue. Unknown result may require rebiopsy. The Markov cycle length was one month, and the time horizon was five years (Fig 1). To simplify the model, it was assumed that cancer treatment was permitted up to the second progression, and best support care (BSC) was provided to control symptoms after the second progression.
Fig 1. Model structure.
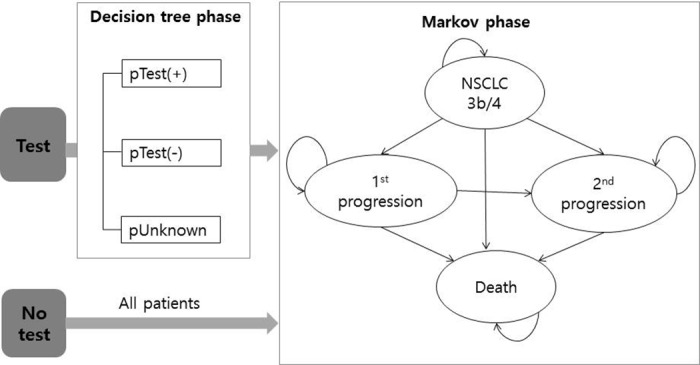
NSCLC, non-small cell lung cancer; pTest+, proportion of test positives; pTest-, proportion of test negatives; pUnknown, proportion of unknown tests. Note: pTest+ = pTP + pFP [pTP = prevalence × sensitivity × (1-pUnknown), pFP = (1-prevalence) × (1-specificity) × (1-pUnknown)]; Test- = pTN + pFN [pTN = (1-prevalence) × specificity × (1-pUnknown), pFN = prevalence × (1-sensitivity) × (1-pUnknown)]. ※ pTP, pFP, pTN, and pFN is the proportion of true positive, false positive, true negative, and false positive results, respectively. Prevalence is the percentage of patients harboring EGFR mutations in stage 3b and 4 lung adenocarcinoma.
The type of drug therapy was selected based on treatment guidelines [6–8], the medical advice of oncologists, and previous cost-effectiveness studies [5,12,14]. In the testing strategy, EGFR mutation-positive patients received first-line erlotinib treatment, second-line chemotherapy with cisplatin plus pemetrexed, and then BSC if all treatments failed. EGFR mutation–negative or test-unknown patients received first-line cisplatin plus pemetrexed treatment, second-line docetaxel, and then BSC. Meanwhile, patients in the no-testing strategy received first-line cisplatin plus pemetrexed treatment, second-line erlotinib, and then BSC. We assumed that all patients were administered four cycles of chemotherapy.
Progression-free survival (PFS), overall survival (OS), and adverse events were included as clinical outcomes. Disutilities for adverse events were applied to the first cycle in the Markov model for a single cycle. To apply time-variant Markov transition probabilities, we used data obtained from the Kaplan-Meier (K-M) curve, which was extracted using an image-digitizing program [16]. We extrapolated up to 60 months, applying frequently used methods: (1) to fit the curve and minimize the sum of squares between actual data obtained from the K-M curve and estimated data (least square method), (2) to regress ln(-ln(S(t)) vs. ln(t) (regression method). The mean of the two methods was used for the base case model.
The study was analyzed from a healthcare–payer perspective and included analysis of direct medical costs and excluded uninsured benefits. Costs were estimated using a macro-cost approach with the claims data of the 2011 Health Insurance Review and Assessment Services-National Patient Sample (HIRA-NPS). All the costs were adjusted to 2014 prices with the Consumer Price Index and were reported in US dollars (1 US dollar = Kor ₩1,012.1). The base case model applied a 3% discount rate.
The data for clinical outcomes and utilities were obtained from published studies. The result was reported as an incremental cost-effectiveness ratio (ICER), and sensitivity analyses were performed to explore uncertainties. Cost data were analyzed using SAS® 8.2 software, and the cost-effectiveness model was analyzed using Treeage® pro 2014 software.
Epidemiologic Data
To investigate the prevalence of EGFR mutations in stage 3b and 4 lung adenocarcinoma in Korea, we performed a literature search using the Medline and Korean search engine (RISS). Inclusion was limited to studies published after 2005 with greater than 50 samples. Only one study by Choi et al. [17] was found to be valid. Choi et al. analyzed retrospective cohorts (n = 1,503) of all patients treated for stage 3b or 4 NSCLC between 2007 and 2010 in the Samsung Medical Center, one of the major hospitals in Korea. Adenocarcinoma composed 75% of the cohorts. The prevalence of EGFR mutations was 36.3% in stage 3b and 4 NSCLC and 39% in stage 3b and 4 adenocarcinoma (Table 1). The proportion with an unknown test result was 11%, which was decided based on expert opinions and was considered to be comparable to results from another study [11] where rebiopsy was not performed in 10% despite inadequate tumor tissue and ‘still unknown test result after rebiopsy‘ was 1.5%.
Table 1. Input parameters.
| Item | Value | 95% CI or SD | Source |
|---|---|---|---|
| Prevalence of EGFR mutations in adenocarcinoma | 39% | [17] | |
| Prevalence of EGFR mutations in stage 3b and 4 NSCLC | 36.3% | [17] | |
| Test sensitivity | 98.4% | [18–22] | |
| Test specificity | 89.2% | [18–22] | |
| Unknown % | 11% | Assumption | |
| Cost of EGFR testing (US $) | 104 | Medical fee | |
| Total treatment cost per one cycle (US $) | (SD) | ||
| During ERT administration (all cycles) | 2,113 | 2,381 | 2011 Claims data |
| During CPEM administration | 4,157 | 3,301 | 2011 Claims data |
| During CPEM non-administration | 503 | 471 | 2011 Claims data |
| During CGEM administration | 3,327 | 2,943 | 2011 Claims data |
| During CGEM non-administration | 454 | 455 | 2011 Claims data |
| During DOX administration | 3,300 | 2,378 | 2011 Claims data |
| During DOX non-administration | 616 | 430 | 2011 Claims data |
| During PEM administration | 4,501 | 2,708 | 2011 Claims data |
| During PEM non-administration | 594 | 603 | 2011 Claims data |
| During BSC (all cycles) | 1,038 | 884 | [23] |
| Utilities | (95% CI) | ||
| NSCLC | 0.6532 | 0.6096/0.6968 | [24] |
| Progression | 0.4734 | 0.4309/0.5159 | [24] |
| BSC | 0.4734 | 0.4309/0.5159 | [24] |
| Disutility by adverse side effect | |||
| - Neutropenia | -0.0897 | 0.0595/0.1200 | [24] |
| - Febrile neutropenia | -0.0900 | 0.0580/0.1220 | [24] |
| - Fatigue | -0.0735 | 0.0373/0.1097 | [24] |
| - Nausea & vomiting | -0.0480 | 0.0163/0.0797 | [24] |
| - Diarrhea | -0.0468 | 0.0164/0.0772 | [24] |
| - Hair loss | -0.0450 | 0.0160/0.0740 | [24] |
| - Rash | -0.0325 | 0.0095/0.0555 | [24] |
| - Anemia | -0.0730 | 0.0377/0.1083 | [14] |
| Utility in PO. | +0.02 | Not reported | [25] |
| Disutility by IV inj. | -0.043 | 0.0038/0.0822 | [14] |
(1 US dollar = Kor ₩1,012.1). 95% CI, 95% confidence interval; SD, standard deviation; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; ERT, erlotinib; DOX, docetaxel; CGEM, cisplatin plus gemcitabine; CPEM, cisplatin plus pemetrexed; PEM, pemetrexed; BSC, best supportive care; PFS, progression-free survival; OS, overall survival.
Diagnostic Accuracy
With regard to the average diagnostic accuracy for representative EGFR mutation testing techniques, we used a systematic review study on diagnostic accuracy by Ellison et al. [18]. Since the study did not include the Therascreen® EGFR RGQ PCR kit or the Cobas® CDx as approved by the FDA, we performed an additional literature search for these two techniques. We used the results of sensitivity and specificity testing as evaluated by direct sequencing (Sanger sequencing) as a reference standard; the average sensitivity and specificity were 98.4% and 89.2%, respectively (Table 1).
Clinical Outcomes
We adopted systematic approaches to literature searches from Medline to obtain the clinical data needed for the Markov branches. Separate searching strategies were developed for the intervention required in each Markov branch, based on first-line or second-line treatment, or mutation status. The search was limited to randomized controlled trials (RCT), published after 2005, and written in English or Korean; however, the type of control was not defined. The major inclusion criteria were studies reporting survival curves for outcomes, a target population of patients with stage 3b and 4 NSCLC, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 ≥ 80% of included patients. The weighted average was calculated by the sample size if more than one study was selected.
Studies meeting the inclusion criteria for most Markov branches are listed as follows: PFS with erlotinib as the first-line treatment in EGFR mutation-positive patients [15,19,26], PFS and OS with cisplatin plus pemetrexed as a second-line treatment in mutation-positive patients [27], PFS with cisplatin plus pemetrexed as a first-line treatment in mutation-negative patients [28], PFS and OS with docetaxel as a second-line treatment in mutation-negative patients [29], PFS and OS with cisplatin plus pemetrexed as a first-line treatment in the no-testing strategy [30,31], PFS and OS with cisplatin plus gemcitabine as a first-line treatment in the no-testing strategy [31–39], PFS and OS with erlotinib as a second-line treatment in the no-testing strategy [40–51], and OS with BSC [52]. The study for second-line cisplatin plus pemetrexed treatment in mutation-positive patients was a single-arm study. The OS of first-line erlotinib treatment in mutation-positive patients and first-line cisplatin plus pemetrexed treatment in mutation-negative patients were replaced with the OS of all patients in the no-testing strategy. According to the results, the PFS of erlotinib in EGFR mutation-positive patients produces a distinctively better result than other cases. The integrated results applied to the model are provided in a S1 and S2 Figs as form of survival curves.
Costs
We considered only reimbursed direct medical costs, which included inpatient, outpatient, and pharmacy costs based on health insurance claims data. To assess the costs, we selected patients diagnosed with lung cancer (C34) using the Korean Standard Classification of Diseases version 6 (KCD-6) codes. To select patients with stage 3b and 4 NSCLC, we used an operational definition of all the cases of patients who received chemotherapy and excluded patients who had surgery. The main assumption in the cost estimation was that costs only depended on the treatment regimen, regardless of first-line or second-line treatment, and regardless of EGFR mutation status.
A total of 378 patients were analyzed for the cost assessment. According to the descriptive analysis, 224 of the patients were men (59.3%), the average age was 64 years, and the majority (64.2%) was in their seventies. Excluding outliers, the total average annual cost for NSCLC treatment was $17,785 USD and the average inpatient cost was $9,584 USD. Inputs of costs for Markov states are presented in Table 1.
Costs for BSC were obtained from a published study [23] that investigated terminal cancer patients under hospice care; the average medical cost for these patients was $1,038 USD per month (Table 1).
Utilities
The two representative studies on the quality of life of NSCLC patients were performed by Nafees et al [24], and Doyle et al. [53]. We applied the results of the former to the model because that study was more relevant in terms of health statuses. The study determined the utilities of the health statuses of patients with stage 3b and 4 NSCLC and the disutilities of grade III-IV toxicities under situations where metastatic NSCLC patients received second-line chemotherapy. Data on disutility in anemia was taken from a study by Westwood et al. [14]. We assumed that the adverse events occurred during the first cycle, and that adverse event rates relied on the type of treatment regimen, regardless of whether it was first-line or second-line treatment. Finally, in case of an oral medication, an adjustment of 0.02, obtained from a study by Tabberer et al. [25], was added to the utilities investigated by Nafees et al., because the study was performed using intravenous treatments of pemetrexed and docetaxel.
Sensitivity Analysis
Sensitivity analyses were conducted for uncertainties in different treatment effects and different utilities. Additionally, one-way sensitivity analyses were conducted for the test cost, analysis cycle, prevalence of EGFR mutations, proportion of unknown tests, test accuracy, and NSCLC treatment costs.
Results
The base case analysis (Table 2) showed that the testing strategy would be the dominant option, with greater effectiveness and lower costs than the no-testing strategy: the average medical costs were $23,952 USD in the no-testing strategy and $23,334 USD in the testing strategy, and quality-adjusted life-years gained (QALYs) were 0.556 and 0.635 respectively. The sensitivity analyses generally demonstrated robust results. However, both the incremental cost and incremental QALY of the testing strategy increased in the model that substituted the OS with erlotinib in mutation-positive patients with the OS achieved with cisplatin plus pemetrexed treatment in all patients of the no-testing strategy, and in the CGEM-PEM model that applied first-line cisplatin plus gemcitabine and second-line pemetrexed for chemotherapy treatment branches. As a result, the testing strategy was not dominant, but still a cost-effective option. In particular, application of the prolonged OS of erlotinib resulted in a greater increase in the cost than the effect, meaning that erlotinib may not be effective enough to offset the increased cost.
Table 2. Results of cost-effectiveness in the base case and sensitivity analyses.
| Strategy | Cost | Incr Cost | Efficacy | Incr Eff | ICER |
|---|---|---|---|---|---|
| (US$) | (US$) | (QALY) | (QALY) | (US$/QALY) | |
| Base case: adenocarcinoma 39%, discount rate 3%, sensitivity 98.4%, specificity 89.2% | |||||
| Test | 23,334 | 0.63522 | |||
| No test | 23,952 | 619 | 0.55621 | -0.07901 | dominated |
| Sensitivity analyses of different probabilities of PFS or OS | |||||
| 1) Extrapolation: Regression ln(-ln(S(t)) vs. ln(t) | |||||
| Test | 23,504 | 0.65240 | |||
| No test | 24,048 | 544 | 0.57575 | -0.07665 | dominated |
| 2) Extrapolation: Least square estimation | |||||
| Test | 22,715 | 0.60107 | |||
| No test | 23,747 | 1,032 | 0.53264 | -0.06843 | dominated |
| 3) Substitution OS of erlotinib in M+ by OS of cisplatin plus pemetrexed in all (no-testing) | |||||
| No test | 23,952 | 0.55621 | |||
| Test | 24,064 | 111 | 0.66774 | 0.11153 | 999 |
| Sensitivity analyses of different treatment regimens (health outcomes and costs) | |||||
| 1) ERT-PEM model (1st line ERT, 2nd line PEM in M+) | |||||
| Test | 23,300 | 0.63087 | |||
| No test | 23,952 | 653 | 0.55621 | -0.07466 | dominated |
| 2) CGEM-PEM model [1st line CGEM, 2nd line PEM in M- and 1st line CGEM in all (no-testing)] | |||||
| No test | 19,954 | 0.55591 | |||
| Test | 21,528 | 1,574 | 0.64013 | 0.08421 | 18,687 |
| Sensitivity analyses of different utilities | |||||
| 1) Side-effect induced disutility during the whole period of drug administration | |||||
| Test | 23,334 | 0.62775 | |||
| No test | 23,952 | 619 | 0.54760 | -0.08015 | dominated |
| 2) Disutility in intravenous medication according to Wang et al. [54] | |||||
| Test | 23,334 | 0.61087 | |||
| No test | 23,952 | 619 | 0.53137 | -0.07950 | dominated |
(Exchange rate: 1 US$ = 1,021 KRW); ICER, Incremental cost-effectiveness ratio; OS, overall survival; M+, EGFR mutation positive; M-, EGFR mutation negative; ERT, erlotinib; PEM, pemetrexed; CPEM, cisplatin plus pemetrexed.
The results of the one-way sensitivity analyses are presented as a tornado diagram in Fig 2. The most sensitive parameter was the medical cost of cisplatin plus pemetrexed therapy, but the testing strategy was still cost-effective within the lower and upper 25% of medical costs. The medical cost of BSC was the least sensitive of all costs. The ICERs increased with increasing cycles, prevalence of EGFR mutations, medical cost of erlotinib or docetaxel therapy, and test cost. A high prevalence of EGFR mutations incurred a greater increase in the cost than in the effect. This resulted in an increase in ICER. The results with regard to the proportion of unknown tests or sensitivity of a test also showed the same pattern, i.e., the ICER increased because of increased costs when the number of patients receiving first-line erlotinib treatment increased.
Fig 2. Tornado diagram of one-way sensitivity analysis.
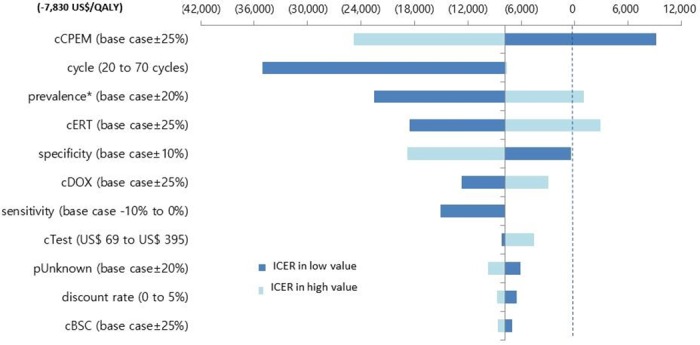
* Prevalence of EGFR mutation. cERT, medical cost of erlotinib; cCPEM, medical cost of cisplatin plus pemetrexed; cDOX, medical cost of docetaxel; cTest, cost of genetic test; cBSC, medical cost of best supportive care in terminal cancer patients; pUnknown, proportion of unknown tests.
Discussion
The study was conducted to investigate the cost-effectiveness of CDx and targeted therapy in patients with stage 3b and 4 lung adenocarcinoma. The base case and sensitivity analyses showed that treating patients as guided by EGFR mutation status was a dominant option. Nevertheless, without a cost-effective targeted therapy, the cost-effectiveness of CDx cannot be attained even if the technique is highly accurate; that is, the cost-effectiveness of CDx heavily depends on the cost and the effect of the targeted therapy in the context of the Korean healthcare system. From sensitivity analyses, we found that ICERs increased in situations where the number of patients receiving first-line erlotinib treatment increased, such as in the improved OS of first-line erlotinib treatment in mutation-positive patients, high prevalence of EGFR mutations, and low proportion of unknown test results. Those increased ICERs came from a much greater increase in the incremental cost than in the incremental effect. However, the benefit of first-line erlotinib treatment may be underestimated in this model, since the OS of first-line erlotinib treatment was substituted with the OS of cisplatin plus gemcitabine treatment in the no-testing strategy. The outcomes of the treatments tend to be better in EGFR-mutated patients than EGFR wild type or all patients, even with chemotherapy treatment [1].
Even though several studies have investigated the cost-effectiveness of EGFR mutation testing with accompanying targeted therapy (including gefitinib), any direct comparison of the results should be carefully made due to huge differences in the models and applied data among the studies, as well as differences among nations in terms of resource utilization, healthcare patterns, and medical costs. Nevertheless, by simply comparing the cost-effectiveness of first-line TKI therapy without considering other differences, our study showed more favorable results for the testing strategy than other studies [5,9–11]: the testing strategy was less costly and more effective than the no-testing strategy. All studies, including ours, demonstrated that the effect was improved in the testing strategy over the no-testing strategy. The incremental effects were 0.0307 QALYs in an Ontario study [5], 0.013 QALYs in a German study [11], 0.036 QALYs in a Japanese study [9], and 0.079 in our study. This greatest incremental effect in our study could be attributed to inclusion of the OPTIMAL trial [15] which reported the most favorable outcome for a TKI relative to other representative trials of TKIs such as the EURTAC trial [26] and the IPASS trial [1]. Varying prevalence of EGFR mutations in the studies may also account for the difference in incremental QALYs: 16.8% in the Ontario study, 12.8% in the study by Schremser et al., 32% in the study by Narita et al., and 39% in our study. However, the costs were more or less varied among the studies: decreased costs in the testing strategy in our study, and increased costs in the other studies. The difference in incremental costs may be due to variations in medical costs across countries and in conventional chemotherapy regimens across studies. The chemotherapy regimen applied in our model, cisplatin plus pemetrexed, was relatively costly compared to other models, such as cisplatin plus docetaxel in the Ontario model [5], carboplatin plus docetaxel and others in a German model [11], or carboplatin plus paclitaxel in a Japanese model [9]. The costly chemotherapy regimen would result in high costs for the no-testing strategy.
The prevalence of EGFR mutations in our study was obtained from a landmark study with large samples. Since all patients with stage 3b and 4 NSCLC were not tested for EGFR mutations in that study [17], the 39% EGFR prevalence reported in adenocarcinoma patients could be considered to be an overestimate. However, this prevalence did not differ greatly among other studies on the Asian population [9,55].
The present study is meaningful in some aspects. Differing from the previous studies [9–13] (except for the Ontario study), all the data available including the clinical outcomes of the intervention required in each Markov branch was obtained via a systematic review approach, instead of using one or two representative studies; as a result, the study provides up-to-date information on clinical efficacy with increased reliability and generalizability. In addition, we tried to reflect results seen in real-world settings by adopting a model of whole-disease progression from first-line treatment of stage 3b and 4 NSCLC to patient death.
However, the study has some limitations, mainly related to the clinical outcomes of the studies used. First, while we included RCT studies, the control effect was ignored because only a single arm from these studies was used, since the comparator was different among the studies available for each Markov branch. Second, no RCT study was found for second-line cisplatin plus pemetrexed treatment in mutation-positive patients, so that we used a single-arm study instead. Third, the study population was limited to patients with stage 3b and 4 lung adenocarcinoma, but most studies were conducted in all patients with stage 3b and 4 NSCLC. As a result, adenocarcinoma patients were analyzed as a subpopulation; thus, the population was not restricted to adenocarcinoma patients. However, these issues are likely to equally affect both strategies, and the cost-effectiveness may not change significantly. In addition, issues related to claims data could occur in the process of cost estimation, such as coding accuracy produced by the intention to maximize payment [56].
Conclusion
From the Korean healthcare payer’s perspective using the Markov model of a one-month Markov cycle and five-year time horizon, the strategy of EGFR mutation testing before first-line erlotinib administration for advanced lung adenocarcinoma was the dominant option in the base case analysis, compared with the no-testing strategy. The incremental QALY was 0.07901, and the cost decrement of the testing strategy was $619 USD. The incremental QALY from our study was greater than other studies, in which the incremental QALYs ranged from 0.013 to 0.036. The sensitivity analyses were generally robust, but the ICERs increased in situations where the number of patients receiving erlotinib increased. Thus, we postulate that the cost-effectiveness of CDx depends on the cost-effectiveness of the targeted therapy. Further study is recommended to support the results of this study due to data limitations regarding clinical effects and costs.
This research was supported by the R&D Program for Society of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant number: 2013M3C8A1075908). Financial support from any other companies was not provided.
Supporting Information
The survival cures were drawn by integrating results obtained from a systematic review. The number of included trials were three for first line erlotinib in M(+), one for second line cisplatin plus pemetrexed in M(+), one for first line cisplatin plus pemetrexed in M(-), one for second line docetaxel in M(-), two for first line cisplatin plus pemetrexed in all and twelve for second line erlotinib in all patients. M+, mutation positive; M-, mutation negative; ERT, erlotinib; CPEM, cisplatin plus pemetrexed; DOX, docetaxel.
(TIFF)
Due to lack of data, the OS of first line erlotinib in M(+) was replaced with the OS of first line cisplatin plus gemcitabine in all patients and first line cisplatin plus pemetrexed in M(-) was replaced with the OS of all patients. The number of included trials were nine for first line cisplatin plus gemcitabine in all, one for second line cisplatin plus pemetrexed in M(+), one for second line docetaxel in M(-), two for first line cisplatin plus pemetrexed in all and twelve for second line erlotinib in all patients. M+, mutation positive; M-, mutation negative; ERT, erlotinib; CPEM, cisplatin plus pemetrexed; DOX, docetaxel.
(TIFF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the R&D Program for Society of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant number: 2013M3C8A1075908). Financial support from any other companies was not provided.
References
- 1.IPASS study. In: IRESSA. 2016; 3: 29. Available: http://www.iressa.com/ipass-study.html.
- 2.Health C for D and R. In Vitro Diagnostics—Laboratory Developed Tests. In: U.S. Food and Drug Administration (FDA). 2016; 3: 29. Available: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm407296.htm.
- 3.Health C for D and R. In Vitro Diagnostics—Companion Diagnostics. In: U.S. Food and Drug Administration (FDA). 2016; 3: 29. Available: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm407297.htm.
- 4.WHO | Cancer. In: WHO. 2016; 3: 29. Available: http://www.who.int/mediacentre/factsheets/fs297/en/.
- 5.Ontario, Ministry of Health and Long-Term Care, Medical Advisory Secretariat. Epidermal growth factor receptor mutation (EGFR) testing for prediction of response to EGFR-targeting tyrosine kinase inhibitor (TKI) drugs in patients with advanced non-small-cell lung cancer an evidence-based analysis Toronto, Ont.: Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care; 2011. Available: http://www.deslibris.ca/ID/227827 [PMC free article] [PubMed]
- 6.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version 4. 2014. In: National Comprehensive Cancer Network (NCCN). 2014; 11: 12. Available: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 7.2010 Korean Medical Guideline: Lung cancer. Korean Association for the Study of Lung Cancer. In: Korean Medical Guideline Information Center (KoMGI) 2014; 11:12. Available: http://www.guideline.or.kr/guideline/guide/guide_renew.php.
- 8.Erlotinib for the first-line treatment of locally advanced or metastatic EGFR-TK mutation-positive non-small-cell lung cancer | Guidance and guidelines | NICE. In: National Institute for Health and Care Excellence (NICE) 2016; 3: 29. Available: https://www.nice.org.uk/guidance/ta258.
- 9.Narita Y, Matsushima Y, Shiroiwa T, Chiba K, Nakanishi Y, Kurokawa T, et al. Cost-effectiveness analysis of EGFR mutation testing and gefitinib as first-line therapy for non-small cell lung cancer. Lung Cancer. 2015;90: 71–77. 10.1016/j.lungcan.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Lieberthal RD, Dudash K, Axelrod R, Goldfarb NI. An economic model to value companion diagnostics in non-small-cell lung cancer. Pers Med. 2013;10: 139–147. 10.2217/pme.13.7 [DOI] [PubMed] [Google Scholar]
- 11.Schremser K, Rogowski WH, Adler-Reichel S, Tufman ALH, Huber RM, Stollenwerk B. Cost-Effectiveness of an Individualized First-Line Treatment Strategy Offering Erlotinib Based on EGFR Mutation Testing in Advanced Lung Adenocarcinoma Patients in Germany. PharmacoEconomics. 2015;33: 1215–1228. 10.1007/s40273-015-0305-8 [DOI] [PubMed] [Google Scholar]
- 12.Borget I, Cadranel J, Pignon J-P, Quoix E, Coudert B, Westeel V, et al. Cost-effectiveness of three strategies for second-line erlotinib initiation in nonsmall-cell lung cancer: the ERMETIC study part 3. Eur Respir J. 2012;39: 172–179. 10.1183/09031936.00201210 [DOI] [PubMed] [Google Scholar]
- 13.Carlson JJ, Garrison LP, Ramsey SD, Veenstra DL. The Potential Clinical and Economic Outcomes of Pharmacogenomic Approaches to EGFR-Tyrosine Kinase Inhibitor Therapy in Non–Small-Cell Lung Cancer. Value Health. 2009;12: 20–27. 10.1111/j.1524-4733.2008.00415.x [DOI] [PubMed] [Google Scholar]
- 14.Westwood M, Joore M, Whiting P, van Asselt T, Ramaekers B, Armstrong N, et al. Epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation testing in adults with locally advanced or metastatic non-small cell lung cancer: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18 10.3310/hta18320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12: 735–742. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 16.WebPlotDigitizer—Extract data from plots, images, and maps. In: Web Plot Digitizer 2016; 2: 29. Available: http://arohatgi.info/WebPlotDigitizer/.
- 17.Choi Y-L, Sun J-M, Cho J, Rampal S, Han J, Parasuraman B, et al. EGFR Mutation Testing in Patients with Advanced Non-Small Cell Lung Cancer: A Comprehensive Evaluation of Real-World Practice in an East Asian Tertiary Hospital. Mittal B, editor. PLoS ONE. 2013;8: e56011 10.1371/journal.pone.0056011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66: 79–89. 10.1136/jclinpath-2012-201194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benlloch S, Botero ML, Beltran-Alamillo J, Mayo C, Gimenez-Capitán A, de Aguirre I, et al. Clinical Validation of a PCR Assay for the Detection of EGFR Mutations in Non–Small-Cell Lung Cancer: Retrospective Testing of Specimens from the EURTAC Trial. Minna JD, editor. PLoS ONE. 2014;9: e89518 10.1371/journal.pone.0089518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Rios F, Angulo B, Gomez B, Mair D, Martinez R, Conde E, et al. Comparison of molecular testing methods for the detection of EGFR mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer. Journal of Clinical Pathology. 2013; 66: 381–5. 10.1136/jclinpath-2012-201240. Epub 2013 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell P, Ferguson J, Shyu J, Current R, Rehage T, Tsai J, et al. Analytic performance studies and clinical reproducibility of a real-time PCR assay for the detection of epidermal growth factor receptor gene mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer. BMC Cancer 2013; 13: 1–10. 10.1186/1471-2407-13-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2013 Therascreen® EGFR RGQ PCR Kit Handbook. 2016; 3: 29. Available: https://www.google.co.kr/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&sqi=2&ved=0ahUKEwiG-MDw2_7NAhVBpZQKHTt8BwQQFgggMAA&url=https%3A%2F%2Fwww.qiagen.com%2Fch%2Fresources%2Fdownload.aspx%3Fid%3Ddb794cae-999b-4362-aba3-455ebfd807a5%26lang%3Den&usg=AFQjCNEpCgF-lJMNDBi55fvHpkdyi2LG3A&sig2=orILzprNXlYNLRDK3Dmt0w&bvm=bv.127521224,d.dGo&cad=것.
- 23.Choi KS, You CH, Lee KH, Kim CY, Heo DS, Yun YH. Comparison of medical care cost between hospice care and conventional care in the last year of life. Korean J Health Policy Adm. 2005;15: 1–15. [Google Scholar]
- 24.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6: 84 10.1186/1477-7525-6-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabberer M, Stamuli E, Walker M, Summerhayes M, Lees M. PCN74 UTILITIES ASSOCIATED WITH NON-SMALL CELL LUNG CANCER (NSCLC): A COMMUNITY STUDY. Value Health. 2006;9: A298. [Google Scholar]
- 26.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13: 239–246. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 27.Zhang G-Z, Jiao S-C, Meng Z-T. Pemetrexed plus cisplatin/carboplatin in previously treated locally advanced or metastatic non-small cell lung cancer patients. J Exp Clin Cancer Res. 2010;29: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Fang S, Guo J, Liu J, Li C, Liu L, et al. Correlation between EGFR mutation status and response to first-line platinum-based chemotherapy in patients with advanced non-small cell lung cancer. OncoTargets Ther. 2014; 7: 1185–93. 10.2147/OTT.S63665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, et al. Randomized phase III trial of erlotinib versus docetaxel as second-or third-line therapy in patients with advanced non–small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol. 2014;32: 1902–1908. 10.1200/JCO.2013.52.4694 [DOI] [PubMed] [Google Scholar]
- 30.Schuette WHW, Gröschel A, Sebastian M, Andreas S, Müller T, Schneller F, et al. A Randomized Phase II Study of Pemetrexed in Combination With Cisplatin or Carboplatin as First-Line Therapy for Patients With Locally Advanced or Metastatic Non–Small-Cell Lung Cancer. Clin Lung Cancer. 2013;14: 215–223. 10.1016/j.cllc.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Lu J, Xu J, Li H, Wang J, Qin Y, et al. Pemetrexed plus platinum or gemcitabine plus platinum for advanced non-small cell lung cancer: final survival analysis from a multicentre randomized phase II trial in the East Asia region and a meta-analysis: Pemetrexed and survival of NSCLC patients. Respirology. 2013;18: 131–139. 10.1111/j.1440-1843.2012.02248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gridelli C, Gallo C, Ceribelli A, Gebbia V, Gamucci T, Ciardiello F, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the Gemcitabine-COxib in NSCLC (GECO) study. Lancet Oncol. 2007;8: 500–512. [DOI] [PubMed] [Google Scholar]
- 33.Hsu C, Kuo S-H, Hu F-C, Cheng A-L, Shih J-Y, Yu C-J, et al. Gemcitabine plus conventional-dose epirubicin versus gemcitabine plus cisplatin as first-line chemotherapy for stage IIIB/IV non-small cell lung carcinoma—A randomized phase II trial. Lung Cancer. 2008;62: 334–343. 10.1016/j.lungcan.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 34.Mok TSK, Wu Y-L, Yu C-J, Zhou C, Chen Y-M, Zhang L, et al. Randomized, Placebo-Controlled, Phase II Study of Sequential Erlotinib and Chemotherapy As First-Line Treatment for Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2009;27: 5080–5087. 10.1200/JCO.2008.21.5541 [DOI] [PubMed] [Google Scholar]
- 35.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III Trial of Cisplatin Plus Gemcitabine With Either Placebo or Bevacizumab As First-Line Therapy for Nonsquamous Non-Small-Cell Lung Cancer: AVAiL. J Clin Oncol. 2009;27: 1227–1234. 10.1200/JCO.2007.14.5466 [DOI] [PubMed] [Google Scholar]
- 36.Mylonakis N, Athanasiou A, Ziras N, Angel J, Rapti A, Lampaki S, et al. Phase II study of liposomal cisplatin (LipoplatinTM) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer. 2010;68: 240–247. 10.1016/j.lungcan.2009.06.017 [DOI] [PubMed] [Google Scholar]
- 37.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JSK, Blasinska-Morawiec M, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2012;23: 72–77. 10.1093/annonc/mdr030 [DOI] [PubMed] [Google Scholar]
- 38.Paz-Ares LG, Biesma B, Heigener D, von Pawel J, Eisen T, Bennouna J, et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Gemcitabine/Cisplatin Alone or With Sorafenib for the First-Line Treatment of Advanced, Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2012;30: 3084–3092. 10.1200/JCO.2011.39.7646 [DOI] [PubMed] [Google Scholar]
- 39.Jänne PA, Paz-Ares L, Oh Y, Eschbach C, Hirsh V, Enas N, et al. Randomized, Double-Blind, Phase II Trial Comparing Gemcitabine-Cisplatin plus the LTB4 Antagonist LY293111 versus Gemcitabine-Cisplatin plus Placebo in First-Line Non–Small-Cell Lung Cancer. J Thorac Oncol. 2014;9: 126–131. 10.1097/JTO.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 40.Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. The Lancet. 2011;377: 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim ST, Uhm JE, Lee J, Sun J, Sohn I, Kim SW, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer. 2012;75: 82–88. 10.1016/j.lungcan.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 42.Natale RB, Thongprasert S, Greco FA, Thomas M, Tsai C-M, Sunpaweravong P, et al. Phase III Trial of Vandetanib Compared With Erlotinib in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;29: 1059–1066. 10.1200/JCO.2010.28.5981 [DOI] [PubMed] [Google Scholar]
- 43.Spigel DR, Burris HA, Greco FA, Shipley DL, Friedman EK, Waterhouse DM, et al. Randomized, Double-Blind, Placebo-Controlled, Phase II Trial of Sorafenib and Erlotinib or Erlotinib Alone in Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;29: 2582–2589. 10.1200/JCO.2010.30.7678 [DOI] [PubMed] [Google Scholar]
- 44.Kelly K, Azzoli CG, Zatloukal P, Albert I, Jiang PY, Bodkin D, et al. Randomized Phase 2b Study of Pralatrexate Versus Erlotinib in Patients With Stage IIIB/IV Non–Small-Cell Lung Cancer (NSCLC) After Failure of Prior Platinum-Based Therapy. J Thorac Oncol. 2012;7: 1041–1048. 10.1097/JTO.0b013e31824cc66c [DOI] [PubMed] [Google Scholar]
- 45.Scagliotti GV, Krzakowski M, Szczesna A, Strausz J, Makhson A, Reck M, et al. Sunitinib Plus Erlotinib Versus Placebo Plus Erlotinib in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Phase III Trial. J Clin Oncol. 2012;30: 2070–2078. 10.1200/JCO.2011.39.2993 [DOI] [PubMed] [Google Scholar]
- 46.Witta SE, Jotte RM, Konduri K, Neubauer MA, Spira AI, Ruxer RL, et al. Randomized Phase II Trial of Erlotinib With and Without Entinostat in Patients With Advanced Non-Small-Cell Lung Cancer Who Progressed on Prior Chemotherapy. J Clin Oncol. 2012;30: 2248–2255. 10.1200/JCO.2011.38.9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aerts JG, Codrington H, Lankheet NAG, Burgers S, Biesma B, Dingemans A-MC, et al. A randomized phase II study comparing erlotinib versus erlotinib with alternating chemotherapy in relapsed non-small-cell lung cancer patients: the NVALT-10 study. Ann Oncol. 2013;24: 2860–2865. 10.1093/annonc/mdt341 [DOI] [PubMed] [Google Scholar]
- 48.Groen HJM, Socinski MA, Grossi F, Juhasz E, Gridelli C, Baas P, et al. A randomized, double-blind, phase II study of erlotinib with or without sunitinib for the second-line treatment of metastatic non-small-cell lung cancer (NSCLC). Ann Oncol. 2013;24: 2382–2389. 10.1093/annonc/mdt212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee DH, Lee JS, Kim S-W, Rodrigues-Pereira J, Han B, Song X-Q, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer. 2013;49: 3111–3121. 10.1016/j.ejca.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 50.Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH, Blumenschein GR, et al. Randomized Phase II Trial of Onartuzumab in Combination With Erlotinib in Patients With Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2013;31: 4105–4114. 10.1200/JCO.2012.47.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregorc V, Novello S, Lazzari C, Barni S, Aieta M, Mencoboni M, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol. 2014;15: 713–721. 10.1016/S1470-2045(14)70162-7 [DOI] [PubMed] [Google Scholar]
- 52.Kim YJ, Kim S-J, Lee JK, Choi W-S, Park JH, Kim HJ, et al. Prediction of survival in terminally ill cancer patients at the time of terminal cancer diagnosis. J Cancer Res Clin Oncol. 2014;140: 1567–1574. 10.1007/s00432-014-1688-1 [DOI] [PubMed] [Google Scholar]
- 53.Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. 2008;62: 374–380. 10.1016/j.lungcan.2008.03.019 [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Peng L, Li J, Zeng X, Ouyang L, Tan C, et al. A trial-based cost-effectiveness analysis of erlotinib alone versus platinum-based doublet chemotherapy as first-line therapy for Eastern Asian nonsquamous non-small-cell lung cancer. PloS One. 2013; 8: e55917 10.1371/journal.pone.0055917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the Epidermal Growth Factor Receptor Gene in Lung Cancer Biological and Clinical Implications. Cancer Res. 2004;64: 8919–8923. 10.1158/0008-5472.CAN-04-2818 [DOI] [PubMed] [Google Scholar]
- 56.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47: S51–S55. 10.1097/MLR.0b013e31819c95aa [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The survival cures were drawn by integrating results obtained from a systematic review. The number of included trials were three for first line erlotinib in M(+), one for second line cisplatin plus pemetrexed in M(+), one for first line cisplatin plus pemetrexed in M(-), one for second line docetaxel in M(-), two for first line cisplatin plus pemetrexed in all and twelve for second line erlotinib in all patients. M+, mutation positive; M-, mutation negative; ERT, erlotinib; CPEM, cisplatin plus pemetrexed; DOX, docetaxel.
(TIFF)
Due to lack of data, the OS of first line erlotinib in M(+) was replaced with the OS of first line cisplatin plus gemcitabine in all patients and first line cisplatin plus pemetrexed in M(-) was replaced with the OS of all patients. The number of included trials were nine for first line cisplatin plus gemcitabine in all, one for second line cisplatin plus pemetrexed in M(+), one for second line docetaxel in M(-), two for first line cisplatin plus pemetrexed in all and twelve for second line erlotinib in all patients. M+, mutation positive; M-, mutation negative; ERT, erlotinib; CPEM, cisplatin plus pemetrexed; DOX, docetaxel.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


