Abstract
Purpose
p53 is mutated in about 50% of human cancers, mostly through missense mutations. Expression of mutant p53 is associated with poor clinical outcomes or metastasis. Although mutant p53 is inherently instable, various stressors such as DNA damage or expression of the oncogenic Kras or c-myc affect the oncogenic properties of mutant p53. However, the effects of inflammation on mutant p53 are largely unknown. Interleukin-27 (IL-27) is an important immunomodulatory cytokine but its impact on mutant p53-driven tumorigenesis has not been reported.
Experimental Design
IL27RA−/− mice were bred with mutant p53 heterozygous (p53R172H/+) mice to obtain IL27RA−/−p53H/+ and IL27RA−/−p53H/H mice. Mouse survival and tumor spectra for the cohort were analyzed. Stability of p53 protein was analyzed via immunohistochemistry and western blot.
Results
this study unraveled that lack of IL-27 signaling significantly shortened the survival duration of mice with tumors expressing both copies of the mutant p53 gene (Li-Fraumeni mouse model). Interestingly, in mice that were heterozygous for mutant p53, lack of IL-27 signaling not only significantly shortened survival time but also doubled the incidence of osteosarcomas. Furthermore, lack of IL-27 signaling is closely associated with increased mutant p53 stability in vivo from early age.
Conclusions
These results suggest that IL-27 signaling modulates the oncogenic properties of mutant p53 in vivo.
Keywords: mutant p53R172H, tumor incidence, survival, IL-27, IL27RA, osteosarcoma
INTRODUCTION
p53, which functions as a tumor suppressor, is mutated in about 50% of tumors, often through missense mutations occurring in the DNA-binding domain. Aside from affecting the transcriptional activity of p53, these missense mutations often are associated with an increase in the half-life of p53, causing mutant p53 to accumulate in the tumor cells and often acquire a gain-of-function phenotype. Although mutant p53 is inherently unstable, various stressors affect the oncogenic activation and properties of this protein. For example, DNA damage and oncogene activation such as RAS mutations, c-myc, or p16INK4a can alter the oncogenic properties of mutant p53 (1, 2).
Interleukin (IL)-27 is known to be an important pro-inflammatory or anti-inflammatory cytokine. IL-27 has been increasingly studied for its role in regulating the intensity and duration of T cell response. Briefly, IL-27 can antagonize IL-2 production (3) and GATA3 expression by limiting Th2 response (4), as well as decrease RoRγ expression by limiting Th17 (5) and increase IL-10 expression by inducing Th10 cells (6–8). IL-27 signaling through STAT1 and T-bet can also enhance Th1-type responses. Furthermore, IL-27 has been shown to have anti-tumor effects in subcutaneously injected neuroblastoma, colon carcinoma, lung carcinoma, and acute myeloid leukemia cells (9) (10, 11) (12) (13).. However, IL-27 can also increase PDL1, an important immune-suppressor checkpoint in both cancer and inflammation (14). Therefore, the biology of IL-27 is complex and context-dependent; IL-27 may either dampen or promote different types of inflammation or cancer.
Although inflammation plays a central role in all aspects of cancer, from tumor initiation to progression and metastasis, it remains unknown if or how inflammatory signals stabilize or promote oncogenic properties, such as tumor development and survival of oncogenes such as mutant p53. Recent mouse models have been developed to better dissect the role of mutant p53. Specifically, mice were bred with mutations at amino acid 172 of p53 (R172H, Li-Fraumeni mice), corresponding to the human p53 hot spot mutation at amino acid 175. However, how endogenous levels of IL-27 affect tumorigenesis or survival in these mice remains largely unknown. In the current study, we found that lack of IL-27 signaling significantly shortens survival duration in mice with both copies of the mutant p53 gene (Li-Fraumeni mice, p53H/H). Interestingly, in mice heterozygous for mutant p53 (p53H/+), lack of IL-27 signaling not only significantly shortened survival but also doubled the incidence of osteosarcomas. This suggests that IL-27 signaling negatively modulates the oncogenic properties of mutant p53 and lack of IL-27 signaling is closely associated with early mutant p53 stability in vivo.
MATERIALS AND METHODS
Transgenic Mice
We bred IL27RA−/− mice with p53R172H/+ (generously donated by Dr. Lozano, University of Texas MD Anderson Cancer Center) mice to create IL27RA−/−p53R172H/HIL27RA−/−p53R172H/+ and IL27RA−/−p53+/+ mice, which were born at a Mendelian ratio. All mouse strains were maintained in C57Bl/6 cohorts. IL27RA−/− mice were previously donated by Dr. Frederic de Sauvage (Genentech). Mice were monitored over time for tumor development. Genotyping was performed as described below for both p53 and IL-27 receptor alpha (IL27RA). For the cohort studies, mice were euthanized when they met the criteria for euthanasia, mainly related to tumor burden or distress. For the time course study, mice from each genotype were euthanized at the indicated times. Tissue was fixed in neutralized paraformaldehyde prior to sectioning. All procedures involving animals were approved by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee.
Hematoxylin-Eosin Staining and Pathologic Scoring
Sections of paraffin-embedded tissues were stained with hematoxylin and eosin. The pathologic readings for various abnormal growths were scored by a pathologist at MD Anderson. Images were captured using a Nikon Eclipse Ti microscope.
Micro-CT image Acquisition and Analysis
Computed tomography (CT) images were acquired as previously described (15). Briefly, images were acquired in 5 100-ms frames at 1° rotation increments with an X-ray tube potential of 80 kVp and a current of 450 µA using an RS-9 micro-CT scanner (GE Healthcare, London, Ontario, Canada). Two adjacent image volumes were acquired with an overlap of 40% of the field of view in the axial direction, and these images were stitched together after reconstruction. Images had a nominal isotropic resolution of 91 µm and required approximately 25 minutes of scan time. The bone was visualized using the GEHC Microview Software (GE Healthcare, London, ON, Canada). All image isosurfaces were visualized and analyzed for bone mineral density using the same image threshold value.
Immunohistochemistry Staining
Briefly, paraffin-embedded unstained slides from IL27RA−/− p53R172H/+ or control (p53R172H/+) mice were processed for heat-induced antigen retrieval in pH 6.0 citrate buffer for 40 minutes in the steamer. Slides were incubated with rabbit monoclonal anti-p53 (1:100, Novus Antibodies) or rabbit anti-p21 (1:50, Santa Cruz) antibodies diluted at 1:100 in goat serum/fish gel/BSA blocking buffer overnight at 4°C. The next day, tissue was washed, blocked for 15 minutes in blocking buffer, and incubated with anti-rabbit IgG-Biotin (diluted at 1:500 in blocking buffer) for 1 hour at room temperature, followed by 30 minutes of incubation in the ABC enhanced Vectastain Kit system (Vector Laboratories, Burlingame, CA). Similarly, other antibodies used for IHC staining included: Ki67 (1:800, Abcam); CD3 (1:100, Abcam), NCR1 (1:100, Abcam), B220 (1:50, BD Pharmingen), Foxp3 (1:100, Cell Signaling), Endoglin (1:100, Vector Laboratories).
Genotyping
DNA was extracted from ear tissue, and polymerase chain reaction (PCR) analysis for the p53 gene was performed using the primer sequence described by Lang et al. (16). The presence of the IL27RA gene was confirmed using the forward primer sequence CAAGAAGAGGTCCCGTGCTG and the reverse primer sequence TTGAGCCCAGTCCACCACAT. PCR primers used to determine the absence of IL27RA were as follows: GCTTTCGTCTCCCGTGTGCT (forward) and TGAGCCCAGAAAGCGAAGGA (reverse).
Invasion and migration assays
The osteosarcoma H318 cell line derived from a p53H/+ mouse and expresses functional levels of IL27RA was used for these studies (17). The invasion assay was performed using Transwell inserts (Greiner Bio-one) in 24-well plates. To perform invasion assays, inserts were pre-coated with 30 µl phenol red-free matrigel (BD Biosciences) for 1 hour at 37 C. Approximately, 105 cells in 100 µl heat-inactivated complete RPMI media (with or without rIL27) were plated on top of the matrigel-coated insert. was placed at each well of 24 well plate. Then the insert was placed on top of the 500 µl complete media containing 10% FBS in each well of 24 well plate overnight at 37C. 48hrs later, the media was removed from lower chamber and the insert was incubated with 500 µl media containing 8 µM Calcein-AM for 1 hour at 37 C. Then the insert was transferred to a new well containing 500 µl of pre-warmed 1 mM EDTA to detach the cells on the outer surface of the insert. 100 µl of cell suspension was transferred to each well (triplicate) of 96 well plate to measure fluorescence using spectramax. The migration assay was performed in a similar fashion with 104 cells in 100 µl (with or without rIL27) without coating the insert with matrigel. Cells inside the inserts were removed with cotton-tipped swabs on next day after overnight incubation.
Proliferation assay
H318 cell lines was serum starved overnight. Approximately 10,000 cells/well were treated with or without rIL27 (20ng/mL) containing heat inactivated serum media in quadruplicate for 24 or 48 hours. For 48 hrs treatment, fresh heat-inactivated media (with or without rIL27) was added to each well after 24hrs. To measure the viability of H318 cells after treating with or without rIL27, 100 µl of CyQUANT NF Cell Proliferation Assay reagent was added to each well after aspiration of medium. After incubation for 1 hour at 37°C, fluorescence was measured (excitation 485 nm, emission 538 nm) on a SpectraMax Geminin EM microplate reader (Molecular Devices) using Softmax Pro version 5.4.
Bone marrow isolation
Bone marrow cells from age-matched wildtype, p53H/+ or IL27RA−/−p53H/+ mice were isolated as previously described (18) and seeded at 3x106 in RPMI media in a 6 well plate. These cells were irradiated with 6Gy or left un-irradiated. These samples were harvested 4 and 24 hours post irradiation.
Flow cytometry analysis
Isolated bone marrow cells from 3 month old mice and were blocked for non-specific staining in 2% BSA and with anti-CD16 (5 µg/mL) for 20′ at 4°C. Afterwards, cells were stained in 2% BSA with anti-CD4-Violet421 (1:100, Pharminogen), anti-CD8-PE-Cy7 (1:100, Biolegend), anti-F4/80- Fitc (1:100, eBiosciences), anti-CD3-e450 (eBiosciences), anti-NK1.1- PE-Cy7 (eBiosciences), anti-CD19-Violet421 (1:100, Biolegend) and anti-B220-PerCP (1:100, Biolegend), anti-CD11c-Violet 421 (1:100, Biolegend), anti-MHCII-PerCP (1:100, Biolegend). At last, the cells were washed twice in 2% BSA and analyzed by flow cytometry using Attune (Invitrogen), and FlowJo.
Western Blot
Briefly, cells were lysed in lysis buffer, and supernatants were quantified for protein amount. Eighty microgram of cell lysate was loaded in 10% SDS-PAGE, transferred to a nitrocellulose membrane and incubated with primary antibody overnight at 4° C (1000x dilution for anti-p53 (Novus), 1000x anti-actin.
Growth of primary tumor cells in NSG mice
Femur, tibia, and lumbar vertebras from mice were cut aseptically in 1.5 mm small pieces, rinsed in PBS, and placed in digestion media consisting 5 mg collagenase, 5 mg pronase, and 1 mg DNAse for 45 minutes at 37° C (19). Following digestion, single cell suspension was passed through a 70 µm strainers twice. And lastly, these cells were rinsed in PBS three times prior to intraosseous (1*107 in 15 µL PBS) or subcutaneous injections (1*107 in 30 µL PBS) as previously described (20).
Statistical Analysis
The Student t test was used to analyze differences between groups. P < 0.05 was considered statistically significant. GraphPad Prism for Windows was used to prepare and analyze the graphs (GraphPad Software, La Jolla, CA).
RESULTS AND DISCUSSION
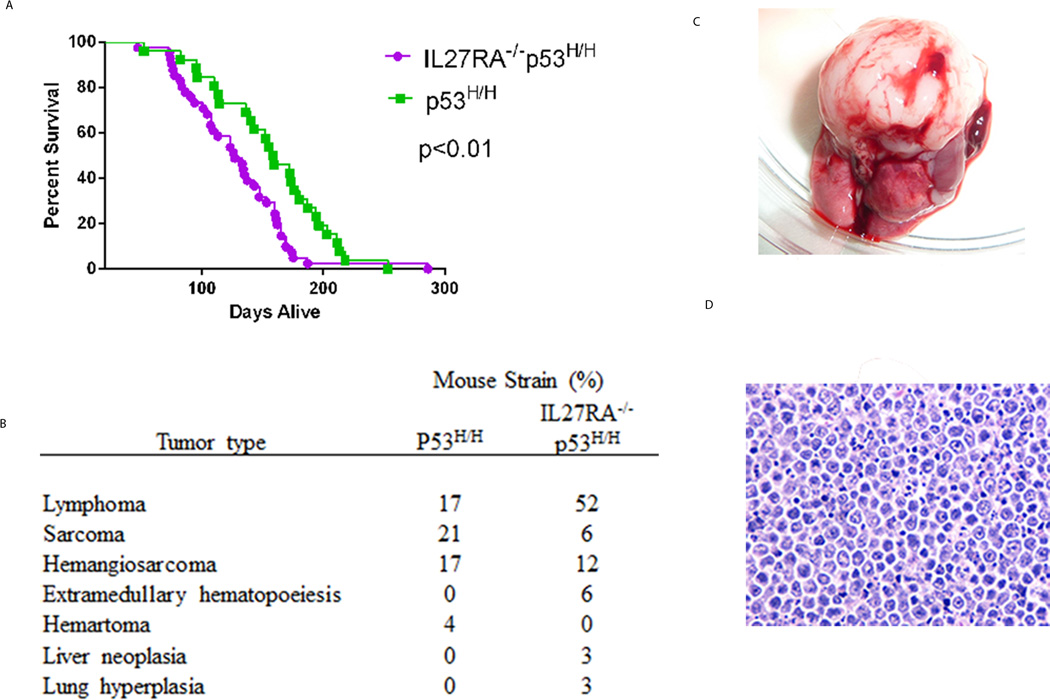
The role of endogenous IL-27 during spontaneous tumorigenesis, and more specifically in a Li-Fraumeni mouse model, is not known. To understand the in vivo significance of IL-27 in regulating mutant p53-driven oncogenic properties, we crossed IL27RA−/− mice with heterozygous p53H/+ mice, which allowed us to examine tumor incidence and survival between IL27RA−/− p53H/H mice and p53H/H mice. Interestingly, we found that mice lacking IL27RA signaling that carried both alleles of mutant p53 (IL27RA−/− p53H/H mice) had significantly shorter survival times than p53H/H mice (Figure 1A), suggesting that endogenous IL-27 signaling has antitumor properties. We observed a similar spectrum of tumor development between IL27RA−/−p53H/H mice and p53H/H mice, including malignancies such as lymphoma and sarcoma (Figure 1B, C, D). However, the incidence of lymphomas in IL27RA−/−p53R172H/H mice was 3-fold higher than in p53R172H/H mice.
Figure 1. Increased tumorigenesis in homozygous p53H/H mice lacking IL27RA signaling.
(A) Kaplan-Meier survival curve of IL27RA−/− p53H/H (N=41) compared with p53H/H mice (N=26). P< 0.05. (B) Tumor spectrum differences in tumor incidence between IL27RA−/− p53R172H/H compared with p53R172H/H mice. (C, D) Photomicrograph and hemotoxylin and eosin (HE) staining of thymus lymphoma tumor that occurs most frequently in IL27RA−/− p53H/H.
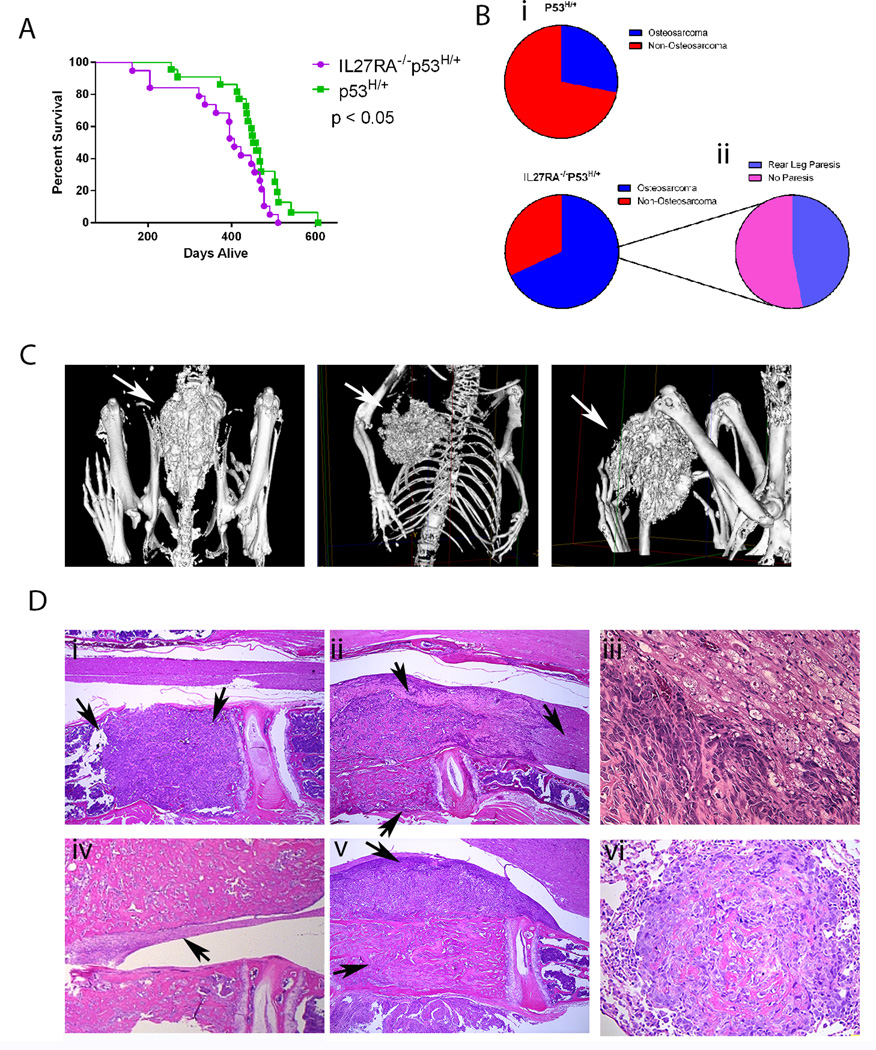
Because IL27RA−/−p53R172H/H mice have a short survival span, some important IL27RA−/−-facilitated oncogenic interactions may be difficult to observe in these mice. Therefore, we sought to investigate the role of IL-27 signaling in p53R127H/+ heterozygote background. Therefore, we compared survival and tumor spectrum between IL27RA−/−p53H/+ and p53H/+ mice (IL27RA−/−p53H/+ mice have a longer survival time than p53H/H mice because these mice carry only 1 mutant p53 allele). Similar to the IL27RA−/− p53H/H mice, IL27RA−/−p53H/+ mice had significantly shorter survival times than p53H/+ mice (Figure 2A). Interestingly, the incidence of osteosarcomas in IL27RA−/− p53R172H/+ mice (68%) was twice that in p53R172H/+ mice (Figure 2B). More than 80% of these IL27RA−/− p53H/+ osteosarcomas were osteblastic and 12% had fibroblastic and giant cell differentiation.
Figure 2. Increased tumorigenesis and doubling of osteosarcoma incidence in heterozygous p53H/+ mice lacking IL27RA signaling when compared to p53H/+ mice.
(A) Kaplan-Meier survival curve of IL27RA−/− p53H/+ (N=18) compared with p53H/+ (N=22) mice. P< 0.05. (B) Difference in osteosarcoma incidence between IL27RA−/− p53H/+ and p53H/+ mice (i) and percentage of mice with osteosarcoma that develop rear leg paresis (ii). (C) micro-CT images portraying osteosarcomas in different anatomical positions. (D) Representative HE photomicrographs of multicentric osteosarcomas developing throughout different vertebral bodies through the spine (i) osteosarcoma from vertebral body infiltrated the spinal cord (ii), osteosarcoma causing Wallerian degeneration (iii), compressive myelopathy (iv), osteosarcoma from vertebral body expended into spinal canal and infiltrated subjacent muscle (v), and HE photomicrographs from metastatic lesions of osteosarcomas in the lungs (vi).
Lastly, 41 % of the IL27RA−/−p53H/+ mice that had osteosarcomas progressed to rear leg paresis (Supplementary Figure 1Figure 2B), while none of p53H/+ mice in our cohort developed any of this phenotype.
Similar to human patients, micro-CT imaging showed that IL27RA−/− p53H/+ mice developed tumors in various anatomical sites, including the spine, ribs, or tibia (Figure 2C). Since 41% of osteosarcoma-positive IL27RA−/−p53H/+ mice developed rear leg paresis, compressive myelopathy and Wallerian degeneration were observed histologically in these mice (Figure 2D).
Interestingly, osteosarcomas occurred in multiple locations simultaneously in the IL27RA−/−p53H/+ mice. Within the same mouse, we found multicentric osteosarcoma in the femur and throughout the spine, including the lumbar, thoracic, and cervical vertebrae (Figure 2D). These multicentric osteosarcomas were found in 41% of the IL27RA−/−p53H/+ mice. And lastly, these osteosarcomas also had the potential not only to invade into the spinal cord, spinal canal, or subjacent muscle, but also to form metastases to the lung (Figure 2D). However, the metastatic potential to the lung was not increased in IL27RA−/−p53H/+ mice when compared to p53H/+. These observations suggest that lack of IL-27 signaling might generate a tumor-promoting environment.
To determine whether these osteosarcomas were aggressive, we transplanted the primary tumors into NSG mice. Briefly, we collected primary bone marrow from the femur or spine of mice that did not have visible tumor growth, minced the bone marrow, and placed it in enzymatic digestion media. Single cell suspensions were injected directly into NSG mice either subcutaneously or orthotopically via intra-osseous injections. Within 6–10 weeks, these mice developed visible tumors, which become aggressive osteosarcomas (Supplementary Figure 2A-C).
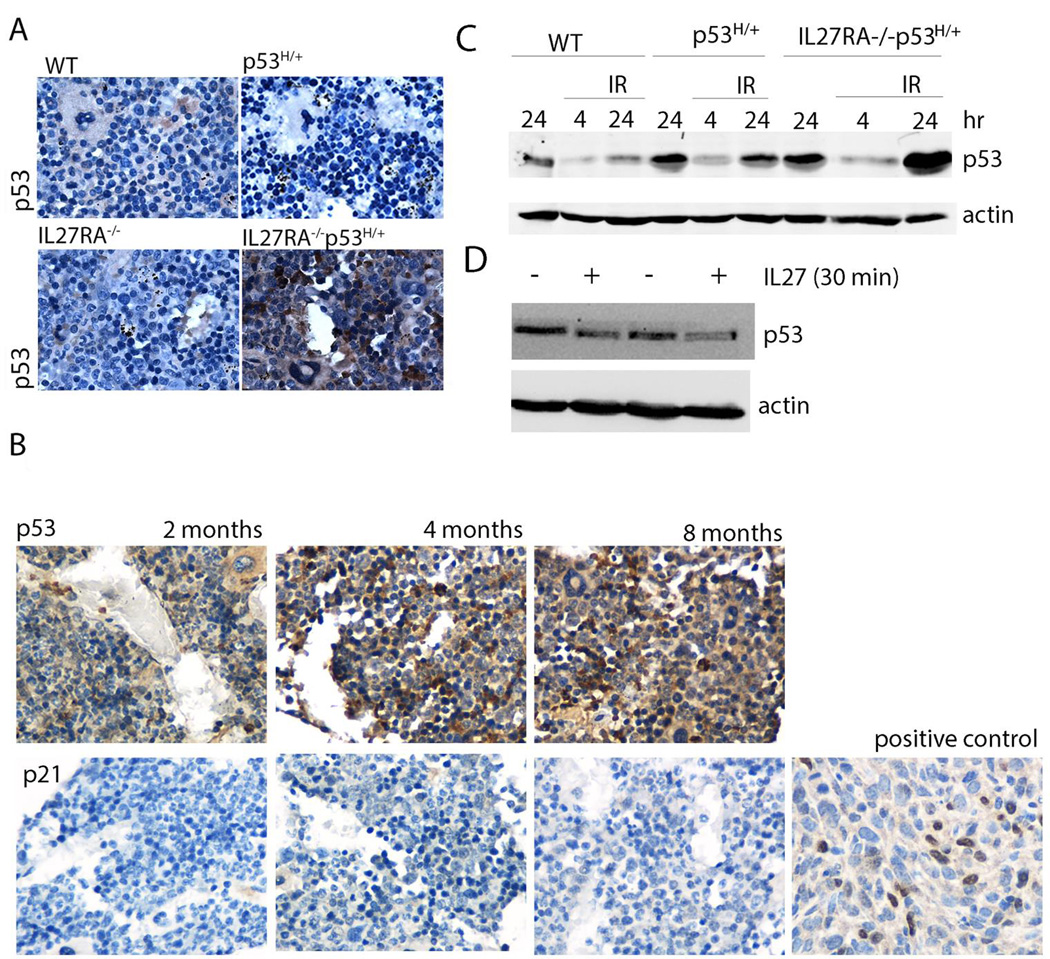
Mutant p53 protein stability is often associated with oncogenic functions of this protein. Because IL27RA−/− p53H/+ mice have a higher incidence of osteosarcomas and shorter survival times than p53H/+ mice, one possibility is that the lack of IL-27 signaling increases the number of cells with stable mutant p53 protein at a faster rate than under normal IL-27 signaling conditions. To examine the stability of mutant p53 over time, we stained for p53 healthy tumor-free bone marrow tissue derived from the spinal vertebrae of 8 month age-matched wild-type, IL27RA−/−p53H/+ and IL27RA−/− p53H/+ mice (Figure 3A). Indeed, we found significant expression of p53 in IL27RA−/− p53H/+ mice but not in controls. Stable p53 was detected as early as 2 months of age, but the levels of p21 were not detectable (Figure 3B). These findings suggest that the absence of IL-27 signaling modulates the oncogenic properties of mutant p53 in vivo and is closely associated with early mutant p53 stability.
Figure 3. Lack of IL27 signaling stabilizes mutant p53 in vivo and in vitro.
(A) p53 staining via immunohistochemistry of bone marrow vertebral bodies from 8 month old wildtype, IL27RA−/− p53R172H/+ , and IL27RA−/− p53H/+ mice. (B) p53 and p21 staining via immunohistochemistry of bone marrow vertebral bodies from 2, 4, and 8 month old IL27RA−/− p53H/+ mice including a positive control for p21. (C) Bone marrow cells derived from aged wildtype, p53H/+ and IL27RA−/−p53H/+ mice were seeded in a 6-well plate and either irradiated (IR) or left un-irradiated. Cells were collected at the indicated time points and were probed for p53 and actin. (D) H318 and H76 osteosarcoma cells were treated with rIL27 for 30 mins at 20ng/ml and collected lysates were probed for p53 levels and actin,
p53 is stabilized in response to stress signals, which could come from either irradiation, or from the stress of exposing primary cells in tissue culture growth and oxygen exposure. To test whether different stressor modulate p53 stability in absence of IL27, we exposed bone marrow cell from wildtype, p53H/+ and IL27RA−/−p53H/+ age-matched mice to irradiation and growth in culture. We hypothesize that bone marrow cells derived from IL27RA−/−p53H/+ may have higher levels of p53 in response to in-vitro culture or due to irradiation. Indeed, bone marrow cells from IL27RA−/−p53H/+ had higher levels of p53 stability when grown in culture (compared to other genotypes at 24 hours) and when exposed to irradiation (See Figure 3C).
Additionally, the authors tested whether recombinant IL27 (rIL27) could reduce p53 levels in osteosarcomas cell lines derived from p53H/+ mice (17). Indeed, short term treatment (30 minutes) with rIL27 has modestly reduced the p53 levels in these cells (Figure 3D).
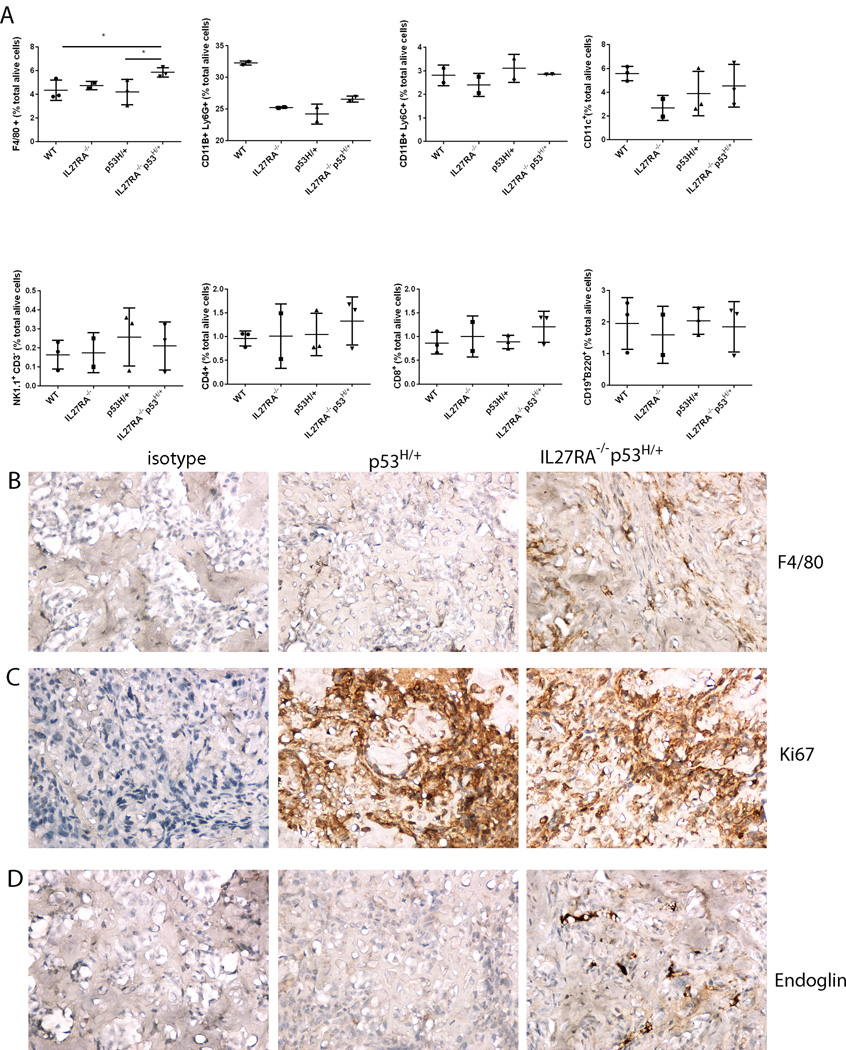
Natividad and colleagues showed that absence of IL27 signaling enhanced tumor growth in a carcinogen-induced fibrosarcoma and oncogene-driven mammary carcinoma. Lack of IL27 signaling was associated with a decrease of interferon-γ-positive CD4 and CD8 T cells and absence of T regulatory cells (21). To understand which immune cell population may be involved in our model in absence of IL27 signaling, we performed an immune cell profiling among different mice at age 3 months in the bone marrow (pre-malignant niche). No significant changes were seen in immune cell subgroups amongst all genotypes, except the number of F4/80-positive cells were significantly increased in the bone marrow of IL27RA−/−p53H/+ mice when compared to the controls (Figure 4A).
Figure 4. Absence of IL27 signaling increases macrophage and neo-angiogenesis marker Endoglin in mutant p53-driven osteosarcomas.
(A) No significant changes were seen in mice of different genotypes in CD4, CD8, B, NK, myeloid, CD11c cells in the bone marrow of 3 month old mice bearing indicated genetic modifications as analyzed by flow cytometry. Significant changes were seen in the number of macrophages in the bone marrow (B) present in the aged IL27RA−/−p53H/+ mice when compared to age-matched control ones analyzed via immunohistochemistry. (C-D) No differences in the proliferation rate of these end-stage tumors were noted (C, Ki67 staining), but a higher number of endoglin-positive cells in osteosarcomas from IL27RA−/−p53H/+. Representative migrographs from at least 3 independent mice. Dots indicate data from the bone marrow of individual mice. Pictures takes at 400X. *, p<0.05
Similarly, in end-stage osteosarcoma tumors derived from p53H/+ vs. IL27RA−/−p53H/+ ones, we found no differences in immune cell infiltrates (CD3, Foxp3, NCR1, or B220-positive cells. We only found differences in the number of macrophages in these established osteosarcomas (Figure 4B). The immune cell infiltrates in these osteosarcomas are different from Natividad which saw a T cell dependent mechanism in carcinogen-induces fibrosarcomas. One possibility for this discrepancy may rely on the fact that carcinogen-induced tumors are more immunogenic than oncogene-induced spontaneously-developing tumors.
To better understand on a mechanistic basis the increased tumorigenicity and shorten survival that occurs in p53H/+ mice lacking IL-27, we compared neo-angiogenesis markers and proliferation markers between p53H/+ vs. IL27RA−/−p53H/+ osteosarcoma cells. We saw no differences in terms of proliferation, but increase in neo-angiogenesis marker endoglin was observed (Figure 4C, 4D). Endoglin has been suggested as an appropriate marker for tumor-related angiogenesis and neovascularization (22).
And lastly, to better understand the role of IL27 in osteosarcoma tumor cells, we determined the effects on proliferative capacity, migration and invasive properties on H318 osteosarcoma that is derived from p53H/+ mice. H318 also contains IL27RA expression. Interestingly, we found that in vitro rIL27 has a modest increase in osteosarcoma cell proliferation and invasion, but not migration (Supplementary Figure 3A-C). However, these effects are derived from cell lines in vitro while neglecting the immune response and in absence of the tumor microenvironment implying the importance of functionally understanding the biological significance of these cytokines in vivo.
Our study is one of the first to report that, in addition to other oncogenes such as Kras or c-myc, inflammatory signals such as IL-27 can negatively regulate the oncogenic properties of mutant p53.
The mouse studies reported here are in concordance with a study in human patients showing that IL27p28 levels were significantly lower in patients with osteosarcoma than in healthy controls (23). Furthermore, in that study, as the disease progressed, the levels of IL27p28 were further reduced. The antitumor properties of IL-27 in the Li-Fraumeni mouse model were also closely associated with mutant p53 stability in our study. One possible explanation is that inflammatory signals such as the absence of IL-27 signaling stabilize p53 in vivo. Another explanation could be that IL-27 promotes immunosurveillance to eradicate cells with excessive levels of stable p53.
In summary, endogenous levels of IL-27 affected the oncogenic properties of mutant p53 in vivo in a Li-Fraumeni mouse model. Lack of IL-27 signaling led to shortened survival times and increased incidence of osteosarcomas, suggesting that lack of IL-27 signaling was closely associated with early mutant p53 stability.
Supplementary Material
TRANSLATIONAL RELEVANCE.
p53 is the most commonly mutated gene in cancer and the oncogenic properties of mutant p53 are highly dependent on protein stability. Understanding and targeting molecules that enhance mutant p53 stability and subsequently p53-driven oncogenesis is of utmost importance in cancer prevention and progression. Inflammatory signals play a crucial role in the initiation of tumorigenesis. It is not understood if and how inflammatory signals affect oncogenic properties of mutant p53. Here shows that immune-regulatory cytokine IL-27 modulates the oncogenic properties of mutant p53 (R172H, or short for H allele) in vivo and that lack of IL-27 signaling is closely associated with early mutant p53 stability in vivo. Of note, this study underlines the significance of IL27 signaling in osteosarcoma initiation by doubling the incidence of osteosarcoma in these IL27RA−/−p53H/+ mice, suggesting that IL27 could be a treatment modality to prevent or reduce osteosarcoma incidence.
Acknowledgments
GRANT SUPPORT
This study was supported by NIH R01 CA120985.
The authors thank the pathologists, Dr. Laura Pageon for her histopathological readings. We also thank Connie Larsson for providing the positive control slide for p21.
Footnotes
Disclosure of potential conflict of interest: The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Conception and design: D. Dibra, G. Lozano, S. Li
Development of methodology: D. Dibra, J. Cutrera
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc): D. Dibra, A. Mitra, M. Newman, J. Cutrera, X. Xia, D. Hughes, S. Li
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): D. Dibra, M. Gagea, X. Xia, S. Li
Writing, review, and/or revision of the manuscript: D. Dibra, S. Li, E. Kleinerman
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S. Li; G. Lozano.
Study supervision: S. Li;
REFERENCES
- 1.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes & development. 2008;22:1337–1344. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh YA, Post SM, Elizondo-Fraire AC, Maccio DR, Jackson JG, El-Naggar AK, et al. Multiple stress signals activate mutant p53 in vivo. Cancer research. 2011;71:7168–7175. doi: 10.1158/0008-5472.CAN-11-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. Journal of immunology. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 4.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4(+) T cells through Stat1-dependent and -independent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, et al. IL-27 Blocks RORc Expression to Inhibit Lineage Commitment of Th17 Cells. Journal of immunology. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi A, Carrier Y, Peron JPS, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature immunology. 2007;8:1372-U6. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 8.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8:1363-U5. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 9.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. International journal of cancer Journal international du cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 10.Dibra D, Cutrera JJ, Xia X, Birkenbach MP, Li S. Expression of WSX1 in tumors sensitizes IL-27 signaling-independent natural killer cell surveillance. Cancer research. 2009;69:5505–5513. doi: 10.1158/0008-5472.CAN-08-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, et al. Potent antitumor activity of interleukin-27. Cancer research. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 12.Zorzoli A, Di Carlo E, Cocco C, Ognio E, Ribatti D, Ferretti E, et al. Interleukin-27 inhibits the growth of pediatric acute myeloid leukemia in NOD/SCID/Il2rg−/− mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1630–1640. doi: 10.1158/1078-0432.CCR-11-2432. [DOI] [PubMed] [Google Scholar]
- 13.Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. Journal of immunology. 2009;183:6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- 14.Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. Interleukin-27 Priming of T Cells Controls IL-17 Production In trans via Induction of the Ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bankson JA, Ji L, Ravoori M, Han L, Kundra V. Echo-planar imaging for MRI evaluation of intrathoracic tumors in murine models of lung cancer. Journal of magnetic resonance imaging : JMRI. 2008;27:57–62. doi: 10.1002/jmri.21221. [DOI] [PubMed] [Google Scholar]
- 16.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Xiong S, Tu H, Kollareddy M, Pant V, Li Q, Zhang Y, et al. Pla2g16 phospholipase mediates gain-of-function activities of mutant p53. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11145–11150. doi: 10.1073/pnas.1404139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. Interleukin-30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology. 2012;55:1204–1214. doi: 10.1002/hep.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rountree CB, Ding W, Dang H, Vankirk C, Crooks GM. Isolation of CD133+ liver stem cells for clonal expansion. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutrera J, Johnson B, Ellis L, Li S. Intraosseous inoculation of tumor cells into bone marrow promotes distant metastatic tumor development: A novel tool for mechanistic and therapeutic studies. Cancer letters. 2013;329:68–73. doi: 10.1016/j.canlet.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natividad KD, Junankar SR, Mohd Redzwan N, Nair R, Wirasinha RC, King C, et al. Interleukin-27 signaling promotes immunity against endogenously arising murine tumors. PloS one. 2013;8:e57469. doi: 10.1371/journal.pone.0057469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer research. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 23.Tang YJ, Wang JL, Nong LG, Lan CG, Zha ZG, Liao PH. Associations of IL-27 polymorphisms and serum IL-27p28 levels with osteosarcoma risk. Medicine. 2014;93:e56. doi: 10.1097/MD.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.