Abstract
Stimulus-reward learning has been heavily linked to the reward-prediction error learning hypothesis and dopaminergic function. However, some evidence suggests dopaminergic function may not strictly underlie reward-prediction error learning, but may be specific to incentive salience attribution. Utilizing a Pavlovian conditioned approach procedure consisting of two stimuli that were equally reward-predictive (both undergoing reward-prediction error learning) but functionally distinct in regard to incentive salience (levers that elicited sign-tracking and tones that elicited goal-tracking), we tested the differential role of D1 and D2 dopamine receptors and nucleus accumbens dopamine in the acquisition of sign- and goal-tracking behavior and their associated conditioned reinforcing value within individuals. Overall, the results revealed that both D1 and D2 inhibition disrupted performance of sign- and goal-tracking. However, D1 inhibition specifically prevented the acquisition of sign-tracking to a lever, instead promoting goal-tracking and decreasing its conditioned reinforcing value, while neither D1 nor D2 signaling was required for goal-tracking in response to a tone. Likewise, nucleus accumbens dopaminergic lesions disrupted acquisition of sign-tracking to a lever, while leaving goal-tracking in response to a tone unaffected. Collectively, these results are the first evidence of an intraindividual dissociation of dopaminergic function in incentive salience attribution from reward-prediction error learning, indicating that incentive salience, reward-prediction error, and their associated dopaminergic signaling exist within individuals and are stimulus-specific. Thus, individual differences in incentive salience attribution may be reflective of a differential balance in dopaminergic function that may bias toward the attribution of incentive salience, relative to reward-prediction error learning only.
Keywords: incentive salience, dopamine, sign-tracking, goal-tracking
1. Introduction
One of the underlying mechanisms proposed to drive the learning of stimulus-reinforcer associations is reward-prediction error, where learning is driven by the magnitude of discrepancy between outcome “expectations” and experienced outcomes (Rescorla and Wagner, 1972; Mackintosh, 1975; Schultz et al. 1997; Sutton and Barto, 1998). In addition to describing associative phenomena at the behavioral level (Miller et al. 1995; Shultz, 2007), reward-prediction error models have also been used successfully to describe the neurobiological changes that occur during stimulus-reward learning. For example, over repeated pairings of the conditioned stimulus (CS) and unconditioned stimulus (US) there is a shift in dopamine neuron activation from the delivery of a US to presentation of the CS (Montague et al. 1996; Schultz, 1998; Schultz, 2006; Schultz, 2007). Since the seminal discovery of dopaminergic signaling changes during CS-US acquisition, reward-prediction error models have been more generally applied to various types of reward-based learning phenomena in both human and non-human animals (Schultz et al. 1998; O’Doherty, 2004; Pessiglione et al. 2006), giving rise to the hypothesis that dopamine signaling carries the reward-prediction error signal during learning.
While reward-prediction error describes how the learning of a CS-US relationship might take place, there are instances where a CS appears to operate beyond its predictive function. For example, there is evidence that a CS can be attributed with “incentive salience”, defined as some form of motivational value that makes the CS “wanted” (Robinson and Berridge, 2008). One common paradigm used to study the attribution of incentive salience or the value attributed to a CS is a Pavlovian conditioned approach (PCA) task, where a single lever reliably predicts non-contingent food delivery. Under these conditions, two distinct conditioned response (CR) topographies can be measured in response to the lever CS, sign-tracking (approach and interaction with the stimulus; Brown and Jenkins, 1968; Hearst and Jenkins, 1974) and goal-tracking (approach to the location of reward delivery; Boakes, 1977). Although both CRs are dependent on the learned relation between the lever CS and food (Robinson and Flagel, 2009), there is evidence that some individuals have a predisposition to display sign-tracking behavior, while others have a predisposition to display goal-tracking behavior (Robinson et al. 2014). According to the reward-prediction error hypothesis, since both sign- and goal-tracking are learned CRs, the lever should serve as an equally effective CS for all individuals, despite the difference in the type of CR that is elicited. Despite being equally effective CSs, a food-associated lever acts as a more robust conditioned reinforcer in individuals that have a propensity to sign-track to the lever CS versus those that goal-track in response to it (Robinson and Flagel, 2009; Meyer et al. 2014); furthermore, stimuli that elicit sign-tracking behavior also serve as more robust conditioned reinforcers relative to stimuli that elicit goal-tracking behavior within individuals (Meyer et al. 2014; Beckmann and Chow 2015). Collectively, the results suggest that while both sign- and goal-tracking responses are indicative of a learned CS-US relationship, sign-tracking responses are also reflective of the attribution of incentive value.
Interestingly, Flagel and colleagues (2011) demonstrated that dopamine appears to preferentially contribute to the attribution of incentive value to a CS. For example, animals selectively bred to preferentially display sign-tracking behavior and outbred animals with a predisposition to sign-track both show an increase in nucleus accumbens core dopamine release during the presentation of the single lever CS, relative to animals that were selectively bred to preferentially display goal-tracking behavior and outbred animals with a predisposition to goal-track. Moreover, the “shift” of phasic dopamine release from the US to the CS during acquisition, as predicted by the reward-prediction error hypothesis, was only observed when the lever elicited sign-tracking. Additionally, the attribution of incentive value can be attenuated by nonspecific dopamine antagonism (e.g., flupenthixol) via systemic injections or microinjections into the nucleus accumbens core, where sign-tracking behavior is more readily attenuated during and after PCA training relative to goal-tracking (Flagel et al. 2011; Saunders and Robinson, 2012). Relatedly, excitotoxic or 6-hydroxdopamine (6-OHDA) lesions of the nucleus accumbens can attenuate sign-tracking to a CS as well (Parkinson et al. 2002; Chang et al. 2012; Chang and Holland, 2013). Collectively, these studies suggest that dopamine may not be underlying the reward-prediction error signal, but may be more reflective of incentive value attribution to reward-predictive stimuli (Berridge, 2007; Flagel et al. 2011; Saunders and Robinson, 2012; Dayan and Berridge, 2014; Robinson et al. 2014).
However, using a single-lever PCA procedure to isolate and compare the neurobehavioral systems that drive sign- and goal-tracking relies heavily upon the post-acquisition identification of CRs to the CS (Meyer et al. 2014), making it difficult to determine how different dopamine manipulations might differentially engage the neurobehavioral systems that underlie sign- and goal-tracking during acquisition. To better dissociate the acquisition of incentive value attribution from reward-prediction error learning, we utilized a 2-CS PCA procedure, where two different but equally-predictive and equally learned conditioned-stimuli (lever and tone) are used to preferentially elicit sign- and goal-tracking within an animal (Beckmann and Chow, 2015). Using a 2-CS PCA procedure, it has been demonstrated that a lever CS associated with sign-tracking engages an incentive value attribution process and a tone CS associated with goal-tracking engages a more general prediction error learning process within individuals (Beckmann and Chow, 2015). We reasoned that the 2-CS procedure would better dissociate the role of D1 and D2 dopamine receptor function and nucleus accumbens dopamine function in the attribution of incentive value from general prediction error learning by directly comparing the effects of systemic pretreatments of SCH- and eticlopride and nucleus accumbens 6-OHDA lesions on sign-tracking to a lever versus goal-tracking in response to a tone within individuals. More importantly, according to the reward-prediction error hypothesis, because sign-tracking to the lever and goal-tracking in response to the tone are both equally learned responses, both CRs should depend on reward-prediction error and be equally affected by dopaminergic manipulation. However, if dopamine were more specifically involved in incentive value attribution, we hypothesized that dopaminergic receptor antagonism and nucleus accumbens dopamine depletion would preferentially affect sign-tracking to the lever CS, leaving goal-tracking in the presence of the tone CS relatively unaffected.
2. Materials and Methods
2.1 Animals
One-hundred and eight adult male Sprague-Dawley rats (Harlan Inc.; Indianapolis, IN, USA), weighing approximately 250–275 g at the beginning of experimentation, were used. Rats were individually housed in a temperature-controlled environment with a 12:12 hr light:dark cycle, with lights on at 0600 h. All rats were first acclimated to the colony environment and handled daily for one week prior to experimentation. All experimentation was conducted during the light phase. All rats had ad libitum access to food and water in their home cage throughout experimentation. All experimental protocols were conducted according to the 2010 NIH Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.2 Apparatus
Experiments were conducted in operant conditioning chambers (ENV-008, MED Associates, St. Albans, VT) that were enclosed within sound-attenuating compartments (ENV-018M, MED Associates). Each chamber was connected to a personal computer interface (SG-502, MED Associates), and all chambers were operated using MED-PC. Within each operant chamber, a 5.1 × 5.1 cm recessed food receptacle (ENV-200R2MA) outfitted with a head-entry detector (ENV-254-CB) was located on the front response panel of the chamber, two retractable response levers were mounted on either side of the food receptacle (ENV-122CM; 6 cm above metal rod floor), two white cue lights (ENV-221M) were mounted at 4.1 cm and 8.2 cm above each response lever, and a Sonalert© tone (ENV-223 AM) was located above the top left cue light and a Sonalert© tone (ENV-223 HAM) was located above the top right cue light. The back response panel was outfitted with a single retractable response lever (ENV-122CM; located directly opposite of the food receptacle); two nosepoke response receptacles (ENV-114BM; 6 cm above metal rod floor and directly opposite to front response levers) were mounted on either side of the retractable response lever, and a house-light (ENV-227M) was located 12 cm above the response lever. Food pellets (45-mg Noyes Precision Pellets; Research Diets, Inc., New Brunswick, NJ) were delivered via a dispenser (ENV-203M-45).
2.3 Drugs
R(+)-SCH-23390 hydrochloride, S-(-)-Eticlopride hydrochloride, pargyline hydrochloride, and desipramine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA) and mixed in sterile saline (0.9% NaCl). The 6-OHDA (Sigma-Aldrich) was mixed fresh in ice-cold saline (0.9% NaCl) containing 0.2 mg/ml ascorbic acid.
2.4 Behavioral Procedures
2.4.1 Magazine Shaping
During the last two days of acclimation to the colony, after animals were handled, 10 to 15 food pellets were dropped into their home cages. After acclimation, animals were trained to retrieve food pellets from the food receptacle for two consecutive days; rats were placed in the operant chambers and given 40 minutes to retrieve and consume 16 food pellets, delivered on a 60-s fixed time schedule.
2.4.2 2-CS PCA Training
To isolate incentive salience from reward-prediction error learning within an individual, rats were trained on a 2-CS PCA task following magazine training. Methods for the 2-CS PCA task were identical to that of Beckmann and Chow (2015). Briefly, during each session, a single response lever adjacent to the food receptacle (balanced for side) was inserted into the chamber for 8s or a 4,500 Hz tone was presented for 8s. Immediately after lever retraction or tone offset, a food pellet was non-contingently delivered into the food receptacle. Stimulus-reward presentations were independently presented and separated by a 90-s VT-ITI, ranging from 12-s to 286-s (Fleshler and Hoffman, 1962). Each session consisted of 32 total trials, consisting of 16 lever insertions and 16 tone presentations in random order, where no more than four presentations of the same stimulus occurred consecutively. Rats were trained for 14 consecutive sessions. Sign-tracking responses were recorded as lever presses, while goal-tracking responses were recorded as breaks of a photo beam within the food receptacle during stimulus presentation. Because the 0.03 mg/kg dose of both SCH-23390 and eticlopride (see supplementary materials; Figure S1) caused non-specific effects (failure to eat food pellets) during the single-lever PCA experiment, we used the 0.01 dose during the 2-CS acquisition experiment. Fifteen minutes prior to each 2-CS PCA session rats (n=12/group) were pretreated (s.c. injection) with saline, SCH-23390 (0.01 mg/kg) or eticlopride (0.01 mg/kg).
Additionally, after 2-CS PCA training with pretreatments of saline, SCH-23390, or eticlopride, animals were randomly assigned and assessed on a CS-only test or conditioned reinforcement test (as described below and in Table 1). Furthermore, there were no significant differences in terminal sign- or goal-tracking behavior between groups.
Table 1.
General experimental conditions and timeline for each experiment. Note: animals in the 2-CS PCA task with pretreatments were randomly assigned to the CS-only and conditioned reinforcement follow-up tests; there were no differences in sign- or goal-tracking behavior upon completion of acquisition between groups for each acquisition treatment (i.e., saline, SCH-23390, and eticlopride).
| Experiment | Pre- training |
Design |
|---|---|---|
| D1 vs D2 receptor function on acquisition |
Magazine Shaping |
 |
| D1 vs D2 receptor function on relative valuation |
Magazine Shaping |
 |
| Role of nucleus accumbens DA |
Magazine Shaping |
 |
2.4.3 CS-only Test
To determine what was learned about the lever CS and tone CS during acquisition, animals (n=6/group; randomly selected from the 2-CS PCA group with pretreatments) pretreated (s.c. injection) with saline, SCH-23390, or eticlopride during acquisition were tested under extinction conditions. Extinction conditions followed the parameters described in the 2-CS PCA training; however, food was not delivered following each of the 8 stimulus presentations (4 lever and 4 tone). Importantly, rats were drug free during the CS test. Additionally, the use of the CS-only test acts as a probe for what was learned under the effects of the drug pretreatments on the CS-US relationships, where it controls for relearning of the CS-US relation under drug-free conditions, US specific effects, such as pseudoconditioning, and new learning, via the limited number of probe trials (Rodnick, 1937; Rescorla 1967; Bouton, 2007).
2.4.4 Conditioned Reinforcement
To determine the relative value associated with the lever CS or tone CS, animals (n=6/group; randomly selected from the 2-CS PCA group with pretreatments) pretreated with saline, SCH-23390, or eticlopride during the acquisition phase were tested under a conditioned reinforcement test. The conditioned reinforcement test took place over two subsequent days immediately following the 14 days of 2-CS PCA training. Conditioned reinforcement tests consisted of two 30-min sessions where rats were presented with an illuminated nosepoke (balanced for side), where a single response (break in photobeam within the nosepoke receptacle) resulted in the simultaneous offset of the nosepoke light and 8s presentation of either the lever or tone (balanced across animals) that was used during initial 2-CS PCA training. After the 8-s stimulus presentation (lever or tone), the nosepoke light turned on again. On the subsequent day, the opposite nosepoke light was illuminated, and a response produced either the lever or tone for 8s, opposite to what stimulus was previously presented during the day before. During each session, only one of the two nosepoke receptacles was illuminated. Nosepoke responses into the illuminated receptacle were recorded as active nosepokes and nosepoke responses into the non-illuminated receptacle were recorded as inactive nosepokes. Furthermore, inactive nosepokes had no consequence. Importantly, rats were drug free during these two conditioned reinforcement tests.
2.4.5 2-CS Choice
To determine the relative valuation or preference for a lever CS or a tone CS, a separate group of animals (n=12) were initially trained on the 2-CS PCA task, then subsequently trained on a 2-CS choice procedure (see Table 1) as described in Beckmann and Chow (2015). Using a choice procedure offers the ability to study conditioned reinforcement, without the limitations associated with extinction conditions (e.g., limited period of study). Moreover, the choice procedure scales the relative value of the lever CS in relation to primary reinforcement, as choices for the lever CS across choice are inversely related to primary reinforcement earned. Finally, the choice procedure allows for a direct comparison of the relative value associated with the different CS-US relationships learned during 2-CS PCA task; thus, it provides a more direct assessment of the value associated with the different neurobehavioral mechanisms that govern sign- versus goal-tracking behavior.
Briefly, the choice procedure consisted of 5 blocks of 13 trials separated by a dark 60-s ITI. Each trial began with the illumination of the house-light, where a single orienting response in the food receptacle resulted in the offset of the house-light and onset of a nosepoke light (left, right, or both, depending on the trial type) on the opposing panel. During forced-choice trials (8/block; 4 lever and 4 tone), only a single nosepoke light (left or right) was available. A single nosepoke into the illuminated receptacle resulted in the offset of the nosepoke light and presentation of the previously conditioned 8-s lever or 8-s tone, followed by a single non-contingent pellet and the initiation of the ITI. During free-choice trials (5/block), both nosepoke lights were illuminated. However, a single nosepoke into either illuminated receptacle resulted in the offset of both nosepoke lights and presentation of the previously conditioned 8-s lever or 8-s tone associated with the selected nosepoke, followed by a single non-contingent pellet and initiation of the ITI. All trial types operated according to a 30-s limited hold; if a nosepoke response did not occur within the 30-s window from onset of nosepoke illumination, the trial ended, an omission was counted, and the ITI began. The probability (p) of pellet delivery following lever choice decreased (100%, 50%, 25%, 12.5%, and 6.25%) in descending order over the 5 blocks. Following stable performance (no statistically significant changes in the discounting parameters over 3 consecutive sessions) on the probabilistic choice task, 15 minutes prior to each session animals were pretreated (s.c. injections) with saline, 0.003 mg/kg SCH-23390, 0.01 mg/kg SCH-23390, 0.017 mg/kg SCH-23390, 0.003 mg/kg eticlopride, 0.01 mg/kg eticlopride, or 0.017 mg/kg eticlopride. Pretreatments were administered according to a Latin-square design where all animals received each drug and dose combination and sessions were separated by a 2-day washout period.
2.5 6-OHDA lesions
To determine the role of dopamine in the nucleus accumbens on reward-prediction error learning and incentive value attribution, animals (n=6/group) were first magazine trained and then underwent stereotaxic surgery. Animals were administered (i.p. injections) the monoamine oxidase inhibitor pargyline (50 mg/ml, 1.0 ml/kg) and the norepinephrine uptake blocker desipramine (25 mg/ml, 1.0 ml/kg) approximately 30 minutes prior to surgery. Animals were then anesthetized with isoflurane, and animals in the lesion group were placed into a stereotaxic instrument and received bilateral injections of 6-OHDA (8.0 µg/2.0µl/side); sham groups underwent similar procedures, but 6-OHDA was not administered. Stereotaxic coordinates for the nucleus accumbens, inclusive of both core and shell, were AP + 1.6 mm, ML ± 1.5 mm, and DV − 7.0 mm, relative to Bregma; the needle was left in place for 5 minutes following injection (Pierce et al. 1990). Following 3 days of post-operative care, animals were placed on the 2-CS PCA task (see Table 1).
2.6 Endogenous DA content assay
Following 14-days of 2-CS PCA animals that received bilateral stereotaxic surgery were sacrificed by rapid decapitation. The brain was removed, placed on an ice-cold dissecting plate, and then the nucleus accumbens, comprising of both core and shell, was collected. All samples were frozen at −80°C between dissection and assay. DA content was assessed via HPLC-EC. Briefly, nucleus accumbens samples from each animal were weighed, after which they were placed within 1 mL of perchloric acid and sonicated. Samples were then centrifuged at 16,400 rpm for 30 min at 4°C. Fifty µL of each sample were injected into the HPLC-EC, equipped with pump and auto-sampler (508 Beckman Coulter, Inc, Fullerton, CA). The samples were separated on an ODS ultra sphere C18 reverse-phase column (80 × 4.6 mm, 3-µm ESA Inc., Chelmsford, MA) with 0.07 M citrate/0.1 M acetate buffer (175 mg/L octylsulfonic acid-sodium salt, 650 mg/L of NaCl and 7% methanol; pH = 4.2). The analytes were then detected with a coulometric-II detector with guard cell (model 5020) maintained at +0.60 V and an analytical cell (model 5011) maintained at potentials E1 = +0.05 V & E2 = +0.35 V (ESA, Inc., Chelmsford, MA). Separations were performed at room temperature at a flow rate of 1.2 ml/min, and 4–5 minutes were required to process each sample. Retention times of DA standards were used to identify peaks. Peak heights were used to quantify the detected amounts on the basis of standard curves. The peak identification, height measurement and analysis were performed by using 32 karat software (Beckman Coulter, Inc, Fullerton, CA).
2.7 Analysis
Data were analyzed using linear mixed-effects modeling (Gelman and Hill, 2006). Due to the absence of sign-tracking to a tone, sign-tracking response rates for 2-CS PCA training and extinction were analyzed alone with session/trial block (continuous) as a within-subject factor, treatment group (nominal) as a between-subject factor, and subject as a random factor (Beckmann and Chow, 2015). Goal-tracking and difference scores (probability of making a sign-tracking response – probability of making a goal-tracking response) for 2-CS PCA training were analyzed with session/trial block (continuous) and stimulus (nominal) as within-subject factors, treatment group (nominal) as a between-subject factor, and subject as a random factor. For the CS test, the first 4 trials per each stimulus was collapsed as an average rate and data were analyzed using two-way mixed effects ANOVA, with stimulus (nominal) as within-subject factor, treatment (nominal) as a between subject factor, and subject as a random factor. Conditioned reinforcement tests data were analyzed using two-way mixed effects ANOVA, with stimulus (nominal) and response type (nominal) as within-subject factors, treatment (nominal) as between-subject factor, and subject as a random factor. Conditioned reinforcement tests for active and inactive nosepoke responses were also analyzed individually using two-way mixed effects ANOVA, with stimulus (nominal) as a within-subject factor, treatment (nominal) as between-subject factor, and subject as a random factor. For the 2-CS choice data, hyperbolic discounting functions (Rachlin et al. 1986, 1991; Mazur, 1987) of the form VLever=(s/(1+(kO) were fit to the data, where VLever represents the subjective value of the lever CS, s represents the sensitivity to stimulus value (lever vs tone) at equal food probability, k represents the discounting rate of lever value, and O represents odds against [(1 – p)/p]. Hyperbolic discounting functions were fit to the data via nonlinear mixed effects modeling (NLME; Pinheiro et al. 2007; Beckmann and Young, 2009; Young et al. 2009; Brooks et al. 2010; Beckmann and Chow, 2015) with treatment (nominal) and dose (continuous) defined as fixed within-subject factors and subject defined as a random factor. A t-test was performed on the data comparing DA concentrations from sham and 6-OHDA lesioned animals. All post hoc tests were conducted with Tukey HSD. For all tests, α was set at 0.05.
3. Results
3.1 Effects of D1 and D2 dopamine receptor antagonism during 2-CS PCA acquisition
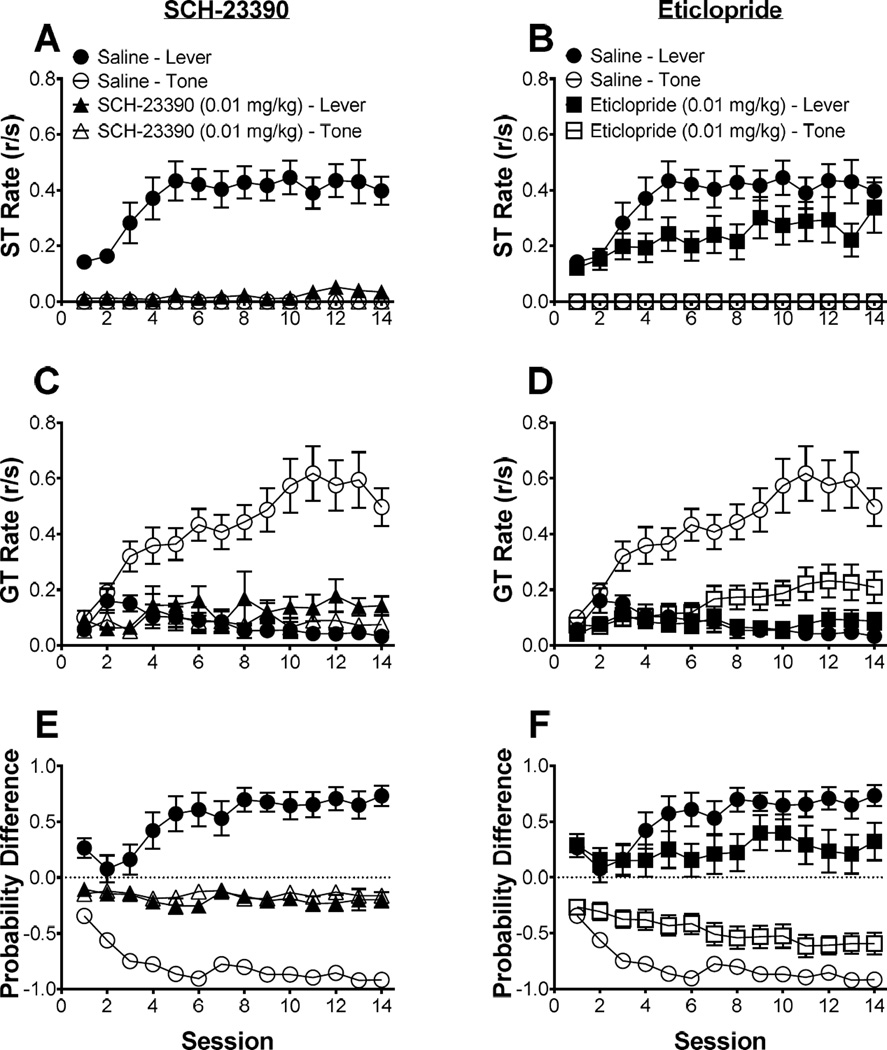
Figure 1 illustrates the acquisition of sign- and goal-tracking response rates to the lever and tone CSs, along with the difference score during the 14-day training period with pretreatments of a saline control or dopamine receptor antagonist. Figure 1A and 1B illustrate sign-tracking rates to a lever CS with daily pretreatments of saline control, SCH-23390, or eticlopride. Linear mixed effects analysis revealed a main effect of treatment [F(2,33) = 17.60, p < 0.05], suggesting that the different dopamine receptor antagonists had an effect on sign-tracking behavior; post hoc analysis revealed that sign-tracking rates for saline and eticlopride pretreatments were higher than that of SCH-23390 pretreatments. Linear mixed effects analysis also revealed there was also a main effect of session [F(1,33) = 18.73, p < 0.05], indicating that sign-tracking rates changed over sessions. Collectively, the results indicate that SCH-23390 suppressed sign-tracking to the lever CS, while eticlopride had less of an effect on sign-tracking, while each drug was onboard.
Figure 1.
The effects of a dopamine receptor antagonist on sign- and goal-tracking during 2-CS PCA acquisition, while the drugs were onboard. Mean (± SEM) response rate (responses/second; r/s) for the effects of SCH-23390 (0.01 mg/kg) on (A) sign-tracking and (C) goal-tracking. Mean (± SEM) response rate (responses/second; r/s) for the effects of eticlopride (0.01 mg/kg) on (B) sign-tracking and (D) goal-tracking. Mean (± SEM) difference in response probability (ST probability – GT probability) for a lever CS and tone CS during 2-CS PCA training for (E) SCH-23390 (0.01 mg/kg) and (F) eticlopride (0.01 mg/kg) compared against a saline control group. 1.0 indicates exclusive sign-tracking while −1.0 indicates exclusice goal-tracking.
Figure 1C and 1D illustrate goal-tracking rates to a lever CS and tone CS with daily pretreatments of saline control, SCH-23390, or eticlopride. Linear mixed effects analysis revealed that goal-tracking rates were significantly affected by stimulus [F(1,33) = 28.76, p < 0.05], indicating a difference in goal-tracking rates to the lever CS and tone CS; there was a main effect of treatment [F(2,33) = 9.45, p < 0.05], revealing that goal-tracking rates varied across the pretreatment groups, and there was a main effect of session [F(1,33) = 22.02, p < 0.05], suggesting that goal-tracking rates changed over the 14-day training period. There was also a significant stimulus × treatment interaction [F(2,33) = 23.45, p < 0.05], indicating that goal-tracking rates to the lever CS or tone CS were dependent on the pretreatments; a significant stimulus × session interaction [F(1,33) = 21.77, p < 0.05] was also observed, indicating that changes in goal-tracking across sessions were stimulus dependent. There was also a significant treatment × session interaction [F(2,33) = 7.93, p < 0.05], indicating that changes in goal-tracking rates over sessions were pretreatment dependent. Finally, there was a significant stimulus × treatment × session interaction [F(2,33) = 15.30, p < 0.05], indicating that changes in goal-tracking rates over the 14-day training sessions were dependent on the CS type and the pretreatment given. Post hoc analysis revealed that goal-tracking rates to the tone CS were higher for saline pretreated groups than SCH-23390 and eticlopride. Moreover, there were no significant effects on the 8-s pre-CS response rate (Figure S2). Collectively, these results indicate that both SCH-23390 and eticlopride attenuated goal-tracking in response to the tone CS relative to saline control, while each drug was onboard.
Figure 1E and 1F illustrate the difference scores for saline control, SCH-23390, and eticlopride. Linear mixed effects analysis revealed a significant main effect of stimulus [F(1,33) = 7.93, p < 0.05], indicating difference scores were dependent on the CS. Additionally, linear mixed effects analysis revealed a stimulus × treatment interaction [F(2,33) = 37.66, p < 0.05], indicating the likelihood of a sign- or goal-tracking response to occur was dependent on the CS and the pretreatment; there was also a stimulus × session interaction [F(1,33) = 33.92, p < 0.05], indicating that changes in difference score across session were dependent on the stimulus presented. Finally, linear mixed effects revealed a stimulus × treatment × session interaction [F(2,33) = 13.58, p < 0.05], indicating that the change in probability of a sign- or goal-tracking response over sessions was dependent on the lever CS or tone CS and the pretreatment administered. Collectively, when the drug was onboard, SCH-23390 attenuated the probability of a sign- or goal-tracking response to the lever CS and tone CS relative to saline control, while eticlopride had a similar but lessened effect.
3.2 Post-acquisition drug-free CS-only test
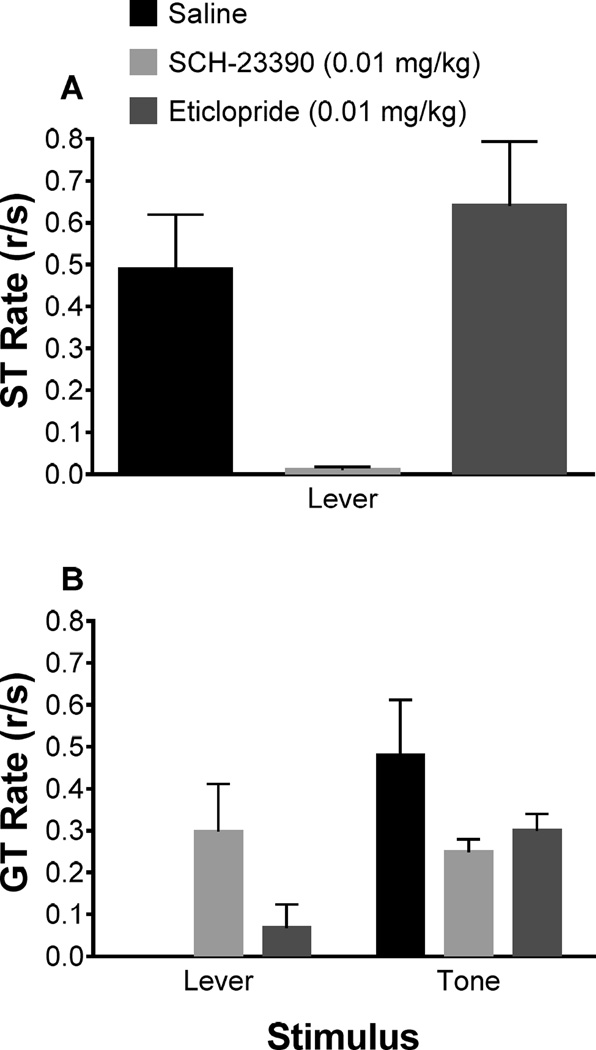
Figure 2 illustrates the sign- and goal-tracking rates to the lever CS and tone CS during a drug-free test session, where each CS, both lever and tone, were presented under extinction conditions without either drug onboard. Figure 2A and Figure 2B illustrate sign- and goal-tracking rates, respectively, to a lever CS or tone CS averaged (4 trials/stimulus to probe learning without inducing extinction (Rescorla, 1967) for saline control, SCH-23390, and eticlopride acquisition treatments. Two-way mixed ANOVAs revealed a main effect of stimulus [F(1,15) = 11.53, p < 0.05], indicating that goal-tracking rates during the CS test were different depending on the CS presented; there was also a significant treatment × stimulus interaction [F(2,15) = 5.92, p < 0.05], indicating that the pretreatment during 2-CS PCA acquisition affected what was learned about each stimulus. Post hoc tests revealed that goal-tracking in the presence of the tone CS following saline pretreatment during acquisition was significantly higher than goal-tracking in the presence of the lever CS for animals pretreated with SCH-23390 or eticlopride during acquisition. Moreover, goal-tracking response rates were significantly higher than the 8-s pre-CS response rate during the CS-test [F(1,17) = 20.53, p < 0.05] (Figure S3). Collectively, the results indicate that when presented with each stimulus alone during the post-acquisition drug-free CS-test prior pretreatments of SCH-23390 during 2-CS PCA acquisition prevented the learning of a CS-US relationship that results in sign-tracking to the lever, instead promoting the learning of a CS-US relationship that resulted in goal-tracking in response to the lever. Additionally, eticlopride pretreatments during 2-CS PCA acquisition did not affect the learning of sign-tracking behavior to a lever relative to saline, nor did it significantly affect the learning of a goal-tracking response to the tone relative to saline.
Figure 2.
Sign- and goal-tracking responses to a lever and tone CS during a drug-free CS-only test to identify what was learned under treatment conditions during 2-CS PCA acquisition. The legend refers to the pretreatments each group received during 2-CS PCA acquisition. Mean (± SEM) response rate (responses/second; r/s) for individuals pretreated with SCH-23390 (0.01 mg/kg), eticlopride (0.01 mg/kg), and saline during acquisition on (A) sign-tracking and (B) goal-tracking. Note: data not present (i.e., no bar in the graph) for goal-tracking in response to a lever CS in saline pretreated animals directly reflects no goal-tracking behavior measured for that group.
3.3 Post-acquisition drug-free conditioned reinforcement tests
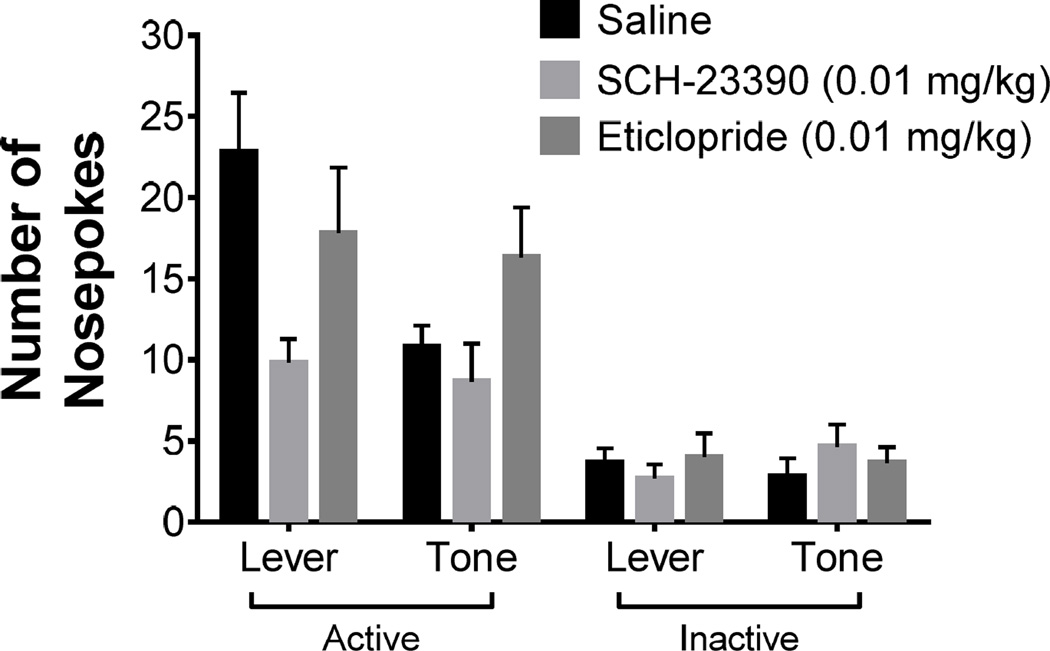
Figure 3 illustrates the active and inactive nosepoke responses during drug-free conditioned reinforcement test for the lever CS and tone CS. Figure 3 illustrates the number of active nosepokes for access to the previously learned lever CS and tone CSs alone and the number of inactive nosepokes, where responses produced no consequence, recorded during the conditioned reinforcement tests. Two-way mixed ANOVAs revealed that there was a main effect of treatment [F(2,15) = 4.25, p < 0.05], indicating that the pretreatments during acquisition affected the value attributed to the CSs; there was a main effect of response type [F(1,15) = 82.68, p < 0.05], indicating that there were more active nosepoke responses than inactive nosepoke responses. Finally, two-way mixed ANOVAs revealed a treatment × response type interaction [F(2,15) = 4.83, p < 0.05], indicating that the pretreatments during 2-CS PCA training affected active and inactive nosepoke responses. Post hoc tests revealed that active responses were higher than inactive responses for all treatment groups, and animals pretreated with SCH-23390 during the acquisition period exhibited lower active responses, relative to animals pretreated with either saline or eticlopride during the acquisition period. Moreover, there were no significant effects in which CS was tested first. Collectively, these results suggest that SCH-23390 pretreatments during acquisition prevented the attribution of incentive value, while eticlopride pretreatments did not.
Figure 3.
The conditioned reinforcing value attributed to the lever or tone CS during drug-free tests post 2-CS PCA training. The legend refers to the pretreatments each group received during 2-CS PCA acquisition. Mean (± SEM) number of active nosepokes that produced the previously learned stimulus for SCH-23390 (0.01 mg/kg), eticlopride (0.01 mg/kg), and saline pretreated groups during 2-CS PCA acquisition and mean (± SEM) number of inactive nosepokes that results in no consequences for SCH-23390 (0.01 mg/kg), eticlopride (0.01 mg/kg), and saline pretreated groups during 2-CS PCA acquisition.
3.4 Effects of D1 and D2 antagonists on lever vs. tone choice
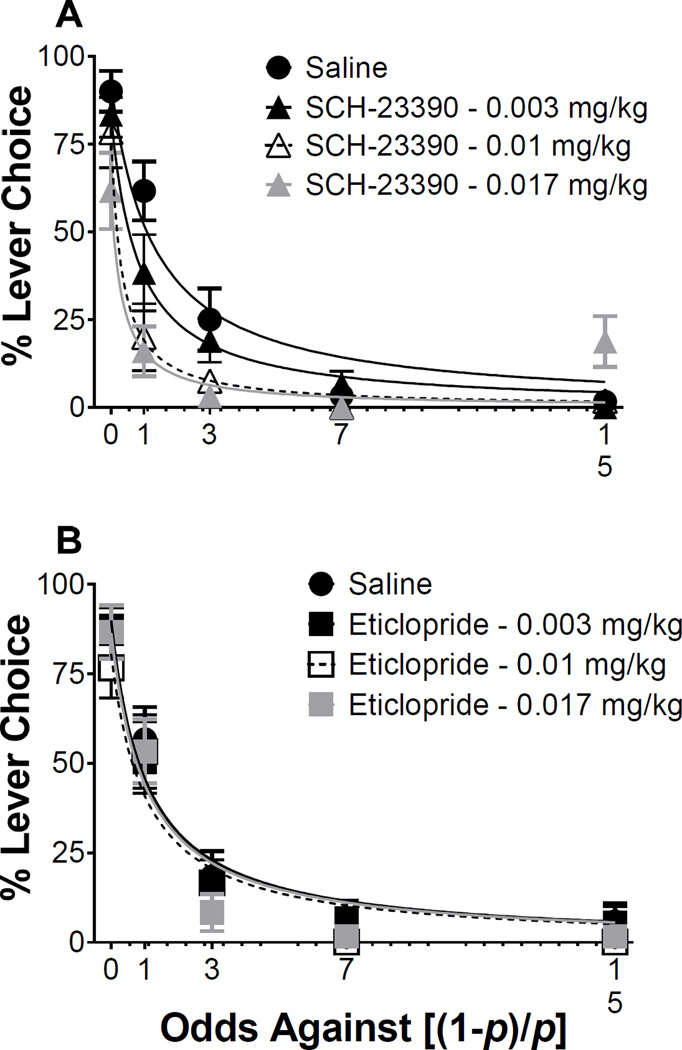
Figure 4 illustrates lever choices as a function of odds against, including the effects of acute SCH-23390 (Figure 4A) and eticlopride (Figure 4B) treatment. NLME analysis revealed a main effect of drug [F(1,453) = 4.77, p < 0.05], a main effect of dose [F(3,453) = 4.28, p < 0.05], and a significant drug × dose interaction [F(1,453) = 3.58, p < 0.05] on initial lever choices, when food probability was equal (s parameter; intercept of function). Post hoc tests revealed a significant decrease in initial lever choices following the 0.017 dose of SCH-23390, relative to saline. There were no significant effects of any dose of either drug on discounting rate (k parameter; slope of function). There were no significant effects of any dose on the number of omissions as well (Figure S4), suggesting the results were not confounded by non-specific effects of the drugs. Collectively, these results demonstrate that under saline controls, a lever CS, which was associated with sign-tracking, can preferentially bias choice towards an option which results in the loss of primary reinforcement. Furthermore, SCH-23390 pretreatments dose-dependently decreased the initial value attributed to the lever CS while eticlopride did not affect relative preference. Finally, by using the 2-CS choice procedure and directly comparing the relative value of a lever CS and tone CS through conditioned reinforcement, it appears that D1 receptor function is necessary in maintaining the value attributed to the CS, thus suggesting the importance of dopamine and valuation.
Figure 4.
The acute effects of (A) SCH-23390 and (B) eticlopride on CS preference, post 2-CS PCA training, where the relative value of the lever CS is directly compared to that of the tone CS. Mean (± SEM) % choice for the lever CS as a function of increasing odds against [(1 – p)/p] food delivery following the lever CS. Lines are choice functions defined by s/(1 + k(odds against)), (s) sensitivity to lever CS value; (k) discounting rate of lever CS value.
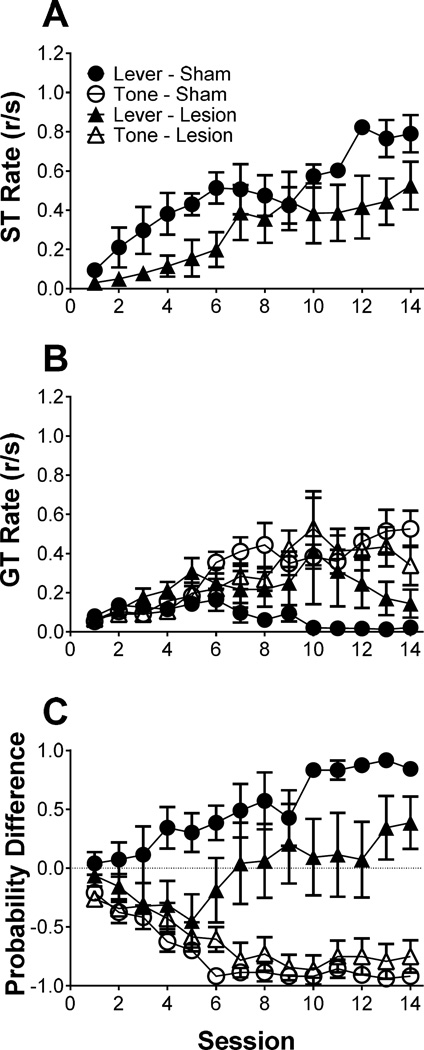
3.5 Effects of nucleus accumbens 6-OHDA lesions on 2-CS PCA acquisition
Figure 5 illustrates the acquisition of sign- and goal-tracking response rates to the lever and tone CSs and the difference score during the 14-day training period following 6-OHDA lesions to the nucleus accumbens. Figure 5A illustrates sign-tracking to a lever CS; linear mixed effects analysis revealed a main effect of session [F(1,10) = 29.53, p < 0.05], indicating that sign-tracking rates changed over session. Figure 5B illustrates goal-tracking rates to a lever CS and tone CS; linear mixed effects analysis revealed a significant main effect of stimulus [F(1,10) = 10.90, p < 0.05], indicating a difference in goal-tracking rates to the lever CS and tone CS. Additionally, there was a main effect of session [F(1,10) = 11.53, p < 0.05], indicating that goal-tracking rates changed over session. Finally, there was a stimulus × session interaction [F(1,10) = 25.71, p < 0.05], indicating that goal-tracking rates were stimulus dependent.
Figure 5.
The effects of 6-OHDA lesion on 2-CS PCA acquisition. Mean (± SEM) response rate (responses/second; r/s) for the (A) sign-tracking and (B) goal-tracking. Mean (± SEM) difference in response probability (ST probability – GT probability) for a lever CS and tone CS during 2-CS PCA training (C). 1.0 indicates exclusive sign-tracking while −1.0 indicates exclusive goal-tracking.
Figure 5C illustrates the difference score for lesion and sham treated animals. Linear mixed effects revealed a main effect of stimulus [F(1,10) = 49.18, p < 0.05], indicating difference scores were dependent on the CS type; a treatment × stimulus interaction [F(1,10) = 5.67, p < 0.05], indicating that difference scores were both lesion and stimulus dependent; post hoc tests revealed that difference scores for the tone CS in both sham and lesion groups were significantly different than those for the lever CS in the lesion group. Finally, there was a stimulus × session interaction [F(1,10) = 97.66, p < 0.05], indicating that difference scores were dependent on the stimulus presented in relation to session. Collectively, the results indicate that while the dopaminergic lesion to the nucleus accumbens did not significantly affect sign- or goal-tracking rates (p > 0.05), it did affect the probability of sign-tracking to the lever CS during 2-CS PCA acquisition. Importantly, dopaminergic lesion had no effect on goal-tracking behavior in response to the tone CS.
HPLC analysis of dopamine content from nucleus accumbens tissue punches following completion of the 14-day PCA training indicated a significant reduction [t(10) = 5.38, p < 0.05) in dopamine content from animals given a 6-OHDA lesion (2.19 ± 0.36 ng/mg) relative to animals given a sham lesion (4.93 ± 0.35 ng/mg), resulting in a 55.48% decrease in dopamine content.
4. Discussion
The results from the present experiments provide several important and novel findings in relation to dissociating the role of D1 and D2 dopamine receptor function and nucleus accumbens dopamine function in incentive salience or value attribution from reward-prediction error learning. First, both systemic D1 and D2 antagonism affect sign-and goal-tracking performance to the lever CS and tone CS when either drug was onboard (Figure 1). Second, D1 antagonism during 2-CS PCA acquisition prevented the learning of a sign-tracking response, while promoting the learning of a goal-tracking response in the presence of a lever CS and leaving goal-tracking in response to a tone within the same animals relatively unaffected, as seen in the post-acquisition drug-free, CS-only test (Figure 2). Third, D1 antagonism during 2-CS PCA acquisition specifically eliminated the attribution of incentive value, as seen in the subsequent drug-free conditioned reinforcement tests (Figure 3). Fourth, D1 antagonism during choice produced a specific, dose-dependent decrease in preference for the lever CS relative to the tone CS (Figure 4). Fifth, nucleus accumbens 6-OHDA lesions specifically disrupted sign-tracking to the lever CS during acquisition, but leaving goal-tracking in response to a tone CS within the same animals unaffected (Figure 5). Finally, by demonstrating all of the lever- and tone-associated results discussed above within subject, these findings suggest that the neurobehavioral processes underlying incentive salience and reward-prediction error are independent, parallel processes that are, at least partially, stimulus-dependent. Collectively, the present results support the literature that indicates dopamine may play a larger role in the attribution of incentive value to a CS than in reward-prediction error learning (Parker et al. 2010; Flagel et al. 2011; Saunders and Robinson 2012; Robinson et al. 2014).
Although the 2-CS PCA procedure is able to produce both sign- and goal-tracking within animals, there have been implications that a tone CS associated with goal-tracking functions differently than a lever CS associated with goal-tracking due to its modality(Saunders and Robinson, 2012; Meyer et al. 2014; Ahrens et al. 2015), calling into question whether or not goal-tracking in the presence of a tone CS is functionally equivalent to goal-tracking in the presence of a lever CS (Dickinson et al. 2000; Dayan and Berridge 2014; Robinson et al. 2014). While the findings herein indicate that goal-tracking in response to a tone CS is a relatively dopamine-independent process, the literature on the role of dopamine in goal-tracking behavior, generally speaking, has been mixed. For example, Flagel et al. (2011, supplemental material) demonstrated that, following PCA acquisition, sign- and goal-tracking to a lever CS were equally sensitive to the performance-disruptive effects of an acute flupenthixol (a non-selective dopamine receptor antagonist) treatment, while the drug was onboard. Others have also found that dopamine antagonists affect the performance of sign- and goal-tracking to a food predictive CS, while the drugs were onboard (Danna and Elmer, 2007; Lopez et al. 2015; Fraser et al. 2016), and performance-disruptive effects have also been reported for goal-tracking in response to a tone CS, while the drugs were onboard (Eyny and Horvitz, 2003; Wassum et al. 2011). However, Flagel et al. (2011) also reported that drug-free testing following chronic flupenthixol treatment during PCA acquisition revealed that chronic flupenthixol treatment during acquisition prevented the learning of sign-tracking to a lever CS in animals genetically predisposed to sign-track and had no effect on the learning of a goal-tracking response to a lever CS in animals genetically predisposed to goal-track, even though the performance of both sign- and goal-tracking were affected by flupenthixol treatment during acquisition. In other words, when the animals were tested while not under the influence of flupenthixol, sign-tracking was still attenuated and goal-tracking was exhibited at rates equivalent to that of saline control, but when flupenthixol was onboard it had equally disruptive effects on the performance of both sign- and goal-tracking. Furthermore, the relative ineffectiveness of D2 on the acquisition of sign- and goal-tracking reported here appears to be at odds with the literature. Danna and Elmer (2010), Lopez et al. (2015), and Fraser et al. (2016) demonstrated that D2 antagonism affected sign- and goal-tracking behavior. Importantly, all of these effects were demonstrated while the antagonists were onboard. Similarly, performance of both sign- and goal-tracking behavior herein were both affected by the D2 antagonist, while the drug was onboard. However, the subsequent CS-only test, which was conducted under drug-free extinction conditions, revealed that D2 antagonism during acquisition had no effect on the learning of the CS-US relationship for either the lever or tone CS, contrary of its effects on performance for each stimulus during 2-CS PCA acquisition. Thus, D2 antagonism appears to disrupt performance of sign- and goal-tracking, while leaving the learned CS-US association intact. Collectively, these results demonstrate the important distinction between dopamine receptor antagonism on the performance of sign- or goal-tracking from the acquisition of CS-US relationships that lead to either sign- or goal-tracking. The similar effects between D1 treatment on goal-tracking in response to a tone CS herein and flupenthixol treatment on goal-tracking in response to a lever CS within Flagel et al. (2011) when tested post-acquisition under drug-free conditions provide further support that goal-tracking in the presence of a lever CS or a tone CS are functionally equivalent.
Saunders and Robinson (2012) demonstrated that nucleus accumbens dopamine receptor antagonism, via flupenthixol, resulted in unaffected goal-tracking behavior to a lever. Additionally, using transgenic mice, Parker et al. (2010) reported that attenuation of phasic dopamine release within the nucleus accumbens via NMDA knock-out of VTA dopamine neurons exclusively had no effect on the acquisition of a goal-tracking response to a lever, relative to wild type. Furthermore, Blaiss and Janak (2009) using GABA receptor reversible inactivation examined the role of nucleus accumbens core versus shell function in a PCA task and found that inactivation of the nucleus accumbens core is not necessary for the learning of goal-tracking behavior, thus suggesting that the nucleus accumbens core is not necessarily required for reward-prediction error learning but possibly more for the attribution of incentive value. Relatedly, using a 2-CS PCA procedure and 6-OHDA lesions of the nucleus accumbens, herein we demonstrated that both lesion and sham animals acquired goal-tracking behavior to a tone CS at the same rate, suggesting that a tone CS is engaging the same relatively dopamine-independent system that is seen in animals that goal-track to a lever CS. Thus, from the present results herein, it appears that while nucleus accumbens dopamine is necessary for the acquisition of a sign-tracking response to a lever CS, it is not necessary for the acquisition of a goal-tracking response to either a lever or tone CS, further supporting the functional equivalence between goal-tracking in the presence of a tone CS and goal-tracking in the presence of a lever CS.
Behaviorally, sign- and goal-tracking have been demonstrated to be associated with differential conditioned reinforcement (Robinson and Flagel 2009; Meyer et al. 2014; Beckmann and Chow 2015), differential resistance to both omission and extinction contingencies (Beckmann and Chow 2015; Ahrens et al. 2016), and differential sensitivity to reward devaluation (Nasser et al. 2015; Morrison et al. 2015). Sign- and goal-tracking have also been demonstrated to be associated with differential dopamine signaling, and this has been interpreted as a preferential role for dopamine signaling in incentive value attribution over reward-prediction error (Flagel et al. 2011). The results reported herein support the literature suggesting a preferential role for dopamine in incentive salience attribution over general reward-prediction error learning; however, an alternative interpretation could be that this dissociation might be reflective of different types of error-related learning (Glascher et al. 2010). Although far from conclusive (see Dayan and Berridge, 2014, for a discussion of model-based versus model-free Pavlovian systems), the neurobehavioral repertoires of sign- and goal-tracking have been formally modeled as different reinforcement learning systems that become engaged during stimulus-reward learning, where sign-tracking may reflect a more model-free, stimulus-response learning process that is dopamine-dependent and goal-tracking may reflect a more model-based, action-outcome learning system that is far less reliant on dopaminergic signaling (LeSaint et al. 2014; Huys et al. 2014). In general, the results reported here support the model-free/model-based distinction and further suggest that these 2 systems can be stimulus-specific and act independently and in parallel within an individual.
In addition to demonstrating the functional differences and differential dopamine dependence of sign- and goal-tracking within subjects, the present results also extend previous findings by implicating D1 dopamine receptors as a receptor of interest in the learning of a sign-tracking response during acquisition; this particular result lends itself to the notion that D1 function is required for model-free learning, and attenuating D1 signaling during acquisition can shift the representation of a stimulus-reward relation towards model-based learning (Dayan and Berridge, 2014). Furthermore, the present results extend previous findings by demonstrating both model-based and model-free learning and their differential dopamine dependency within individuals, indicating that both learning systems can take place independently within a single system. Interestingly, when sign-tracking and goal-tracking associated stimuli were pit against each other within a choice procedure, animals consistently chose the stimulus associated with sign-tracking over the stimulus associated with goal-tracking, even though each stimulus was paired with the same food pellet. D1 antagonism dose-dependently reduced the value associated with the sign-tracking associated stimulus, increasing choices for the goal-tracking associated stimulus. The choice results have interesting implications in regard to the relative value associated with the 2 different learning systems, suggesting that products of stimulus-response, model-free learning are more valuable than their action-outcome, model-based counterparts, and this suggests that incentive salience attribution may be indicative of a stimulus-response learning processes. The choice results also indicate that D1 receptor signaling may play a potential role in determining the relative weighting (ω; Dayan et al. 2006; LeSaint et al. 2014) of model-free versus model-based learning systems during stimulus-reward learning. However, there is much to be done in regard to the dissociation of incentive salience, reward-prediction error, model-free, and model-based hypotheses (Dayan and Berridge, 2014); but, the results reported herein do suggest that the within-subject 2-CS PCA procedure may be a good method to dissociate competing hypotheses regarding stimulus-reward learning in future research, and a combination of the 2-CS procedure used here with more advanced neuroscience techniques (e.g., DREADDs, optogenetics, fast-scan cyclic voltammetry; etc.) and models of individual differences in incentive salience attribution may aid in furthering this dissociation.
Individual differences in what is learned about a conditioned stimulus has predictive value regarding substance abuse-related behavior between individuals (Tomie et al. 2008). For example, Saunders and Robinson (2010) demonstrated animals that sign-tracked to a lever CS were more affected by a cocaine-associated CS during cue-induced reinstatement. The same group (Saunders et al. 2014) later demonstrated that animals that goal-tracked to a lever CS were more affected by a cocaine-associated discriminative stimulus during context-induced reinstatement, relative to those that sign-tracked to a lever CS. These findings suggest that the type of stimulus-reinforcer relationship learned during drug taking could determine individual vulnerability to future drug-seeking, where some individuals are more heavily influenced by products of stimulus-response learning processes while others are more heavily influenced by products of response-outcome learning processes. Most importantly, both of these stimulus-reinforcer relationships can produce drug-seeking behavior, and this fact highlights the great importance of understanding the functional role a stimulus plays within a stimulus-reinforcer relation when attempting to understand stimulus control of drug-seeking behavior. In other words, not all stimulus-reinforcer relationships that induce drug-seeking behavior are the same; it is dependent upon the functional role a stimulus serves (c.f., Robinson et al. 2014). Using a 2-CS PCA procedure and isolating the different neurobehavioral systems associated with sign- and goal-tracking and extending a 2-CS procedure to drug-taking models may provide future insight into different aspects of substance abuse-related behavior, specifically how drug-associated stimuli can come to function differently and engage different neurobehavioral systems within individuals.
One standing issue with investigating any hypotheses related to acquisition and sign- and goal-tracking response predispositions, which partially served as the impetus for the development of the 2-CS PCA procedure (Beckmann and Chow, 2015), is that the experimenter does not know if an animal is a “sign-tracker” or a “goal-tracker”, until the CS-US relation has already been acquired (i.e., post-acquisition; Meyer et al. 2012). Thus, any manipulations during acquisition in a large cohort of outbred animals trained under a single-lever PCA procedure cannot be attributed directly to a pre-existing response predisposition. Although there are selective-breeding models of sign- and goal-tracking (e.g., Flagel et al. 2010, 2011), possible uncharacterized, inadvertent neurobehavioral changes due to selective-breeding make interpretation problematic, especially given that the existing model is selected on a response (locomotor responding to a novel environment) known not to be related to sign-tracking or goal-tracking in outbred animals (Robinson and Flagel, 2009; Beckmann et al. 2011). However, within the 2-CS PCA procedure, we have never witnessed a single “goal-tracker”; that is, in our hands, this procedure elicits exclusive sign-tracking to the lever CS, a consequence we believe might be due to the nature of the lever CS used here (retractable lever only) versus that of the compound lever and light CS (described as an “illuminated lever”) used in other laboratories (e.g., Lovic et al. 2011; Flagel et al. 2011; Saunders and Robinson, 2011; Meyer et al. 2012; Fitzpatrick et al. 2013; Meyer et al. 2014). More specifically, the individual differences in sign- and goal-tracking to the illuminated lever could be reflective of individual differences in the learning of the lever or light element of the compound CS used due to individual differences in CS element associability (cf. Pearce and Hall, 1982). Other factors that may impact the lack of “goal-trackers” within the 2-CS procedure include the possible added influence of animal vendor (Fitzpatrick et al. 2013; a small effect not likely to be the sole source the exclusivity of sign-tracking we have witnessed), the multiple-stimulus design (cf. Holland et al. 2014; Beckmann and Chow, 2015), and training time (14 days vs. 5–7). Furthermore, there are a number of environmental factors known to influence whether a sign- or a goal-tracking response is elicited by a lever CS, including motivational establishing operations (Davey and Cleland, 1982, 1984; Robinson and Berridge, 2013), learning history (Davey and Cleland, 1984), and the distance of the lever CS from the location of food delivery (Silva et al. 1992). Thus, any predispositions regarding sign- and goal-tracking would be heavily influenced or even overridden by any of the factors discussed above, raising questions regarding the generality of their functional importance; clearly, the data presented here and within Meyer et al. (2014) indicate a limit on such generalization (i.e., animals that sign-track and attribute incentive salience to a lever CS do not generalize this process to all reward-predictive CSs, including associated dopaminergic signaling). Therefore, it seems pertinent that future research aims toward defining the boundary conditions under which a CS-US relationship that leads to sign-tracking is likely to be learned, as individual differences in incentive salience attribution might best be captured by the range magnitude of said boundary conditions.
In conclusion, the results found here suggest that D1 and D2 dopamine receptor function and nucleus accumbens dopamine have dissociable roles in the acquisition of incentive salience attribution to reward-associated stimuli, and they suggest that dopamine plays a preferential role in attributing incentive value to reward-associated stimuli. Furthermore, the present results suggest that using a within-subject, 2-CS PCA approach in combination with more advanced neuroscience techniques may help in the future dissociation of various proposed neurobehavioral systems thought to underlie stimulus-reward learning, and extending this approach to self-administration models of drug use may help in identifying the role these different systems have in the stimulus control of drug-seeking behavior, including individual differences.
Supplementary Material
Highlights.
Both D1 and D2 receptor inhibition affected sign- and goal-tracking performance
D1 receptor inhibition specifically prevented the acquisition of incentive salience
Nucleus acccumbens dopamine lesions prevented sign-tracking but not goal-tracking
Showed a within-subject incentive salience vs. reward-prediction error dissociation
Acknowledgments
We would like to thank William T. McCuddy, Joshua N. Lavy, and Andrew Edel for their technical support. We also thank Linda P. Dwoskin for her support. Finally, we thank Steven B. Harrod for his insightful feedback on an earlier draft of the manuscript. Funding was provided by the National Institute for Drug Abuse (NIDA), DA033373 and DA016176.
Abbreviations
- CS
conditioned stimulus
- US
unconditioned stimulus
- CR
conditioned response
- 6-OHDA
6-hydroxdopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors report no conflicts of interest.
References
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behavioural brain research. 2016;296:418–430. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Young ME. Stimulus dynamics and temporal discrimination: implications for pacemakers. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35(4):525. doi: 10.1037/a0015891. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioural brain research. 2011;216(1):159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learn. Mem. 2015;22:116–127. doi: 10.1101/lm.037382.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behavioural brain research. 2009;200(1):22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. Operant-Pavlovian interactions. 1977:67–97. [Google Scholar]
- Bouton ME. Learning and behavior: A contemporary synthesis. Sinauer Associates; 2007. [Google Scholar]
- Brooks DI, Rasmussen IP, Hollingworth A. The nesting of search contexts within natural scenes: evidence from contextual cuing. Journal of Experimental Psychology: Human Perception and Performance. 2010;36(6):1406. doi: 10.1037/a0019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. AUTO-SHAPING OF THE PIGEON’S KEY-PECK1. Journal of the experimental analysis of behavior. 1968;11(1):1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiology of learning and memory. 2012;97(4):441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Holland PC. Effects of nucleus accumbens core and shell lesions on autoshaped lever-pressing. Behavioural brain research. 2013;256:36–42. doi: 10.1016/j.bbr.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Hollon NG, Phillips PE. Pavlovian valuation systems in learning and decision making. Current opinion in neurobiology. 2012;22(6):1054–1061. doi: 10.1016/j.conb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacology Biochemistry and Behavior. 2010;96(1):40–47. doi: 10.1016/j.pbb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GC, Cleland GG. TOPOGRAPHY OF SIGNAL-CENTERED BEHAVIOR IN THE RAT: EFFECTS OF DEPRIVATION STATE AND REINFORCER TYPE. Journal of the experimental analysis of behavior. 1982;38(3):291–304. doi: 10.1901/jeab.1982.38-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GC, Cleland GG. Food anticipation and lever-directed activities in rats. Learning and Motivation. 1984;15(1):12–36. [Google Scholar]
- Dayan P, Berridge KC. Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(2):473–492. doi: 10.3758/s13415-014-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behavioral neuroscience. 2000;114(3):468. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Eyny YS, Horvitz JC. Opposing roles of D1 and D2 receptors in appetitive conditioning. The Journal of neuroscience. 2003;23(5):1584–1587. doi: 10.1523/JNEUROSCI.23-05-01584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, Saunders BT, Parker CC, Gonzales NM, Aryee E, Flagel SB. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PloS one. 2013;8(10):e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35(2):388. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5(4):529. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KM, Haight JL, Gardner EL, Flagel SB. Examining the role of dopamine D 2 and D 3 receptors in Pavlovian conditioned approach behaviors. Behavioural Brain Research. 2016 doi: 10.1016/j.bbr.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press; 2006. [Google Scholar]
- Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66(4):585–595. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Psychonomic Society; 1974. [Google Scholar]
- Holland PC, Asem JS, Galvin CP, Keeney CH, Hsu M, Miller A, Zhou V. Blocking in autoshaped lever-pressing procedures with rats. Learning & behavior. 2014;42(1):1–21. doi: 10.3758/s13420-013-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Tobler PT, Hasler G, Flagel SB. The role of learning-related dopamine signals in addiction vulnerability. Prog. Brain Res. 2014;211:31–77. doi: 10.1016/B978-0-444-63425-2.00003-9. [DOI] [PubMed] [Google Scholar]
- Lesaint F, Sigaud O, Flagel SB, Robinson TE, Khamassi M. Modelling individual differences in the form of pavlovian conditioned approach responses: a dual learning systems approach with factored representations. PLoS Comput Biol. 2014;10(2):e1003466. doi: 10.1371/journal.pcbi.1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JC, Karlsson RM, O’Donnell P. Dopamine D2 modulation of sign and goal tracking in rats. Neuropsychopharmacology. 2015;40(9):2096–2102. doi: 10.1038/npp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural brain research. 2011;223(2):255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: variations in the associability of stimuli with reinforcement. Psychological review. 1975;82(4):276. [Google Scholar]
- Mazur JE. Commons, ML.; Mazur, JE.; Nevin, JA. 1987. An adjusting procedure for studying delayed reinforcement; pp. 55–73. [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS one. 2012;7(6):e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PloS one. 2014;9(6):e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Barnet RC, Grahame NJ. Assessment of the Rescorla-Wagner model. Psychological bulletin. 1995;117(3):363. doi: 10.1037/0033-2909.117.3.363. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. The Journal of neuroscience. 1996;16(5):1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Bamkole MA, Nicola SM. Sign tracking, but not goal tracking, is resistant to outcome devaluation. Frontiers in neuroscience. 2015;9:468. doi: 10.3389/fnins.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser HM, Chen YW, Fiscella K, Calu DJ. Individual variability in behavioral flexibility predicts sign-tracking tendency. Frontiers in behavioral neuroscience. 2015;9:289. doi: 10.3389/fnbeh.2015.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current opinion in neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proceedings of the National Academy of Sciences. 2010;107(30):13491–13496. doi: 10.1073/pnas.1007827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behavioural brain research. 2002;137(1):149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Kaye H, Hall G. Predictive accuracy and stimulus associability: Development of a model for Pavlovian learning. Quantitative analyses of behavior. 1982;3:241–256. [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R package version. 2007;3:57. [Google Scholar]
- Rachlin H, Logue AW, Gibbon J, Frankel M. Cognition and behavior in studies of choice. Psychological review. 1986;93(1):33. [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the experimental analysis of behavior. 1991;55(2):233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological review. 1967;74(1):71. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical conditioning II: Current research and theory. 1972;2:64–99. [Google Scholar]
- Robinson MJ, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Current Biology. 2013;23(4):282–289. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological psychiatry. 2009;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnick EH. Does the interval of delay of conditioned responses possess inhibitory properties? Journal of Experimental Psychology. 1937;20(6):507. [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36(4):2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology. 2014;39(12):2816–2823. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in neurosciences. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Silva FJ, Silva K, Pear JJ. SIGN-VERSUS GOAL-TRACKING: EFFECTS OF CONDITIONED-STIMULUS-TO-UNCONDITIONED-STIMULUS DISTANCE. Journal of the Experimental Analysis of Behavior. 1992;57(1):17–31. doi: 10.1901/jeab.1992.57-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: An introduction. MIT press; 1998. [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain research reviews. 2008;58(1):121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learning & memory. 2011;18(7):475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: Advantages and cautionary notes. Learning and Motivation. 2009;40(2):160–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.