Abstract
Background and Objectives
Genome-wide association studies (GWAS) have identified several genetic regions involved in immune-regulatory mechanisms to be associated with celiac disease. Previous GWAS also revealed an over-representation of genes involved in type 2 diabetes and anorexia nervosa associated with celiac disease, suggesting involvement of common metabolic pathways for development of these chronic diseases. The aim of this study was to extend these previous analyses to study the gene expression in the gut from children with active celiac disease.
Material and Methods
Thirty six target genes involved in type 2 diabetes and four genes associated with anorexia nervosa were investigated for gene expression in small intestinal biopsies from 144 children with celiac disease at median (range) age of 7.4 years (1.6–17.8) and from 154 disease controls at a median (range) age 11.4.years (1.4–18.3).
Results
A total of eleven of genes were differently expressed in celiac patients compared with disease controls of which CD36, CD38, FOXP1, SELL, PPARA, PPARG, AGT previously associated with type 2 diabetes and AKAP6, NTNG1 with anorexia nervosa remained significant after correction for multiple testing.
Conclusion
Shared genetic factors involved in celiac disease, type 2 diabetes and anorexia nervosa suggest common underlying molecular pathways for these diseases.
Introduction
The prevalence of autoimmune disease including celiac disease has increased among the population in many high-income countries worldwide [1, 2]. Celiac disease is an autoimmune intestinal disorder triggered by intolerance to gluten in genetic susceptible individuals carrying the HLA-DR3-DQ2 and DR4-DQ8 risk haplotypes [3]. Another feature of celiac disease is the presence of autoantibodies directed against tissue transglutaminase (tTGA), the serological marker for the disease [4, 5] as well as to protein kinase C delta (PRKCD) [6]. It is well established that HLA-DQ heterodimers present gluten peptides to CD4+ T-cells causing an inflammation of the gut mucosa leading to villous atrophy with malabsorption of vitamins and nutrients as a consequence [5, 7].
Most chronic inflammatory disorders have a multifactorial etiology caused by a cumulative effect of environmental factors triggering the disease in genetically susceptible individuals [8]. To disentangle which of environmental and/or genetic factors that are involved in disease development and to provide a deeper understanding of the pathogenesis, linkage analysis, allele-sharing methods, genetic association studies in human populations, or genetic analysis of large crosses in model organisms have been developed [9]. Genome-wide association studies (GWAS) enable testing of the whole genome in order to identify statistical association between genetic variants and a trait of interest to compare the frequency of genetic variants (alleles) in affected and unaffected individuals [10].
To date, GWAS has identified more than forty genome-wide significant non-HLA risk loci linked to celiac disease [7, 11–15]. Many of these celiac disease associated loci exhibit an overlap with those of other immune-related diseases such as type 1 diabetes (T1D) [13]. In a previous family GWAS on celiac disease, an overlap between genes implicated in type 2 diabetes (T2D) and anorexia nervosa and gene-regions associated with celiac disease was revealed and a new model behind disease was suggested [11]. Although there is an overlap of celiac disease and other autoimmune conditions, few studies have been performed on the possible common aetiology between celiac disease and metabolic conditions such as T2D and anorexia. Due to the large number of associated polymorphisms and possible bias towards common metabolic pathways, the overlap which was identified in the previous GWAS could be just a chance finding. However, there are also data supporting a connection between these metabolic pathways and celiac disease. A population study from India recently demonstrated an increased frequency of tTGA in T2D patients [16]. Furthermore, a change in metabolism may affect the risk for disease. When a gluten-free diet was introduced in patients diagnosed with celiac disease, it reduced the risk of T2D, even when corrected for BMI [17].
Despite the fact that there can be a high variability of expression of genes in different tissues and between individuals making it a challenge to use this type of data for diagnostic purposes, targeting of genes and expression of them in the affected organ may lead to better understanding of how genes are involved in signaling pathways that eventually lead to chronic diseases such as celiac disease and T2D. The aim of this study was therefore to investigate potential common genetic factors contributing to the development of celiac disease, T2D and anorexia nervosa by analyzing gene expression in intestinal biopsies from children with active celiac disease as compared with disease controls.
Results
A total of 46 target genes involved in T2D or in anorexia nervosa were identified by pathway enrichment analysis in our previous GWAS study[11]. Six of these genes were analyzed for gene expression previously in our material [11, 18] and the remaining forty genes were selected in this study for gene expression analysis. A pilot gene expression experiment was run on all 40 target genes involved in T2D or anorexia nervosa using 51 celiac disease cases and 64 controls. Out of these 40 target genes, 16 genes showed nominally significant differences in gene expression and were picked along with reference genes for the main experiment including 144 cases and 154 controls. The list of all 46 target genes and control genes are presented in S1 Table (including the six identified T2D genes which were analyzed for gene expression previously [11].)
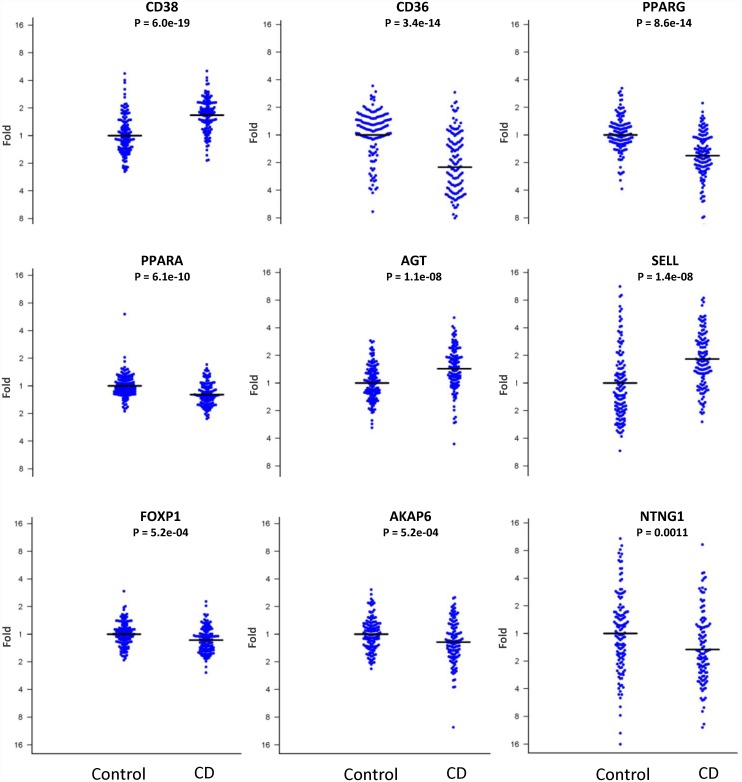
Results from the 16 target genes from the main experiment and the fold change expression difference between the target genes and the reference “housekeeping” gene IPO8 are presented in Table 1. Eleven genes reached a nominally significant p-value below 0.05, of which nine remained significant after adjusting for multiple comparisons using Bonferroni correction (Fig 1). Of those nine genes, AKAP6 and NTNG1 were genes associated with anorexia nervosa and the remaining seven (AGT, CD36, CD38, FOXP1, PPARA, PPARG and SELL) were selected for their relationship with T2D (S1 Table).
Table 1. Results from the main gene expression analysis.
The Delta-Delta CT (ΔΔCT) relative quantification method was used to estimate mRNA levels of target genes relative to a reference gene (IPO8) in the small intestinal biopsies from patients with celiac disease and compared with controls. A total of 144 cases and 154 control samples were analyzed. The p-value is calculated using the independent samples t-test for equality of means (equal variances assumed).
| Gene | P-value | Mean Ct Difference | Std. Error | Lower | Upper | Bonferroni corrected p-value | FC | Percent change | Direction in CD vs Control |
|---|---|---|---|---|---|---|---|---|---|
| CD38 | 6.00E-19 | 0.74 | 0.08 | 0.59 | 0.89 | 2.40E-17 | 1.67 | 67% | UP |
| CD36 | 3.40E-14 | 1.19 | 0.15 | -1.48 | -0.9 | 1.36E-12 | 2.28 | 128% | DOWN |
| PPARG | 8.60E-14 | 0.83 | 0.09 | -1 | -0.65 | 3.44E-12 | 1.77 | 77% | DOWN |
| PPARA | 6.10E-10 | 0.33 | 0.05 | -0.42 | -0.23 | 2.44E-08 | 1.25 | 25% | DOWN |
| AGT | 1.10E-08 | 0.59 | 0.09 | 0.42 | 0.76 | 4.40E-07 | 1.5 | 50% | UP |
| SELL | 1.40E-08 | 0.88 | 0.15 | 0.59 | 1.17 | 5.60E-07 | 1.84 | 84% | UP |
| FOXP1 | 5.70E-05 | 0.22 | 0.05 | -0.32 | -0.12 | 2.28E-03 | 1.17 | 17% | DOWN |
| AKAP6 | 5.20E-04 | 0.29 | 0.08 | -0.45 | -0.12 | 0.02 | 1.22 | 22% | DOWN |
| NTNG1 | 1.10E-03 | 0.57 | 0.18 | -0.92 | -0.22 | 0.04 | 1.48 | 48% | DOWN |
| BCL2L11 | 1.70E-03 | 0.23 | 0.07 | -0.38 | -0.09 | 0.07 | 1.18 | 18% | DOWN |
| PIEZO2 | 6.70E-03 | 0.29 | 0.1 | -0.49 | -0.09 | 0.27 | 1.22 | 22% | DOWN |
| FTO | 0.08 | 0.09 | 0.05 | -0.01 | 0.19 | 1 | 1.07 | 7% | UP |
| HFE | 0.09 | 0.19 | 0.11 | -0.02 | 0.41 | 1 | 1.14 | 14% | UP |
| ZNF804B | 0.11 | 0.43 | 0.28 | -0.99 | 0.13 | 1 | 1.35 | 35% | DOWN |
| ADRA1D | 0.18 | 0.36 | 0.22 | -0.8 | 0.08 | 1 | 1.29 | 29% | DOWN |
| KCNJ11 | 0.53 | 0.1 | 0.12 | -0.33 | 0.14 | 1 | 1.07 | 7% | DOWN |
FC = Fold Change, CD = Celiac Disease
Fig 1. Nine target genes showing significant difference in gene expression between celiac disease (CD) patients and control patients.
The y-axis shows the fold change gene expression and the mean expression in control patients is set to one.
Discussion
In this study we found that seven genes involved in T2D (CD36, CD38, FOXP1, SELL, PPARA, PPARG, AGT) and two genes involved in anorexia (AKAP6, NTNG1) were differently expressed in the small intestinal tissue of celiac patients compared with control patients, suggesting common genetic pathways leading to the disease among these phenotypically different chronic disorders. A plausible explanation could be that the genes discovered in this study are just an artifact of an inflamed tissue. However, except for CD38, AGT and SELL, the remaining genes are lower expressed in patients and therefore at least unlikely to be the result of an increased representation of immune cells in the intestinal tissue of celiac patients compared with controls.
The T2D related gene, CD36, is down regulated in celiac patients in this study. Studies on this gene showed its various functions in many processes including angiogenesis, inflammation, lipid metabolism, atherosclerosis, and platelet activation [19]. It has been shown that the CD36 gene is linked with increased risk of T2D [20] and the CD36 protein level may serve as a biological marker of T2D [21]. Transgenic mice overexpressing CD36 have reduced blood lipids and deficiency of CD36 could lead to insulin resistance [22]. Our data show down regulation of CD36 in celiac patients and the CD36 protein has also been previously shown to be significantly reduced in active as compared to inactive celiac disease and normal mucosal samples [23]. If this is a consequence of inflammation or a risk for developing both diseases needs to be addressed.
This study also showed that CD38 was up-regulated in children with celiac disease. However, when the expression of this gene was normalized to CD3D, which is a part of the T-cell receptor, the difference was only nominally significant. The CD38 protein is like CD36, a surface molecule and CD38 is expressed in human T and B cells during different stages of their development. A function of CD38 is mediating insulin secretion [24] and an immune response with auto-antibodies to CD38 protein is present in T2D patients [24, 25].
Another finding from this study was the down-regulation of mRNA levels of both PPARA and PPARG in celiac disease children as compared with controls. Peroxisome Proliferator-Activated Receptors (PPAR)-alpha and PPAR-gamma are two of three known subtypes of PPARs acting as transcription factors activated by ligands. Peroxisomes contain enzymes necessary for the oxidation process [26]. PPARs are known to regulate target genes of inflammatory responses as well as energy balance [27]. Importantly, PPARs have been found to act as anti-inflammatory and for these reasons PPARs have been considered for the development of new therapies for chronic inflammatory diseases [28]. Previous studies on PPARG revealed its contribution in numerous diseases, such as: obesity [29], T2D [30], and atherosclerosis [27] as well as in celiac disease [31]. It has also been shown that tTGA drives inflammation via PPARG down-regulation in celiac patients [31] and that down-regulation of proteins involved in PPAR signaling are associated with the highest celiac disease histological score [32].
Angiotensin-2, or the AGT gene was more expressed in the small intestinal mucosa of celiac disease children as compared with controls. AGT encodes a potent vasoconstrictor and acts directly on vascular smooth muscle and it is associated with T1D and T2D as well as with cardiovascular disease [33–35]. Interestingly, olmesartan, an angiotensin II receptor antagonist, has been shown to cause a severe form of sprue-like enteropathy [36]. SELL or L-selectin, encodes a cell surface adhesion protein which mediates the adherence of lymphocytes to endothelial cells. SELL is associated to both T2D [37] and amyotrophic lateral sclerosis (ALS) [38]. Both AGT and SELL are expressed at higher levels in celiac cases compared with controls indicating a high activity of adherence and interaction between leucocytes and endothelial cells.
The forkhead box P1 (FOXP1) is an essential transcriptional regulator for thymocyte development and the generation of quiescent naive T cells [39]. BCL2L11 (which was slightly above the corrected p-value of 0.05) is important for apoptosis (cell death) and thymocytes lacking this pro-apoptotic Bcl-2 family member (also known as Bim) are refractory to apoptosis induced by TCR-CD3 stimulation [40]. BCL2L11 has been identified as an essential initiator of apoptosis in thymocyte-negative selection of autoreactive T-cells [40]. BCL2L11 and FOXP1 seems to have an important role in normal thymocyte apoptosis and are both significantly down regulated in celiac cases compared with controls in this study.
AKAP6 and NTNG1 were previously reported in a GWAS of anorexia nervosa [41]. AKAP6 is a member of A-kinase anchoring protein. Following the name, its main role is to attach enzymes and transport it near the target substrate. It is mostly expressed in brain and cardiac region as well as in skeletal muscles [42]. NTNG1 (Netrin-G1) is a member of protein family playing an important role in the development of the human nervous system [43]. Previous studies revealed that mutations in NTNG1 are associated with schizophrenia [44] and Parkinson’s disease [45].
In conclusion, even though the common mechanisms for the development of celiac disease, T2D or anorexia nervosa remains unresolved, the present study shows that several genes associated with T2D and anorexia nervosa are differentially expressed in children with active celiac disease as compared with controls, indicating a connection between these diseases.
Materials and Methods
Ethics Statement
This study has been conducted according to the principles expressed in the Declaration of Helsinki and approved by the Regional ethics board in Gothenburg. All guardians and study participants (when appropriate) gave their written informed consent. All personal data as well as results obtained from the research are coded and will remain confidential to all except for the treating physician at each hospital.
Biological material
All children who were investigated at the Departments of Pediatrics with an upper endoscopy were consecutively asked to participate in the study as previously described [18, 46].
There were no exclusion criteria for participation. A child with a Marsh score >1 was considered to have CD and included as a case. A child with a Marsh score ≤1 was considered not to have CD and included as a disease control. In all, small intestinal biopsies and blood samples were collected from 144 cases with CD at a median age of 7.4 years (range 1.6–17.8) and from 154 disease controls at median 11.4.years (range 1.4–18.3). Among the disease controls, the most common diagnoses were gastroesophageal reflux disease and helicobacter pylori gastritis, whereas only eleven children had Crohn´s disease and four children ulcerative colitis, respectively. Another eight disease controls were detected with moderately elevated tTG antibody levels in a screening study and were considered to have potential CD.
RNA extraction
Small intestinal biopsies were immediately put in a RNA stabilizing reagent, RNAlater solution (Life Technologies, CA, USA) and put in -4°C overnight in order to allow the reagent to penetrate the tissue. The biopsy was further frozen in -80°C until RNA extraction was carried out. Total RNA was extracted using the miRNeasy Mini Kit (QIAGEN, Germany) or the Maxwell® 16 Total RNA Purification Kit (Promega) together with the Maxwell®16 instrument. The RNA quality and quantity was checked with a NanoDrop 2000 spectrophotometer and a 2100 Bioanalyzer (Agilent Technologies). RNA was converted to cDNA using the Vilo kit (Life Technologies, CA, USA).
Reference gene validation
A total of 23 reference (housekeeping) genes were tested with the GeNorm algorithm [47]. The IPO8 gene had the highest stability value (m<0.5) and was chosen as reference gene for normalization.
Gene Expression Analysis
Quantitive gene expression analysis was performed by means of quantitative Polymerase Chain Reaction (qPCR) with TaqMan chemistry (Life Technologies, CA, USA). A total of 1 ng/reaction cDNA together with Master Mix was added to all genes simultaneously using a Nanodrop II dispenser (GC biotech, Netherlands), for a final reaction volume of 2 μl per gene and sample. QPCR reaction was run on the real-time PCR, ABI PRISM 7900HT Sequence Detection System (Life Technologies, CA, USA). Raw data was analyzed with the SDS 2.4 and RQ manager 1.2.1 software provided by the instrument.
Gene selection criteria
The T2D risk genes were defined by the IPA analysis (Ingenuity Inc., CA, USA) in our previous GWAS [11]. All of these genes, except PPARG, were located in potential risk regions of celiac disease, with one or more nominally associated SNPs [11]. PPARG was chosen due to its relation to PPARA. We also included the nearest gene from four regions associated with anorexia nervosa [41] and overlapping with the results from our GWAS in celiac disease [11].
Statistical analysis
Gene expression data was analyzed using the Delta-Delta CT (ΔΔCT) relative quantification method[48]. This approach enables the expression of target genes to be normalized to reference genes, and then compared between cases and control samples (the ΔΔCT value). To normalize the qPCR reaction for the amount of RNA added, the use of an internal reference gene (housekeeping gene) is used [48]. The threshold cycle (CT) or the cycle of quantification (Cq), is the PCR cycle when the amplification reaches a set threshold. Delta Ct (ΔCT) is calculated by the difference between the cycle of quantification for the target gene compared with the reference gene(-s). The t-test was used to calculate if there was a significant difference between the mean delta CT value of the controls compared to the mean delta CT of cases.
URLs
Protein Database—UniProtKb/SwissProt: http://www.uniprot.org/
Entrez Cross Database: http://www.ncbi.nlm.nih.gov/sites/gquery
Gene Cards: http://www.genecards.org/
A Catalog of Published Genome-Wide Association Studies: www.genome.gov/gwastudies.
Supporting Information
This file gives information regarding CD diagnosis.
(TXT)
This file gives the Ct value for each gene and patient.
(TXT)
A list of all type 2 Diabetes and anorexia genes (including six genes were run as previously described. Forty-two genes were involved in type 2 diabetes and four were associated with anorexia. A total of forty target genes were analyzed in this study.
(DOCX)
Acknowledgments
We thank the Gothenburg Genomic Core Facility and the Biobanking and Molecular Resource Infrastructure (BBMRI.se) for support. This study would not be possible without the generous participation of all the families and patients who contributed to the study.
Abbreviations
- GWAS
Genome-wide association studies
- HLA
Human leukocyte antigen
- PCR
Polymarease chain reaction
- qPCR
Quantitative polymerase chain reaction
- SNP
Single nucleotide polymorphism
- T2D
Type 2 diabetes
- T1D
Type 1 diabetes
- tTGA
Tissue transglutaminase antibodies
- TG2
Tissue transglutaminase
Data Availability
Original expression data and phenotype information for samples is freely available and added as supplementary information.
Funding Statement
Bengt Ihre foundation ÅTN http://www.sls.se/Forskning--utbildning/Forskningsdelegationen--Prioriteringskommitten-/Bengt-Ihres-fond-/, Swedish Society of Medicine ÅTN http://www.sls.se/, Professor Nanna Svartz foundation ÅTN http://www.stiftelseansokan.se/Pages/Svartz.aspx, Ruth and Richard Julin foundation ÅTN https://internwebben.ki.se/en/ruth-and-richard-julin-foundation, Clas Groschinskys foundation ÅTN http://www.groschinsky.org/, Tore Nilson's foundation ÅTN http://www.torenilsonsstiftelse.nu/, Nilsson-Ehle's foundation ÅTN http://www.fysiografen.se/sv/stipendier/, and Åke Wiberg's foundation ÅTN http://ake-wiberg.se/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D'Amato M, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139(1):112–9. 10.1053/j.gastro.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88–93. 10.1053/j.gastro.2009.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55(7):1037–46. 10.1136/gut.2005.075119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hrdlickova B, Westra HJ, Franke L, Wijmenga C. Celiac disease: moving from genetic associations to causal variants. Clin Genet. 2011;80(3):203–313. 10.1111/j.1399-0004.2011.01707.x . [DOI] [PubMed] [Google Scholar]
- 5.Hodgson HJF, Boulton R, Cousins C, Gupta S. A Colour Handbook of Gastroenterology: Manson Publishing, Limited; 2000. [Google Scholar]
- 6.Byrne G, Freeley M, Feighery C, Whelan A, Long A. Protein kinase C delta is a substrate of tissue transglutaminase and a novel autoantigen in coeliac disease. Clin Immunol. 2013;147(1):1–8. 10.1016/j.clim.2013.01.007 . [DOI] [PubMed] [Google Scholar]
- 7.Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nature genetics. 2010;42(4):295–302. Epub 2010/03/02. 10.1038/ng.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strachan T, Read AP. Human molecular genetics. New York: Wiley-Liss Bios Scientific Publishers, an imprint of Taylor & Francis Group; 1999. [Google Scholar]
- 9.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265(5181):2037–48. . [DOI] [PubMed] [Google Scholar]
- 10.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–7. Epub 1996/09/13. . [DOI] [PubMed] [Google Scholar]
- 11.Östensson M, Montén C, Bacelis J, Gudjonsdottir AH, Adamovic S, Ek J, et al. A Possible Mechanism behind Autoimmune Disorders Discovered By Genome-Wide Linkage and Association Analysis in Celiac Disease. PLoS ONE. 2013;8(8):e70174 10.1371/journal.pone.0070174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, Inouye M, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nature genetics. 2007;39(7):827–9. 10.1038/ng2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trynka G, Hunt KA, Bockett NA, Romanos J, Mistry V, Szperl A, et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nature genetics. 2011;43(12):1193–201. 10.1038/ng.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garner C, Ahn R, Ding YC, Steele L, Stoven S, Green PH, et al. Genome-wide association study of celiac disease in North America confirms FRMD4B as new celiac locus. PLoS One. 2014;9(7):e101428 10.1371/journal.pone.0101428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Liu X, Hadley D, Hagopian W, Liu E, Chen WM, et al. Identification of Non-HLA Genes Associated with Celiac Disease and Country-Specific Differences in a Large, International Pediatric Cohort. PLoS One. 2016;11(3):e0152476 10.1371/journal.pone.0152476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanungo A, Shtauvere-Brameus A, Samal KC, Sanjeevi CB. Autoantibodies to tissue transglutaminase in patients from eastern India with malnutrition-modulated diabetes mellitus, insulin-dependent diabetes mellitus, and non-insulin-dependent diabetes mellitus. Ann N Y Acad Sci. 2002;958:232–4. . [DOI] [PubMed] [Google Scholar]
- 17.Kabbani TA, Kelly CP, Betensky RA, Hansen J, Pallav K, Villafuerte-Galvez JA, et al. Patients with celiac disease have a lower prevalence of non-insulin-dependent diabetes mellitus and metabolic syndrome. Gastroenterology. 2013;144(5):912–7.e1. 10.1053/j.gastro.2013.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monten C, Gudjonsdottir AH, Browaldh L, Arnell H, Nilsson S, Agardh D, et al. Genes involved in muscle contractility and nutrient signaling pathways within celiac disease risk loci show differential mRNA expression. BMC medical genetics. 2015;16:44 10.1186/s12881-015-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. The Journal of clinical investigation. 2001;108(6):785–91. Epub 2001/09/19. 10.1172/jci14006 ; PubMed Central PMCID: PMCPmc200943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW. Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. The Journal of clinical endocrinology and metabolism. 2010;95(4):1939–46. Epub 2010/02/09. 10.1210/jc.2009-2002 . [DOI] [PubMed] [Google Scholar]
- 21.Alkhatatbeh MJ, Enjeti AK, Acharya S, Thorne RF, Lincz LF. The origin of circulating CD36 in type 2 diabetes. Nutrition & diabetes. 2013;3:e59 Epub 2013/02/06. 10.1038/nutd.2013.1 ; PubMed Central PMCID: PMCPmc3584987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nature genetics. 1999;21(1):76–83. 10.1038/5013 . [DOI] [PubMed] [Google Scholar]
- 23.Cupi ML, Sarra M, De Nitto D, Franze E, Marafini I, Monteleone I, et al. Defective expression of scavenger receptors in celiac disease mucosa. PLoS One. 2014;9(6):e100980 10.1371/journal.pone.0100980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallone R, Ortolan E, Baj G, Funaro A, Giunti S, Lillaz E, et al. Autoantibody response to CD38 in Caucasian patients with type 1 and type 2 diabetes: immunological and genetic characterization. Diabetes. 2001;50(4):752–62. . [DOI] [PubMed] [Google Scholar]
- 25.Ikehata F, Satoh J, Nata K, Tohgo A, Nakazawa T, Kato I, et al. Autoantibodies against CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) that impair glucose-induced insulin secretion in noninsulin- dependent diabetes patients. The Journal of clinical investigation. 1998;102(2):395–401. 10.1172/JCI1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Chen L, Zhang X, Zhou Y, Zhang D, Huo M, et al. PPARs and Female Reproduction: Evidence from Genetically Manipulated Mice. PPAR research. 2008;2008:723243 Epub 2008/04/11. 10.1155/2008/723243 ; PubMed Central PMCID: PMCPmc2288756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Yang G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR research. 2014;2014:653017 Epub 2014/04/03. 10.1155/2014/653017 ; PubMed Central PMCID: PMCPmc3945976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. The Journal of endocrinology. 2001;169(3):453–9. Epub 2001/05/26. . [DOI] [PubMed] [Google Scholar]
- 29.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutrition journal. 2014;13(1):17 Epub 2014/02/15. 10.1186/1475-2891-13-17 ; PubMed Central PMCID: PMCPmc3943808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Z, Sun Y, Yang G, Zhang A, Huang S, Heiney KM, et al. New Insights into the PPAR Agonists for the Treatment of Diabetic Nephropathy. PPAR research. 2014;2014:818530 Epub 2014/03/14. 10.1155/2014/818530 ; PubMed Central PMCID: PMCPmc3927865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luciani A, Villella VR, Vasaturo A, Giardino I, Pettoello-Mantovani M, Guido S, et al. Lysosomal accumulation of gliadin p31-43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut. 2010;59(3):311–9. 10.1136/gut.2009.183608 . [DOI] [PubMed] [Google Scholar]
- 32.Simula MP, Cannizzaro R, Canzonieri V, Pavan A, Maiero S, Toffoli G, et al. PPAR signaling pathway and cancer-related proteins are involved in celiac disease-associated tissue damage. Molecular medicine. 2010;16(5–6):199–209. 10.2119/molmed.2009.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi F, Yamamoto K, Katsuya T, Sugiyama T, Nabika T, Ohnaka K, et al. Reevaluation of the association of seven candidate genes with blood pressure and hypertension: a replication study and meta-analysis with a larger sample size. Hypertension research: official journal of the Japanese Society of Hypertension. 2012;35(8):825–31. 10.1038/hr.2012.43 . [DOI] [PubMed] [Google Scholar]
- 34.Ilic V, Ilic M, Soldatovic I, Popovic S, Magic Z. Association of renin-angiotensin system genes polymorphism with progression of diabetic nephropathy in patients with type 1 diabetes mellitus. Vojnosanitetski pregled Military-medical and pharmaceutical review. 2014;71(7):627–33. . [DOI] [PubMed] [Google Scholar]
- 35.Konopka A, Szperl M, Piotrowski W, Roszczynko M, Stepinska J. Influence of renin-angiotensin system gene polymorphisms on the risk of ST-segment-elevation myocardial infarction and association with coronary artery disease risk factors. Molecular diagnosis & therapy. 2011;15(3):167–76. 10.2165/11590650-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 36.Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wu TT, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clinic proceedings. 2012;87(8):732–8. 10.1016/j.mayocp.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamiuchi K, Hasegawa G, Obayashi H, Kitamura A, Ishii M, Yano M, et al. Leukocyte-endothelial cell adhesion molecule 1 (LECAM-1) polymorphism is associated with diabetic nephropathy in type 2 diabetes mellitus. Journal of diabetes and its complications. 2002;16(5):333–7. . [DOI] [PubMed] [Google Scholar]
- 38.Landers JE, Melki J, Meininger V, Glass JD, van den Berg LH, van Es MA, et al. Reduced expression of the Kinesin-Associated Protein 3 (KIFAP3) gene increases survival in sporadic amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):9004–9. 10.1073/pnas.0812937106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng X, Ippolito GC, Tian L, Wiehagen K, Oh S, Sambandam A, et al. Foxp1 is an essential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood. 2010;115(3):510–8. 10.1182/blood-2009-07-232694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415(6874):922–6. 10.1038/415922a . [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Zhang H, Bloss CS, Duvvuri V, Kaye W, Schork NJ, et al. A genome-wide association study on common SNPs and rare CNVs in anorexia nervosa. Molecular psychiatry. 2011;16(9):949–59. 10.1038/mp.2010.107 [DOI] [PubMed] [Google Scholar]
- 42.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. Journal of cell science. 1999;112 (Pt 16):2725–36. Epub 1999/07/22. . [DOI] [PubMed] [Google Scholar]
- 43.Nakashiba T, Ikeda T, Nishimura S, Tashiro K, Honjo T, Culotti JG, et al. Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(17):6540–50. Epub 2000/08/31. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohtsuki T, Horiuchi Y, Koga M, Ishiguro H, Inada T, Iwata N, et al. Association of polymorphisms in the haplotype block spanning the alternatively spliced exons of the NTNG1 gene at 1p13.3 with schizophrenia in Japanese populations. Neuroscience letters. 2008;435(3):194–7. Epub 2008/04/04. 10.1016/j.neulet.2008.02.053 . [DOI] [PubMed] [Google Scholar]
- 45.Lin L, Lesnick TG, Maraganore DM, Isacson O. Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends in neurosciences. 2009;32(3):142–9. Epub 2009/01/24. 10.1016/j.tins.2008.11.006 ; PubMed Central PMCID: PMCPmc2954610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monten C, Bjelkenkrantz K, Gudjonsdottir AH, Browaldh L, Arnell H, Naluai AT, et al. Validity of histology for the diagnosis of paediatric coeliac disease: a Swedish multicentre study. Scand J Gastroenterol. 2015:1–7. 10.3109/00365521.2015.1101486 . [DOI] [PubMed] [Google Scholar]
- 47.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002;3(7):RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file gives information regarding CD diagnosis.
(TXT)
This file gives the Ct value for each gene and patient.
(TXT)
A list of all type 2 Diabetes and anorexia genes (including six genes were run as previously described. Forty-two genes were involved in type 2 diabetes and four were associated with anorexia. A total of forty target genes were analyzed in this study.
(DOCX)
Data Availability Statement
Original expression data and phenotype information for samples is freely available and added as supplementary information.