Abstract
A phase I study of a new cancer vaccine (KRM‐10), consisting of a mixture of 10 different short peptides, was conducted for patients with advanced gastrointestinal cancers. Primary or secondary endpoints included the dose‐limiting toxicity (DLT), or safety and immune responses, respectively. Peptide‐specific cytotoxic T lymphocytes (CTL) and immunoglobulin G (IgG), together with soluble inflammatory factors, were measured before and after vaccination. Twenty‐one patients were vaccinated with KRM‐10 at dose levels of 10 (n = 6), 20 (n = 8) or 30 mg (n = 7) of peptides every week for 6 weeks. No DLT were observed in the dose range evaluated. Common treatment‐related adverse events were a grade 1 injection site reaction in 15 patients, and fever in three patients (grade 1 in two patients and grade 2 in one patient). CTL activity to at least one peptide at the time of the third and sixth vaccination increased in 2 and 3 of 6 (10 mg), 2 of 8 and 4 of 6 (20 mg), or 2 and 1 of 6 (30 mg) patients, respectively. IgG levels, at the third and sixth vaccination, were also increased in 1 and 1 of 6 (10 mg), 2 of 8 and 4 of 6 (20 mg), or 1 and 3 of 6 (30 mg) patients, respectively. The KRM‐10 vaccine consisting of 20 mg of peptides was determined as the optimal dose for a coming phase II trial because of its safety, and also for demonstrating the most potent activity for augmenting the immune response of the three doses tested. This trial was registered at the UMIN Clinical Trials Registry as UMIN000008820.
Keywords: Cancer vaccine, cytotoxic T‐lymphocytes, human leukocyte antigen, peptide, phase I
Immune checkpoint blockers can achieve durable clinical responses in at least one‐fifth of patients with various types of advanced cancer.1, 2 However, clinical benefits cannot be expected in cancer patients whose tumors display no or few tumor‐infiltrating lymphocytes. We previously reported that personalized peptide vaccination rapidly induced the proliferation of CD45RO+‐activated lymphocytes at tumor sites, in association with various clinical benefits, in patients with advanced cancer.3, 4 These results suggest that the combined therapy of a cancer vaccine and immune checkpoint blockers could be more efficacious than monotherapy.
In contrast to immune checkpoint blockers, however, cancer vaccines tested in the past two decades have not yet yielded optimal clinical outcomes for drug approval.5, 6, 7 The large heterogeneity of tumor‐associated antigens and the diversity in both human leukocyte antigen (HLA) and T cell subsets may hamper the successful development of a cancer vaccine.8, 9
An appropriate dose setting for each vaccine, as well as suitable predictive biomarkers in early phase studies, may have hampered previous trials to address clinical benefits.5, 6, 7, 8, 9 To overcome these problems, we have aimed to develop a new type of CTL‐epitope peptide vaccine consisting of 10 mixed peptides (KRM‐10) that can be applied to the vast majority of cancer patients with different HLA alleles, including the HLA‐A2, A24 and A3 supertypes (A3, A11, A31 and A33) or A26. This study presents the results of a phase I study of peptide vaccination using these 10 peptides that were frequently chosen in previous clinical trials of personalized peptide vaccination for patients with advanced cancers.10, 11, 12
Patients and Methods
Patients and eligibility
Eligible patients were aged between 20 and 80 years, with histologically confirmed gastrointestinal cancer, for whom standard therapies were ineffective or inappropriate. Patients were required to have a life expectancy of at least 3 months, an Eastern Cooperative Oncology Group performance status of 0–1, the ability to eat, and adequate organ function as assessed by a platelet (≥75 000/mm3) count, an absolute lymphocyte (≥1000/mm3) count, serum total bilirubin (≤2.0 mg/dL), serum creatinine (≤1.5 mg/dL) and alanine aminotransferase and aspartate aminotransferase (≤100 IU/L, or ≤200 IU/L in patients with hepatic metastasis) levels. Having a target lesion or lesions according to the response evaluation criteria for solid tumors was not mandatory. Exclusion criteria included patients with active infection, significant cardiovascular disease, psychogenic disorders, uncontrolled diabetes, pulmonary fibrosis or active pneumonitis, treatment with systemic corticosteroids, active gastrointestinal bleeding, treatment for pleural effusions and/or ascites, active concomitant malignancy, pregnancy or lactation, and hepatitis B infection. This study was approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan and conducted in accordance with the Declaration of Helsinki. All patients provided written, informed consent prior to study entry. This study was registered with the UMIN Clinical Trials Registry as UMIN000008820.
Study design and vaccinations
This was an open‐label, single‐center, phase I, dose‐escalation study undertaken to determine the recommended dose of KRM‐10 based on its dose‐limiting toxicity (DLT) and immunological response. The primary objective was to identify the maximum tolerated dose (MTD) of KRM‐10. Secondary objectives were to assess safety and immune responses as assessed by peptide‐specific immunoglobulin (IgG) and CTL levels. The KRM‐10 vaccine was supplied by the Kurume University School of Medicine (Fukuoka, Japan).
Patients were injected subcutaneously with the KRM‐10 vaccine at a dose of 10 mg/0.5 mL (1 mg of each peptide), 20 mg/1.0 mL (2 mg of each peptide) or 30 mg/1.5 mL (3 mg of each peptide), once a week for 6 weeks. These three dose levels were chosen based on a previously conducted personalized peptide vaccine study in which 3 mg of each peptide (total of four peptides per injection) was considered an acceptable dose according to safety and immunological responses.13 The sample size for each cohort was six on completion of the protocol treatment, allowing the adequate evaluation of safety and tolerability while minimizing exposure to a new cancer vaccine.
Dose‐limiting toxicities were defined as any of the following events that were considered by the investigator to be related to the KRM‐10 treatment and which occurred up until the end of the first week after the sixth vaccination, irrespective of whether the observed adverse events were eliminated or reduced: grade 3 skin induration; skin ulceration; injection site reaction; other non‐hematological grade 3 or 4 toxicities except for anorexia, nausea, vomiting and fatigue, constipation and dehydration; hyperglycemia; and electrolyte abnormality.
Patients with non‐progressive disease (PD), after a protocol treatment period involving the six KRM‐10 vaccinations, were allowed to continue with KRM‐10 treatment on compassionate grounds until disease progression. During this period of continued use, patients were allowed to receive the vaccine six times every 2 weeks, followed by six times every 4 weeks, up to a total of 18 times.
Peptides
KRM‐10 consisted of the following 10 peptides: SART3302–310, Lck246–254 and HNRPL140–148 for patients with HLA‐A2; Lck488–497, MRP3503–511 and EGFR800–809 for patients with HLA‐A24; SART3734–742, and Lck90–99 for patients with the HLA‐A3 supertype; SART3109–118 for patients with the HLA‐A24 and HLA‐A3 supertypes or HLA‐A26; and WHSC2103–111 for HLA‐A2 and HLA‐A3 supertypes or HLA‐A26; their abilities to induce HLA‐class IA‐restricted CTL activity have been reported previously.10, 11, 12, 14 These 10 peptides were prepared under the conditions outlined by the code of Good Manufacturing Practice using an automated multiple peptide synthesizer (Multiple Peptide Systems, San Diego, CA, USA) and the services of the American Peptide Company (Vista, CA, USA). Ten peptides were mixed with Incomplete Freund's adjuvant (Montanide ISA‐51VG; Seppic, Paris, France).
Although the HLA types were shown at two digits in this study, we had reported that the 10 peptides employed are applicable to patients with HLA‐A2404, HLA‐A0201, HLA‐A0206, HLA‐A0207, HLA‐A1101, HLA‐A3101, HLA‐A3303, HLA‐A2601, HLA‐A2602 or HLA‐A2603 at four digits. The HLA restriction of 10 peptides of KRM‐10 are shown in Table S1. Because these four‐digit HLA types are expected to cover 99.94% of the Japanese population, we think that it could be worthwhile using these 10 peptides for all Japanese patients without screening for HLA genotypes.
Prior to the initiation of this phase I study, we had tested the CTL and IgG responses to more than 500 peptide candidates derived from the six mother antigens using pre‐vaccination samples of cancer patients, as reported previously.11, 12 The 10 peptides employed in the present study were chosen from those >500 peptides based upon the higher reactivity in pre‐vaccination samples of advanced gastrointestinal cancer patients with regard to peptide‐specific CTL and IgG responses. In addition, both CTL and IgG responses to these 10 peptides were shown to be well boosted after vaccination in patients enrolled in the phase II clinical trials of personalized peptide vaccinations as reported previously.10, 11, 12, 13, 14, 15 Therefore, these 10 peptides of KRM‐10 might be recognized by the immune system of pre‐vaccination patients with advanced gastrointestinal cancers through natural presentation to peptide‐reactive T and B cells.
It might be important to examine the expression of molecules, from which each peptide is derived, in the original tumors in each patient. However, we could not test the original tumors, mainly because surgical tumor samples or biopsy samples just before or after vaccination were unavailable from most of the enrolled patients with far advanced gastrointestinal cancers, who had no surgical indication. Instead, we examined the expression of six different mother antigens in resected tumors from non‐vaccinated esophageal (n = 10), gastric (n = 15) or colorectal (n = 10 or n = 15)10 cancer patients (Table 1). Representative results of immunohistochemical staining are shown in Figure 1. All of the six mother antigens were expressed in adenocarcinoma tissues from gastric and colorectal cancer patients at different frequencies, but 2 of 6 antigens (LCK and MRP3) were not detectable in any of 10 squamous cell carcinoma tissues tested from esophageal cancer patients.
Table 1.
Expression of original proteins in esophageal, gastric or colorectal tumors
| Original protein | Peptide name | Esophagusa | Stomacha | Colorectuma |
|---|---|---|---|---|
| EGFR | EGF‐R‐800 | 8/10 (80%) | 9/15 (60%) | 8/15 (53%) |
| HNRPL | HNRPL‐140 | 10/10 (100%) | 15/15 (100%) | 10/10 (100%) |
| p56Lck | Lck‐90 | 0/10 (0%) | 1/15 (7%) | 4/15 (27%) |
| Lck‐246 | ||||
| Lck‐488 | ||||
| MRP3 | MRP3‐503 | 0/10 (0%) | 9/15 (60%) | 9/15 (60%) |
| SART3 | SART3‐109 | 10/10 (100%) | 15/15 (100%) | 10/10 (100%) |
| SART3‐302 | ||||
| SART3‐734 | ||||
| WHSC2 | WHSC2‐103 | 9/10 (90%) | 15/15 (100%) | 10/10 (100%) |
Frequency of original protein expression (positive cases/examined cases [percentage]) was determined by immunohistochemistry in resected tumors from non‐vaccinated esophageal (n = 10), gastric (n = 15) or colorectal (n = 10 or n = 15) cancer patients.
Figure 1.
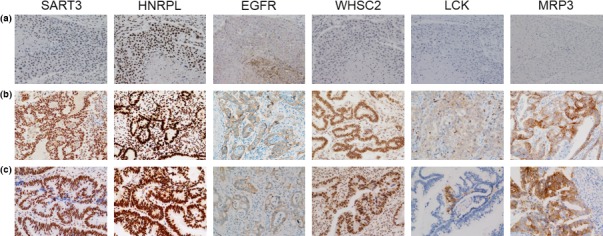
The expression levels of the six vaccine antigens that code the peptides were examined by immunohistochemical staining in tumor tissues from non‐vaccinated esophageal (n = 10), gastric (n = 15) or colorectal (n = 10 or n = 15) cancer patients.10 Paraffin‐embedded tissue samples were cut into 4‐μm sections, and examined on a coated slide glass. Detailed methods including the antibodies used for immunohistochemistry (IHC) have been described previously.10, 15 Representative results of immunohistochemical staining are shown: (a) esophageal cancer; (b) gastric cancer and (c) colorectal cancer.
Measurement of cytotoxic T lymphocytes and immunoglobulin G, and soluble inflammatory factors
Cytotoxic T lymphocyte activity specific to each of the HLA‐matched peptides and the 10 mixed peptides (KRM‐10) was evaluated by interferon‐γ (IFN‐γ) enzyme‐linked immunospot (ELISPOT) assay using peripheral blood mononuclear cell (PBMC) as reported previously.10, 14 All assays were carried out in triplicate and analyzed with an ELISPOT reader (CTL‐ImmunoSpot S5 Series; Cellular Technology, Shaker Heights, OH, USA). CTL activity was evaluated by the difference between spot numbers in response to the corresponding peptide and those of the control peptide. The cut‐off level was set as 10 IFNγ‐spots per 105 PBMC. If the spot numbers, in response to the corresponding peptide in post‐vaccination PBMC, were more than twofold higher than those in pre‐vaccination PBMC, the changes were considered to be positive immune responses, as reported previously.10, 11, 12, 13, 14, 15 The changes were also considered to be positive if the spot numbers were under 10 in the pre‐vaccination samples and became detectable after the vaccination. An IgG response specific to HLA‐matched peptides was determined by peptide‐specific IgG levels using a Luminex system (Luminex, Austin, TX, USA).10, 11, 12, 13, 14, 15 The cut‐off level of FIU titers was set as 10. If titers of peptide‐specific IgG in the post‐vaccination plasma were more than twofold higher than those in the pre‐vaccination plasma, the increases were considered to be positive immune responses, as reported previously.10, 11, 12, 13, 14, 15
Acute‐phase inflammatory factors (C‐reactive protein, haptoglobin, beta2‐microglobulin and Gc globulin) in pre‐vaccination and post‐vaccination plasmas were examined in the present study using Invitrogen's Multiplex Bead Immunoassay Kit (Invitrogen Thermo Fisher Scientific, Waltham, MA, USA). Frozen plasma samples were thawed, diluted and assayed in accordance with the manufacturer's instructions. If the levels of inflammatory factors in the post‐vaccination plasma were more than twofold higher than those in the pre‐vaccination plasma, the increases were considered to be significant.
Safety and tumor assessments
Adverse events were evaluated using the Common Terminology Criteria for Adverse Events version 4.0 throughout the treatment period until a minimum of 28 days after the last dose, or until all drug‐related adverse events had recovered to baseline or were deemed irreversible. Tumor assessments by computed tomography or magnetic resonance imaging scans were carried out at baseline and after the sixth vaccination, and evaluated according to the Response Evaluation Criteria In Solid Tumors version 1.1.16
Statistical analysis
All patients who received vaccinations were included in the analysis of its safety and efficacy. All statistical analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Twenty‐one patients were enrolled in this study: 6, 8 and 7 patients received 10, 20 and 30 mg of KRM‐10 vaccine (1, 2 or 3 mg of each peptide, respectively) once a week for 6 weeks, respectively. Patient characteristics are shown in Table 2. HLA‐class IA types determined by genotyping were A24 (n = 10), A2 (9), A31 (6), A26 (4), A33 (3), A11 (2) and A3 (1). At least four peptides of the 10 mixed peptides were matched in each of the 20 patients with 4 peptides for 11 patients, 5 for 2, 7 for 3, and 8 for 4 patients (Table 3). Eighteen patients completed the protocol treatment as planned in the 6‐week period; three patients discontinued treatment due to early tumor progression and were excluded from subjects used to assess DLT: 2 in the 20 mg, and 1 in the 30‐mg cohort. The median number of vaccinations for the 10, 20 and 30‐mg cohorts was 6 (range 6–14), 6 (range 3–9) and 6 (range 2–17), respectively.
Table 2.
Baseline characteristics
| 10‐mg peptide (n = 6) | 20‐mg peptide (n = 8) | 30‐mg peptide (n = 7) | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 5 | 5 | 4 |
| Female | 1 | 3 | 3 |
| Age, years | |||
| Median (range) | 71.5 (63–77) | 65.5 (49–77) | 65 (59–74) |
| ECOG PS, n (%) | |||
| 0 | 2 | 5 | 2 |
| 1 | 4 | 3 | 5 |
| HLA expression, n (%) | |||
| HLA‐A2 | 5 | 2 | 2 |
| HLA‐A3 | 1 | 0 | 0 |
| HLA‐A11 | 0 | 1 | 1 |
| HLA‐A24 | 3 | 4 | 3 |
| HLA‐A26 | 0 | 1 | 3 |
| HLA‐A31 | 2 | 1 | 3 |
| HLA‐A33 | 2 | 1 | 0 |
| Previous therapy, n (%) | |||
| Chemotherapy | 6 | 8 | 7 |
| Radiotherapy | 3 | 1 | 2 |
| Surgery | 3 | 8 | 4 |
| Chemotherapy | |||
| 1 regimen | 1 | 0 | 1 |
| 2 regimens | 1 | 0 | 1 |
| 3+ regimens | 4 | 8 | 5 |
| Type of tumor, n (%) | |||
| Esophageal squamous cell carcinoma | 4 | 0 | 0 |
| Gastric adenocarcinoma | 2 | 1 | 1 |
| Small intestinal adenocarcinoma | 0 | 1 | 0 |
| Colorectal adenocarcinoma | 0 | 6 | 4 |
| Anal canal squamous cell carcinoma | 0 | 0 | 1 |
ECOG, Eastern Cooperative Oncology Group; HLA, human leukocyte antigens; PS, performance status.
Table 3.
Immune responses
| Pt no. | Dose of KRM‐10 (mg) | HLA type | HLA matching peptides or KRM10 | CTL response (pg/mL) | IgG response (FIU) | Response | OS(m) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination | Post third† | Post sixth† | Prevaccination | Post third† | Post sixth† | ||||||
| L1‐1 | 10 | A33 | WHSC2‐103 | 0 | 0 | 0 | 0 | 0 | 0 | SD | 13.9 |
| SART3‐734 | 0 | 0 | 0 | 159 | 147 | 156 | |||||
| Lck‐90 | 0 | 28 | 0 | 0 | 0 | 0 | |||||
| SART3‐109 | 0 | 18 | 0 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 22 | 0 | ND | ND | ND | |||||
| L1‐2 | 10 | A2/A24 | SART2‐302 | 0 | 0 | 0 | 42 | 58 | 92 | PD | 8.7 |
| Lck‐246 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| HNRPL‐140 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| EGFR‐800 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Lck‐488 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| MRPP3‐503 | 0 | 0 | 227 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 260 | ND | ND | ND | |||||
| L1‐3 | 10 | A11/A31 | WHSC2‐103 | 0 | 0 | 0 | 0 | 0 | 0 | PD | 2.5 |
| SART3‐734 | 0 | 0 | 0 | 5939 | 3685 | 2456 | |||||
| Lck‐90 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L1‐4 | 10 | A2/A24 | SART3‐302 | 0 | 0 | 0 | 0 | 0 | 0 | SD | 20.1 |
| Lck‐246 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| HNRPL‐140 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| EGFR‐800 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Lck‐488 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| MRP3‐503 | 0 | 0 | 33 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L1‐5 | 10 | A24 | EGFR‐800 | 0 | 0 | 0 | 0 | 0 | 0 | PD | 7.6 |
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Lck‐488 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| MRP3‐503 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L1‐6 | 10 | A24/A26 | EGFR‐800 | 0 | 0 | 0 | 0 | 0 | 0 | PD | 3.0 |
| SART3‐109 | 0 | 0 | 0 | 0 | 21 | 25 | |||||
| Lck‐488 | 20 | 0 | 0 | 74 | 130 | 123 | |||||
| MRP3‐503 | 0 | 74 | 42 | 111 | 141 | 144 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 0 | 10 | 0 | |||||
| KRM‐10 | 0 | 59 | 30 | ND | ND | ND | |||||
| L2‐1 | 20 | A3/A33 | WHSC2‐103 | 0 | 0 | 32 | 0 | 0 | 0 | PD | 7.6 |
| SART3‐734 | 0 | 0 | 131 | 22 | 44 | 18 | |||||
| Lck‐90 | 26 | 0 | 0 | 0 | 0 | 0 | |||||
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 1090 | |||||
| KRM‐10 | 0 | 241 | 148 | ND | ND | ND | |||||
| L2‐2 | 20 | A2 | SART3‐302 | 0 | 0 | – | 20 | 22 | – | PD | 3.4 |
| Lck‐246 | 0 | 0 | – | 0 | 0 | – | |||||
| WHSC2‐103 | 0 | 0 | – | 0 | 0 | – | |||||
| HNRPL‐140 | 0 | 0 | – | 0 | 0 | – | |||||
| KRM‐10 | 0 | 0 | – | ND | ND | – | |||||
| L2‐3 | 20 | A31 | WHSC2‐103 | 0 | 0 | 0 | 0 | 14 | 19 | PD | 9.8 |
| SART3‐734 | 0 | 0 | 0 | 0 | 36 | 36 | |||||
| Lck‐90 | 0 | 0 | 0 | 0 | 16 | 41 | |||||
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 24 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L2‐4 | 20 | A2/A33 | SART3‐302 | 0 | 0 | 0 | 0 | 0 | 0 | SD | 5.1 |
| Lck‐246 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| WHSC2‐103 | 22 | 0 | 0 | 15 | 14 | 0 | |||||
| HNRPL‐140 | 0 | 0 | 24 | 27 | 20 | 0 | |||||
| SART3‐734 | 0 | 0 | 0 | 14 | 11 | 0 | |||||
| Lck‐90 | 0 | 0 | 0 | 32 | 27 | 135 | |||||
| SART3‐109 | 0 | 0 | 0 | 14 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L2‐5 | 20 | A11 | SART3‐302 | 0 | 0 | 0 | 1682 | 1595 | 1088 | PD | 4.0 |
| Lck‐246 | 0 | 0 | 0 | 16 | 11 | 22 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 24 | 19 | 23 | |||||
| HNRPL‐140 | 0 | 20 | 16 | 20 | 15 | 16 | |||||
| EGFR‐800 | 0 | 0 | 0 | 13 | 11 | 11 | |||||
| SART3‐109 | 0 | 0 | 0 | 20 | 12 | 23 | |||||
| Lck‐488 | 0 | 82 | 0 | 74 | 62 | 61 | |||||
| MRP3‐503 | 0 | 131 | 134 | 13 | 10 | 148 | |||||
| KRM‐10 | 0 | 194 | 177 | ND | ND | ND | |||||
| L2‐6 | 20 | A24 | EGFR‐800 | 0 | 0 | – | 77 | 73 | – | PD | 1.4 |
| SART3‐109 | 0 | 0 | – | 75 | 79 | – | |||||
| Lck‐488 | 0 | 59 | – | 429 | 410 | – | |||||
| MRP3‐503 | 0 | 0 | – | 37 | 39 | – | |||||
| KRM‐10 | 0 | 0 | – | ND | ND | – | |||||
| L2‐7 | 20 | A11/A31 | WHSC2‐103 | 0 | 0 | 0 | 18 | 14 | 13 | SD | 19.9 |
| SART3‐734 | 0 | 0 | 0 | 8125 | 8454 | 8639 | |||||
| Lck‐90 | 0 | 0 | 0 | 18 | 17 | 22 | |||||
| SART3‐109 | 0 | 0 | 0 | 16 | 14 | 14 | |||||
| KRM‐10 | 0 | 31 | 0 | ND | ND | ND | |||||
| L2‐8 | 20 | A24 | EGFR‐800 | 0 | 0 | 0 | 0 | 0 | 0 | PD | 6.6 |
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Lck‐488 | 0 | 0 | 0 | 42 | 34 | 30 | |||||
| MRP3‐503 | 0 | 0 | 42 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 57 | ND | ND | ND | |||||
| L3‐1 | 30 | A2/A31 | SART3‐302 | 0 | 0 | 0 | 273 | 243 | 24947 | PD | 6.2 |
| Lck‐246 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 12 | 10 | 11 | |||||
| HNRPL‐140 | 0 | 0 | 0 | 11 | 9 | 10 | |||||
| SART3‐734 | 0 | 0 | 0 | 57 | 43 | 80 | |||||
| Lck‐90 | 0 | 0 | 0 | 24 | 21 | 566 | |||||
| SART3‐109 | 0 | 0 | 0 | 10 | 0 | 10 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L3‐2 | 30 | A26/A31 | WHSC2‐103 | 0 | 82 | 0 | 0 | 0 | 0 | PD | 3.7 |
| SART3‐109 | 0 | 0 | 0 | 0 | 10 | 11 | |||||
| SART3‐734 | 0 | 0 | 0 | 1206 | 975 | 454 | |||||
| Lck‐90 | 0 | 0 | 0 | 23 | 30 | 565 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L3‐3 | 30 | A2/A24 | SART3‐302 | 0 | 0 | 0 | 1337 | 1715 | 814 | PD | 12.8 |
| Lck‐246 | 0 | 0 | 0 | 19 | 20 | 11 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 24 | 26 | 20 | |||||
| HNRPL‐140 | 91 | 0 | 0 | 22 | 26 | 18 | |||||
| EGFR‐800 | 0 | 0 | 0 | 18 | 17 | 14 | |||||
| SART3‐109 | 0 | 0 | 0 | 21 | 26 | 19 | |||||
| Lck‐488 | 0 | 0 | 0 | 98 | 108 | 93 | |||||
| MRP3‐503 | 0 | 0 | 0 | 15 | 18 | 12 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L3‐4 | 30 | A24/A33 | EGFR‐800 | 0 | 0 | 84 | 0 | 0 | 0 | PD | 10.2 |
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Lck‐488 | 0 | 0 | 0 | 18 | 11 | 15 | |||||
| MRP3‐503 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 17 | 11 | 11 | |||||
| SART3‐734 | 0 | 0 | 0 | 3049 | 2124 | 3182 | |||||
| Lck‐90 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L3‐5 | 30 | A24/A26 | EGFR‐800 | 0 | 0 | 0 | 0 | 0 | 0 | SD | 16.6 |
| SART3‐109 | 0 | 0 | 0 | 932 | 833 | 480 | |||||
| Lck‐488 | 0 | 0 | 0 | 743 | 652 | 405 | |||||
| MRP3‐503 | 0 | 0 | 0 | 643 | 560 | 320 | |||||
| WHSC2‐103 | 0 | 0 | 0 | 74 | 61 | 0 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
| L3‐6 | 30 | A26/A31 | NA | NA | NA | NA | NA | NA | PD | 6.1 | |
| L3‐7 | 30 | A11 | WHSC2‐103 | 0 | 0 | 0 | 19 | 20 | 21 | PD | 10.8 |
| SART3‐734 | 0 | 0 | 0 | 1042 | 1010 | 1003 | |||||
| Lck‐90 | 0 | 12 | 0 | 22 | 22 | 23 | |||||
| SART3‐109 | 0 | 0 | 0 | 0 | 0 | 11 | |||||
| KRM‐10 | 0 | 0 | 0 | ND | ND | ND | |||||
A blood test was performed after the third/sixth vaccination or disease progression, whichever occurred first. Pt no., patient number; OS(m), overall survival (months); SD, stable disease; PD, progressive disease; CTL, cytotoxic T lymphocytes; FIU, fluorescent intensity units; ND, not detected; NA, not available. –, Cases with the dashes had no post sixth sample.
Safety and tolerability
No DLT were reported in this study. Adverse events are summarized in Table 4. The most frequent treatment‐related adverse event was a dermatological reaction to the peptide vaccine at injection sites in 15 patients (71%) and fever in three patients (14%). Two patients had either a grade 2 herpes zoster or herpes labialis. No treatment‐related serious adverse events were observed. One patient in the 20‐mg cohort experienced a serious adverse event (increased grade 3 bilirubin) that was considered to be due to disease progression, not vaccination, by the independent review board.
Table 4.
Adverse event
| 10‐mg peptide | 20‐mg peptide | 30‐mg peptide | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 6 | n = 8 | n = 7 | n = 21 | ||||||||||
| G1 | G2 | ≥G3 | G1 | G2 | ≥G3 | G1 | G2 | ≥G3 | G1 | G2 | ≥G3 | All (%) | |
| Any AE | |||||||||||||
| Anemia | 4 | 2 | 5 | 4 | 13 | 2 | 15 (71%) | ||||||
| Injection site skin reaction | 5 | 7 | 3 | 15 | 15 (71%) | ||||||||
| Fever | 2 | 1 | 2 | 1 | 3 (14%) | ||||||||
| Increased ALT and AST | 1 | 1 | 1 | 1 | 1 | 3 | 2 | 5 (24%) | |||||
| Blood bilirubin increased | 1 | 1 | 1 | 1 | 2 (9%) | ||||||||
| Hyponatremia | 4 | 3 | 2 | 9 | 9 (43%) | ||||||||
| Diarrhea | 2 | 2 | 2 (9%) | ||||||||||
| Bladder infection | 1 | 1 | 1 (5%) | ||||||||||
| Dysgeusia | 1 | 1 | 1 (5%) | ||||||||||
| Hoarseness | 1 | 1 | 1 (5%) | ||||||||||
| Bronchopulmonary hemorrhage | 1 | 1 | 1 (5%) | ||||||||||
| Neuropathy‐sensory | 1 | 1 | 1 (5%) | ||||||||||
| Increased creatinine | 2 | 1 | 1 | 4 | 4 (19%) | ||||||||
| Herpes labialis | 1 | 1 | 1 (5%) | ||||||||||
| Herpes zoster | 1 | 1 | 1 (5%) | ||||||||||
| Treatment‐related AE | |||||||||||||
| Fever | 2 | 1 | 2 | 1 | 3 (14%) | ||||||||
| Injection site skin reaction | 5 | 7 | 3 | 15 | 15 (71%) | ||||||||
| Dysgeusia | 1 | 1 | 1 (5%) | ||||||||||
| Bladder infection | 1 | 1 | 1 (5%) | ||||||||||
| Herpes labialis | 1 | 1 | 1 (5%) | ||||||||||
| Herpes zoster | 1 | 1 | 1 (5%) | ||||||||||
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; G, grade.
Cytotoxic T lymphocytes and immunoglobulin G responses, and inflammatory cytokines
Cytotoxic T lymphocyte responses to each of the vaccinated peptides were detectable in only 4 of 20 patients, with 4 of 111 peptides tested prior to vaccination (Table 3). However, CTL activity to at least one peptide at the third and sixth vaccination increased in 2 and 3 of 6 (10 mg), 2 of 8 and 4 of 6 (20 mg), or 2 and 1 of 6 (30 mg) patients, respectively. In addition, CTL activity to the 10‐peptide mix was increased in 2 and 2 of 6 (10 mg), 3 of 8 and 3 of 6 (20 mg), or 0 and 0 of 6 (30 mg) patients tested, respectively. IgG levels at the third and sixth vaccinations were also increased in 1 and 1 of 6 (10 mg), 2 of 8 and 4 of 6 (20 mg), or 1 and 3 of 6 (30 mg) patients, respectively. C‐reactive protein levels at the time of the sixth vaccination increased in 2 of 5, 1 of 6 or 1 of 6 patients receiving 10, 20 or 30 mg of peptides, while haptoglobin levels increased in 2, 0 or 0 patients receiving 10, 20 or 30 mg of peptides, respectively (Table 5). Beta2‐microglobulin and Gc globulin increased in 2 and 3 of 5 patients tested in the 10‐mg cohort, respectively, but not in the other cohorts.
Table 5.
Soluble inflammatory factors
| Pt no. | C‐reactive protein | Haptoglobin | Beta‐2 microglobulin | Gc globulin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination | Post third† | Post sixth† | Prevaccination | Post third† | Post sixth† | Prevaccination | Post third† | Post sixth† | Prevaccination | Post third† | Post sixth† | |
| L1‐1 | 0.56 | 0.19 | 2.96 | 379.3 | 668.9 | 966.6 | 10.2 | 19.6 | 22.7 | 8.7 | 21.2 | 21.2 |
| L1‐2 | 0.08 | 0.04 | 0.07 | 3118.8 | 2946.8 | 1921.1 | 10.1 | 9.1 | 8.6 | 21.2 | 15.8 | 7.0 |
| L1‐3 | 0.91 | 1.39 | 2.96 | 1762.7 | 2798.0 | 3430.1 | 8.2 | 10.4 | 12.2 | 8.4 | 16.0 | 17.7 |
| L1‐4 | 0.05 | 0.06 | 0.08 | 1536.3 | 1749.1 | 3236.4 | 9.9 | 13.6 | 22.8 | 10.5 | 12.7 | 21.2 |
| L1‐5 | 0.14 | NA | NA | 2167.8 | NA | NA | 21.9 | NA | NA | 14.3 | NA | NA |
| L1‐6 | 0.66 | 0.88 | 1.00 | 964.8 | 1172.5 | 1104.0 | 21.9 | 21.1 | 15.9 | 21.2 | 20.3 | 16.4 |
| L2‐1 | 0.32 | 0.17 | 0.25 | 699.7 | 444.4 | 613.1 | 7.4 | 4.5 | 6.2 | 9.9 | 4.1 | 6.3 |
| L2‐2 | 0.07 | 0.11 | NA | 730.2 | 1157.9 | NA | 7.0 | 8.2 | NA | 4.7 | 5.2 | NA |
| L2‐3 | 0.03 | 0.01 | 0.03 | 1054.2 | 479.1 | 639.8 | 4.3 | 2.3 | 2.6 | 13.0 | 4.0 | 4.6 |
| L2‐4 | 0.98 | 0.12 | 0.18 | 1559.0 | 735.1 | 680.8 | 8.5 | 4.6 | 4.5 | 10.4 | 5.1 | 4.2 |
| L2‐5 | 0.01 | 0.12 | 0.06 | 614.1 | 611.6 | 581.1 | 2.3 | 3.3 | 2.3 | 4.5 | 3.9 | 3.7 |
| L2‐6 | 1.16 | 2.99 | NA | 1396.2 | 984.3 | NA | 7.2 | 9.0 | NA | 4.4 | 2.3 | NA |
| L2‐7 | 1.90 | 17.60 | 2.00 | 1180.0 | 1950.0 | 1540.0 | NA | NA | NA | NA | NA | NA |
| L2‐8 | 0.70 | 1.10 | 148.10 | 1480.0 | 1510.0 | 1970.0 | NA | NA | NA | NA | NA | NA |
| L3‐1 | 0.03 | 0.07 | 0.08 | 346.3 | 418.1 | 413.0 | 1.7 | 2.4 | 2.9 | 2.6 | 1.9 | 1.7 |
| L3‐2 | 0.26 | 0.41 | 0.24 | 500.5 | 564.7 | 428.9 | 4.1 | 5.1 | 4.1 | 1.8 | 1.6 | 1.4 |
| L3‐3 | 0.10 | 0.21 | 0.29 | 485.8 | 495.9 | 629.4 | 2.5 | 3.0 | 3.8 | 1.9 | 2.0 | 2.0 |
| L3‐4 | 0.01 | 0.00 | 0.01 | 361.2 | 313.8 | 272.4 | 2.0 | 1.9 | 1.6 | 5.8 | 2.1 | 2.0 |
| L3‐5 | 0.02 | 0.01 | 0.01 | 201.3 | 167.3 | 153.6 | 2.1 | 2.5 | 2.2 | 2.1 | 2.2 | 2.3 |
| L3‐6 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| L3‐7 | 1.50 | 1.50 | NA | 1960.0 | 1890.0 | NA | NA | NA | NA | NA | NA | NA |
A blood test was performed after the third/sixth vaccination or disease progression, whichever occurred first. Pt no., patient number; na, not available.
Clinical outcomes
Of the 21 patients evaluated in this study, six had stable disease (SD) and 15 showed PD (Table 6). Six cases with SD were observed after the sixth vaccination (3, 2 and 1 patient received 10, 20 and 30 mg). Among the six patients with SD, two had esophageal squamous cell carcinoma, two had colorectal adenocarcinoma, one had gastric adenocarcinoma, and one had anal canal squamous cell carcinoma. Three of five SD patients, who had been allowed to continue with peptide vaccinations on compassionate grounds, experienced a long, consistent SD for 26, 35 and 37 weeks, respectively. One patient (L1‐4) with metastatic esophageal squamous cell carcinoma showed an initial disease progression, followed by regression at 3.5 months after initial treatment (Fig. 2).
Table 6.
Patient characteristics, clinical response and immune response
| Patient number | Dose of KRM‐10 (mg) | Age/gender | PS | Primary lesion | Prior therapy | Number of vaccinations | Response† | CTL response‡ | IgG response | OS (m) | Between (x) and (y), (week) | Post‐treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1‐1 | 10 | 71/M | 1 | Esophagus | FU, CDDP, RT, Ope | 14 | SD | + | − | 13.9 | 26 | BSC |
| L1‐2 | 10 | 77/M | 1 | Esophagus | FU, CDDP, Ope, PTX | 6 | PD | + | − | 8.7 | 6 | BSC |
| L1‐3 | 10 | 63/M | 1 | Stomach | S‐1, CDDP, CPT, MMC | 6 | PD | − | − | 2.5 | 6 | BSC |
| L1‐4 | 10 | 72/F | 0 | Esophagus | FU, CDDP, RT, PTX | 16 | SD | + | − | 20.1 | 35 | CDK4/6 inhibitor |
| L1‐5 | 10 | 72/M | 1 | Stomach | Ope, FU, S‐1, L‐OHP | 6 | SD | − | − | 7.6 | 6 | RT |
| L1‐6 | 10 | 68/M | 0 | Esophagus | FU, CDDP, MEK inhibitor, PTX | 6 | PD | + | + | 3.0 | 6 | BSC |
| L2‐1 | 20 | 62/M | 1 | Colorectum | S‐1, CPT, Capecitabine, L‐OHP, BV, Pmab, Cmab | 6 | PD | + | + | 7.6 | 6 | BSC |
| L2‐2 | 20 | 72/M | 1 | Small intestine | Ope, FU, L‐OHP | 5 | PD | NA | NA | 3.4 | 5 | BSC |
| L2‐3 | 20 | 72/F | 0 | Colorectum | Ope, FU, L‐OHP, BV, CPT | 6 | PD | − | + | 9.8 | 6 | Regorafenib |
| L2‐4 | 20 | 70/F | 1 | Colorectum | Ope, UFT, FU, L‐OHP, BV, CPT, Pmab | 8 | SD | + | + | 5.1 | 11 | BSC |
| L2‐5 | 20 | 49/F | 0 | Colorectum | Ope, Capecitabine, CPT, S‐1, L‐OHP | 6 | PD | + | + | 4.0 | 6 | BSC |
| L2‐6 | 20 | 61/M | 0 | Colorectum | Ope, S‐1, CPT, BV, Capecitabine, L‐OHP | 3 | PD | NA | NA | 1.4 | 3 | BSC |
| L2‐7 | 20 | 69/M | 0 | Colorectum | Ope, FU, CPT, Pmab, L‐OHP, BV, TAS102, Cmab | 9 | SD | + | − | 19.9 | 13 | Regorafenib |
| L2‐8 | 20 | 77/M | 0 | Stomach | S‐1, CDDP, Ope, CPT, PTX | 6 | PD | + | − | 6.6 | 6 | BSC |
| L3‐1 | 30 | 65/M | 1 | Colorectum | Ope, FU, L‐OHP, BV | 6 | PD | − | + | 6.2 | 6 | BSC |
| L3‐2 | 30 | 75/F | 1 | Colorectum | FU, L‐OHP, CPT, Cmab | 6 | PD | + | + | 3.7 | 6 | BSC |
| L3‐3 | 30 | 61/M | 1 | Colorectum | Ope, UFT, FU, L‐OHP, CPT | 6 | PD | − | − | 12.8 | 6 | Regorafenib |
| L3‐4 | 30 | 72/M | 0 | Stomach | S‐1, CDDP, PTX | 6 | PD | + | − | 10.2 | 6 | CPT |
| L3‐5 | 30 | 63/F | 1 | Anal canal | UFT, RT, Ope, FU, CDDP, CPT, Pmab, PTX | 17 | SD | − | − | 16.6 | 37 | Ope |
| L3‐6 | 30 | 66/M | 0 | Colorectum | Ope, RT, S‐1, L‐OHP, BV, CPT | 2 | PD | NA | NA | 6.1 | 2 | CPT+Cmab |
| L3‐7 | 30 | 59/F | 1 | Stomach | S‐1, CDDP, Ope, DTX, CPT | 6 | PD | + | + | 10.8 | 6 | BSC |
†Clinical responses were evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1. ‡CTL responses were classified according to the number of peptides inducing positive CTL responses in patients. CPT, irinotecan; CTL, cytotoxic T lymphocytes; F, female; M, male; OS(m), overall survival (months); PD, progressive disease; PS, performance status; SD, stable disease; (x), start of vaccination; (y), completion of last treatment. Prior therapy: BV, bevacizumab; CDDP, cisplatin; Cmab, cetuximab; DTX, docetaxel; FU, 5‐fluorouracil; L‐OHP, oxaliplatin; MMC, mitomycin C; Ope, surgery; Pmab, panitumumab; PTX, paclitaxel; RT, radiation; UFT, tegafur/uracil.
Figure 2.
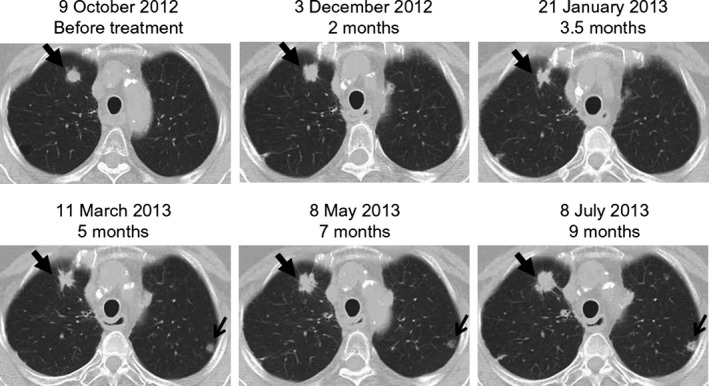
A closed arrow indicates the initial progression (2 months), followed by regression (3.5 months), and then progression again (7 and 9 months) of a pulmonary lesion in a patient (L1‐4). An open arrow shows a new pulmonary lesion.
Discussion
Optimal clinical outcomes have not ensued after the testing of cancer vaccines in the past two decades, with a consequent lack of drug approvals.5, 6, 7 Therefore, we aimed to develop and test different doses of a new type of CTL‐epitope peptide vaccine consisting of 10 mixed peptides (KRM‐10) that can be applied to a majority of cancer patients with different HLA alleles. KRM‐10 was well tolerated at doses up to 30 mg of peptides (3 mg per peptide) in gastrointestinal cancer patients who were refractory to standard chemotherapy. The most common adverse event observed was an injection site reaction. DLT were not observed in all three cohorts during 6 weeks of treatment. In addition, no cumulative and delayed toxicities were observed during the use of KRM‐10 on compassionate grounds after its initial (six times) use. These apparent safety characteristics of KRM‐10 are consistent with previously conducted evaluations of peptide‐based cancer vaccines.3, 4, 5, 6, 7, 10, 11, 12
Most previously conducted phase I studies of cancer vaccines failed to show either clear evidence of an appropriate dose setting or a definitive predictive biomarker, which, in turn, hampered the further development of clinical trials.3, 4, 5, 6, 7, 10, 11, 12 The present study showed that both CTL and IgG responses, favorable markers for a cancer vaccine as reported previously,10, 11, 12 were more frequently increased after the vaccination of patients in the 20‐mg cohort as compared with the other two groups. In contrast, soluble inflammatory factors, including C‐reactive protein and haptoglobin, which are unfavorable markers for cancer vaccines as previously reported,10, 11, 12, 17, 18 were somewhat increased after the vaccination of patients in the 10‐mg group. These results suggest that a dose of 20 mg of KRM‐10 should be recommended for any future phase II study for not only its observed safety but also because of the demonstration of having the most potent activity that augments the immune response, with minimal effects on soluble inflammatory factors among the three different doses tested.
However, of note, the 10‐mg group was dominated by advanced esophageal cancer patients, whereas the other groups were dominated by colorectal cancer patients. This difference might influence the immune responses to the KRM‐10. This issue shall be considered in the next step of clinical study, although each of these 10 peptides equally boosted the peptide‐specific immune responses for the majority (>more than 50%) of both advanced esophageal cancer and colorectal cancer patients, who received these peptides in the clinical study of personalized peptide vaccination regimens (unpublished results).10
It has been well documented that efficiently primed CTL induced by cancer vaccines often lose their responsiveness to tumor cells, primarily due to immunosuppression by Tregs and myeloid‐derived suppressor cell (MDSC), and also by T cell inhibition mediated by checkpoint molecules, such as CTL‐associated protein 4 (CTLA‐4) and programmed cell death 1 (PD‐1).1, 2, 11, 12 Acute‐phase inflammatory factors, including C‐reactive protein, haptoglobin, serum amyloid A and IL‐6, are well known soluble mediators for Treg‐induced and MDSC‐induced suppression against vaccine‐induced immune activation.10, 11, 12, 14, 17, 18 Precise mechanisms involved in the phenomenon of lower and higher peptide doses (10 and 30 mg of peptides), but not the modest dose (20 mg of peptides), inducing lower levels of CTL and IgG responses are presently unknown. These results, however, are consistent with a recently conducted phase I dose setting study of a 20‐mixed peptide vaccine for advanced hormone‐refractory prostate cancer patients in which lower and higher peptide doses (6 and 60 mg total doses with 0.3 and 3 mg of each peptide, respectively), but not a modest dose (20 mg total dose with 1 mg of each peptide), induced lower levels of CTL and IgG responses.14 One possible explanation could be that both lower, as well as excess, amounts of antigens often induce immune tolerance or suppressive regulating activity rather than immune activation, respectively. Although this issue will be further investigated, these results suggest that approximately 20 mg of the CTL epitope peptide per injection would be appropriate for peptide‐specific immune induction.
At least 4 of the 10 mixed peptides were matched to each of the 21 enrolled patients in terms of HLA‐class IA types, as shown in Table 3. After vaccination with 10 mixed peptides, HLA‐matched peptides would be recognized by CTL, but peptides with differing HLA alleles would, theoretically, be metabolized without any apparent biological effects. There may be some concern in regard to the competition between peptides in the KRM‐10 peptide mix to bind to the same HLA restriction element. Although peptide competition was not directly evaluated here, we found that the frequency of a CTL response to each of the 10 peptides was similar to that of the 10‐peptide mix. This is consistent with what has been reported for other multiple peptide vaccines, suggesting that competition for binding to the same HLA molecule is not significant enough to limit immunogenicity.14, 19 Indeed, Hazama et al.20 have also recently reported that the CTL induction was not different between separate injections and mixed injections.
Clinical efficacy was not an endpoint of this small scale, phase I study. However, information on clinical efficacy is of the utmost importance in the development of a cancer vaccine. The histological type of squamous cell cancer came from the esophagus or anal canal, while adenocarcinoma was derived from the stomach, intestine or colorectal tissues. Patients did not exhibit a complete or partial response, but some patients displayed SD, regardless of a previous history of intensive treatment for gastrointestinal cancers. All three patients with a prolonged SD were being treated for squamous cell carcinomas of the esophagus and anal canal. Although the reasons involved in this issue were presently unclear, EGFR800–80 contained in the KRM‐10 might be in part involved in better responses of three squamous cell carcinomas patients, because squamous cell cancer of the esophagus showed higher frequency of EGFR expression, compared to adenocarcinoma of stomach or colorectum, as shown in Table 1, and better clinical efficacy of anti‐EGFR therapy for squamous cell cancer of the esophagus or head and neck is often reported. In contrast, colorectal cancer patients entered in this study mostly received anti‐EGFR therapy and became refractory to the treatment. Further studies are needed to better understand this issue.
The progression free survival and overall survival of a few patients under KRM‐10 vaccination was somewhat longer than those under best supportive care. No patients received other than KRM‐10 during the stable disease, suggesting the contribution of KRM‐10 vaccination to at least progression free survival among a part of the patients. After disease progression, 13 of 21 patients received only best supportive care, four patients received targeted therapies, and the other four received other anti‐cancer therapies, as shown in Table 6. Clinical benefits should be carefully evaluated in the next step of clinical trials.
It is also of importance to note the limitations of the present study. First, advanced gastrointestinal cancer patients had a relatively large tumor burden resistant to standard therapies. Second, the median number of times of vaccination was only six in this study, which may not have been sufficient for the induction of potent immune responses with regard to the history of previously conducted peptide‐based cancer vaccines.5, 6, 7, 10, 11, 12
In conclusion, a 20‐mg KRM‐10 vaccine was determined to be a recommended phase II dose because of its safety and because it exhibited the most potent activity to augment the immune response of the three different doses tested.
Disclosure Statement
Akira Yamada has a leadership position and stock ownership from Green Peptide. Kyogo Itoh has stock ownership from Green Peptide, and received research fund from Taiho Pharmaceutical. The other authors have no conflict of interest to declare. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Supporting information
Table S1. HLA restriction of 10 peptides of KRM‐10.
Acknowledgments
The authors wish to thank Yushi Nagai and Chika Asami for data management, Tomoyo Manita and Tsukasa Shinohara for providing technical assistance, and Mayumi Ayabe and Kumiko Hirayama for collecting clinical data. This study was supported in part by a grant from a research program of The Project for Development of Innovative Research on Cancer Therapeutics (P‐DIRECT) from The Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Sendai Kousei Hospital.
Cancer Sci 107 (2016) 590–600
Funding Information
The Ministry of Education, Culture, Sports, Science and Technology, (Grant/Award Number: ‘The Project for Development of Innovative Research’).
References
- 1. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powles T, Eder JP, Fine GD et al MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. [DOI] [PubMed] [Google Scholar]
- 3. Noguchi M, Yao A, Harada M et al Immunological evaluation of neoadjuvant peptide vaccination before radical prostatectomy for patients with localized prostate cancer. Prostate 2007; 67: 933–42. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto K, Noguchi M, Satoh T et al A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int 2011; 108: 831–8. [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg SA, Yang JC, Schwartzentruber DJ et al Immunologic and therapeutic evaluation of synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4: 321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartzentruber DJ, Lawson DH, Richards JM et al gp100 peptide vaccine and interleukin‐2 in patients with advanced melanoma. N Engl J Med 2011; 364: 2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez SA, Hofe E, Kallinteris NL et al A new era in anticancer peptide vaccines. Cancer 2010; 116: 2071–80. [DOI] [PubMed] [Google Scholar]
- 8. Cheever MA, Allison JP, Ferris AS et al The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15: 5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014; 14: 135–46. [DOI] [PubMed] [Google Scholar]
- 10. Kibe S, Yutani S, Motoyama S et al Phase II study of personalized peptide vaccination for previously treated advanced colorectal cancer. Cancer Immunol Res 2014; 2: 1154–62. [DOI] [PubMed] [Google Scholar]
- 11. Noguchi M, Sasada T, Itoh K. Personalized peptide vaccination: a new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol Immunother 2013; 62: 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem 2014; 21: 2332–45. [DOI] [PubMed] [Google Scholar]
- 13. Noguchi M, Uemura H, Naito S, Akaza H, Yamada A, Itoh K. A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low‐dose estramustine in HLA‐A24‐positive patients with castration‐resistant prostate cancer. Prostate 2011; 71: 470–9. [DOI] [PubMed] [Google Scholar]
- 14. Noguchi M, Arai G, Matsumoto K et al Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration‐resistant prostate cancer: dose‐related immune boosting and suppression. Cancer Immunol Immunother 2015; 64: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamada T, Terasaki Y, Sakamoto S et al Feasibility study of personalized peptide vaccination for advanced non‐small cell lung cancer patients who failed two or more treatment regimens. Int J Oncol 2015; 46: 55–62. [DOI] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guileline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 17. Pang X, Tashiro K, Eguchi R et al Haptoglobin is a prognostic biomarker for cancer vaccine in peripheral blood of patients with advanced castration‐resistant prostate cancer. Biosci Biotechnol Biochem 2013; 77: 766–70. [DOI] [PubMed] [Google Scholar]
- 18. Yoshiyama K, Terazaki Y, Matsueda S et al Personalized peptide vaccination in patients with refractory non‐small cell lung cancer. Int J Oncol 2012; 40: 1492–500. [DOI] [PubMed] [Google Scholar]
- 19. Chianese‐Bullock KA, Irvin WP Jr et al A multipeptide vaccine is safe and elicits T‐cell responses in participants with advanced stage ovarian cancer. J Immunother 2008; 31: 420–30. [DOI] [PubMed] [Google Scholar]
- 20. Hazama S, Nakamura Y, Takenouchi H et al A phase I study of combination vaccine treatment of five therapeutic epitope‐peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J Transl Med 2014; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. HLA restriction of 10 peptides of KRM‐10.


