Abstract
We surveyed the Trichoderma (Hypocreales, Ascomycota) biodiversity in agricultural fields in four major agricultural provinces of East China. Trichoderma strains were identified based on molecular approaches and morphological characteristics. In three sampled seasons (spring, summer and autumn), 2078 strains were isolated and identified to 17 known species: T. harzianum (429 isolates), T. asperellum (425), T. hamatum (397), T. virens (340), T. koningiopsis (248), T. brevicompactum (73), T. atroviride (73), T. fertile (26), T. longibrachiatum (22), T. pleuroticola (16), T. erinaceum (16), T. oblongisporum (2), T. polysporum (2), T. spirale (2), T. capillare (2), T. velutinum (2), and T. saturnisporum (1). T. harzianum, T. asperellum, T. hamatum, and T. virens were identified as the dominant species with dominance (Y) values of 0.057, 0.052, 0.048, and 0.039, respectively. The species amount, isolate numbers and the dominant species of Trichoderma varied between provinces. Zhejiang Province has shown the highest diversity, which was reflected in the highest species amount (14) and the highest Shannon–Wiener diversity index of Trichoderma haplotypes (1.46). We observed that relative frequencies of T. hamatum and T. koningiopsis under rice soil were higher than those under wheat and maize soil, indicating the preference of Trichoderma to different crops. Remarkable seasonal variation was shown, with summer exhibiting the highest biodiversity of the studied seasons. These results show that Trichoderma biodiversity in agricultural fields varies by region, crop, and season. Zhejiang Province (the southernmost province in the investigated area) had more T. hamatum than Shandong Province (the northernmost province), not only in isolate amounts but also in haplotype amounts. Furthermore, at haplotype level, only T. hamatum showed a gradient distribution from south to north in correspondence analysis among the four dominant species. The above results would contribute to the application of Trichoderma biocontrol strains.
Introduction
Species in the fungal genus Trichoderma (Hypocreales, Ascomycota) possess rapid growth and high stress-tolerance. They are widely distributed and have a variety of biological activities. Some species, such as T. harzianum form the basis for commercial products applied for biological control of plant pathogenic fungi [1–3]. Some Trichoderma species have beneficial effects on plant growth and development [4, 5]. And some species, such as T. reesei have important industrial applications as producer of cellulolytic and hemicellulolytic enzymes [6].
New species of Trichoderma have recently been reported [7–9] and 254 named species were previously known [10, 11]. A broad array of secondary metabolites, many of which have novel bioactivity, are produced by Trichoderma species isolated from varied environments [12, 13]. Native Trichoderma strains in agricultural soils are likely to be more effective biocontrol agents, since they are already adapted to variable field conditions [14]. The prevalence of Trichoderma in different ecological niches contributes to the high diversity of genetic and metabolic variability [15]. Exploration of genetic diversity in this genus has been conducted in several regions [16–20].
Trichoderma has shown a considerably high genetic biodiversity not only at the species level but also at the under species level. For instance, T. harzianum and T. asperellum were represented by several genotypes or haplotypes [21, 22]. Trichoderma diversity has been explored in China, but only in a limited number of climatic regions (excluding major agricultural provinces) or only in a limited number of seasons in species level [21, 23]. We therefore focused our research on Trichoderma species and haplotype biodiversity within the major agricultural areas of China, investigated the influence of regional, seasonal and cropping variability and examined the species and amounts of Trichoderma to find the potential distribution pattern.
Materials and Methods
No specific permissions were required for sampling locations/activities for native research institutes or researchers, and the studies did not involve endangered or protected species.
Sampling
Trichoderma isolates in this study derived from soils of cultivated lands, orchards, and gardens in 20 regions of East China. The sampling sites were located in four major agricultural provinces (Anhui, Jiangsu, Shandong and Zhejiang). We collected 737 samples in total during May, August, and October of 2014 and 2015. The sampling sites at different months were the same, and the geographical coordinates were shown in S1 Table. Among these, 312 samples were from soil under rice cultivation, followed by maize (94) and wheat (75). Each sample contained about 200 g of soil from a depth of approximately 20 cm. Samples were placed into sterile polyethylene bags, transported to the laboratory, and stored at 4°C until isolation.
Isolation and storage of Trichoderma strains
Rose Bengal Agar was the selective medium for Trichoderma and we used the soil dilution plating method. Putative Trichoderma colonies were purified by two rounds of subculturing on potato-dextrose agar (PDA). Pure cultures were suspended in cryopreservation liquid (15% v/v glycerol, 10 g/L glucose, 1 g/L yeast extract and 1 g/L casein hydrolysate) and stored at -70°C.
DNA extraction, amplification, and sequencing
The mycelium of pure cultures was scraped directly from plates after 3 d growth on PDA at 25°C and used to extract DNA according to the method of [24]. To amplify internal transcribed spacer regions 1 and 2 (ITS1 and ITS2) of the ribosomal nucleotide sequence, a pair of general primers ITS5 (5'GGAAGTAAAAGTCGTAACAAGG3') and ITS4 (5'TCCTCCGCTTATTGATATGC3') were used. The PCR reaction was performed in a thermal cycler with the following parameters: 3 min initial denaturation at 94°C, followed by 30 cycles of 1 min denaturation at 94°C, 30 s primer annealing at 56°C, 30 s extension at 72°C, and a final extension period of 10 min at 72°C.
A 0.9-kb fragment of the 5' end of the translation elongation factor-1α (tef) gene (eEF1a1) containing three major introns was amplified using the primer pair tef71f (C AAA ATG GGT AAG GAG GAS AAG AC) and tef997R (CA GTA CCG GCR GCR ATR ATS AG) in the case where the ITS sequence did not provide unambiguous identification. The amplification program reference protocol was submitted by John Bissett (http://www.isth.info/isth/methods/method.php?method_id=9).
Phylogenetic analysis
ITS sequences of all isolated Trichoderma strains were used to obtain haplotypes by DnaSP ver. 5.1 [25]. Sequences of known species including type strains and outgroup were downloaded in NCBI database. The alignment of type haplotypes and the known species was produced by Clustal W [26]. After the exclusion of leading and trailing gap regions, the nucleotide substitution model was selected using Jmodeltest [27]. A phylogenetic tree was obtained with the combination of Bayesian analysis and maximum parsimony analysis, which was performed by MrBayes v. 3.0b4 [28] and PAUP* 4.0 b10 [29], respectively.
Statistical analysis
The dominance values were calculated using the following formula:
| (1) |
where N is the total strain count, ni is the count of genus (species) i, and fi is the frequency that genus (species) i appears in the samples. The genus i is dominant while Y > 0.02. SPSS version 20.0 (IBM Corp, Armonk, NY, USA) was used for multiple correspondence analysis of sampling sites, based on the existence of the dominant species, respectively, and the CA bioplots were used for results analysis.
Results
Trichoderma isolation and Phylogenetic analyses
A total of 737 samples distributed across three seasons were collected from East China. All putative Trichoderma isolates were separated from Rose Bengal Agar to obtain a full-scale Trichoderma diversity profile. These isolates were preliminarily identified using morphological characters and ITS1 and 2 oligonucleotide barcode. In some cases, species pairs occurred because ITS1 and 2 sequences did not provide sufficient available species-specific diagnostic characters. If so, the translation elongation factor 1-alpha (tef1-α) gene was amplified for additional characters. A total of 2078 isolates were identified as Trichoderma. Among these, 2075 isolates were classified into 17 species: T. harzianum (429 isolates), T. asperellum (425), T. hamatum (397), T. virens (340), T. koningiopsis (248), T. brevicompactum (73), T. atroviride (73), T. fertile (26), T. longibrachiatum (22), T. pleuroticola (16), T. erinaceum (16), T. oblongisporum (2), T. polysporum (2), T. spirale (2), T. capillare (2), T. velutinum (2), and T. saturnisporum (1). The remaining three isolates were not identified at the species level.
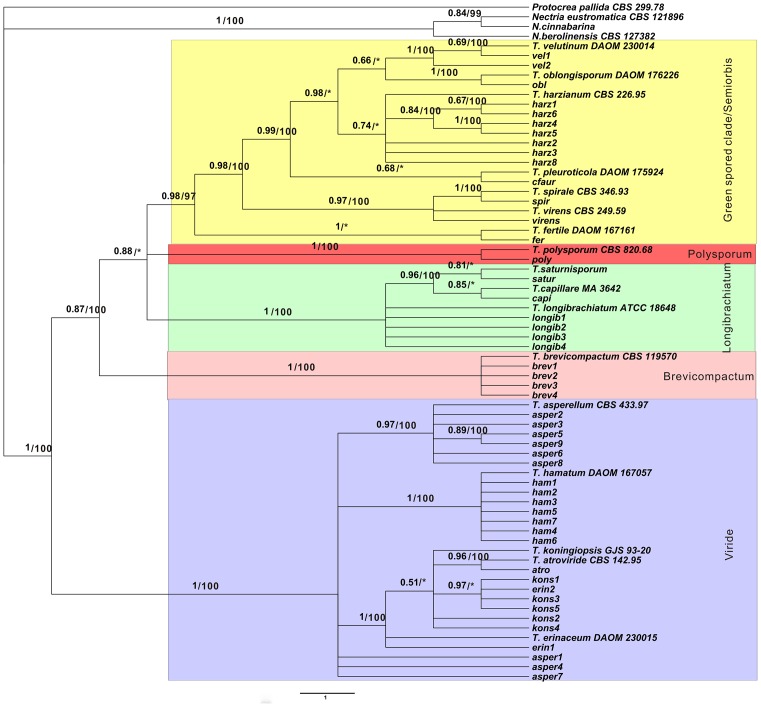
To establish a phylogenetic tree, we first calculated haplotypes from 2078 ITS1 and 2 sequences. Finally, 8 haplotypes (Table 1) were subjected to parsimony and Bayesian analysis, which used Protocrea pallida, Nectria eustromatica, N. berolinensis and N. cinnabarina as the outgroup to identify the situation of the root. The result of this phylogenetic analysis is shown in Fig 1. The 48 haplotypes belonging to the 17 Trichoderma species were positioned into five clusters with strong bootstrap supports. The phylogenetic structure was consistent with previously established sections and clades in most cases [30, 31].
Table 1. Haplotypes (48) of Trichoderma identified in this study.
| Haplotype Code | Species | Number of isolated strains | Representative strains | |
|---|---|---|---|---|
| Code | GenBank Accession | |||
| asper1 | T. asperellum | 237 | CTCCSJ-A-SD31000 | KT314289 |
| asper2 | T. asperellum | 173 | CTCCSJ-A-SC31024 | KT314294 |
| asper3 | T. asperellum | 1 | CTCCSJ-A-SC31096 | KT314300 |
| asper4 | T. asperellum | 1 | CTCCSJ-A-SD31174 | KT314302 |
| asper5 | T. asperellum | 9 | CTCCSJ-A-SC3140 | KT314305 |
| asper6 | T. asperellum | 1 | CTCCSJ-A-GS31670 | KT314308 |
| asper7 | T. asperellum | 1 | CTCCSJ-A-SC3254 | KT314313 |
| asper8 | T. asperellum | 1 | CTCCSJ-A-XM337 | KT314317 |
| asper9 | T. asperellum | 1 | CTCCSJ-A-SD3908 | KT314330 |
| atro | T. atroviride | 73 | CTCCSJ-A-SC350 | KT314321 |
| brev1 | T. brevicompactum | 67 | CTCCSJ-A-SD31063 | KT314299 |
| brev2 | T. brevicompactum | 3 | CTCCSJ-A-XM3257 | KT314314 |
| brev3 | T. brevicompactum | 2 | CTCCSJ-A-GS3259 | KT314315 |
| brev4 | T. brevicompactum | 1 | CTCCSJ-A-GS3847 | KT314329 |
| erin1 | T. erinaceum | 15 | CTCCSJ-A-SC31615 | KT314307 |
| erin2 | T. erinaceum | 1 | CTCCSJ-A-SC31443 | KT314306 |
| fer | T. fertile | 26 | CTCCSJ-A-YM3122 | KT314304 |
| ham1 | T. hamatum | 340 | CTCCSJ-A-SD31002 | KT314290 |
| ham2 | T. hamatum | 6 | CTCCSJ-A-SD31012 | KT314293 |
| ham3 | T. hamatum | 44 | CTCCSJ-A-SD31214 | KT314303 |
| ham4 | T. hamatum | 1 | CTCCSJ-A-SD3711 | KT314327 |
| ham5 | T. hamatum | 1 | CTCCSJ-A-QT3177 | KT314309 |
| ham6 | T. hamatum | 1 | CTCCSJ-A-SD3937 | KT314332 |
| ham7 | T. hamatum | 4 | CTCCSJ-A-SD3550 | KT314322 |
| harz1 | T. harzianum | 235 | CTCCSJ-A-SD31009 | KT314292 |
| harz2 | T. harzianum | 66 | CTCCSJ-A-SD31031 | KT314296 |
| harz3 | T. harzianum | 21 | CTCCSJ-A-XM31791 | KU375457 |
| harz4 | T. harzianum | 41 | CTCCSJ-A-MH31042 | KT314298 |
| harz5 | T. harzianum | 26 | CTCCSJ-A-SD3109 | KT314301 |
| harz6 | T. harzianum | 33 | CTCCSJ-A-SD31059 | KU375456 |
| harz8 | T. harzianum | 7 | CTCCSJ-A-SD3775 | KU375462 |
| satur | T. saturnisporum | 1 | CTCCSJ-A-SD3604 | KT314324 |
| kons1 | T. koningiopsis | 79 | CTCCSJ-A-YC3100 | KT314288 |
| kons2 | T. koningiopsis | 164 | CTCCSJ-A-SD31029 | KT314295 |
| kons3 | T. koningiopsis | 2 | CTCCSJ-A-XM323 | KT314311 |
| kons4 | T. koningiopsis | 2 | CTCCSJ-A-SD3388 | KT314319 |
| kons5 | T. koningiopsis | 1 | CTCCSJ-A-SD3644 | KT314325 |
| longib1 | T. longibrachiatum | 18 | CTCCSJ-A-SC3195 | KT314310 |
| longib2 | T. longibrachiatum | 1 | CTCCSJ-A-SC378 | KT314328 |
| longib3 | T. longibrachiatum | 2 | CTCCSJ-A-YM32425 | KU375460 |
| longib4 | T. longibrachiatum | 1 | CTCCSJ-A-YM32819 | KU375461 |
| obl | T. oblongisporum | 2 | CTCCSJ-A-GS3677 | KT314326 |
| cfaur | T. pleuroticola | 16 | CTCCSJ-A-YM3383 | KT314318 |
| poly | T. polysporum | 2 | CTCCSJ-A-SD3918 | KT314331 |
| capi | T. capillare | 1 | CTCCSJ-A-SD3940 | KT314333 |
| spir | T. spirale | 2 | CTCCSJ-A-SC3230 | KT314312 |
| vel | T. velutinum | 2 | CTCCSJ-A-SD3579 | KT314323 |
| virens | T. virens | 340 | CTCCSJ-A-SD31006 | KT314291 |
Fig 1. Phylogenetic tree inferred from ITS rDNA sequences.
Bayesian posterior probabilities followed by maximum parsimony bootstrap values above 50 with 1000 replications are shown at the branch. Branches marked with an asterisk indicated that the branches were not present or have bootstrap values below 50 in the maximum parsimony tree. The bar represents 1.0 substitution per site.
The first cluster includes clade Viride with T. asperellum, T. hamatum, T. atroviride, T. erinaceum, and T. koningiopsis. For the mutation at 115 and 371 nt (shown in S1 Fig) haplotypes of asper1, 4 and 7 do not cluster in the branch, which includes the other haplotypes and ex-type of T. asperellum. The four haplotypes of T. brevicompactum constitute the second cluster with Bayesian posterior probabilities of 1 and a maximum parsimony bootstrap value of 100. The third cluster (clade Longibrachiatum) includes T. longibrachiatum, T. capillare, and T. saturnisporum, and is strongly supported. The fourth cluster is clade polysporum, which only includes one species (T. polysporum). The remaining species were clustered with a Bayesian posterior probability of 0.98 and a maximum parsimony bootstrap value of 97. These species are distributed in two clades: green spored clade and semiorbis clade. The seven haplotypes of T. harzianum formed a moderately well-supported (0.74) clade.
Species diversity
A total of 557 soil samples (isolation rate = 76%) produced Trichoderma isolates from 737 varied soil samples, and 2078 Trichoderma isolates (relative rate = 3%) were identified from 63426 fungal colonies. The dominance (Y) value was 0.025 (> 0.02), indicating that the genus Trichoderma was dominant in the soil samples. T. harzianum, T. asperellum, T. hamatum, and T. virens were the dominant species with dominance (Y) values of 0.057, 0.052, 0.047, and 0.039, respectively. The Shannon–Wiener diversity index of haplotypes was similar between the four provinces: 1.46 (Zhejiang), 1.43 (Jiangsu), 1.40 (Anhui), and 1.32 (Shandong). However, the proportion and composition of Trichoderma species varied between provinces.
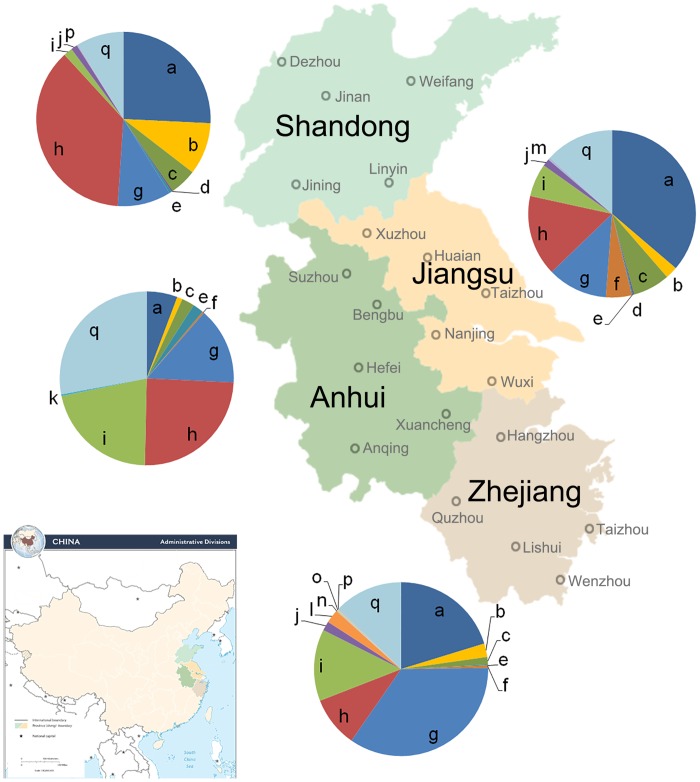
A total of 14 species were identified from Zhejiang Province: T. asperellum, T. atroviride, T. brevicompactum, T. erinaceum, T. fertile, T. hamatum, T. harzianum, T. saturnisporum, T. koningiopsis, T. pleuroticola, T. spirale, T. virens, T. longibrachiatum, and T. velutinum (Fig 2); Among these, T. asperellum, T. hamatum, T. koningiopsis, and T. virens were the dominant species with dominance (Y) values of 0.086, 0.217, 0.025, and 0.045, respectively. Trichoderma isolates from Jiangsu Province comprised 12 species: T. asperellum, T. atroviride, T. brevicompactum, T. erinaceum, T. fertile, T. hamatum, T. harzianum, T. koningiopsis, T. polysporum, T. virens, T. longibrachiatum, and T. capillare (Fig 2); T. asperellum, T. hamatum, T. harzianum, and T. virens were dominant with dominance (Y) values of 0.159, 0.025, 0.05, and 0.029, respectively. T. asperellum, and T. harzianum were the dominant species with dominance (Y) values of 0.091 and 0.219, respectively, in 11 species that were found in Shandong Province; Other detected species include T. atroviride, T. brevicompactum, T. capillare, T. erinaceum, T. hamatum, T. koningiopsis, T. longibrachiatum, T. velutinum, and T. virens (Fig 2). T. virens, T. hamatum, T. harzianum, and T. koningiopsis were the dominant species with dominance (Y) values of 0.130, 0.038, 0.102, and 0.086, respectively, in the 10 species that were found in Anhui. Other detected species include T. asperellum, T. atroviride, T. brevicompactum, T. erinaceum, T. fertile, and T. oblongisporum (Fig 2).
Fig 2. Distribution of Trichoderma species in East China.
Individual species are abbreviated as follows: a—T. asperellum, b—T. atroviride, c—T. brevicompactum, d—T. capillare, e—T. erinaceum, f—T. fertile, g—T. hamatum, h—T. harzianum, i—T. koningiopsis, j—T. longibrachiatum, k—T. oblongisporum, l—T. pleuroticola, m—T. polysporum, n—T. saturnisporum, o—T. spirale, p—T. velutinum, and q—T. virens.
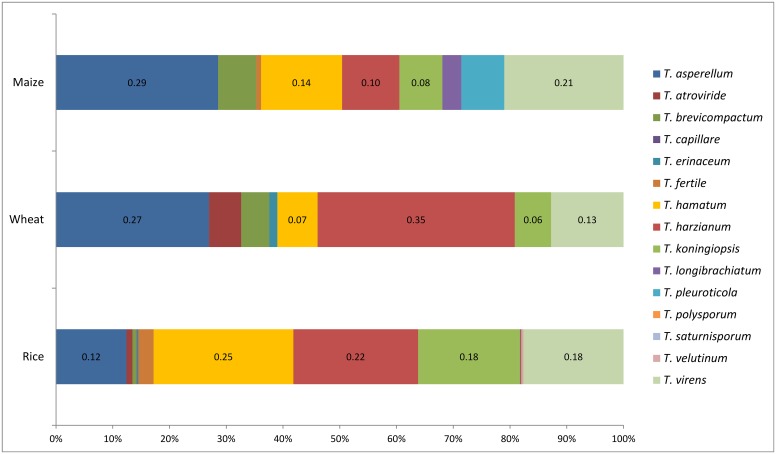
There were significant differences in community of Trichoderma species from soils under different crop types (Fig 3). Rice soil yielded the highest species numbers, with 14 species being isolated from 942 strains. A total of nine and eight species were separated from maize and wheat soils, respectively. In addition, different Trichoderma species showed different relative frequencies in soils under different crop cultivation. The relative frequencies of T. asperellum ranged from 12 to 29%, and T. harzianum from 10 to 35%. The relative frequencies of T. virens exceeded 13% in all three cultivation types. We observed that relative frequencies of T. hamatum and T. koningiopsis under rice soil were higher than those under wheat and maize soil. Furthermore, the noticeable discrepancies indicate the preference of the Trichoderma to the crop soils.
Fig 3. Relative frequencies of Trichoderma species isolated from rice, wheat, and maize soils.
The relative frequencies of the main Trichoderma species are shown at the center of the bar.
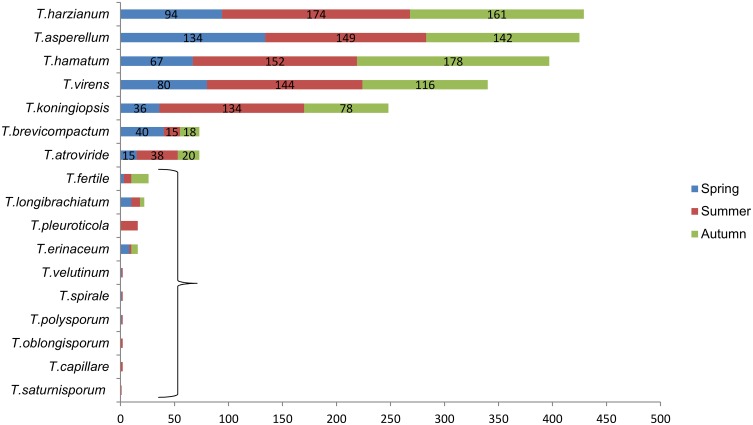
The 2078 Trichoderma strains were collected over three seasons and the remarkable seasonal differences are shown in Fig 4. The amount of Trichoderma isolated during spring was clearly lower than during summer and autumn. For instance, the amounts of T. virens, T. koningiopsis, T. hamatum and T. harzianum in spring were lower than the amounts in summer and autumn. In terms of the number of diverse species, 17 species were observed in summer, followed by 13 in spring and 9 in autumn. T. capillare, T. saturnisporum, T. oblongisporm, and T. pleuroticola were only found in summer.
Fig 4. Distribution of Trichoderma species in different seasons.
Inside box indicates the distribution of T. fertile, T. longibrachiatum, T. pleuroticola, T. erinaceum, T. velutinum, T. spirale, T. polysporum, T. oblongisporum, T. saturnisporum, and T. capillare. The numbers written on the bar charts correspond to the number of isolates per season.
Distribution of the dominant species T. hamatum in East China
There were 397 Trichoderma isolates, belonging to seven haplotypes, identified as T. hamatum. Haplotypes ham1 (337 isolates) and ham3 (44 isolates) accounted for the vast majority of isolates. Haplotypes ham4, ham5, and ham6 were monotypic. Five T. hamatum haplotypes were distributed in Zhejiang Province, which was the southernmost province in the investigated area. In addition, only haplotype ham1, which was the most distributed, was found in Shandong Province (the northernmost province in the investigated area). Furthermore, the number of T. hamatum isolates in Shandong Province was 42, much less than in Zhejiang Province (222 isolates) and Anhui Province (84).
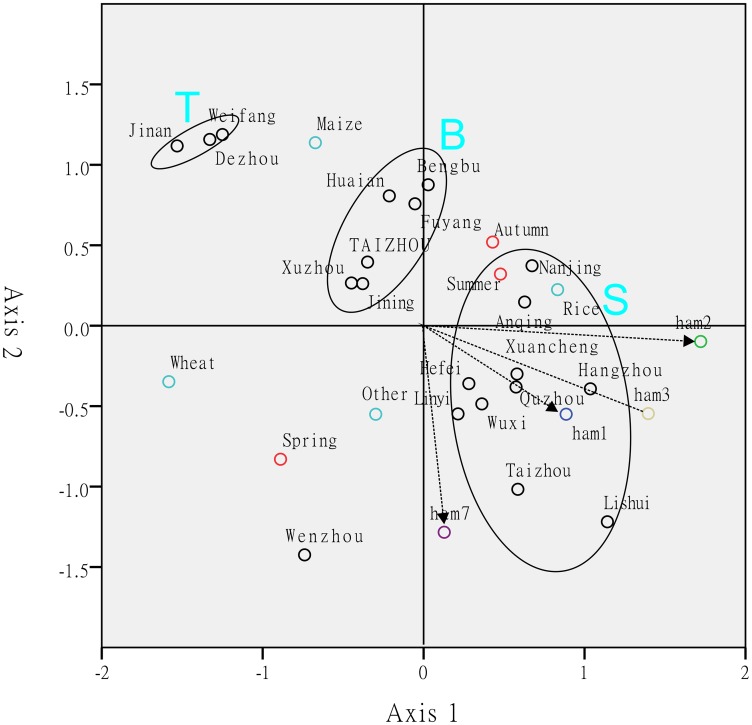
The distribution of T. hamatum was indicated by indirect correspondence analysis (CA) based on the existence of T. hamatum. In CA bioplot, the closer two points appear, the closer their profile patterns are to each other. Fig 5 shows that Jinan, Weifang, and Dezhou, which belong to the temperate monsoon climate (T), were clustered together. Nanjing, Wuxi, Anqing, Quzhou, Taizhou, Linyi, Hangzhou, Hefei, Xuancheng, and Lishui formed an ellipsoid, and all belong to the lower latitude regions and subtropical monsoon climate (S), except for Linyi. Bengbu, Fuyang, Huaian, Xuzhou, Jining, and Taizhou, which clustered in an ellipsoid (B), were near the boundary between the subtropical monsoon climatic region and the temperate monsoon climatic region. Ellipsoid S was closer to main haplotypes of T. hamatum than Ellipsoids T and B, which indicates a strong correlation between Ellipsoid S and the main haplotypes of T. hamatum. When the magnitudes to haplotypes of T. hamatum were drawn, we found that the regions (ellipsoids) are arranged on a gradient from south (subtropical monsoon climate) to north (temperate monsoon climate) along the axis. Furthermore, in the bioplots of other dominant species (T. virens, T. asperellum, and T. harzianum), this distribution pattern was not seen. According to the distance between points in the bioplot (Fig 5), different crops yielded different T. hamatum profile patterns. Furthermore, summer and autumn present a similar T. hamatum profile pattern, whereas that of spring is different.
Fig 5. Multiple correspondence analysis of sampling sites based on the existence of T. hamatum.
The inertia of the first and second axes is 0.33 and 0.28, respectively. Ellipsoids show the centre of the regions. S is the region of subtropical monsoon climate, T is the region of temperate monsoon climate, and B is the boundary region between the subtropical monsoon and the temperate monsoon climatic regions. Red circles indicate the seasons and cyan circles indicate the crop types. Other colored circles indicate the main haplotypes of T. hamatum.
Discussion
Morphological analysis is a poor method for Trichoderma species identification because of character plasticity [32]. Therefore, all putative Trichoderma isolates in this study were identified from selective medium. ITS rDNA sequences were also submitted to the oligonucleotide barcode program TrichOKEY [30] for identification. The accuracy and convenience of this method has enabled the completion of many studies over large regions such as China [21, 23], Colombia [22, 33], and Europe [7, 16, 34–36]. Although similar research has been completed, noticeable differences were still found. Additional details of Trichoderma biodiversity appear in our study.
In our study, the composition and proportion of Trichoderma species and the dominant species were distinct between different regions and cropping types. Zhejiang Province had 14 species and the most dominant species in Zhejiang Province was T. hamatum, followed by Jiangsu (12, T. asperellum), Shandong (11, T. harzianum) and Anhui (10, T. virens). The Shannon–Wiener diversity index of haplotypes also suggested that Zhejiang Province has the greatest level of Trichoderma diversity. Environmental preference of Trichoderma has also been found in many studies [37–40].
Significant differences of Trichoderma communities in different crop soil were observed in our study. Variation in the amount and chemical composition of the plant root exudation may contribute to the significant differences of Trichoderma communities. These plant root exudation, including carbohydrates, amino acids, organic acids, secondary metabolite and signaling molecules, can alter physico-chemical properties of soil, provide carbon and nitrogen sources and interacting with Trichoderma [41]. As Trichoderma have been considered as plant symbionts, the plant root may show a stronger influence to Trichoderma. The remarkable effects of plant root exudation on bacteria and phytopathogenic fungi communities have been observed in other studies [42]. Thus, we have reason to consider that the root exudation may be responsible for plant types-specific Trichoderma communities. In our study, the diversity of Trichoderma in rice soil is higher than wheat and corn. This may not only be attributed to the more beneficial exudation that rice roots exudates, but may also be associated with the soil moisture, pH, oxygen content and particle morphology that notably affected by cultivation measures in rice growth period. For example, we observed the preference of T. hamatum and T. koningiopsis to rice soil.
We found that fungal diversity was influenced by seasonal effects, supporting previous studies [19]. In our study, the amount and composition of Trichoderma were diverse in different seasons. Nelson et al. showed that T. hamatum was more abundant in spring than in summer [43], which was contradictory to our results. This result may be attributed to differences in climate, soil depth or plant type. More likely, the misidentified T. hamatum accounts for a greater proportion since the concept of aggregate species was adopted at that time [44]. Of interest is that T. asperellum has few clear changes between seasons.
Compared with the report of Sun et al.[23], a similar diversity pattern with several species forming a co-dominant group was observed in soil samples from East China. In both sun et al. and our studies, T. asperellum, T. virens, T. harzianum, and T. hamatum were the most frequent species. However, T. koningiopsis was frequently isolated in our study while only one strain was found by Sun. T. viride was on an exception. These results may be related to the latitudinal difference of the sample sites. T. koningiopsis is essentially a tropical species [45] and T. viride is a representation of the temperate Trichoderma biota [46]. We found that uncommon species were different in proportion and composition; for example, T. citrioviride and T. koningii were not found in our study. Instead of focusing on the description of Trichoderma biodiversity, we explored the distribution pattern at the haplotype level and attempted to identify the factors.
Based on our study, T. hamatum was the dominant species in farmland soil. The dominance of T. hamatum in farmland soil may be related to higher stress-tolerance and excellent competition at soil temperatures of 20–25°C [47–49]. We also found that the south to north distribution of T. hamatum, which was consistent with the change of climate types. The decreasing precipitation from south to north is the main feature of East China. The increased tolerance of T. hamatum to excessive moisture above other species may contribute to this situation.
T. harzianum has a worldwide distribution and is often the dominant species in other environments, such as Egypt [50, 51], Asia [39] and Europe [34]. As the most dominant species in our study, T. harzianum constituted of seven haplotypes, which were strongly supported by the phylogenetic tree. High genetic diversity may contribute to the higher abundance of T. harzianum. Haplotypes harz1 and asper1 are able to adapt well to a varied environment and are dominant species, so they are potentially important agents for use in biological control.
A level of Trichoderma biodiversity in agricultural soil was reported in this study. Trichoderma biodiversity in agricultural fields varies with season, region, and crop type. Of the four dominant species identified, only T. hamatum showed a south to north distribution. However, some species which grow slowly or sporulate later in the year may not have been isolated due to the limitations of the cultivation-dependent methods that we used. A combination of cultivation-dependent and cultivation-independent methods should be used to provide further information in future exploration of Trichoderma biodiversity in agricultural soils.
Supporting Information
At the position of 115 and 371, base deletion or transversion was observed in asper1, 4 and 7.
(TIF)
(DOCX)
Acknowledgments
We would like to thank Jia-Ying Wang for her help in establishing a phylogenetic tree, Huan-Bin Shi for him help in performing the experiments. We would also like to thank Erika Kothe and an anonymous reviewer for their precious suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Special Foundation for the Basic Work of Science and Technology, the Ministry of Science and Technology, China (2014FY120900) and the National Key Technology Research and Development Program, the Ministry of Science and Technology, China (2015BAC02B03). CLZ and FCL received the funding, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Benitez T, Rincon AM, Limon MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. International microbiology: the official journal of the Spanish Society for Microbiology. 2004;7(4):249–60. . [PubMed] [Google Scholar]
- 2.Zafari D, Koushki MM, Bazgir E. Biocontrol evaluation of wheat take-all disease by Trichoderma screened isolates. Afr J Biotechnol. 2008;7(20):3650–6. WOS:000260457800026. [Google Scholar]
- 3.Asad SA, Ali N, Hameed A, Khan SA, Ahmad R, Bilal M, et al. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Polish journal of microbiology / Polskie Towarzystwo Mikrobiologow = The Polish Society of Microbiologists. 2014;63(1):95–103. . [PubMed] [Google Scholar]
- 4.Yedidia I, Srivastva AK, Kapulnik Y, Chet I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil. 2001;235(2):235–42. 10.1023/A:1011990013955. WOS:000171298500011. [DOI] [Google Scholar]
- 5.Harman GE. Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. The New phytologist. 2011;189(3):647–9. 10.1111/j.1469-8137.2010.03614.x . [DOI] [PubMed] [Google Scholar]
- 6.Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nature biotechnology. 2008;26(5):553–60. 10.1038/nbt1403 . [DOI] [PubMed] [Google Scholar]
- 7.Jaklitsch WM, Voglmayr H. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Studies in mycology. 2015;80:1–87. 10.1016/j.simyco.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu ZX, Zhuang WY. Three new species of Trichoderma with hyaline ascospores from China. Mycologia. 2015;107(2):328–45. 10.3852/14-141 . [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Oh SY, Cho HJ, Fong JJ, Cheon WJ, Lim YW. Trichoderma songyi sp. nov., a new species associated with the pine mushroom (Tricholoma matsutake). Antonie van Leeuwenhoek. 2014;106(4):593–603. 10.1007/s10482-014-0230-4 . [DOI] [PubMed] [Google Scholar]
- 10.Atanasova L, Druzhinina IS, Jaklitsch WM, Mukherjee P, Horwitz B, Singh U, et al. Two hundred Trichoderma species recognized on the basis of molecular phylogeny. Trichoderma: biology and applications CABI, Wallingford: 2013:10–42. [Google Scholar]
- 11.Bissett J, Gams W, Jaklitsch W, Samuels GJ. Accepted Trichoderma names in the year 2015. Ima Fungus. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang CL, Liu SP, Lin FC, Kubicek CP, Druzhinina IS. Trichoderma taxi sp. nov., an endophytic fungus from Chinese yew Taxus mairei. FEMS microbiology letters. 2007;270(1):90–6. 10.1111/j.1574-6968.2007.00659.x . [DOI] [PubMed] [Google Scholar]
- 13.Nielsen KF, Grafenhan T, Zafari D, Thrane U. Trichothecene production by Trichoderma brevicompactum. Journal of agricultural and food chemistry. 2005;53(21):8190–6. 10.1021/jf051279b . [DOI] [PubMed] [Google Scholar]
- 14.Joshi D, Misra SC. Characterization of Trichoderma Isolates from Sugarcane Agro-Ecosystem and their Efficacy Against Colletotrichum falcatum Causing Red Rot of Sugarcane. Sugar Tech. 2013;15(2):192–6. 10.1007/s12355-013-0208-y [DOI] [Google Scholar]
- 15.Schuster A, Schmoll M. Biology and biotechnology of Trichoderma. Applied microbiology and biotechnology. 2010;87(3):787–99. 10.1007/s00253-010-2632-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaklitsch WM. European species of Hypocrea part II: species with hyaline ascospores. Fungal diversity. 2011;48(1):1–250. 10.1007/s13225-011-0088-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadfi-Zouaoui N, Chaabani S, Rouaissi M, Hedi A, Hajlaoui MR, Boudabous A. Analysis of the diversity of Trichoderma spp. in soil horizons using digested ITS regions. Ann Microbiol. 2009;59(3):459–63. WOS:000271346700010. [Google Scholar]
- 18.Kamala T, Devi SI, Sharma KC, Kennedy K. Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma Biodiversity hot spot region with special reference to Manipur. BioMed research international. 2015;2015:285261 10.1155/2015/285261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagn A, Pritsch K, Schloter M, Munch JC. Fungal diversity in agricultural soil under different farming management systems, with special reference to biocontrol strains of Trichoderma spp. Biology and Fertility of Soils. 2003;38(4):236–44. 10.1007/s00374-003-0651-0 [DOI] [Google Scholar]
- 20.Maina PK, Wachira PM, Okoth SA, Kimenju JW. Distribution and Diversity of Indigenous Trichoderma species in Machakos County, Kenya. British Microbiology Research Journal. 2015;9:1–15. [Google Scholar]
- 21.Zhang CL, Druzhinina IS, Kubicek CP, Xu T. Trichoderma biodiversity in China: evidence for a North to South distribution of species in East Asia. FEMS microbiology letters. 2005;251(2):251–7. 10.1016/j.femsle.2005.08.034 . [DOI] [PubMed] [Google Scholar]
- 22.Hoyos-Carvajal L, Orduz S, Bissett J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal genetics and biology: FG & B. 2009;46(9):615–31. 10.1016/j.fgb.2009.04.006 . [DOI] [PubMed] [Google Scholar]
- 23.Sun RY, Liu ZC, Fu K, Fan L, Chen J. Trichoderma biodiversity in China. Journal of applied genetics. 2012;53(3):343–54. 10.1007/s13353-012-0093-1 . [DOI] [PubMed] [Google Scholar]
- 24.Chi MH, Park SY, Lee YH. A Quick and Safe Method for Fungal DNA Extraction. Plant Pathology J. 2009;25(1):108–11. 10.5423/Ppj.2009.25.1.108. WOS:000263834200017. [DOI] [Google Scholar]
- 25.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–2. 10.1093/bioinformatics/btp187 . [DOI] [PubMed] [Google Scholar]
- 26.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. 10.1093/bioinformatics/btm404 . [DOI] [PubMed] [Google Scholar]
- 27.Santorum JM, Darriba D, Taboada GL, Posada D. jmodeltest.org: selection of nucleotide substitution models on the cloud. Bioinformatics. 2014;30(9):1310–1. 10.1093/bioinformatics/btu032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- 29.Swofford D. PAUP* version 4.0 b10. Sinauer, Sunderland, MA. 2002.
- 30.Druzhinina IS, Kopchinskiy AG, Komon M, Bissett J, Szakacs G, Kubicek CP. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal genetics and biology: FG & B. 2005;42(10):813–28. 10.1016/j.fgb.2005.06.007 . [DOI] [PubMed] [Google Scholar]
- 31.Druzhinina IS, Komon-Zelazowska M, Ismaiel A, Jaklitsch W, Mullaw T, Samuels GJ, et al. Molecular phylogeny and species delimitation in the section Longibrachiatum of Trichoderma. Fungal genetics and biology: FG & B. 2012;49(5):358–68. 10.1016/j.fgb.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Druzhinina I, Kubicek CP. Species concepts and biodiversity in Trichoderma and Hypocrea: from aggregate species to species clusters? Journal of Zhejiang University Science B. 2005;6(2):100–12. 10.1631/jzus.2005.B0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Quintero CA, Atanasova L, Franco-Molano AE, Gams W, Komon-Zelazowska M, Theelen B, et al. DNA barcoding survey of Trichoderma diversity in soil and litter of the Colombian lowland Amazonian rainforest reveals Trichoderma strigosellum sp. nov. and other species. Antonie van Leeuwenhoek. 2013;104(5):657–74. 10.1007/s10482-013-9975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaszczyk L, Popiel D, Chelkowski J, Koczyk G, Samuels GJ, Sobieralski K, et al. Species diversity of Trichoderma in Poland. Journal of applied genetics. 2011;52(2):233–43. 10.1007/s13353-011-0039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaklitsch WM. European species of Hypocrea Part I. The green-spored species. Studies in mycology. 2009;63:1–91. 10.3114/sim.2009.63.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migheli Q, Balmas V, Komon-Zelazowska M, Scherm B, Fiori S, Kopchinskiy AG, et al. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environmental microbiology. 2009;11(1):35–46. 10.1111/j.1462-2920.2008.01736.x . [DOI] [PubMed] [Google Scholar]
- 37.Friedl MA, Druzhinina IS. Taxon-specific metagenomics of Trichoderma reveals a narrow community of opportunistic species that regulate each other's development. Microbiology. 2012;158(Pt 1):69–83. 10.1099/mic.0.052555-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zachow C, Berg C, Muller H, Meincke R, Komon-Zelazowska M, Druzhinina IS, et al. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): relationship to vegetation zones and environmental factors. The ISME journal. 2009;3(1):79–92. 10.1038/ismej.2008.87 . [DOI] [PubMed] [Google Scholar]
- 39.Kubicek CP, Bissett J, Druzhinina I, Kullnig-Gradinger C, Szakacs G. Genetic and metabolic diversity of Trichoderma: a case study on South-East Asian isolates. Fungal Genetics and Biology. 2003;38(3):310–9. 10.1016/s1087-1845(02)00583-2 [DOI] [PubMed] [Google Scholar]
- 40.Kullnig-Gradinger CM, Szakacs G, Kubicek CP. Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycological Research. 2002;106(7):757–67. 10.1017/s0953756202006172 [DOI] [Google Scholar]
- 41.Garbeva P, van Veen JA, van Elsas JD. Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Annual review of phytopathology. 2004;42:243–70. 10.1146/annurev.phyto.42.012604.135455 . [DOI] [PubMed] [Google Scholar]
- 42.Lareen A, Burton F, Schafer P. Plant root-microbe communication in shaping root microbiomes. Plant molecular biology. 2016;90(6):575–87. 10.1007/s11103-015-0417-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson EE. Occurrence of Trichoderma in a Douglas-fir Soil. Mycologia. 1982;74(2):280–4. 10.2307/3792896 [DOI] [Google Scholar]
- 44.Rifai MA. A Revision of the Genus Trichoderma: Commonwealth Mycological Institute; 1969. [Google Scholar]
- 45.Samuels GJ, Dodd SL, Lu BS, Petrini O, Schroers HJ, Druzhinina IS. The Trichoderma koningii aggregate species. Studies in mycology. 2006;56:67–133. 10.3114/sim.2006.56.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubicek CP, Komon-Zelazowska M, Druzhinina IS. Fungal genus Hypocrea/Trichoderma: from barcodes to biodiversity. Journal of Zhejiang University Science B. 2008;9(10):753–63. 10.1631/jzus.B0860015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danielson R, Davey C. The abundance of Trichoderma propagules and the distribution of species in forest soils. Soil Biology and Biochemistry. 1973;5(5):485–94. [Google Scholar]
- 48.Harman GE, Kubicek CP. Trichoderma And Gliocladium: Basic Biology, Taxonomy and Genetics: CRC Press; 2002. [Google Scholar]
- 49.Widden P, Scattolin V. Competitive Interactions And Ecological Strategies Of Trichoderma Species Colonizing Spruce Litter. Mycologia. 1988;80(6):795–803. 10.2307/3807557. WOS:A1988R999300007. [DOI] [Google Scholar]
- 50.Gherbawy Y, Druzhinina I, Shaban GM, Wuczkowsky M, Yaser M, El-Naghy MA, et al. Trichoderma populations from alkaline agricultural soil in the Nile valley, Egypt, consist of only two species. Mycological Progress. 2004;3(3):211–8. 10.1007/s11557-006-0091-y [DOI] [Google Scholar]
- 51.Sadfi-Zouaoui N, Hannachi I, Rouaissi M, Hajlaoui MR, Rubio MB, Monte E, et al. Biodiversity of Trichoderma strains in Tunisia. Can J Microbiol. 2009;55(2):154–62. 10.1139/w08-101 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
At the position of 115 and 371, base deletion or transversion was observed in asper1, 4 and 7.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.