Abstract
Pancreatic ductal adenocarcinoma (PDAC) remains the most deadly disease worldwide, with the lowest survival rate among all cancer types. Recent evidence suggests that hyaluronan (HA), a major component of ECM, provides a favorable microenvironment for cancer progression. Pancreatic ductal adenocarcinoma is typically characterized by a dense desmoplastic stroma containing a large amount of HA. Accumulation of HA promotes tumor growth in mice and correlates with poor prognosis in patients with PDAC. Because HA is involved in various malignant behaviors of cancer (such as increased cell proliferation, migration, invasion, angiogenesis, and chemoresistance), inhibiting HA synthesis/signaling or depleting HA in tumor stroma could represent a promising therapeutic strategy against PDAC. In this review article, we summarize our current understanding of the role of HA in the progression of PDAC and discuss possible therapeutic approaches targeting HA.
Keywords: Desmoplasia, hyaluronan, pancreatic ductal adenocarcinoma, tumor stroma, tumor–stroma interaction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and intractable solid tumors, which often invades surrounding stromal components, including lymphatic, vascular, and perineural systems, ultimately metastasizing to distant organs. Despite recent advances in the clinical management, the survival rate in patients with PDAC remains the lowest among all cancer types, emphasizing the need for a better understanding of its biology. In particular, identification of molecular mechanisms underlying the aggressive behaviors of PDAC can provide the basis for the development of novel therapeutic intervention.1 Although substantial progress has been made in our understanding of the genetic and epigenetic alterations in PDAC, the identification of these molecular defects predominantly in cancer cells has led to little progress in developing new treatment strategies.2, 3, 4
The progression of cancer is governed by complex mechanisms and is significantly accelerated by the tumor microenvironment, composed of a variety of stromal cells and ECM.5 The tumor–stroma interactions play a critical role in the progression of PDAC, which is typically characterized by a dense desmoplastic stroma.6, 7 Of the major ECM components detected in tumor stroma, hyaluronan (HA) has been extensively studied in its relation to cancer progression. In normal physiological conditions, the amount of HA is controlled by a balance between synthesis and degradation; however, HA has been shown to be abundantly accumulated in the surrounding stroma of malignant tumor.8, 9 The HA‐rich microenvironment may promote tumor progression by enhancing cell proliferation, migration, invasion, metastasis, angiogenesis, and resistance to chemotherapeutic agents.8, 9
Several studies have shown increased expression of HA and its receptors in PDAC.10, 11, 12, 13, 14, 15, 16, 17 Importantly, the abnormal accumulation of HA correlates with worsened prognosis in patients with PDAC.14, 15 In an experimental model of PDAC, accumulation of extracellular HA by forced expression of synthesizing genes accelerated tumor growth.18 Taken together, these findings strongly suggest that HA may play a critical role in the progression of PDAC and could be a therapeutic target. In this review article, we summarize the current understanding of the role of HA in PDAC and discuss its potential therapeutic applications.
Role of HA in Initiation and Progression of Human Cancers
Regulatory mechanisms of HA
Hyaluronan is a linear glycosaminoglycan that consists of repeating disaccharide subunits of glucuronic acid and N‐acetylglucosamine, with molecular weights usually ranging from 105 to 107 Da.19 Hyaluronan is synthesized by HA synthases (HAS1, HAS2, and HAS3). Newly synthesized HA molecules are extruded directly onto the cell surface for assembly into pericellular or extracellular matrices. Hyaluronan is degraded by hyaluronidases (HYAL1, HYAL2, HYAL3, HYAL4, HYALP1, and PH20).19 Among the known HYALs, HYAL1 and HYAL2 are widely distributed throughout tissues and are most likely to play key roles in degrading HA.20, 21 HYAL2 initially cleaves high‐molecular‐weight (HMW)‐HA into ~20‐kDa fragments, which are further digested into small fragments of ~0.8 kDa by HYAL1.22, 23 Extracellular HA binds to and interacts with specific cell surface receptors including CD44 and receptor for HA‐mediated motility (RHAMM).24 CD44 is a major receptor for HA and is also a multifunctional receptor, having diverse roles in cell–cell and cell–matrix interactions.25 Due to alternative splicing, multiple forms of CD44 variants (CD44v) are generated that are further modified by N‐ and O‐linked glycosylation. Importantly, variants of CD44, specifically CD44v6, have been shown to promote tumor progression and metastatic spread in lung, breast, and colon cancer.26 RHAMM, another major receptor for HA, is located intracellularly in the cytoplasm, in the nucleus, and on the cell surface.27 The interactions between HA and CD44 and/or RHAMM induce a wide range of signals that are required to modulate a variety of cellular processes, including cell adhesion, migration, invasion, survival, and proliferation. In addition, both CD44 and cell‐surface RHAMM function as a co‐receptors for activating transmembrane tyrosine kinases (including epidermal growth factor receptor, c‐MET, and platelet‐derived growth factor receptor) and ERK1,2.24
Hyaluronan in cancer: clinical findings
Hyaluronin is distributed ubiquitously throughout human tissue and plays an important role in structuring tissue architecture based on its characteristic hydrodynamic properties. However, HA is also involved in various inflammatory and pathological conditions. In many types of malignant tumors, HA is often overexpressed or highly concentrated in tumor cells and, particularly, in their surrounding ECM.19, 28 Furthermore, tumoral and/or stromal HA accumulation has been shown to correlate with poor prognosis in patients with various cancer types. For example, HA expression in tumor cells is associated with poor survival in patients with gastric and colorectal cancers.29, 30 Furthermore, high levels of HA in the stroma are associated with decreased survival rates in patients with prostate, breast, and ovarian cancers.31, 32 These findings suggest that, at least in certain cancer types, tumors containing high HA are more aggressive than those containing low HA.
Role of HA in cancer: experimental findings
In addition to these clinical findings, there is considerable experimental evidence supporting the role of HA in cancer initiation and progression. For example, overexpression of Has2 in mammary glands resulted in the development of mammary tumors in a transgenic mouse model.33 Likewise, other investigators showed that forced expression of HAS2 and HAS3 results in overproduction of HA and enhances the tumorigenic ability of fibrosarcoma34 and prostate cancer.35 Together, these studies provided evidence for an involvement of HA in cancer initiation. In terms of cancer progression, suppression of HA production by antisense inhibition of HAS blocked growth of prostate cancer36 and invasion of colon cancer cells.37 Furthermore, abnormal HA production in non‐malignant cells diminishes contact inhibition and enhances cell migration,38 suggesting that HA is required for the aggressive behaviors of cancer cells. A close link between HA and epithelial–mesenchymal transition also supports the role of HA in cancer initiation and progression.33, 39, 40
Interestingly, the size of HA is important in terms of its effects on cancer initiation, growth, and progression, although the actions of different sizes of HA are diverse and complex. High‐molecular‐weight HA may play, at least in a certain species, an inhibitory role in cancer initiation.41 However, other studies have shown cancer‐promoting effects of HMW‐HA. For example, HMW‐HA enhanced cell migration and angiogenesis of hepatocellular carcinoma through accelerating CXCL12‐induced CXCR4 activation.42 Binding of HMW‐HA to CD44 downregulated tumor suppressor protein PDCD4, leading to anti‐apoptosis and chemotherapy resistance43 and enhanced invasion44 in breast cancer cells.
However, several studies have shown that low‐molecular‐weight HA (LMW‐HA) or HA oligosaccharides promote angiogenesis,45 suggesting that LMW‐HA accelerates tumor progression through the stimulation of angiogenesis. In addition, LMW‐HA has been shown to promote cancer growth and metastasis.46, 47 We also showed that direct addition of LMW‐HA (25–75 kDa) to cultured PDAC cells increased their migration more robustly than HMW‐HA (400–600 kDa) (Fig. 1).48 Thus, the effects of HA on cell behavior may depend on its size and cell type; however, the mechanism underlying these different effects of HA is not clearly understood.
Figure 1.
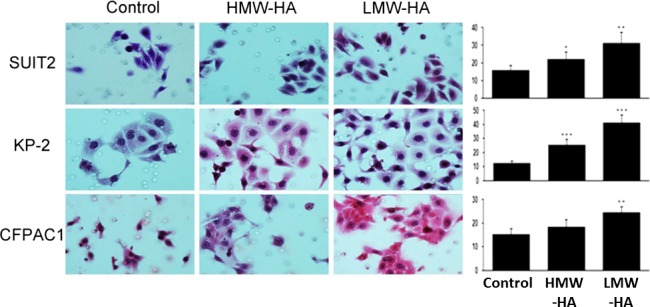
Effects of high‐molecular‐weight hyaluronan (HMW‐HA) and low‐molecular‐weight (LMW)‐HA on the migration of pancreatic ductal adenocarcinoma cells assessed by Transwell assay. Left, photograph of migrated cells on the lower surface of the membrane under microscope. Right, average number of migrated cells counted in six randomly selected fields. Addition of exogenous HMW‐HA and, more robustly, LMW‐HA increases the migration of pancreatic ductal adenocarcinoma cells. *P < 0.05, **P < 0.01, ***P < 0.001, paired t‐test.
Expression and distribution of HA and its receptors in PDAC
There have been several studies investigating the degree of HA concentration and/or pattern of HA expression in PDAC. For example, a previous study showed that HA is secreted from cultured human pancreatic cancer cell lines.10 In addition, the amount of HA is increased in human PDAC tissues (12‐fold increase) compared to the normal pancreas.13 Using a biotinylated HA‐binding protein isolated from bovine cartilage, Fries et al.11 showed that, in primary PDAC tissues, HA was found predominantly in the connective tissue immediately around tumor cells or at the border between the tumor and normal pancreatic tissue. A comprehensive analysis of the HA content in a variety of human malignant tumors revealed that PDAC had the highest incidence of detectable HA content, which was predominantly associated with the desmoplastic stroma rather than with tumor cells.49 We also used immunohistochemistry to analyze the expression of HA and its regulators (including HAS2 and HYAL1) in primary PDAC.15 We found that HA is strongly expressed in 80% of primary PDAC tissues, with positive staining being detected both in tumor and stromal components. Furthermore, strong expression of HAS2 and weak expression of HYAL1 were significantly associated with shorter survival time after surgery.15 Whatcott et al.14 reported that ECM components, such as collagen and HA, are found in high levels in both primary tumors and metastatic lesions in patients with PDAC. Importantly, we showed that strong HA expression was an independent prognostic factor in patients with PDAC undergoing resection, suggesting a prognostic significance of HA in PDAC.15 Similarly, Whatcott et al.14 reported that patients having primary PDAC tumors with low‐level HA showed median survival times of 24.3 months compared with 9.3 months in those patients having tumors with high‐level HA (P < 0.05).
It has also been shown that HA receptors are overexpressed in PDAC. Previous studies using immunohistochemistry have shown that CD44 is highly expressed on the membrane (mainly on the basolateral membrane) of PDAC cells.50, 51 Interestingly, while the expression of standard CD44 (CD44s) was frequently observed in both PDAC and intraductal papillary neoplasms of the pancreas, the expression of CD44v5 and CD44v6 was exclusively seen in PDAC, suggesting a role of these splice variants in the invasive phenotype.52 Similarly, CD44v6 was not detected in any of the normal tissue or chronic pancreatitis specimens, whereas 54% of primary PDAC and 55% of metastases expressed this variant.53 Furthermore, the expression of CD44v6 and CD44v2 was correlated with decreased overall survival in patients with PDAC (P = 0.0160 and P = 0.0125, respectively).54 These results suggest that CD44, especially CD44v6, plays a role in the aggressive phenotype in PDAC. Similarly, RHAMM mRNA is overexpressed in PDAC cell lines showing a poorly differentiated phenotype and a high metastatic potential.12 Recently, we also showed that RHAMM is overexpressed in primary PDAC tissues with both membranous and cytoplasmic staining.17 Furthermore, high RHAMM expression correlated with poor survival in PDAC patients who underwent surgical resection.17
Functional significance of HA in PDAC progression
There is only little experimental evidence supporting the role of HA in the progression of PDAC. Teranishi et al.55 reported that addition of exogenous HA increases the migratory ability of PDAC cells. Kultti et al.18 showed that forced production and accumulation of HA by HAS3 overexpression promoted the growth of PDAC tumor in mice through initiation of epithelial–mesenchymal transition, as evidenced by loss of plasma membrane E‐cadherin and accumulation of cytoplasmic β‐catenin. We used different models to investigate the effect of HA on PDAC cell motility by wound healing and Transwell migration assays.48 Inhibition of HA by 4‐methylumbelliferone (4‐MU) significantly decreased migration, whereas promotion of HA by 12‐O‐tetradecanoylphorbol‐13‐acetate or co‐culture with tumor‐derived fibroblasts significantly increased the migration of PDAC cells. Furthermore, addition of exogenous HA significantly increased the migration of PDAC cells. These studies may provide the first experimental evidence for a relationship between increased HA and progression of PDAC. Further studies are required to determine the exact mechanism by which HA promotes the progression of PDAC.
Regulation of HA in PDAC and therapeutic strategies targeting HA
Hyaluronan synthesis
The exact mechanism underlying the accumulation of HA in cancer is unclear. Because HA is synthesized by HAS and is degraded by HYALs, one possible mechanism may be overproduction of HA.19, 21, 56 In fact, many previous studies have shown overexpression of HAS in various cancers.57, 58, 59, 60, 61, 62 Our previous study showed overexpression of one of the HA synthases, HAS2, in PDAC tissues.15 We recently investigated a possible association between DNA methylation and HA synthesis in PDAC cells using two different models, including treatment with a DNA methylation inhibitor (5‐aza‐2′‐deoxycytidine) and knockdown of the DNMT1 gene by siRNA.63 In both models, decreased DNA methylation resulted in enhanced HA production in PDAC cells and was associated with increased expression of HAS2 and HAS3. These findings suggest, for the first time, that an epigenetic mechanism (namely DNA methylation) is involved in the regulation of HA synthesis in PDAC. Previous studies have reported increased expression of DNA methyltransferase in PDAC,64, 65 raising concerns about a discrepancy between increased DNA methyltransferase activity and overexpression of HAS in PDAC. This could be explained partly by the fact that DNA methylation affects not only tumor suppressor genes but also cancer‐promoting genes. It could be possible that HAS expression in PDAC cells is suppressed, to some extent, by increased DNA methyltransferase activity and is therefore reactivated by DNA methylation inhibition. In fact, a previous study reported a number of similar genes that are commonly overexpressed in PDAC but regulated by DNA methylation.66, 67 Further studies are required to elucidate the complex mechanism regulating HA production in PDAC cells.
Another mechanism may be related to the enhanced secretion of HA from stromal cells, including fibroblasts. In support of this, it has been shown that HA staining was predominantly found within the desmoplastic stroma rather than tumor cells in human PDAC tissues.14, 49 Interestingly, Knudson et al.68 reported that direct coculture between tumor cells (including PDAC cells) and normal fibroblasts promotes the production of HA into the culture medium. Furthermore, a global gene expression analysis using microarrays identified an increased expression of HAS2 mRNA in PDAC cells in response to coculture with stromal fibroblasts.69 Taken together, these findings suggest that HA can be secreted from both tumor and stromal cells and that tumor–stroma interactions may play a pivotal role in the increased HA accumulation in PDAC.
Based on these findings, it is reasonable to suppose that inhibition of HA synthesis may be an ideal and straightforward treatment strategy. One agent that has been extensively studied as a potent inhibitor for HA is 4‐MU, also known as hymecromone, which is already used in several countries as a drug to improve liver function or to treat biliary spasm.70 4‐Methylumbelliferone inhibits HA synthesis by acting as a competitive substrate for UDP‐glucuronosyltransferase and by downregulating HAS2 and HAS3.71, 72 Potent anticancer effects of 4‐MU have been reported in various malignant tumors, including melanoma,73 breast cancer,74 esophageal cancer,75 and liver cancer (hepatocellular carcinoma).76 Previous studies have shown that 4‐MU and its derivatives (including 4‐methylesculetin) inhibit the growth and metastasis of PDAC in vitro and in vivo.77, 78 In addition, Nakazawa et al.79 showed that 4‐MU enhanced the anticancer activity of a commonly used chemotherapeutic drug, gemcitabine, in a PDAC cell line and animal model. This finding is consistent with a recent study showing that chemotherapy with carboplatin induces HA production, which can contribute to chemoresistance by regulating ABC transporter expression in ovarian cancer.80 Recently, in addition to its anticancer efficacy, the chemopreventive efficacy of 4‐MU has been shown in prostate cancer animal models.81 Therefore, this drug should be tested as a chemopreventive agent in individuals at high risk of developing PDAC and as a chemotherapeutic drug in patients with PDAC in the future.
Another HA inhibitor is Minnelide, a water‐soluble pro‐drug of triptolide, with antitumor activities against variety of cancers.82 Banerjee et al.83 showed that treatment with Minnelide decreases tumor HA thorough downregulating HAS activity, resulting in improved drug delivery and survival in both spontaneous KPC mice (a genetically engineered mouse model of PDAC) and patient‐derived xenografts tumors.
Hyaluronan‐receptor signaling
According to a number of previous studies, there are a variety of intracellular signaling pathways that are triggered by HA‐receptor interactions (Fig. 2).19, 24, 84 Binding of HA to CD44 has been shown to activate MAPK, Rac, and phosphoinositide 3‐kinase (PI3K) signaling, leading to enhanced cell survival, proliferation, migration, and invasion. The activated PI3K pathway also activates MRD1 to mediate multidrug resistance. RHAMM is known to induce focal adhesion kinase and also interacts with CD44 to enhance the signaling pathways, including the MAPK pathway. Furthermore, HA binding to CD44v10 induces CD44v10–EphA2 complex formation, recruitment, and activation of Src and Src‐mediated EphA2 tyrosine phosphorylation, resulting in RhoA activation and angiogenesis.85
Figure 2.
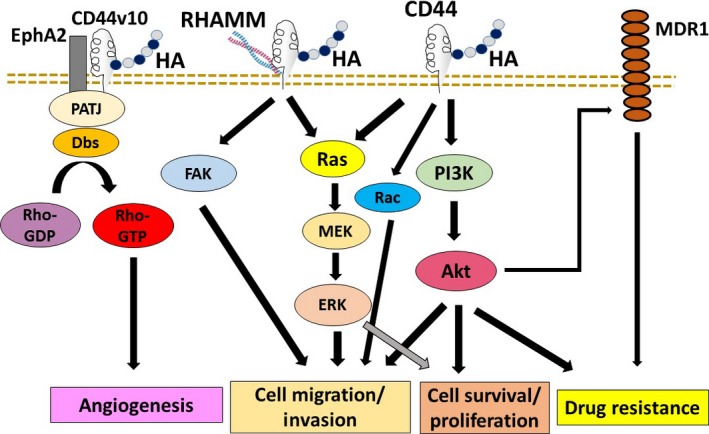
Regulation of cancer cell functions by hyaluronan (HA)‐receptor interactions. Binding of HA to CD44 has been shown to activate MAPK, Rac, and phosphoinositide 3‐kinase (PI3K) signaling, leading to enhanced cell survival, proliferation, migration, and invasion. The activated PI3K pathway also activates multidrug resistance 1 (MDR1) to mediate multidrug resistance. Receptor for HA‐mediated motility (RHAMM) is known to induce focal adhesion kinase (FAK) and also interacts with CD44 to enhance MAPK activity. HA binding to CD44 variant 10 (CD44v10) induces CD44v10–ephrinA2 receptor (EphA2) complex formation, resulting in RhoA activation and angiogenesis. Akt, protein kinase B; PATJ, Pals1‐associated tight junction.
An alternative approach of targeting HA is to block signal transduction pathways induced by HA‐receptor interactions. In many cancers including PDAC, HA–CD44 interactions are known to play an important role in tumor cell growth, survival, migration, invasion, multidrug resistance, and cancer stem cell self‐renewal.24, 26, 50, 84, 86, 87 Therefore, blocking this interaction may prevent progression of PDAC. Several studies have reported the efficacy of anti‐CD44 therapies. For example, Li et al.88 used antibody against CD44s (standard isoform) to inhibit pancreatic tumor initiation and post‐radiation recurrence in mice. Interestingly, anti‐CD44s downregulated the stem cell self‐renewal genes Nanog, Sox‐2, and Rex‐1, suggesting an important role of CD44 in cancer stem cell signatures.88 Furthermore, a recent study used xenograft tumors in mice from human PDAC tissues to show that a residual PDAC tumor originated from a small number of CD44‐positive cells present in the tumor and that systemic administration of anti‐CD44 antibodies inhibited the growth of relapsed tumors, but not primary tumors.89 RHAMM may also be a promising target to block HA signaling but has not yet been investigated in terms of its therapeutic efficacy in PDAC.
Downstream of CD44–HA interactions, Ras–MAPK and PI3K–protein kinase B signaling pathways are known to be involved in the cancer‐promoting effects of HA.90 These signaling pathways are thus a target for therapy. Teranishi et al.55 reported that enhanced cell motility and peritoneal metastasis of PDAC induced by HA were blocked by the PI3K inhibitor wortmannin in mice.
Stromal HA accumulation
Another major effect of HA on PDAC progression is its role as a barrier to access of chemotherapeutic agents to tumor cells. It has been suggested that abundant HA in cancer tissues causes elevated interstitial fluid pressure, vascular collapse, and decreased vascular permeability, thereby leading to impaired drug delivery.49, 91 Consequently, targeting the components of ECM, particularly HA, has been considered an attractive therapeutic strategy to overcome chemoresistance.92, 93, 94 In recent years, several stroma‐targeted agents have been developed for the treatment of PDAC. For example, a stroma‐targeted drug, nab‐paclitaxel, was tested in a pivotal phase III trial, which showed a significantly prolonged survival in the combination of nab‐paclitaxel and gemcitabine as compared to the gemcitabine alone.95
There are several other stroma‐targeted agents under preclinical or clinical evaluation. One best characterized agent to deplete stromal HA is a pegylated recombinant human hyaluronidase PH20 (PEGPH20), which is currently being evaluated in clinical trials.96 In several preclinical models, administration of PEGPH20 depleted stromal HA, reduced interstitial fluid pressure, re‐expanded tumor microvessels, improved macromolecular permeability, and subsequently enhanced the effects of chemotherapeutic agents.49, 91, 97 In addition, treatment with PEGPH20 increases the efficacy of mAb therapy98 and bacterial‐based therapy targeting indoleamine 2,3‐dioxygenase 99 in murine PDAC models, supporting the potential utility of this agent as a sensitizer of various anticancer therapies.
Based on these promising results of preclinical studies, PEGPH20 is now being tested in clinical trials to determine its efficacy when used in combination with nab‐paclitaxel plus gemcitabine (NCT01839487) and in combination with modified FOLFIRINOX (leucovorin calcium, 5‐fluorouracil, irinotecan hydrochloride, and oxaliplatin) (NCT01959139) in patients with metastatic PDAC.96 These studies will reveal the clinical efficacy of PEGPH20 as an adjunct to anticancer therapies and offer a novel treatment option for otherwise untreatable patients with PDAC.
Summary
In PDAC tissues, HA is secreted from tumor cells and stromal cells through upregulation of HAS in response to tumor–stroma interactions. The secreted HA, or its smaller fragments, may promote tumor cell proliferation, migration, invasion, and angiogenesis through HA‐receptor signaling and also serve as a barrier to access of chemotherapeutic agents to tumor cells. Given these links of HA with cancer progression, targeting HA represents a potential therapeutic approach for treatment of PDAC. There are different approaches of HA‐targeted therapies that include strategies aimed at inhibiting HA synthesis, blocking HA‐receptor signaling, and depleting stromal HA (Fig. 3).
Figure 3.
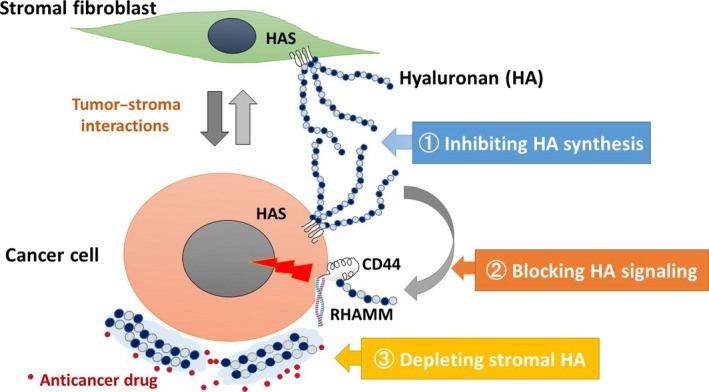
Proposed model of hyaluronan (HA) processing in pancreatic ductal adenocarcinoma (PDAC) and therapeutic strategies targeting HA. In PDAC tissues, HA is secreted from cancer cells and stromal fibroblasts through upregulation of HAS in response to tumor–stroma interactions. The secreted HA, or its smaller fragments, may promote tumor progression through HA‐receptor signaling and also serve as a barrier to access of chemotherapeutic agents to tumor cells. In this regard, three different therapeutic approaches may be considered: (1) inhibiting HA synthesis, (2) blocking HA signaling, and (3) depleting the stromal HA barrier in PDAC to improve chemosensitivity. HAS, HA synthase; RHAMM, receptor for HA‐mediated motility.
Disclosure Statement
There authors have no conflict of interest.
Acknowledgments
We thank Ms. Yuko Ueda for her technical assistance. This study was supported in part by a grant‐in‐aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (26462076).
Cancer Sci 107 (2016) 569–575
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362: 1605–17. [DOI] [PubMed] [Google Scholar]
- 2. Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002; 2: 897–909. [DOI] [PubMed] [Google Scholar]
- 3. Sato N, Goggins M. The role of epigenetic alterations in pancreatic cancer. J Hepatobiliary Pancreat Surg 2006; 13: 286–95. [DOI] [PubMed] [Google Scholar]
- 4. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell–stroma interface. Annu Rev Cell Dev Biol 2009; 25: 567–95. [DOI] [PubMed] [Google Scholar]
- 6. Mahadevan D, Von Hoff DD. Tumor–stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2007; 6: 1186–97. [DOI] [PubMed] [Google Scholar]
- 7. Whatcott C, Han H, Posner RG, Von Hoff DD. Tumor–stromal interactions in pancreatic cancer. Crit Rev Oncog 2013; 18: 135–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Itano N, Zhuo L, Kimata K. Impact of the hyaluronan‐rich tumor microenvironment on cancer initiation and progression. Cancer Sci 2008; 99: 1720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM. Hyaluronan in human malignancies. Exp Cell Res 2011; 317: 383–91. [DOI] [PubMed] [Google Scholar]
- 10. Mahlbacher V, Sewing A, Elsasser HP, Kern HF. Hyaluronan is a secretory product of human pancreatic adenocarcinoma cells. Eur J Cell Biol 1992; 58: 28–34. [PubMed] [Google Scholar]
- 11. Fries H, Elsasser HP, Mahlbacher V, Neumann K, Kern HF. Localisation of hyaluronate (HA) in primary tumors and nude mouse xenografts of human pancreatic carcinomas using a biotinylated HA‐binding protein. Virchows Arch 1994; 424: 7–12. [DOI] [PubMed] [Google Scholar]
- 12. Abetamann V, Kern HF, Elsasser HP. Differential expression of the hyaluronan receptors CD44 and RHAMM in human pancreatic cancer cells. Clin Cancer Res 1996; 2: 1607–18. [PubMed] [Google Scholar]
- 13. Theocharis AD, Tsara ME, Papageorgacopoulou N, Karavias DD, Theocharis DA. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta 2000; 1502: 201–6. [DOI] [PubMed] [Google Scholar]
- 14. Whatcott CJ, Diep CH, Jiang P et al Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res 2015; 21: 3561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng XB, Sato N, Kohi S, Yamaguchi K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS ONE 2013; 8: e80765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li XP, Zhang XW, Zheng LZ, Guo WJ. Expression of CD44 in pancreatic cancer and its significance. Int J Clin Exp Pathol 2015; 8: 6724–31. [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng XB, Sato N, Kohi S, Koga A, Hirata K. Receptor for hyaluronic acid‐mediated motility is associated with poor survival in pancreatic ductal adenocarcinoma. J Cancer 2015; 6: 1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kultti A, Zhao C, Singha NC et al Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. Biomed Res Int 2014; 2014: 817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004; 4: 528–39. [DOI] [PubMed] [Google Scholar]
- 20. Csoka AB, Frost GI, Stern R. The six hyaluronidase‐like genes in the human and mouse genomes. Matrix Biol 2001; 20: 499–508. [DOI] [PubMed] [Google Scholar]
- 21. Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol 2004; 83: 317–25. [DOI] [PubMed] [Google Scholar]
- 22. Lepperdinger G, Mullegger J, Kreil G. Hyal2–less active, but more versatile? Matrix Biol 2001; 20: 509–14. [DOI] [PubMed] [Google Scholar]
- 23. Harada H, Takahashi M. CD44‐dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase‐1 and ‐2. J Biol Chem 2007; 282: 5597–607. [DOI] [PubMed] [Google Scholar]
- 24. Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 2015; 6: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells 1991; 3: 347–50. [PubMed] [Google Scholar]
- 26. Misra S, Heldin P, Hascall VC et al Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J 2011; 278: 1429–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maxwell CA, McCarthy J, Turley E. Cell‐surface and mitotic‐spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci 2008; 121: 925–32. [DOI] [PubMed] [Google Scholar]
- 28. Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell‐associated and stromal hyaluronan. Semin Cancer Biol 2008; 18: 288–95. [DOI] [PubMed] [Google Scholar]
- 29. Ropponen K, Tammi M, Parkkinen J et al Tumor cell‐associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998; 58: 342–7. [PubMed] [Google Scholar]
- 30. Setala LP, Tammi MI, Tammi RH et al Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br J Cancer 1999; 79: 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Auvinen P, Tammi R, Parkkinen J et al Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 2000; 156: 529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anttila MA, Tammi RH, Tammi MI, Syrjanen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 2000; 60: 150–5. [PubMed] [Google Scholar]
- 33. Koyama H, Hibi T, Isogai Z et al Hyperproduction of hyaluronan in neu‐induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG‐M. Am J Pathol 2007; 170: 1086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage‐independent growth and tumorigenicity. Cancer Res 1999; 59: 1141–5. [PubMed] [Google Scholar]
- 35. Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res 2001; 61: 5207–14. [PubMed] [Google Scholar]
- 36. Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol 2002; 161: 849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim HR, Wheeler MA, Wilson CM et al Hyaluronan facilitates invasion of colon carcinoma cells in vitro via interaction with CD44. Cancer Res 2004; 64: 4569–76. [DOI] [PubMed] [Google Scholar]
- 38. Itano N, Atsumi F, Sawai T et al Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci U S A 2002; 99: 3609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zoltan‐Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem 2003; 278: 45801–10. [DOI] [PubMed] [Google Scholar]
- 40. Toole BP, Zoltan‐Jones A, Misra S, Ghatak S. Hyaluronan: a critical component of epithelial–mesenchymal and epithelial–carcinoma transitions. Cells Tissues Organs 2005; 179: 66–72. [DOI] [PubMed] [Google Scholar]
- 41. Tian X, Azpurua J, Hine C et al High‐molecular‐mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013; 499: 346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fuchs K, Hippe A, Schmaus A, Homey B, Sleeman JP, Orian‐Rousseau V. Opposing effects of high‐ and low‐molecular weight hyaluronan on CXCL12‐induced CXCR4 signaling depend on CD44. Cell Death Dis 2013; 4: e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan–CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA‐21, leading to down‐regulation of the tumor suppressor protein PDCD4, anti‐apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem 2009; 284: 26533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan–CD44 interaction promotes c‐Src‐mediated twist signaling, microRNA‐10b expression, and RhoA/RhoC up‐regulation, leading to Rho‐kinase‐associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem 2010; 285: 36721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. West DC, Kumar S. Hyaluronan and angiogenesis. Ciba Found Symp 1989; 143: 187–201; discussion‐7, 81–5. [DOI] [PubMed] [Google Scholar]
- 46. Wu M, Cao M, He Y et al A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J 2015; 29: 1290–8. [DOI] [PubMed] [Google Scholar]
- 47. Schmaus A, Klusmeier S, Rothley M et al Accumulation of small hyaluronan oligosaccharides in tumour interstitial fluid correlates with lymphatic invasion and lymph node metastasis. Br J Cancer 2014; 111: 559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheng XB, Kohi S, Koga A, Hirata K, Sato N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget 2015. doi: 10.18632/oncotarget.6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jacobetz MA, Chan DS, Neesse A et al Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2012; 62: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takada M, Yamamoto M, Saitoh Y. The significance of CD44 in human pancreatic cancer: II. The role of CD44 in human pancreatic adenocarcinoma invasion. Pancreas 1994; 9: 753–7. [DOI] [PubMed] [Google Scholar]
- 51. Immervoll H, Hoem D, Steffensen OJ, Miletic H, Molven A. Visualization of CD44 and CD133 in normal pancreas and pancreatic ductal adenocarcinomas: non‐overlapping membrane expression in cell populations positive for both markers. J Histochem Cytochem 2011; 59: 441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Satoh K, Shimosegawa T, Koizumi M, Toyota T. Expression of CD44 in duct cell carcinomas and in intraductal neoplasms of the pancreas. Anticancer Res 1997; 17: 215–9. [PubMed] [Google Scholar]
- 53. Castella EM, Ariza A, Ojanguren I et al Differential expression of CD44v6 in adenocarcinoma of the pancreas: an immunohistochemical study. Virchows Arch 1996; 429: 191–5. [DOI] [PubMed] [Google Scholar]
- 54. Gotoda T, Matsumura Y, Kondo H et al Expression of CD44 variants and its association with survival in pancreatic cancer. Jpn J Cancer Res 1998; 89: 1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Teranishi F, Takahashi N, Gao N et al Phosphoinositide 3‐kinase inhibitor (wortmannin) inhibits pancreatic cancer cell motility and migration induced by hyaluronan in vitro and peritoneal metastasis in vivo. Cancer Sci 2009; 100: 770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5: 3–14. [DOI] [PubMed] [Google Scholar]
- 57. Itano N, Sawai T, Atsumi F et al Selective expression and functional characteristics of three mammalian hyaluronan synthases in oncogenic malignant transformation. J Biol Chem 2004; 279: 18679–87. [DOI] [PubMed] [Google Scholar]
- 58. Yamada Y, Itano N, Narimatsu H et al Elevated transcript level of hyaluronan synthase1 gene correlates with poor prognosis of human colon cancer. Clin Exp Metastasis 2004; 21: 57–63. [DOI] [PubMed] [Google Scholar]
- 59. Yabushita H, Noguchi M, Kishida T et al Hyaluronan synthase expression in ovarian cancer. Oncol Rep 2004; 12: 739–43. [PubMed] [Google Scholar]
- 60. Kramer MW, Escudero DO, Lokeshwar SD et al Association of hyaluronic acid family members (HAS1, HAS2, and HYAL‐1) with bladder cancer diagnosis and prognosis. Cancer 2011; 117: 1197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chi A, Shirodkar SP, Escudero DO et al Molecular characterization of kidney cancer: association of hyaluronic acid family with histological subtypes and metastasis. Cancer 2012; 118: 2394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Auvinen P, Rilla K, Tumelius R et al Hyaluronan synthases (HAS1‐3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat 2014; 143: 277–86. [DOI] [PubMed] [Google Scholar]
- 63. Kohi S, Sato N, Cheng XB, Koga A, Higure A, Hirata K. A novel epigenetic mechanism regulating hyaluronan production in pancreatic cancer cells. Clin Exp Metastasis 2016; 33: 225–30. [DOI] [PubMed] [Google Scholar]
- 64. Gao J, Wang L, Xu J et al Aberrant DNA methyltransferase expression in pancreatic ductal adenocarcinoma development and progression. J Exp Clin Cancer Res 2013; 32: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peng DF, Kanai Y, Sawada M et al Increased DNA methyltransferase 1 (DNMT1) protein expression in precancerous conditions and ductal carcinomas of the pancreas. Cancer Sci 2005; 96: 403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sato N, Maehara N, Su GH, Goggins M. Effects of 5‐aza‐2′‐deoxycytidine on matrix metalloproteinase expression and pancreatic cancer cell invasiveness. J Natl Cancer Inst 2003; 95: 327–30. [DOI] [PubMed] [Google Scholar]
- 67. Sato N, Maitra A, Fukushima N et al Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res 2003; 63: 4158–66. [PubMed] [Google Scholar]
- 68. Knudson W, Biswas C, Toole BP. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A 1984; 81: 6767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sato N, Maehara N, Goggins M. Gene expression profiling of tumor–stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res 2004; 64: 6950–6. [DOI] [PubMed] [Google Scholar]
- 70. Nagy N, Kuipers HF, Frymoyer AR et al 4‐methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol 2015; 6: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kultti A, Pasonen‐Seppanen S, Jauhiainen M et al 4‐Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP‐glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res 2009; 315: 1914–23. [DOI] [PubMed] [Google Scholar]
- 72. Kakizaki I, Kojima K, Takagaki K et al A novel mechanism for the inhibition of hyaluronan biosynthesis by 4‐methylumbelliferone. J Biol Chem 2004; 279: 33281–9. [DOI] [PubMed] [Google Scholar]
- 73. Yoshihara S, Kon A, Kudo D et al A hyaluronan synthase suppressor, 4‐methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett 2005; 579: 2722–6. [DOI] [PubMed] [Google Scholar]
- 74. Urakawa H, Nishida Y, Wasa J et al Inhibition of hyaluronan synthesis in breast cancer cells by 4‐methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int J Cancer 2012; 130: 454–66. [DOI] [PubMed] [Google Scholar]
- 75. Twarock S, Freudenberger T, Poscher E et al Inhibition of oesophageal squamous cell carcinoma progression by in vivo targeting of hyaluronan synthesis. Mol Cancer 2011; 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Piccioni F, Malvicini M, Garcia MG et al Antitumor effects of hyaluronic acid inhibitor 4‐methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 2012; 22: 400–10. [DOI] [PubMed] [Google Scholar]
- 77. Morohashi H, Kon A, Nakai M et al Study of hyaluronan synthase inhibitor, 4‐methylumbelliferone derivatives on human pancreatic cancer cell (KP1‐NL). Biochem Biophys Res Commun 2006; 345: 1454–9. [DOI] [PubMed] [Google Scholar]
- 78. Hajime M, Shuichi Y, Makoto N et al Inhibitory effect of 4‐methylesculetin on hyaluronan synthesis slows the development of human pancreatic cancer in vitro and in nude mice. Int J Cancer 2007; 120: 2704–9. [DOI] [PubMed] [Google Scholar]
- 79. Nakazawa H, Yoshihara S, Kudo D et al 4‐methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother Pharmacol 2006; 57: 165–70. [DOI] [PubMed] [Google Scholar]
- 80. Ricciardelli C, Ween MP, Lokman NA, Tan IA, Pyragius CE, Oehler MK. Chemotherapy‐induced hyaluronan production: a novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013; 13: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yates TJ, Lopez LE, Lokeshwar SD et al Dietary supplement 4‐methylumbelliferone: an effective chemopreventive and therapeutic agent for prostate cancer. J Natl Cancer Inst 2015; 107: djv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett 2013; 335: 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Banerjee S, Modi S, McGinn O et al Impaired synthesis of stromal components in response to Minnelide improves vascular function, drug delivery and survival in pancreatic cancer. Clin Cancer Res 2015; 22: 415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Toole BP. Hyaluronan–CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res 2009; 15: 7462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lennon FE, Mirzapoiazova T, Mambetsariev N, Mambetsariev B, Salgia R, Singleton PA. Transactivation of the receptor‐tyrosine kinase ephrin receptor A2 is required for the low molecular weight hyaluronan‐mediated angiogenesis that is implicated in tumor progression. J Biol Chem 2014; 289: 24043–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chanmee T, Ontong P, Kimata K, Itano N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol 2015; 5: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiang W, Zhang Y, Kane KT et al CD44 regulates pancreatic cancer invasion through MT1‐MMP. Mol Cancer Res 2015; 13: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li L, Hao X, Qin J et al Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology 2014; 146: 1108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Molejon MI, Tellechea JI, Loncle C et al Deciphering the cellular source of tumor relapse identifies CD44 as a major therapeutic target in pancreatic adenocarcinoma. Oncotarget 2015; 6: 7408–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sohara Y, Ishiguro N, Machida K et al Hyaluronan activates cell motility of v‐Src‐transformed cells via Ras‐mitogen‐activated protein kinase and phosphoinositide 3‐kinase‐Akt in a tumor‐specific manner. Mol Biol Cell 2001; 12: 1859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21: 418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov 2011; 1: 291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yu M, Tannock IF. Targeting tumor architecture to favor drug penetration: a new weapon to combat chemoresistance in pancreatic cancer? Cancer Cell 2012; 21: 327–9. [DOI] [PubMed] [Google Scholar]
- 94. Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer 2013; 108: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Von Hoff DD, Ervin T, Arena FP et al Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shepard HM. Breaching the castle walls: hyaluronan depletion as a therapeutic approach to cancer therapy. Front Oncol 2015; 5: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Thompson CB, Shepard HM, O'Connor PM et al Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther 2010; 9: 3052–64. [DOI] [PubMed] [Google Scholar]
- 98. Singha NC, Nekoroski T, Zhao C et al Tumor‐associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol Cancer Ther 2015; 14: 523–32. [DOI] [PubMed] [Google Scholar]
- 99. Manuel ER, Chen J, D'Apuzzo M et al Salmonella‐based therapy targeting indoleamine 2,3‐dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immunol Res 2015; 3: 1096–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


