Abstract
Antibodies targeting programmed death 1 (PD-1) help prevent tumor cells from escaping immune-mediated destruction. We conducted this systematic review and meta-analysis to gain insight into the efficacy of PD-1 antibodies for the treatment of melanoma. Five trials involving 2,828 adult patients were included in this meta-analysis. In patients with previously untreated or refractory melanoma, treatment with PD-1 antibodies significantly improved the six-month progression-free survival (PFS) (HR 0.55, 95% CI 0.50–0.60, P<0.00001) and the overall response rate (OR 3.89, 95% CI 3.12–4.83, P<0.00001). This meta-analysis indicated that anti-PD-1 treatment might provide a significant survival benefit in patients with melanoma. In addition, we found that patients treated with nivolumab reported significantly fewer treatment-related adverse events (OR 0.74, 95% CI 0.57–0.97, P = 0.03) than those treated with other agents, but there was a dose-dependent increase in the frequency of adverse events in patients treated with pembrolizumab.
Introduction
Malignant melanoma is a type of cancer that develops from pigment-containing cells known as melanocytes. In 2016, an estimated 76,380 new cases will be diagnosed, and 10,130 people will die of melanoma in the United States [1]. A clinical diagnosis of malignant melanoma is confirmed by skin biopsy. Typically, melanoma cells are histologically characterized by the expression of S100, HMB45 and Melan A. The optimal treatment for melanoma remains undetermined, but surgery may be associated with a high cure rate for melanoma in situ. However, patients with high-risk melanoma may require adjuvant treatment, and the prognosis associated with these malignancies is very poor. The estimated five-year disease-free survival rate for advanced melanoma (AM), i.e., stage IIIC and IV disease, is less than 16% [2].
Tumor cells evade immune recognition through multiple mechanisms. One key interaction between cancer cells and the immune system is mediated by programmed death ligand-1 (PD-L1) and programmed death 1 (PD-1) signaling. PD-1 is a member of the CD28 superfamily and is expressed on the surface of activated T-cells and B-cells [3,4]. The human PD-1 gene is located at 2q37.3 and encodes a protein of 288 amino acid residues [5,6]. There are two ligands for the PD-1 receptor, PD-L1 and PD-L2. PD-L1 is mostly present on the surface of hematopoietic and parenchymal cells, whereas PD-L2 is usually present on the surface of macrophages and DCs [7]. PD-1 was first confirmed as a negative regulator of immune responses in a mouse model with a PD-1 null mutation in 1999 [8]. In normal tissue, the combination of PD-1 and PD-L1 protectively inhibits the proliferation of immune cells and induces dysfunction of activated T cells, eventually decreasing autoimmunity and promoting self-tolerance [7]. Upregulation of PD-L1 expression has been reported in many types of tumors, including melanoma, lung cancer, renal carcinoma, and hematological malignancies [9,10]. Binding of PD-L1 to upregulated PD-1 induces apoptosis of tumor-specific cytotoxic T cells and an immunosuppressive effect that promotes tumor cell evasion of immune-mediated destruction [5,6]. PD-1 antibodies inhibit the interaction between PD-1 and its ligands on tumor cells to promote immune-mediated destruction.
PD-1 antibodies have recently emerged as a promising immunotherapeutic approach for the treatment of malignant melanoma, non-small-cell lung cancer, renal cancer cell and hematological malignancies. In a phase 1 study, 296 patients with malignant melanoma, non-small-cell lung cancer, prostate cancer, renal cell cancer or colorectal cancer received nivolumab with different dosages. The rate of PFS at 24 weeks was 30–55% in patients with melanoma and 16–41% in patients with non-small-cell lung cancer [11]. Both nivolumab and pembrolizumab have yielded exciting results for the treatment of different types of malignancies in phase 2 and 3 studies [12–15]. In 2014, pembrolizumab, a humanized IgG4 anti-PD-1 antibody, and nivolumab, a fully human IgG4 anti-PD-1 monoclonal antibody (mAb), were approved in the United States for second- or third-line treatment of patients with AM that was refractory to ipilimumab (BRAF wild-type melanoma) or to ipilimumab and BRAF inhibitors (BRAF V600-mutated melanoma).
To gain further insight into the efficacy and safety of PD-1 antibody treatment, we conducted a systematic review and meta-analysis to compare the efficacy of PD-1 antibody monotherapy with other therapeutic strategies for the treatment of malignant melanoma.
Methods
This systematic review and meta-analysis was conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement and the Cochrane Handbook (S1 Table).
Search strategy
We searched the MEDLINE, EMBASE, and Cochrane Library databases without language restrictions. There are three anti-PD-1 agents with the most clinical information: nivolumab, pembrolizumab and pidilizumab. BMS-936558 and MDX-1106 are bynames of nivolumab. MK-3475 is a byname of pembrolizumab, and CT-011 is a byname of pidilizumab. Thus, we used various combinations of the following MeSH terms and keywords to search for studies of interest: anti-PD-1, PD-1 antibody, anti-programmed death 1 antibody, pembrolizumab, MK-3475, nivolumab, BMS-936558, MDX-1106, pidilizumab and CT-011. We also searched for the following medical conditions of interest: melanoma, malignant melanoma and melanomas.
Inclusion and exclusion criteria
We included all prospective, randomized controlled trials (RCTs) that reported the overall response rate, survival data and treatment-related adverse events associated with anti-PD-1 monotherapy in patients with histologically confirmed melanoma. Melanoma patients who had not previously received systemic treatment for advanced disease and patients with relapsed/refractory disease were included. We excluded all non-comparative, in vitro and animal studies. In addition, we excluded poor quality studies and those with incomplete data or duplicate reports.
Data extraction and outcomes of interest
The titles and abstracts of all the studies identified in the literature search were screened by two reviewers (Zhijuan Lin and Xing Chen) to verify compliance with the inclusion and exclusion criteria. The same reviewers independently extracted the data and assessed the quality of the publications. Disagreements between the two reviewers were resolved by consensus after a joint second review.
The predefined data extraction form consisted of the publication reference, patient characteristics, the PD-1 antibody regimen [i.e., first-line treatment or relapsed/refractory disease and dosing (mg/m2)], the years the trial began and ended, and the chemotherapy regimen used. The following information was also recorded for patients in each treatment arm: number of enrolled patients, median age, female to male ratio, number of events and survival data.
The primary outcome was the six-month progression-free survival (PFS) rate, and the secondary outcomes were the overall response rate and treatment-related adverse events. The overall response rate included complete responses (CRs) and partial responses (PRs). If certain data were not included in the articles, we asked the authors for additional information.
Quality assessment
The Cochrane Collaboration's tool was used to assess the risks of selection, performance, detection, attrition and reporting biases in the RCTs selected for analysis. Trials with more than two high-risk components were deemed to have a moderate risk of bias, and trials with more than four high-risk components were deemed to have a high risk of bias.
Data synthesis and analysis
We used Review Manager 5.3 (Cochrane Collaboration) to conduct the meta-analysis. We evaluated the six-month PFS rates using the hazard ratio (HR) and the 95% confidence interval (95% CI). HR values less than 1.0 indicated that anti-PD-1 treatment was associated with a survival benefit and a P-value less than 0.05 was considered statistically significant. We also assessed the overall response rate and treatment-related adverse events using the odds ratio (OR) and the 95% CI. An OR greater than 1.0 indicated a favorable overall response rate in the anti-PD-1 group or a greater incidence of treatment-related adverse events in the anti-PD-1 group; P-values less than 0.05 were considered statistically significant. If significant heterogeneity was observed, the random-effects model was used. In all other cases, the fixed-effects model was used.
Results
Description of studies
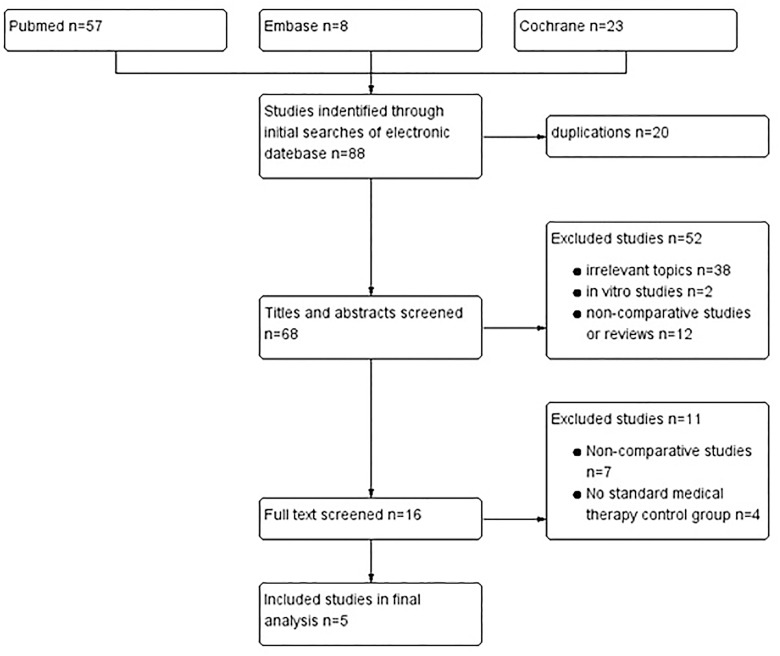
The literature search identified a total of 88 publications (57 studies from PubMed, 8 from Embase and 23 from Cochrane), and five of the reported trials fulfilled our selection criteria [16–20] (Fig 1). These five trials were multicenter RCTs that included a total of 2,828 patients (1,715 randomized to PD-1 antibody treatment and 1,113 to other treatments). Additional details regarding these five clinical trials are provided in Table 1. In all eligible trials, patients were 18 years of age or older and had histologically confirmed Stage III or IV melanoma. In two trials, patients were previously untreated (n = 2, corresponding to 1049 patients) [16,18]. In the other three trials, patients had progression after anti-CTLA-4 treatment (n = 2, corresponding to 945 patients) [17,20] or had received no more than one previous systemic therapy including chemotherapy, immunotherapy, BRAF or MEK inhibitor (n = 1, corresponding to 834 patients) [19]. Patients in the experimental groups were exposed to nivolumab or pembrolizumab with different dosages, while patients in the control groups received ipilimumab or chemotherapy.
Fig 1. Flow diagram of the literature search.
Table 1. Descriptive summary of the included patients and randomized trials.
| Study | ClinicalTrials.gov number | Recruitment period | Median duration of follow-up (months) | Median age (range) | Patient sex (M/F) | Disease stage | First-line therapy | Intervention | Overall response rate (%) | Median progression-free survival (mo.) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-1 group | Control group | PD-1 group | Control group | PD-1 group | Control group | ||||||||
| Larkin J 2015 [19] | NCT01844505 | 2013–2014 | 12.2–12.5 | 60(18–90) | 404/227 | stage III or IV | Yes | Nivolumab 3 mg/kg IV every 2 weeks | Ipilimumab 3 mg/kg every 3 weeks; 4 doses | 43.7 | 19.0 | 6.9 | 2.9 |
| Ribas A 2015 [17] | KEYNOTE-002 | 2012–2013 | 6–14 | 62(18–89) | 327/213 | stage III or IV | No (ipilimumab-refractory) | Pembrolizumab 2 mg/kg or 10 mg/kg IV every 3 weeks | Investigator-choice chemotherapy | 23.3 | 4.5 | 3.7 (2 mg/kg group) 5.4 (10 mg/kg group) | 2.6 |
| Robert C 2015 [18] | NCT01721772 | 2013–2014 | 5.2–16.7 | 65(18–87) | 246/172 | stage III or IV | Yes | Nivolumab 3 mg/kg IV every 2 weeks | Dacarbazine 1000 mg/m2 every 3 weeks | 40.0 | 13.9 | 5.1 | 2.2 |
| Robert C 2015 [19] | NCT01866319 | 2013–2014 | 6.1–11.5 | 62(18–88) | 497/337 | stage III or IV | Y/N (received no more than one previous systemic therapy) | Pembrolizumab 10 mg/kg either every 2 or 3 weeks | Ipilimumab 3 mg/kg every 3 weeks; 4 doses | 33.2 | 11.9 | 5.5 (2-week group) 4.6 (3-week group) | 2.8 |
| Weber J 2015 [20] | CheckMate 037 | 2012–2014 | 5.6–11.2 | 59(23–88) | 261/144 | stage IIIC or IV | No (progression after anti-CTLA4 treatment) | Nivolumab 3 mg/kg IV every 2 weeks | Investigator-choice chemotherapy | 31.6 | 10.6 | 4.7 | 4.2 |
Risk of bias analysis
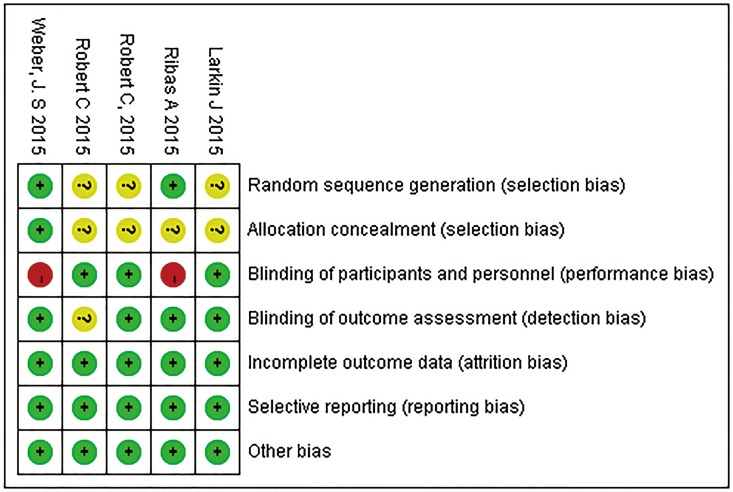
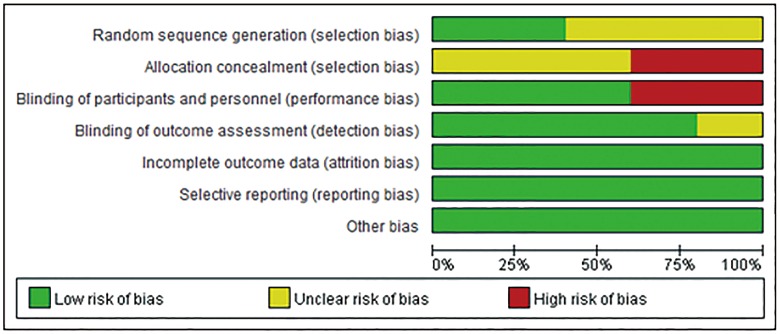
Based on the Cochrane Collaboration tool, the risk of bias was rated as low in all eligible studies (Figs 2 and 3). Two of the five RCTs were open-label studies. Information regarding random sequence generation and allocation concealment was not provided in three and four RCTs, respectively.
Fig 2. Risk of bias summary.
The overall risk of bias was rated as low in all eligible studies.
Fig 3. Risk of bias graph.
The overall risk of bias was rated as low in all eligible studies.
Efficacy of anti-PD-1 agents
Six–month progression-free survival rate
According to Table 1, anti-PD-1 agents prolonged median PFS. The median PFS was more than 4.7 months in the nivolumab group and more than 3.7 months in the pembrolizumab group (Table 1). A high dosage or short intermission of pembrolizumab extended the median progression-free survival.
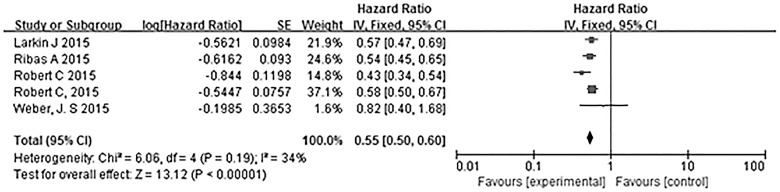
Fig 4 summarizes the comparisons of the six-month PFS rates among the different trials. Overall, patients who received PD-1 antibodies had a significantly greater six-month PFS rate than those who received other treatments, such as chemotherapy and ipilimumab (HR 0.55, 95% CI 0.50–0.60, P<0.00001) (Fig 4). Heterogeneity among the trials was not statistically significant (χ2 = 9.06, P = 0.19, I2 = 34%). Weber et al. reported differences between the experimental groups and control groups, but the weight of the trial was too low to meet statistical significance (HR 0.82, 95% CI 0.40–1.68) [20]. In the other four trials, there were significant differences between the experimental groups and control groups.
Fig 4. Meta-analysis of the 6-month PFS rates in the PD-1 antibody monotherapy groups and the other groups.
The six-month PFS rate was greater among patients who received PD-1 antibodies than among those who received other treatments.
Overall response rate
According to Table 1, anti-PD-1 agents were associated with a higher overall response rate than the control groups for both untreated and relapsed/refractory patients. The overall response rate was greater than 40.0% in patients who received nivolumab 3 mg/kg IV every two weeks as front-line therapy and was 31.6% in patients who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Different dosages of pembrolizumab also improved the overall response rate in both untreated and relapsed/refractory patients. In two trials, the overall response rate of pembrolizumab was between 23.3% and 33.2% [17,19].
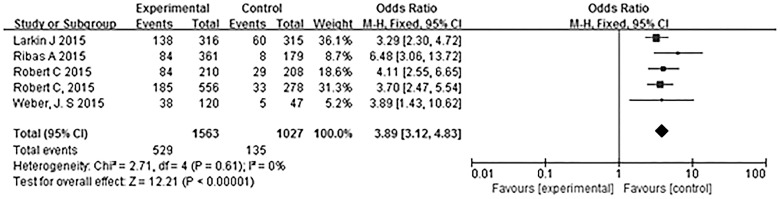
Fig 5 summarizes the comparisons of the overall response rates among the different trials. In all five trials, there were significant differences between anti-PD-1 groups and control groups. In the meta-analysis of the five trials, PD-1 antibody treatment was associated with a significantly better overall response rate (OR 3.89, 95% CI 3.12–4.83, P<0.00001) (Fig 5). Heterogeneity among the trials was not statistically significant (χ2 = 2.71, P = 0.61, I2 = 0%).
Fig 5. Meta-analysis of the overall response rate in the PD-1 antibody monotherapy groups and the other treatment groups.
PD-1 antibody treatment was associated with a higher overall response rate.
Treatment-related adverse events
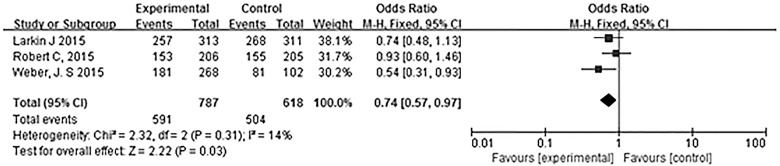
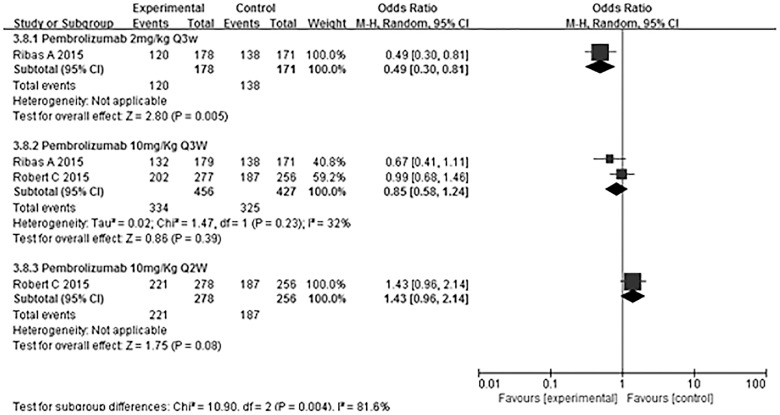
The most commonly reported adverse events associated with PD-1 antibody treatment were fatigue, diarrhea, pruritus, rash and nausea. Table 2 summarizes the treatment-related adverse events in the 5 trials. We also evaluated treatment related-adverse events specific to different PD-1 antibodies and different drug dosages. Larkin et al. [16], Robert et al. [18] and Weber et al. [20] reported treatment-related adverse events associated with nivolumab (Fig 6); specifically, patients treated with this anti-PD-1 antibody reported significantly fewer adverse events (OR 0.74, 95% CI 0.57–0.97, P = 0.03). Heterogeneity among the trials was not statistically significant (χ2 = 2.32, P = 0.31, I2 = 14%). Ribas et al. [17] and Robert et al. [19] reported treatment-related adverse events associated with pembrolizumab (Fig 7), and a subgroup analysis of different doses revealed a significant dose-dependent increase in adverse events with pembrolizumab (χ2 = 10.90, P = 0.004, I2 = 81.6%).
Table 2. Descriptive summary of treatment-related adverse events.
| Study | Intervention in PD-1 group | Adverse events | Intervention in control group | Adverse events | ||
|---|---|---|---|---|---|---|
| Any | Grade 3 or 4 | Any | Grade 3 or 4 | |||
| Larkin J 2015 [16] | Nivolumab 3 mg/kg IV every 2 weeks | 257/313 | 51/313 | Ipilimumab 3 mg/kg every 3 weeks; 4 doses | 268/311 | 85/311 |
| Ribas A 2015 [17] | Pembrolizumab 2 mg/kg every 3 weeks | 120/178 | 19/178 | Investigator-choice chemotherapy | 138/171 | 45/171 |
| Pembrolizumab 10 mg/kg every 3 weeks | 132/179 | 25/179 | Investigator-choice chemotherapy | 138/171 | 45/171 | |
| Robert C 2015 [18] | Nivolumab 3 mg/kg IV every 2 weeks | 153/206 | 24/206 | Dacarbazine 1000 mg/m2 every 3 weeks | 155/205 | 36/205 |
| Robert C 2015 [19] | Pembrolizumab 10 mg/kg every 2 weeks | 221/278 | 37/278 | Ipilimumab 3 mg/kg every 3 weeks; 4 doses | 187/256 | 51/256 |
| Pembrolizumab 10 mg/kg every 3 weeks | 202/279 | 28/279 | Ipilimumab 3 mg/kg every 3 weeks; 4 doses | 187/256 | 51/256 | |
| Weber J 2015 [20] | Nivolumab 3 mg/kg IV every 2 weeks | 181/268 | 24/268 | Investigator-choice chemotherapy | 81/102 | 32/102 |
Fig 6. Meta-analysis of the overall treatment-related adverse events associated with nivolumab.
Patients treated with nivolumab had a lower incidence of adverse events.
Fig 7. Meta-analysis of the overall treatment-related adverse events associated with pembrolizumab.
Subgroup analysis of patients treated with different doses of pembrolizumab revealed a dose-dependent increase in adverse events.
Discussion
Malignant melanoma develops from pigment-containing cells known as melanocytes. Standard systemic treatment options were previously limited to cytotoxic chemotherapy or interleukin 2 therapy [21]. Dacarbazine, one of the important medications in chemotherapy regimens, is commonly used as monotherapy or in combinations. The median PFS of dacarbazine-treated patients is less than six months, and six-year overall survival is less than 2% [22]. The overall objective response rate of high-dose IL-2 treatment was 16% (95% CI 12%-21%) in a retrospective study of 270 patients [21]. The previous mortality rate for metastatic melanoma was high.
In recent years, the inhibition of melanogenesis has gained wide interest and should be a valid method for anti-melanoma treatment. In normal bodies, melanocytes produce melanin pigments, which can protect the body from solar radiation through their antioxidative and free-radical scavenging actions [23]. Through a series of oxidoreduction reactions, L-tyrosine is transformed into melanin via multiple steps. Tyrosinase is the key enzyme regulating melanin synthesis. The melanocortin/MC1R complex, endothelins, histamine, eicosanoids, sex steroids and vitamin D stimulate melanin synthesis, whereas serotonin, dopamine, acetylcholine and some antagonisms with stimulating agents inhibit melanin synthesis [24]. Nevertheless, in melanoma, the synthesis of melanin could generate an oxidative environment, which would lead to mutations in melanoma, and the capacity of the end-product melanin to scavenge free radicals could generate an hypoxic environment that helps melanoma remain resistant to radio- and chemotherapy [24]. Slominski et al. confirmed that the inhibition of melanogenesis could revert the resistance to chemotherapeutic agents or immunotoxic activity of lymphocytes in vitro [25]. Brozyna et al. confirmed that the inhibition of melanin synthesis could increase the sensitivity of melanoma cells to gamma rays in vitro [23]. Moreover, melanogenesis could shorten the survival time of patients with metastatic melanoma. Patients with amelanotic metastatic melanomas present a significant longer DFS and OS than patients with melanotic metastatic melanomas [26,27].
Another improvement in melanoma treatment is due to the successful clinical development of therapies targeting the MAPK pathway and immune checkpoint inhibitors that reactivate the anticancer immune response [28]. The three-year overall survival of ipilimumab-treated patients with advanced melanoma is greater than 22% [29].
PD-1 is a cell surface receptor that is expressed on CD4−/CD8− thymocytes during thymic development and on activated mature hematopoietic cells, such as T-cells, B-cells, NKT cells and monocytes, after prolonged antigen exposure [30]. The human PD-1 gene is located on chromosome 2q37, and the full-length PD-1 cDNA encodes a 288 amino acid protein [31]. PD-1 binds to its ligands (PD-L1 or PD-L2) on the surface of tumor cells, thus down-regulating T-cell activation and impairing tumor cell recognition. In addition, PD-L1 binding to PD-1 inhibits T-cell proliferation, Bcl-xL (an anti-apoptotic molecule) expression, cytokine production and mTOR pathway activity in immune cells [32,33]. These mechanisms help tumor cells escape immune-mediated destruction. However, anti-PD-1 antibodies inhibit PD-1 binding to PD-L1, thereby promoting T-cell activation and the immune-mediated destruction of tumor cells.
In recent years, PD-1 antibodies have yielded many positive results in tumors, including malignant melanoma, non-small-cell lung cancer, renal cancer cell and hematological malignancies. In an open-label, randomized, phase III study, median overall survival was 9.2 months (95% CI 7.3–13.3) in nivolumab-treated patients with squamous cell non-small-cell lung cancer and 6.0 months (95% CI 5.1 to 7.3) in docetaxel-treated patients. Grade 3 or 4 treatment-related adverse events were only 7% in the nivolumab group versus 55% in the docetaxel group [13]. In non-squamous cell non-small-cell lung cancer, median overall survival was 12.2 months in nivolumab-treated patients and 9.4 months in docetaxel-treated patients. Treatment-related adverse events were also lower in the nivolumab group [12]. In a Phase 1 study of patients with relapsed or refractory Hodgkin's lymphoma treated with nivolumab, the 24-week PFS rate was 86% [15]. In renal cell cancers, breast cancers and ovarian cancers, PD-1 antibodies have also emerged as a promising immunotherapeutic option [14,34,35].
Our meta-analysis evaluated the efficacy and treatment-related adverse events reported in five clinical trials (2,828 cases) that investigated PD-1 antibody treatment in patients with melanoma. Patients who were treated with PD-1 antibodies demonstrated an increased survival rate compared to those who received other treatments, such as chemotherapy and ipilimumab. PD-1 antibody treatment was also associated with an increased overall response rate, regardless of its use as first-line treatment or for refractory/relapsed melanoma. The primary treatment-related adverse events associated with anti-PD-1 antibodies were fatigue, diarrhea, pruritus, rash and nausea. Nivolumab (3 mg/kg IV every two weeks) was associated with significantly fewer adverse events than the other treatment options (OR 0.74, 95% CI 0.57–0.97, P = 0.03). In contrast, pembrolizumab was associated with a dose-dependent increase in adverse events. Overall, in our analysis, anti-PD-1 treatment exhibits efficacy, and the risk of treatment-related adverse events is lower with nivolumab than with the other treatment options.
Another published meta-analysis discusses anti-PD-1 in melanoma. Gandini et al. performed a meta-analysis that evaluated the efficacy of PD-1/PD-L1 antibodies in cancer patients with different PD-L1 expression levels. The analysis of patients with melanoma revealed that PD-L1-positive patients not only present a better objective response rate but also have a lower risk of mortality than PD-L1-negative patients [36]. This meta-analysis reminds us that the PD-L1 status may influence the efficacy of anti-PD-1/PD-L1 therapy, but the analysis did not separately analyze PD-1 and PD-L1 antibodies.
Some limitations of our meta-analysis are worth noting. First, the patients evaluated in this study presented with different stages of disease (untreated or refractory), which may have influenced the efficacy of PD-1 antibody treatment. Second, different chemotherapy regimens were administered to the control groups in different trials; patients in the control groups in two of the five studies were treated with ipilimumab (a CTLA-4 inhibitor), while those in the other studies received chemotherapy. The use of different treatments in the control groups may have affected our assessment of PD-1 efficacy. Third, patients in different studies were treated with different dosages of pembrolizumab; therefore, this analysis cannot provide evidence to support a specific pembrolizumab dosage. Fourth, few trials reported overall survival data, and the follow-up times were not sufficiently long for an analysis of late-stage and fatal complications.
Conclusion
In summary, our results showed that PD-1 antibody monotherapy significantly improved the survival of melanoma patients with previously untreated or refractory disease. In addition, we found that nivolumab was associated with a lower risk of adverse events, yet there was a trend toward a dose-dependent increase in adverse events with certain PD-1 antibodies. However, the follow-up times of the studies were not sufficiently long to facilitate an analysis of late-stage and fatal complications. We look forward to extending this type of analysis to multicenter, prospective, double-blind and well-designed RCTs to verify the findings of this study and to gain further insight into the efficacy of PD-1 antibody treatment in patients with melanoma.
Supporting Information
(DOC)
Acknowledgments
We thank all of our colleagues who contributed to this systematic review and meta-analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Caers J, Fernandez de Larrea C, Leleu X, Heusschen R, Zojer N, Decaux O, et al. The changing landscape of smoldering multiple myeloma: a European perspective. Oncologist. 2016;21: 333–342. 10.1634/theoncologist.2015-0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65: 5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 3.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8: 793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 5.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74: 181–273. [DOI] [PubMed] [Google Scholar]
- 6.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99: 12293–12297. 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem ML, El-Badawy A. Programmed death-1/programmed death-L1 signaling pathway and its blockade in hepatitis C virus immunotherapy. World J Hepatol. 2015;7: 2449–2458. 10.4254/wjh.v7.i23.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11: 141–151. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110: 296–304. 10.1182/blood-2006-10-051482 [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101: 17174–17179. 10.1073/pnas.0406351101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366: 2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373: 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373: 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373: 1803–1813. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372: 311–319. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373: 23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16: 908–918. 10.1016/s1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372: 320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 19.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372: 2521–2532. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 20.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16: 375–384. 10.1016/s1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 21.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17: 2105–2116. [DOI] [PubMed] [Google Scholar]
- 22.Ancuceanu R, Neagu M. Immune based therapy for melanoma. Indian J Med Res. 2016;143: 135–144. 10.4103/0971-5916.180197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brozyna AA, VanMiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123: 1448–1456. 10.1002/ijc.23664 [DOI] [PubMed] [Google Scholar]
- 24.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84: 1155–1228. 10.1152/physrev.00044.2003 [DOI] [PubMed] [Google Scholar]
- 25.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124: 1470–1477. 10.1002/ijc.24005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brozyna AA, Jozwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7: 17844–17853. 10.18632/oncotarget.7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brozyna AA, Jozwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44: 2071–2074. 10.1016/j.humpath.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarhini AA. The future of systemic therapy of melanoma: combinations, predictive biomarkers. Oncology. 2015;29: 94, 107. [PubMed] [Google Scholar]
- 29.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33: 1889–1894. 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11: 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics. 1994;23: 704–706. 10.1006/geno.1994.1562 [DOI] [PubMed] [Google Scholar]
- 32.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26: 677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206: 3015–3029. 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33: 4015–4022. 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 35.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib Keynote-012 study. J Clin Oncol 2016. 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100: 88–98. 10.1016/j.critrevonc.2016.02.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.