Abstract
Purpose of review
To review current data on HIV-1 resistance arising from the use of fixed dose combination tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for pre-exposure prophylaxis (PrEP) to prevent HIV-1 infection.
Recent findings
Resistance to tenofovir (TNV) or FTC is infrequently selected by TDF/FTC PrEP if started before HIV-1 infection has occurred, but is much more common when inadvertently started during undiagnosed acute infection. Mathematical modeling predicts that the number of HIV-1 infections averted by the use of PrEP far exceeds the increase in drug-resistant infections that could occur from PrEP. Studies in macaques show that TNV-resistant virus but not FTC-resistant virus can cause breakthrough infection despite TDF/FTC PrEP. FTC resistance with M184V/I occurs more frequently than TFV resistance with K65R in seroconverters from clinical trials of TDF/FTC PrEP.
Summary
The benefit of preventing HIV-1 infections with TDF/FTC PrEP far outweighs the risk of drug-resistant infection, provided PrEP is not started in persons with undiagnosed HIV-1 infection. We should respect but not fear HIV-1 resistance from TDF/FTC PrEP and recognize that most TNV or FTC resistance will arise from its use for ART. Preventing ART failure or detecting it early is most important for preventing the spread of HIV-1 resistance to TDF/FTC and preserving its effectiveness for both PrEP and ART.
Keywords: pre-exposure prophylaxis, TDF, tenofovir, emtricitabine, FTC, Truvada, HIV-1 drug resistance, K65R, M184I/V
INTRODUCTION
The HIV-1 epidemic continues to devastate Sub-Saharan Africa where over 25 million people are already living with HIV-1 and 1.6 million new infections occurred in 2014 (1, 2). Prevention strategies that include antiretroviral agents have the greatest potential for reducing HIV-1 incidence. In 2012, a fixed dosed combination of tenofovir disoproxil fumarate and emtricitabine (TDF/FTC; marketed as Truvada) became the first drug to be approved by the United States Food and Drug Administration (FDA) for pre-exposure prophylaxis (PrEP). The approval was based on data from Phase III HIV prevention trials that showed a 44–75% reduction in HIV acquisition in seronegative individuals using daily, oral TDF/FTC (3, 4). Concurrently, the World Health Organization (WHO) strongly recommended TDF with FTC or lamivudine (3TC) plus a nonnucleoside reverse transcriptase inhibitor as the preferred regimen for first-line antiretroviral therapy (ART) (5), creating concern that the same drugs used for treatment and prevention would increase the spread of drug resistance (6). Breakthrough infection and subsequent selection of resistance with continued use of TDF/FTC PrEP during acute infection could compromise the effectiveness of first-line ART containing TDF/FTC. Conversely, the efficacy of TDF/FTC for PrEP could be reduced if the transmitted variant is from a partner failing a TDF/FTC-based ART regimen. Here, we review the current knowledge about resistance selection with the use of TDF/FTC for HIV prevention and treatment, focusing on resistance analyses from recent prevention trials of TDF or TDF/FTC, on animal and mathematical models of drug resistance, and on the frequency of transmitted drug resistance from failure of ART in sub-Saharan Africa. We discuss ways in which resistance from TDF/FTC PrEP can be minimized and emphasize that the benefit from using TDF/FTC for HIV prevention far exceeds the risk of drug resistance.
RESISTANCE TO FTC OR TDF IS RARELY SELECTED IN SEROCONVERTERS FROM TDF/FTC PREP TRIALS
To date, 5 placebo-controlled, Phase III trials were done to assess the effectiveness of daily oral TDF/FTC PrEP in preventing HIV infection in various populations, including men who have sex with men (MSM) and transgendered women in iPrEx (7), at-risk men and women in TDF2 (8), discordant couples in Partners PrEP (9), and women of reproductive age in FEM-PrEP (10) and VOICE (11*). All studies included an active arm in which participants were assigned a once-daily regimen of oral TDF/FTC, and all participants underwent monthly rapid testing for HIV seroconversion.
FTC and TDF Resistance Selection is Infrequent in Seroconverters in Active Product Arms from TDF/FTC PrEP Trials
Only 5 cases of resistance with M184I/V or K65R were identified by standard genotype analysis in a combined total of 160 seroconverters assigned daily oral TDF/FTC in the five trials listed above and again in Table 1 (7, 8, 9, 10, 11*). Four of these cases were in FEM-PrEP, but only 1 of the 4 is likely to have had M184V selection from PrEP failure despite adherence to TDF/FTC (intracellular TNV-DP concentrations were equivalent to taking 4 or more tablets per week). Two of the three other cases of M184I and V were detected within 4 and 8 weeks of study enrollment (one with detectable TNV-DP and one without, respectively) for whom acute infection at enrollment could not be ruled out, and the fourth case occurred in a participant who seroconverted 48 weeks after discontinuing TDF/FTC, thus FTC resistance was likely transmitted from the participant’s partner. In all cases, the resistance mutation became undetectable after stopping study product – M184I by 4 weeks and M184V by 24–36 weeks (10, 12**), reflecting the negative effect of the 184 mutations on viral replication fitness. The fifth case of resistance occurred in VOICE: 1 of 61 participants who became infected on the TDF/FTC arm developed FTC resistance with a mixed population of M184M/V. This participant had been on oral TDF/FTC 309 days from enrollment (68 days since last negative plasma collection) and had detectable plasma TNV-TP within 6 weeks of her first quarterly plasma sample collection (11*, 13).
TABLE 1.
HIV-1 Resistance to Tenofovir (TNV) or FTC in Oral TDF/FTC PrEP Trials
| Study | TDF/FTC Arm | Tenofovir Resistancea | FTC Resistanceb | ||
|---|---|---|---|---|---|
| Standard Genotype | Sensitive Genotype | Standard Genotype | Sensitive Genotype | ||
| Infection After Enrollment | |||||
| FEM-PrEP | 33 | 0 | 0 | 4c | 1 |
| iPrEx | 36 | 0 | 0 | 0 | 2 |
| TDF2 | 9 | 0 | 0 | 0 | 0 |
| Partners PrEP | 21 | 0 | 1 | 0 | 5 |
| VOICE | 61 | 0 | 0 | 1 | 2 |
| Total | 160 | 0 (0%) | 1 (0.6%) | 5 (3%) | 10 (6%) |
|
| |||||
| Acute Infection at Enrollment | |||||
| FEM-PrEP | 1 | 0 | - | 0 | - |
| iPrEx | 2 | 0 | - | 2 | - |
| TDF2 | 1 | 1 | - | 1 | - |
| Partners PrEP | 4 | 0 | - | 2 | - |
| VOICE | 9 | 0 | - | 2 | - |
| Total | 17 | 1 (6%) | - | 7 (41%) | |
K65R or K70E detected by standard population genotype or above 1% frequency by sensitive genotype (allele-specific PCR or 454 sequencing).
M184V or M184I detected by standard population genotype, or above 1% frequency by sensitive genotype (allele-specific PCR or 454 sequencing).
More sensitive testing for resistance using allele-specific PCR or next generation sequencing identified an additional 10 cases of M184I or V in active arms from the five TDF/FTC PrEP trials. Low-frequency M184I variants were identified in one participant from FEM-PrEP (0.66%), two from iPrEX (0.75 and 0.53%) and two from VOICE (5.2 and 0.7%), although only one of the participants from iPrEx had detectable intracellular PMBC levels of FTC-TP (12**, 14**, 15). Sensitive testing revealed 5 cases of M184I or V present at >1% in Partners PrEP, with 1 of those 5 also having >1% K65R. In Partners PrEP, the estimated median time of taking product during the window period between infection and detection of seroconversion was 45 days (16**). It should be noted that the detection of these low frequency mutants may be intermittent and there is no proof that they were selected by TDF/FTC. The clinical significance of these low frequency drug-resistant variants is also unknown. With the exception of one participant in Partners PrEP with low frequency K65R from the TDF/FTC arm as noted above, no cases of TDF resistance with K65R or K70E (above 1%) have occurred in any seroconverter on a once-daily oral TDF/FTC arm (7, 8, 9, 10, 11*, 12**, 13, 14**, 15, 16**, 17**).
FTC Resistance is Frequently Selected in Participants with Undetected Acute Infection at Enrollment in Active Product Arms from TDF/FTC PrEP Trials
By contrast to the low frequency of TDF or FTC resistance among participants who became infected during the 5 PrEP trials, resistance selection was more frequent among those enrolled into the trials in the seronegative window of acute HIV infection. Specifically, 7 of 17 participants in the TDF/FTC arms of the PrEP trials who had undetected acute infection at enrollment (seronegative with detectable HIV-1 RNA) had FTC resistance with M184V or I and one of those 7 also had TDF resistance with K65R (7, 8, 9, 10, 11*). In the 2 participants with FTC resistance in iPrEx, the M184V/I mutations waned to undetectable levels (<0.5%) by week 4 in one case and by week 14 in the second case, and did not reappear through 52 weeks of follow-up (14**). One participant with acute HIV-1 infection at enrollment into TDF2 had resistance to both TDF and FTC after continuing to take TDF/FTC for 7 months after enrollment. M184V developed 1 month after study entry and A62V and K65R developed between 4–7 months, with all mutations present at high frequency (17**). In Partners PrEP, 2 of 4 participants on the TDF/FTC arm who were retrospectively found to be HIV infected at enrollment had >1% M184V (9, 16**). Finally, 2 of 9 participants in VOICE who were enrolled during seronegative acute HIV infection developed FTC resistance with M184V or M184I/V after being on TDF/FTC for 26 and 29 days, respectively (11*, 13).
RESISTANCE TO TDF IS RARELY SELECTED IN SEROCONVERTERS FROM TENOFOVIR PREP TRIALS
Tenofovir resistance has been infrequently detected in seroconverters in four relevant Phase III trials of products containing tenofovir only – CAPRISA 004 and VOICE for tenofovir gel, and Bangkok Tenofovir, Partners PrEP and VOICE for oral TDF. No cases of K65R or K70E occurred in studies of 1% tenofovir gel, including 0 of 35 seroconverters in CAPRISA 004 and 0 of 60 in VOICE (11*, 18). In addition, in CAPRISA 004, low frequency K65R was not detected in plasma collected within 30 days of estimated seroconversion, or in vaginal swabs collected a median of 19 days from the time of infection (19**). In VOICE, one seroconverter from the 1% tenofovir gel arm had 1.2% K65R detected by allele-specific PCR (15). Resistance results from FACTS 001 have not yet been reported (20).
Resistance from oral TDF PrEP has also been rarely detected. In the Bangkok tenofovir study, no cases of K65R or K70E were found in 17 seroconverters in the TDF arm, and in Partners PrEP, only 1 of 30 seroconverters on TDF and 1 of 8 participants who enrolled with unrecognized acute infection had low frequency K65R or K70E (16**, 21). In VOICE, no instances of K65R or K70E were detected in the 58 seroconverters on the TDF arm (11*, 13, 15).
In the United States, oral TDF is a recommended alternative to TDF/FTC PrEP, although the relative efficacy of the two is unknown. Selection of K65R in clinical trials has occurred rarely with TDF and TDF/FTC PrEP. Resistance was more frequent with TDF/FTC PrEP due to selection of M184I/V which is known to increase HIV susceptibility to TNV and reduce risk of selecting K65R (22, 23), but studies to test this hypothesis are needed. There is also insufficient data to assess comparative risk of resistance with TDF versus TDF/FTC PrEP; therefore, the decision to use TDF over TDF/FTC must be made with individual clinical considerations.
TRANSMISSION OF RESISTANT STRAINS IN BREAKTHROUGH INFECTION WITH TDF/FTC PREP: ANIMAL MODELS
The low-dose repeat-challenge SHIV pigtail macaque model has been a valuable tool to study PrEP scenarios that cannot be addressed in human trials. Resistance could not be assessed in two macaque studies evaluating the impact of DMPA on the efficacy of TDF/FTC PrEP because the 24-hour pre- + 2-hour post-infection dosing regimen conferred 100% efficacy (24*, 25). Breakthrough infection did occur in a macaque study of intermittent TDF/FTC PrEP, but resistance to TDF or FTC was not selected (26). In macaques given a weekly dose of TDF/FTC 3 days before exposure and 2 hours after challenge with a K65R-containing SHIV to simulate transmitted resistance, infection was significantly delayed compared to untreated controls, but 4 of 6 macaques did get infected (27). By contrast, the same dosing regimen protected 5 of 5 animals from M184V-containing SHIV. This may be due to M184V having increased susceptibility to tenofovir or reduced transmission fitness compared to wild type virus (28).
MATHEMATICAL MODELS PREDICT THAT PREP IS NOT A MAJOR CONTRIBUTOR TO DRUG RESISTANCE
Mathematical models have been used to study PrEP efficacy and the spread of HIV drug resistance in scenarios in which the same antiretovirals, e.g. TDF/FTC, are used for both PrEP and ART (29, 30). Modeling predicts that PrEP used in conjunction with ART but not overlapping with ART would reduce HIV prevalence to a greater extent than ART alone, but also modestly increase drug resistance. However, several models agree that the relative contribution of ART to resistance far exceeds that from PrEP, with PrEP contributing <5% to the total burden of resistance (29, 30, 31, 32*). The most important factors contributing to drug resistance from PrEP are the frequency of breakthrough HIV infection while taking PrEP and the frequency of inadvertent PrEP use among individuals who are already infected with HIV. The persistence of transmitted resistance and duration of PrEP use by seroconverters are also major contributors to drug resistance prevalence (33, 34, 35).
TRANSMITTED RESISTANCE AND RESISTANCE IN INDIVIDUALS FAILING TDF/FTC-CONTAINING FIRST-LINE ART
Patients failing ART are the largest contributors to prevalent drug-resistant virus. PharmAccess African Studies to Evaluate Resistance (PASER) conducted a multi-country 13-site cohort study and found that although 70% of patients on first-line ART achieved HIV RNA suppression, 71% of ART failures had drug resistance. In patients on tenofovir-based regimens, almost 30% failed with K65R and half with M184V (36). In South African patients failing first line ART with TDF/FTC, K65R was detected in 5 of 40 (12%) and M184V/I in 12 of 40 (28%) (37). Another South African study looking at 80 patients failing on TDF-based ART with at least one resistance mutation found 10 (12.5%) had K70E, 43 (54%) had K65R and 72 (90%) had M184I/V (38*). By contrast, less than 1% of breakthrough infections on TDF/FTC PrEP had K65R (7, 8, 9, 10, 11*).
As in the macaque models described above, drug-resistant HIV with K65R originating from individuals on failing TDF/FTC-containing ART could compromise the efficacy of TDF/FTC PrEP. Fortunately, the majority of transmitted nucleoside reverse transcriptase inhibitor (NRTI) resistance in Sub-Saharan Africa is due to M184V, whereas rates of transmitted K65R are still low. In a large meta-analysis evaluating 298 sequences from treatment-naïve individuals in Sub-Saharan Africa, only 6 (2%) had K65R while 54 (18%) had M184V (39). The World Health Organization (WHO) threshold surveillance classification for most regions in sub-Saharan Africa for NRTI resistance still remained low (<5%) except in KwaZulu-Natal, South Africa, where NRTI resistance has been reported as both low and moderate (5–15%) (40, 41). In a cross-sectional study of women screening for a prevention trials but ineligible to participate because of pre-existing HIV infection, only 1 of 400 (<1%) had K65R, and 4 of 400 (1%) had M184V (42). These observations suggest that the risk of PrEP breakthrough infection with TDF- resistant HIV containing K65R is low but continued surveillance is warranted.
WHAT CAN WE DO TO MINIMIZE RESISTANCE FROM PREP?
As demonstrated by both clinical and mathematical modelling studies, the greatest risk of resistance from PrEP occurs when PrEP is used during unrecognized acute HIV infection. A point-of-care rapid antibody or nucleic acid detection test that reduces undiagnosed acute infection is critically needed both to avoid prescribing TDF/FTC PrEP to users who are already HIV infected, and to monitor PrEP users to minimize the time on PrEP after seroconverting (43*).
Reducing the overall prevalence of drug-resistant HIV will reduce the risk of PrEP breakthrough infection with resistant virus. PrEP is likely to be highly effective against wild type strains, but may have reduced efficacy against strains resistant to the PrEP agent being used. Real-time global surveillance for HIV-1 drug resistance is needed (44*, 45*), and strengthening the ARV supply chain and preventing drug stock-outs would help reduce resistance from intermittent treatment (46*, 47*).
Point-of-care HIV RNA assays are also urgently needed for monitoring of individuals on ART so that viral breakthrough can be detected early before transmissions occur, rather than waiting for CD4+ T cell counts to decline. Lack of HIV RNA monitoring of individuals on ART can lead to the accumulation of drug resistance mutations as a result of prolonged time on the failing therapy (48). In addition, point of care tests for ARV levels or common drug resistance mutations are urgently needed so that non-adherence can be differentiated from drug resistance as the cause of treatment failure and managed appropriately (49). Improved detection of treatment failure can prolong the lifespan of first-line therapy, limit the spread of drug-resistant HIV and preserve second line therapy for those who have confirmed resistance to first-line.
CONCLUSION
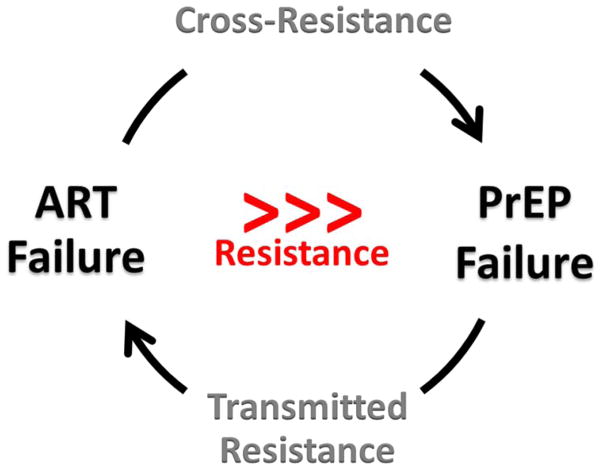
Recent data from clinical trials has shown that the potential for TDF/FTC PrEP to prevent HIV infection far exceeds the risk of resistance that could occur with its use. Resistance has been rare in PrEP trials because when drug pressure from PrEP is high, infection is unlikely to occur, but when drug pressure is low or absent, such as from non-adherence to PrEP, the risk of resistance selection is also low. Careful monitoring of resistance from TDF/FTC PrEP is required but fear of resistance is unwarranted and should not impede its implementation to prevent HIV infection. Resistance from treatment failure of TDF/FTC-containing ART will generate far more resistance than that from TDF/FTC PrEP (Figure 1). The challenge of preserving the efficacy of TDF/FTC PrEP by preventing the spread of drug-resistant HIV can be met by improving individual and epidemiological monitoring for ART failure and drug resistance, simplifying ART to single tablet regimens for first, second and third-line therapy (50*), maintaining a drug strong supply chain (47*), improving HIV diagnostics to reduce unrecognized acute HIV infection, and gaining a better understanding of cross-resistance between ART and PrEP through analysis of patient-derived viruses. Major individual and public health benefits from both PrEP and ART can be realized and sustained if appropriate actions are taken to limit the spread of HIV drug resistance.
FIGURE 1.
Global Threat of Resistance
KEY POINTS.
Resistance to tenofovir (TNV) and emtricitabine (FTC) is infrequent (3%) from use of oral TDF/FTC for PrEP if HIV-1 infection is not present at the time PrEP is started (5 cases in 160 seroconverters assigned to TDF/FTC in 5 PrEP trials).
Resistance to TNV and FTC is much more common (41%) if TDF/FTC PrEP is started during undiagnosed acute HIV-1 infection (7 cases in 17 participants); Acute HIV-1 infections should be excluded before starting PrEP.
Mathematical modeling shows that the number of HIV-1 infections that would be averted by PrEP greatly exceeds the number of drug-resistant infections that could occur.
The risk of HIV-1 resistance to TNV or FTC is far greater when used it is used for ART than for PrEP.
Acknowledgments
Financial support and sponsorship
The authors acknowledge grant support from the National Institutes of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number LC UM1AI106707.
Footnotes
Conflicts of interest
J.W.M. is a consultant to Gilead Sciences and holds share options in Co-Crystal, Inc. No other conflicts are reported. U.M.P reports no potential conflicts.
References
- 1.WHO. HIV/AIDS Fact Sheet N360. 2015 Jul 20; http://www.who.int/mediacentre/factsheets/fs360/en/2015.
- 2.UNAIDS. Global Report: UNAIDS Report on the Global AIDS epidemic 2013. 2013 http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 3.Roehr B. FDA approves first drug to prevent HIV infection. BMJ. 2012;345:e4879. doi: 10.1136/bmj.e4879. [DOI] [PubMed] [Google Scholar]
- 4.Plosker GL. Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis. Drugs. 2013;73(3):279–91. doi: 10.1007/s40265-013-0024-4. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2013 http://www.who.int/hiv/pub/guidelines/arv2013/en/ [PubMed]
- 6.Hurt CB, Eron JJ, Jr, Cohen MS. Pre-exposure prophylaxis and antiretroviral resistance: HIV prevention at a cost? Clin Infect Dis. 2011;53(12):1265–70. doi: 10.1093/cid/cir684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. The findings from the MTN-003 (VOICE) study, which evaluated safety and effectiveness of daily use of an antiretroviral (ARV) tablet (tenofovir or Truvada) or daily use of a vaginal gel (tenofovir gel) two different HIV prevention approaches among 5,029 women in Uganda, South Africa and Zimbabwe, are presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Grant RM, Liegler T, Defechereux P, Kashuba AD, Taylor D, Abdel-Mohsen M, et al. Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. AIDS. 2015;29(3):331–7. doi: 10.1097/QAD.0000000000000556. This paper describes in detail both the standard and sensitive resistance analysis of the seroconverters from the FEM-PrEP trial, including a plasma drug level reporting. Four seroconverters were detected from the TDF/FTC arm with the M184V or I in HIV-1 reverse transcriptase. [DOI] [PubMed] [Google Scholar]
- 13.Parikh UM, Eskay K, Hardesty R, Kelly C, Magaret C, Molitor C, et al. HIV-1 Resistance Outcomes in Seroconverters from the MTN 003 (VOICE) Study. Conference On Retroviruses And Opportunistic Infections (CROI); 2014; Boston. p. MA2014. [Google Scholar]
- 14**.Liegler T, Abdel-Mohsen M, Bentley LG, Atchison R, Schmidt T, Javier J, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis. 2014;210(8):1217–27. doi: 10.1093/infdis/jiu233. A detailed analysis of the genotypic and phenotypic drug resistance results from the iPrEx trial are presented. No participants randomized to TDF/FTC had resistance detected by standard genotype but two had minor variants M184I or I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panousis C, Halvas EK, Kelly C, Marrazzo JM, Chirenje ZM, Mellors JW, et al. Minor Drug-Resistant Variants Infrequently Detected in Seroconverters from MTN-003 (VOICE). Conference On Retroviruses And Opportunistic Infections (CROI); 2015; Seattle. p. WA2015. [Google Scholar]
- 16**.Lehman DA, Baeten JM, McCoy CO, Weis JF, Peterson D, Mbara G, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211(8):1211–8. doi: 10.1093/infdis/jiu677. The detailed Partners PrEP resistance analysis is described in this paper, including analysis of the 5 participants found to have virus with resistance mutations associated with their PrEP regimen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Chirwa LI, Johnson JA, Niska RW, Segolodi TM, Henderson FL, Rose CE, et al. CD4(+) cell count, viral load, and drug resistance patterns among heterosexual breakthrough HIV infections in a study of oral preexposure prophylaxis. AIDS. 2014;28(2):223–6. doi: 10.1097/QAD.0000000000000102. This paper provides details on the drug resistance evulation of the Botswana TDF/FTC Oral HIV Prophylaxis Trial (TDF2) [DOI] [PubMed] [Google Scholar]
- 18.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Wei X, Hunt G, Abdool Karim SS, Naranbhai V, Sibeko S, Abdool Karim Q, et al. Sensitive tenofovir resistance screening of HIV-1 from the genital and blood compartments of women with breakthrough infections in the CAPRISA 004 tenofovir gel trial. J Infect Dis. 2014;209(12):1916–20. doi: 10.1093/infdis/jiu026. This paper describes the results of standard and sensitive resistance analyses of seroconverters from the CAPRISA 004 trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rees H, Delany-Moretlwe S, Lombard C, Baron D, Panchia R, Myer L, et al. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women Conference on Retroviruses and Opportunistic Infections (CROI); 2015; Seatlle. p. WA2015. [Google Scholar]
- 21.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 22.Xu HT, Martinez-Cajas JL, Ntemgwa ML, Coutsinos D, Frankel FA, Brenner BG, Wainberg MA. Effects of the K65R and K65R/M184V reverse transcriptase mutations in subtype C HIV on enzyme function and drug resistance. Retrovirology. 2009;6:14. doi: 10.1186/1742-4690-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly JK, Margot NA, MacArthur HL, Hung M, Miller MD, White KL. The balance between NRTI discrimination and excision drives the susceptibility of HIV-1 RT mutants K65R, M184V and K65r+M184V. Antivir Chem Chemother. 2007;18(6):307–16. doi: 10.1177/095632020701800603. [DOI] [PubMed] [Google Scholar]
- 24*.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins L, et al. Depot-medroxyprogesterone acetate does not reduce the prophylactic efficacy of emtricitabine and tenofovir disoproxil fumarate in macaques. J Acquir Immune Defic Syndr. 2014;67(4):365–9. doi: 10.1097/QAI.0000000000000340. Using a macaque model, this study addresses the concern that the injectable contraceptive depot-medroxyprogestorone (DMPA) increases the risk of HIV acquisition and could impact PrEP. Twelve pigtail macaques treated with DMPA and TDF/FTC PrEP remained uninfected compared to 6 placebo controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One. 2012;7(12):e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Lerma JG, Cong ME, Mitchell J, Youngpairoj AS, Zheng Q, Masciotra S, et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2(14):14ra4. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 27.Cong ME, Mitchell J, Sweeney E, Bachman S, Hanson DL, Heneine W, et al. Prophylactic efficacy of oral emtricitabine and tenofovir disoproxil fumarate combination therapy against a tenofovir-resistant simian/human immunodeficiency virus containing the K65R mutation in macaques. J Infect Dis. 2013;208(3):463–7. doi: 10.1093/infdis/jit189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cong ME, Youngpairoj AS, Zheng Q, Aung W, Mitchell J, Sweeney E, et al. Protection against rectal transmission of an emtricitabine-resistant simian/human immunodeficiency virus SHIV162p3M184V mutant by intermittent prophylaxis with Truvada. J Virol. 2011;85(15):7933–6. doi: 10.1128/JVI.00843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Vijver DA, Nichols BE, Abbas UL, Boucher CA, Cambiano V, Eaton JW, et al. Preexposure prophylaxis will have a limited impact on HIV-1 drug resistance in sub-Saharan Africa: a comparison of mathematical models. AIDS. 2013;27(18):2943–51. doi: 10.1097/01.aids.0000433237.63560.20. [DOI] [PubMed] [Google Scholar]
- 30.Abbas UL, Glaubius R, Mubayi A, Hood G, Mellors JW. Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis. 2013;208(2):224–34. doi: 10.1093/infdis/jit150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrov D, Boily MC, Masse BR, Brown ER. Impact of Pill Sharing on Drug Resistance Due to a Wide-Scale Oral Prep Intervention in Generalized Epidemics. J AIDS Clin Res. 2012;Suppl 5(4) doi: 10.4172/2155-6113.s5-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Abbas UL, Glaubius R, Hood G, Mellors JW. Antiretroviral treatment, preexposure prophylaxis, and drug resistance in sub-Saharan Africa: a consensus among mathematical models. J Infect Dis. 2014;209(1):164–6. doi: 10.1093/infdis/jit545. This modelling study evaluates major concerns about the same antiretrovirals being used for both ART and PrEP. [DOI] [PubMed] [Google Scholar]
- 33.Nichols BE, Boucher CA, van Dijk JH, Thuma PE, Nouwen JL, Baltussen R, et al. Cost-effectiveness of pre-exposure prophylaxis (PrEP) in preventing HIV-1 infections in rural Zambia: a modeling study. PLoS One. 2013;8(3):e59549. doi: 10.1371/journal.pone.0059549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbas UL, Hood G, Wetzel AW, Mellors JW. Factors influencing the emergence and spread of HIV drug resistance arising from rollout of antiretroviral pre-exposure prophylaxis (PrEP) PLoS One. 2011;6(4):e18165. doi: 10.1371/journal.pone.0018165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiri T, Welte A. Modelling the impact of acute infection dynamics on the accumulation of HIV-1 mutations. J Theor Biol. 2011;279(1):44–54. doi: 10.1016/j.jtbi.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Hamers RL, Sigaloff KC, Wensing AM, Wallis CL, Kityo C, Siwale M, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54(11):1660–9. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann CJ, Ledwaba J, Li JF, Johnston V, Hunt G, Fielding KL, et al. Resistance to tenofovir-based regimens during treatment failure of subtype C HIV-1 in South Africa. Antivir Ther. 2013;18(7):915–20. doi: 10.3851/IMP2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Skhosana L, Steegen K, Bronze M, Lukhwareni A, Letsoalo E, Papathanasopoulos MA, et al. High prevalence of the K65R mutation in HIV-1 subtype C infected patients failing tenofovir-based first-line regimens in South Africa. PLoS One. 2015;10(2):e0118145. doi: 10.1371/journal.pone.0118145. Patients in South Africa infected with subtype C HIV-1 and failing first-line ART with TDF were more likely to have K65R than those given d4T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet infectious diseases. 2010;10(3):155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 40.WHO. WHO HIV Drug Resistance Report 2012. 2012 http://apps.who.int/iris/bitstream/10665/75183/1/9789241503938_eng.pdf.
- 41.Parboosing R, Naidoo A, Gordon M, Taylor M, Vella V. Resistance to antiretroviral drugs in newly diagnosed, young treatment-naive HIV-positive pregnant women in the province of KwaZulu-Natal, South Africa. J Med Virol. 2011;83(9):1508–13. doi: 10.1002/jmv.22143. [DOI] [PubMed] [Google Scholar]
- 42.Parikh UM, Kiepiela P, Ganesh S, Gomez K, Horn S, Eskay K, et al. Prevalence of HIV-1 drug resistance among women screening for HIV prevention trials in KwaZulu-Natal, South Africa (MTN-009) PLoS One. 2013;8(4):e59787. doi: 10.1371/journal.pone.0059787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Guanira JV, Leigler T, Kallas E, Schechter M, Sharma U, Glidden D, et al. Streamlining HIV testing for HIV preexposure prophylaxis. J Clin Microbiol. 2015;53(1):179–83. doi: 10.1128/JCM.01540-14. This paper discusses the optimization of HIV testing algorthms to minimize drug resistance with the use of PrEP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Van Laethem K, Theys K, Vandamme AM. HIV-1 genotypic drug resistance testing: digging deep, reaching wide? Curr Opin Virol. 2015;14:16–23. doi: 10.1016/j.coviro.2015.06.001. This review explores new drug resistance technologies with the potential to be low cost and implemented at a larger scale in resource-poor settings. [DOI] [PubMed] [Google Scholar]
- 45*.Parkin NT. Measurement of HIV-1 viral load for drug resistance surveillance using dried blood spots: literature review and modeling of contribution of DNA and RNA. AIDS Rev. 2014;16(3):160–71. New technologies for increasing drug resistance surveillance using viral load are explored in this review. [PubMed] [Google Scholar]
- 46*.Bekker LG, Venter F, Cohen K, Goemare E, Van Cutsem G, Boulle A, et al. Provision of antiretroviral therapy in South Africa: the nuts and bolts. Antivir Ther. 2014;19(Suppl 3):105–16. doi: 10.3851/IMP2905. Issues with antiretroviral provision and operational support are discussed in this paper. [DOI] [PubMed] [Google Scholar]
- 47*.Ripin DJ, Jamieson D, Meyers A, Warty U, Dain M, Khamsi C. Antiretroviral procurement and supply chain management. Antivir Ther. 2014;19(Suppl 3):79–89. doi: 10.3851/IMP2903. This paper describes key aspects of ART distribution and delivery with particular emphasis on supply chain issues. [DOI] [PubMed] [Google Scholar]
- 48.Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58(1):23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 49.Lessells RJ, Avalos A, de Oliveira T. Implementing HIV-1 genotypic resistance testing in antiretroviral therapy programs in Africa: needs, opportunities, and challenges. AIDS Rev. 2013;15(4):221–9. [PMC free article] [PubMed] [Google Scholar]
- 50*.Vitoria M, Ford N, Doherty M, Flexner C. Simplification of antiretroviral therapy: a necessary step in the public health response to HIV/AIDS in resource-limited settings. Antivir Ther. 2014;19(Suppl 3):31–7. doi: 10.3851/IMP2898. Issues with ART scale-up are discussed, including drug supply and simplification of regimens to minimize adverse effects. [DOI] [PubMed] [Google Scholar]