Summary
Objective
Newer ‘smart’ scales that transmit participants' body weights directly to data collection centres offer the opportunity to simplify weight assessment in weight management research; however, little data exist on the concordance of these data compared with weights measured at in‐person assessments.
Methods
We compared the weights of 58 participants (mean ± SD, body mass index = 31.6 ± 4.8, age = 52.1 ± 9.7 years, 86.2% Caucasian, 65.5% female) measured by study staff at an in‐person assessment visit to weights measured on the same day at home using BodyTrace ‘smart’ scales. These measures occurred after 3 months of an internet‐based weight management intervention.
Results
Weight (mean ± SD) measured at the 3‐month in‐person assessment visit was 81.5 ± 14.7 kg compared with 80.4 ± 14.5 kg measured on the same day using in‐home body‐weight scales; mean bias = 1.1 ± 0.8 kg, 95% limits of agreement = −0.5–2.6. Two outliers in the data suggest that there may be greater variability between measurements for participants weighing above 110 kg.
Conclusion
Results suggest good concordance between the measurements and support the use of the BodyTrace smart scale in weight management research. Future trials using BodyTrace scales for outcome assessment should clearly define protocols for measurement and associated instructions to participants (e.g. instruct individuals to weigh at the same time of day, similarly clothed). Finally, measure concordance should be investigated in a group of individuals weighing more than 110 kg.
Keywords: Body‐weight measurement, research methods, smart scales, weight management
Introduction
As newer technology has helped move weight management interventions out of the clinic and research centre and into the home (e.g. through Internet, smartphone and other technology‐based programmes) 1, 2, it is increasingly important to identify valid means of outcomes measurement that can be conducted without requiring participants to attend in‐person assessment visits. The development of in‐home body‐weight scales (often referred to as ‘smart’ scales) that use cellular networks to send participant body weights directly to data collection centres has offered promise for decreasing participant and staff burden related to in‐person weight outcome assessment. Further, these technologies offer avenues for participants who cannot attend a scheduled in‐person assessment visit (e.g. due to vacation, family sickness and work schedule) to provide weight outcome data.
Increasingly, these new smart scales have been integrated into behavioural weight management treatment protocols 3, 4; however, limited evidence exists regarding the concordance of weights measured by these scales with weights measured at traditional in‐person assessments. Thus, the current study assessed the concordance of weight measurements, collected on the same day, using in‐person, staff‐measured assessment measurements and in‐home measurements collected by participants using a smart scale.
Methods
Participants
Participants in the current study were 58 adult employees or dependents of Lifespan, a large healthcare corporation in Providence, Rhode Island, which enrolled in an Internet‐based weight loss intervention as part of a larger worksite wellness programme 5. Individuals were eligible for the current study if they were between 18 and 70 years old, had body mass indexes of at least 25 kg m−2 (and weight < 180 kg due to weight limits of the provided in‐home body‐weight scales), had a computer/Internet at home and did not self‐report medical conditions that would contraindicate weight loss or changes in eating/activity habits for weight loss (e.g. uncontrolled hypertension or diabetes, undergoing treatment for cancer, recent history of coronary heart disease and inability to walk two blocks without stopping) or factors that would render completion of the weight management programme unlikely (e.g. plans to relocate, terminal illness, severe psychiatric conditions or dementia). Further details regarding recruitment and the protocol of the weight management intervention have been described elsewhere 5.
Measures
Demographics
Demographic information was collected using a self‐report questionnaire at baseline.
Height
Height was measured at baseline by trained research assistants, using a wall‐mounted stadiometer and shoes removed.
Weight
Assessment weights were measured in‐person by trained research assistants, using a Tanita BWB‐800 (Arlington Heights, Illinois, USA) digital scale, in light indoor clothing and with shoes removed. Weights were measured twice, in kilograms, with the results of the two weights averaged. The BWB‐800 has been previously used in large weight loss trials (including large clinical trials such as a Look AHEAD, a large multisite weight loss trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases) 6 and is reportedly Tanita's bestselling physician and hospital body‐weight scale 7. The BWB‐800 was calibrated professionally once each year and calibrated using a known reference weight weekly by centre staff.
Weight was also measured on the same day at home by participants using BodyTrace smart scales. BodyTrace scales have similarly been used in several large weight loss trials 3, 4 and have stated accuracy of weight to 0.1 kg 8; however, published data comparing these scales to other gold‐standard measures are not available. These scales used cellular networks to transmit data back to a data collection web server, from which the researchers downloaded the data directly. Participants were instructed to weigh themselves once daily during the Internet‐based weight management intervention, preferably first thing in the morning after voiding and before having anything to eat or drink. Participants were not given additional reminders to weigh themselves the morning of their assessment visit. Participants who did not weigh themselves first thing in the morning on the day of their assessment visit were still included if they weighed themselves at all during that day (as confirmed by date/time stamps on the scale data). Data from the BodyTrace scales were transmitted as pounds and were converted to kilograms using the following formula: weight in lb × 0.453592.
Analyses
Baseline differences between included and excluded participants were assessed using t‐tests for continuous variables and Fisher's exact test (due to small expected cell counts) for categorical variables. Bias in the concordance measure was assessed using a mean difference and 95% limits of agreement around this difference. A Bland–Altman plot 9 demonstrating the difference between measures compared with the average of the measures was used to visually inspect bias and assess whether bias was associated with measurement (e.g. if concordance was affected by participant body weight). Finally, to model utility of smart scale use within clinical practice (wherein smart scales may be used to capture weights for individuals unable to attend an in‐person assessment visit), a sensitivity analyses was conducted to assess the impact of modelling varying levels of missing assessment data (10%, 15% and 20%) on intervention weight change. For this analysis, assessment weights were randomly selected to be ‘missing’, and BodyTrace scale weights were substituted. Mean weights at 3 months were then compared between the full dataset and the datasets with BodyTrace scale substitution. All analyses were conducted using sas version 9.4 (SAS Institute, Cary, NC, USA) for Windows 10, and the Bland–Altman plot was created using GraphPad Prism 11.
Results
Of the 75 participants who enrolled an Internet‐based weight management intervention, 58 had both in‐home BodyTrace scale weights and in‐office assessment weights on the same day at their 3‐month assessment (five participants did not complete an in‐office weight for their scheduled assessment visit, and an additional 12 participants did not have BodyTrace scale weights from the day of their assessment). Table 1 presents demographic data for the 58 participants included in all analyses as well as the 17 participants who were excluded because of missing data. There were no statistically significant differences between included and excluded participants in terms of baseline body mass index, sex, race/ethnicity, marital status, income or education; however, excluded participants were slightly younger than included participants, t(75) = −2.09, p = 0.040.
Table 1.
Demographic characteristics of included and excluded participants
| Included participants | Excluded participants | ||||
|---|---|---|---|---|---|
| n = 58 | n = 17 | ||||
| Mean | SD | Mean | SD | p | |
| Age, years | 52.09 | 9.69 | 46.24 | 11.66 | 0.040 |
| BMI, kg m−2 * | 31.57 | 4.75 | 29.94 | 3.20 | 0.189 |
| n | % | n | % | ||
| Gender | 0.240 | ||||
| Female | 38 | 65.5 | 14 | 82.4 | |
| Male | 20 | 34.5 | 3 | 17.7 | |
| Ethnicity (%) | 0.390 | ||||
| African–American | 2 | 3.5 | 2 | 11.8 | |
| Asian | 1 | 1.7 | 0 | 0.0 | |
| Caucasian | 50 | 86.2 | 13 | 76.5 | |
| Hispanic | 2 | 3.5 | 0 | 0.0 | |
| Other/multiple | 3 | 5.2 | 2 | 11.8 | |
| Marital Status | 0.196 | ||||
| Single | 2 | 3.4 | 3 | 17.6 | |
| Married or living with a partner | 49 | 84.5 | 12 | 70.6 | |
| Separated/divorced | 7 | 12.1 | 2 | 11.8 | |
| Household income, dollars | 0.270 | ||||
| 25,000–50,000 | 6 | 10.3 | 1 | 5.9 | |
| 50,001–75,000 | 9 | 15.5 | 7 | 41.2 | |
| 75,001–100,000 | 15 | 25.9 | 3 | 17.6 | |
| 100,001–125,000 | 9 | 15.5 | 1 | 5.9 | |
| 125,001+ | 18 | 31.0 | 4 | 23.5 | |
| Not reported | 1 | 1.7 | 1 | 5.9 | |
| Education | 0.261 | ||||
| High school or less | 5 | 8.6 | 1 | 5.9 | |
| Vocational training | 2 | 3.4 | 0 | 0.0 | |
| Some college | 8 | 13.8 | 6 | 35.3 | |
| College or university degree | 26 | 44.8 | 8 | 47.1 | |
| Graduate degree | 17 | 29.3 | 2 | 11.8 | |
BMI at intervention baseline.
BMI, body mass index.
Mean (±SD) weight measured at the in‐person assessment was 81.46 ± 14.68 kg, compared with the 80.41 ± 14.50 kg measured on the same day using the in‐home BodyTrace scales. Correlation between the measures was high, at r = 0.999, p < 0.0001. The mean (±SE) difference between the measured (representative of overall bias) was 1.05 ± 0.80 kg (95% limits of agreement: −0.52–l2.62 kg). The Bland–Altman plot in Figure 1 suggests that this bias was stable regardless of participant body weight; however, two outliers on the far right of the figure demonstrate that there may be some additional variability in measurements for individuals with body weights over 110 kg. Excluding these two participants led to a mean bias of 0.96 ± 0.63 kg, with 95% limits of agreement ranging from −0.28 to 2.20 kg.
Figure 1.
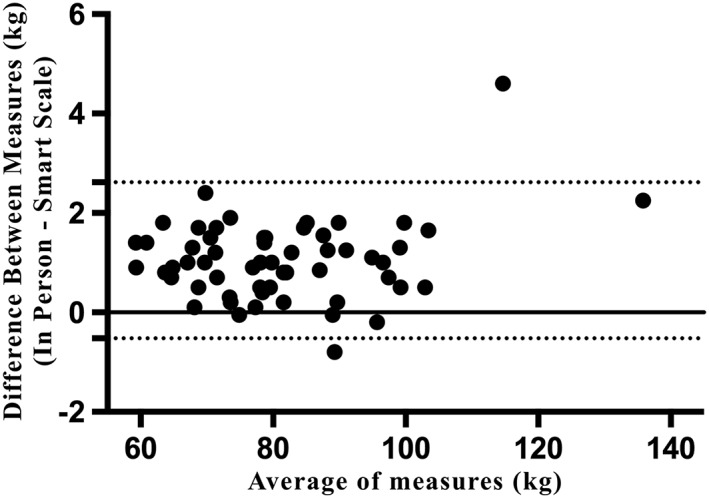
Bland–Altman plot of agreement between in‐home smart scale weights and in‐person clinic assessment weights.
A sensitivity analysis was conducted to assess the impact of substituting scale weights for randomly missing assessment weights (modelled to achieve at least 10%, 15% and 20%; real missingness was 10.3%, 15.5% and 20.7%, respectively). When 10% of the 3‐month assessment data were modelled as missing, substituting scale weights for the missing assessment weights led to a (mean ± SE) 0.08 ± 0.04 kg increase in mean weight in the sample, compared with a mean increase of 0.10 ± 0.04 kg for 15% missing data and a mean increase of 0.18 ± 0.06 kg for 20% missing data.
Discussion
The results from the current analyses demonstrate minimal bias for the use of in‐home BodyTrace scales to measure body weight compared with weight measured at an in‐person assessment. While there was a trend for a positive bias of about 1 kg for in‐person‐measured weights compared with weights measured at‐home, the limits of agreement crossed 0, indicating non‐significant bias. This 1 kg difference may be due, in part, to the varied protocols used for the two measurements. Participants who weighed themselves in‐home were asked to weigh themselves first thing in the morning, before eating or drinking anything and after voiding. Many participants likely weighed themselves at home with only undergarments or no clothes on. At our in‐person visits, participants were scheduled to come in to our research centre between 9 am and 8 pm, at times which they had likely already consumed meals or snacks over the course of the day. Further, participants were weighed in light, indoor clothing with shoes removed. Both participant food/drink consumption and difference in clothing likely contributed to the difference observed in weight measurements. Finally, the increased difference between measurements demonstrated by the two participants with body weights over 110 kg suggests that there may be more error between measurement methods in individuals with weights over 110 kg.
The current study represents the first attempt to validate the use of in‐home BodyTrace smart scales against in‐person assessment methods for body‐weight measurement. Compared with the use of self‐reported weights, the use of in‐home smart scales have the advantage of minimizing potential social desirability bias, rounding errors or more general reporting errors. Strengths of this study include the use of assessments measures within an existing weight management trial (versus a controlled laboratory study with limited generalizability), the use of the two measures on the exact same day and good adherence by participants to the use of in‐home scales, which allowed for a sample of 58 from which to compare the measures. A primary limitation to the current study was the inconsistency in assessment time for in‐person‐measured weights that likely introduced variability into the weight measurements; however, this protocol is similar to existing assessment protocols used in many behavioural weight management intervention research studies and thus provides results that are generalizable to existing research. Furthermore, the sample included only two participants with body weights over 110 kg, leaving us unable to investigate the possibility of increased variability between measures in this population. Finally, given the limited sample size, we were unable to detect statistically significant differences between the participants who were included (n = 58) and excluded (n = 17) from the current analyses; results should be generalizable to populations similar to the included sample. We did not record information regarding why 12 participants were missing in‐home scale weights on assessment days; thus, the missing data could be a result of scale failure (e.g. failure to connect to the cellular network) or from non‐adherence to self‐weighing by participants. Several of these participants had weights on the day prior to or the day after assessments; however, using weights from the day previous to or following assessment would likely increase variability between measures further, and thus, these data were excluded. Reducing missing data due to participant non‐adherence could likely be limited in future studies by providing participants with a reminder to use their in‐home scale on the day of assessment (which was not carried out in the current study).
Conclusion
The current paper demonstrated good concordance between weights measured using in‐home BodyTrace scales and in‐person weight measurements by trained assessment staff. As the use of in‐home smart scales to measure body weight can decrease assessment burden for participants and study staff (in comparison with having participants drive to a research centre to have a staff member measure their weight), future research should focus on continued validation of newer in‐home scale technology and investigate concordance in individuals with heavier body weights.
Conflict of Interest Statement
The authors report no conflict of interest.
Funding
Support for this study is provided by the Lifespan Corporation, and by the National Institute of Diabetes Digestive and Kidney Diseases (National Institutes of Health), under award number F32DK100069 awarded to K. M. R.
Ross, K. M. , and Wing, R. R. (2016) Concordance of in‐home ‘smart’ scale measurement with body weight measured in‐person. Obesity Science & Practice, 2: 224–228. doi: 10.1002/osp4.41.
References
- 1. Coons MJ, DeMott A, Buscemi J, et al Technology interventions to curb obesity: a systematic review of the current literature. Curr Cardiovasc Risk Rep 2012; 6: 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas JG, Bond DS. Review of innovations in digital health technology to promote weight control. Curr Diab Rep 2014; 14: 1–10. [DOI] [PubMed] [Google Scholar]
- 3. Krukowski RA, Hare ME, Talcott GW, et al Dissemination of the Look AHEAD intensive lifestyle intervention in the United States Air Force: study rationale, design and methods. Contemp Clin Trials 2015; 40: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madigan CD, Jolly K, Roalfe A, et al Study protocol: the effectiveness and cost effectiveness of a brief behavioural intervention to promote regular self‐weighing to prevent weight regain after weight loss: randomised controlled trial (The LIMIT Study). BMC Public Health 2015; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross KM, Wing RR. Implementation of an Internet weight loss program in a worksite setting. Manuscript under review. [DOI] [PMC free article] [PubMed]
- 6. The Look AHEAD Research Group . Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity 2014; 22: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanita . BWB800s doctors scale. 2014. Available at: http://www.tanita.com/en/bwb‐800s/. Accessed February 22, 2016.
- 8. BodyTrace, Inc . BodyTrace scale: frequently asked questions. Available at: http://www.bodytrace.com/medical/faq.html. Accessed February 22, 2016.
- 9. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. J R Stat Soc Ser Stat 1983; 32: 307–317. DOI: 10.2307/2987937. [Google Scholar]
- 10. SAS Institute Inc. , SAS version 9.4 . Cary, NC, 2013.
- 11. GraphPad Software, Inc . Prism 6. La Jolla, CA, 2012.


