Abstract
A recent whole-genome sequencing study in search of variation associated with adult areal bone mineral density (aBMD) identified rare variants near EN1, with markedly large effect sizes, and a common variant near SOX6. To understand the developmental effects of these loci, we sought to determine if they were associated with pediatric dual-energy X-ray absorptiometry–derived aBMD and bone mineral content (BMC) and if the associations were modified by sex. Our sample comprised 733 females and 685 males of European ancestry enrolled in the longitudinal Bone Mineral Density in Childhood Study (up to 7 annual study visits). Sex- and age-specific Z-scores, adjusted for height, were calculated for the total hip, femoral neck, spine, and distal radius. Total body less head (TBLH) BMC Z-scores were also calculated. The previously reported single nucleotide polymorphisms (SNPs) near EN1 and SOX6 were derived from our imputed data set. Linear mixed-effects models were used to test associations between each SNP and bone Z-scores, plus interactions with sex were explored. The rare T allele of lead EN1 SNP rs11692564 was associated with higher aBMD Z-score for total hip (beta = 0.62, p = 9.0 × 10−4) and femoral neck (beta = 0.53, p = 0.010). In sex-stratified analyses, this variant was associated with higher bone Z-scores in females only, with the associations being strongest for total hip (sex interaction p = 1.9 × 10−4; beta females = 0.86, p = 6.6 × 10−6) and femoral neck (sex interaction p = 0.016; beta females = 0.73, p = 0.001). The common G allele of SOX6 SNP rs11024028 was associated with higher aBMD Z-score for total hip (beta 0.12, p = 0.009), femoral neck (beta = 0.13, p = 0.003), and TBLH-BMC (beta = 0.09, p = 0.007); furthermore, this association strengthened in males in the sex-stratified analyses. Our findings reveal that rare genetic variation near EN1 and common variation near SOX6 operates in childhood and has implications for the lifelong risk of osteoporosis and fracture. The sex differences observed need to be independently replicated.
Keywords: DXA, GENETIC RESEARCH, GENERAL POPULATION STUDIES, CHILDHOOD, PUBERTY
Introduction
Osteoporosis is a leading noncommunicable disease, characterized by low areal bone mineral density (aBMD).(1) It is estimated that the heritability of aBMD is between 60% and 80% and genome-wide association studies (GWAS) have implicated common variants at 63 loci with adult aBMD.(2) A recent landmark whole-genome sequencing study by Zheng and colleagues identified rare variation near EN1 that is associated with aBMD in European adults.(3) Strikingly, the rare variant effect sizes were 4 times higher than the average effect size of common variants identified by GWAS.(2,3) Furthermore, Zheng and colleagues observed associations between a common variant near SOX6 and adult aBMD. Although this locus was one of the GWAS-implicated aBMD loci, the Zheng and colleagues signal is independent of the original GWAS signal. Adult bone fragility can have its origins in early life, yet it cannot be assumed that a genetic association in adults, when bone loss is occurring, also associates with pediatric aBMD, when bone is being accrued.
The youngest cohort included in the study by Zheng and colleagues comprised 19-year-old young adults from the Avon Longitudinal Study of Parents and Children (ALSPAC)(3) with femoral neck aBMD measurements. Bone fragility is more common among females,(1) and we have shown that there are sex differences in the association of adult GWAS variants and pediatric aBMD.(4–6) We, therefore, aimed to determine if rare variants near EN1 and a common variant near SOX6 associated with pediatric aBMD and bone mineral content (BMC) at multiple skeletal sites and if the associations were modified by sex and maturation.
Materials and Methods
We analyzed data from the longitudinal Bone Mineral Density in Childhood Study (BMDCS).(7,8) A summary of past genetic-based BMDCS studies is given in Supplemental Table S1. Our sample included BMDCS participants who were genotyped and completed up to 7 annual study visits (n = 932) and a cross-sectional sample of participants from our replication cohort (n = 486). Overall, our sample comprised 733 females and 685 males of European ancestry. Principal components analysis of the whole-genome genotype data and ADMIXTURE (>50%) was used to define ancestry. Written informed consent (and assent when applicable) was obtained for all participants and Institutional Review Boards at each institution approved the study.
Dual-energy X-ray absorptiometry (DXA) was used to estimate aBMD at the total hip, femoral neck, spine, and distal radius (Hologic, Inc., Bedford, MA, USA; models QDR4500A, QDR4500W, Delphi A, and Apex). We calculated sex- and age-specific aBMD Z-scores, adjusted for height Z-score.(9) Total body less head bone mineral content (TBLH-BMC) Z-scores were also calculated.(9)
The DNA extracted from blood or saliva was genome-wide SNP genotyped DNA using the Illumina Infinium II OMNI Express plus Exome BeadChip technology (Illumina, San Diego, CA, USA).(4,5) We included 4 variants near EN1, including the lead variant identified by Zheng and colleagues (rs11692564) as well as rs188303909, rs6542457, and rs55983207.(3) Note, rs188303909 was partially in linkage disequilibrium with rs11692564 (r2 = 0.61 in our data set). Zheng and colleagues also identified a common variant near SOX6 (rs11024028) that associated with adult aBMD;(3) we also included this SNP in our study. All were imputed to the 1000 Genome Phase I Integrated Release Version 3 reference panel, using SHAPEIT for haplotype phasing and IMPUTE2 for imputation. All were in Hardy Weinberg Equilibrium (p > 10−3) and had INFO scores >0.4 (rs11692564 = 0.59, rs188303909 = 0.58, rs6542457 = 0.99, rs55983207 = 0.80, and rs11024028 = 0.87). directly genotyped SNPs in the BMDCS, needed for imputation, had call rates >95%, minor allele frequency >1%, missing rate per person <2%, and were in Hardy-Weinberg equilibrium.
Linear mixed-effects models were used to test associations between each SNP and bone Z-scores, using random intercepts, maximum likelihood estimation, and robust standard errors. In our sex-combined analyses, all models were adjusted for age, sex, Tanner stage, body mass index, dietary calcium, and physical activity. We performed sex-stratified analyses, and we statistically tested for SNP-sex interactions in analyses with the older of a sibling pair removed from the discovery (n = 112) and replication cohorts (n = 1). We also tested for SNP-age and SNP-Tanner stage interactions to determine if any SNP associations with bone Z-scores changed as a function of maturation. Associations were considered statistically significant after correcting for the multiple testing of the 5 variants (p≤0.01). All analyses were performed using Stata 14.0 (StataCorp LP, College Station, TX, USA).
Results
Nineteen participants were heterozygous (11 females and 8 males), carrying one copy of the rare T allele of lead SNP rs11692564 (98 observations across 7 study visits), and 1 homozygous female participant carried two copies (7 observations across 7 study visits). In the sex-combined analyses, this variant was associated with higher aBMD Z-scores for the total hip (beta = 0.62, p = 9.0×10−4) and femoral neck (beta 0.53, p = 0.010). In our sex-stratified analyses, the variant was associated with higher bone Z-scores in females only (Table 1), with the aBMD Z-score associations strongest for total hip (beta = 0.86, p = 6.6 × 10−6) and femoral neck (beta = 0.73, p = 0.001). In support of our a priori hypothesis that genetic variants could associate with pediatric aBMD differently for males and females, we also observed statistical evidence of rs11692564-T sex differences (interaction p values, Table 1). The rs11692564-T associations reported in Table 1 did not change as a function of chronological age or puberty status. Furthermore, the results remained similar after adjusting for 10 principal components to correct for any population stratification (Supplemental Table S1).
Table 1.
Variants Near EN1 and SOX6 and Associations With Pediatric Bone Z-Scores
| SNP (effect allele) | EAF | Nearest gene | Site | Both sexes (N = 1301)a
|
Females (n = 730)
|
Males (n = 684)
|
Sex interaction
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% Cl | p Value | Beta | 95% CI | p Value | Beta | 95% CI | p Value | p Value | ||||
| rs11692564 (T) | 0.01 | EN1 | Total hip | 0.62 | (0.25, 0.98) | 9.0 × 10−4 | 0.86 | (0.48, 1.23) | 6.6 × 10−6 | −0.15 | (−0.62, 0.31) | 0.517 | 1.9 × 10−4 |
| rs11692564 (T) | 0.01 | EN1 | Fem neck | 0.53 | (0.12, 0.93) | 0.010 | 0.73 | (0.29, 1.18) | 0.001 | −0.29 | (−0.88, 0.30) | 0.334 | 0.016 |
| rs11692564 (T) | 0.01 | EN1 | Spine | 0.21 | (−0.16, 0.59) | 0.266 | 0.40 | (−0.00, 0.80) | 0.052 | −0.40 | (−0.94, 0.13) | 0.141 | 0.052 |
| rs11692564 (T) | 0.01 | EN1 | Radius | 0.01 | (−0.38, 0.40) | 0.956 | 0.39 | (−0.02, 0.80) | 0.064 | −0.48 | (−0.96, −0.01) | 0.045 | 0.001 |
| rs11692564 (T) | 0.01 | EN1 | TBLH | 0.20 | (−0.07, 0.46) | 0.150 | 0.37 | (0.12, 0.62) | 0.004 | −0.28 | (−0.77, 0.21) | 0.261 | 0.041 |
| rs188303909 (T) | 0.01 | EN1 | Total hip | 0.46 | (0.04, 0.87) | 0.030 | 0.66 | (0.20, 1.12) | 0.005 | −0.02 | (−0.47, 0.43) | 0.930 | 0.013 |
| rs188303909 (T) | 0.01 | EN1 | Fem neck | 0.35 | (−0.04, 0.73) | 0.080 | 0.49 | (0.08, 0.91) | 0.019 | −0.12 | (−0.70, 0.47) | 0.691 | 0.125 |
| rs188303909 (T) | 0.01 | EN1 | Spine | −0.03 | (−0.39, 0.34) | 0.881 | 0.09 | (−0.27, 0.46) | 0.620 | −0.28 | (−0.80, 0.25) | 0.299 | 0.270 |
| rs188303909 (T) | 0.01 | EN1 | Radius | −0.22 | (−0.64, 0.21) | 0.323 | 0.23 | (−0.23, 0.69) | 0.324 | −0.41 | (−0.90, 0.09) | 0.105 | 0.002 |
| rs188303909 (T) | 0.01 | EN1 | TBLH | 0.10 | (−0.13, 0.33) | 0.402 | 0.22 | (0.04, 0.40) | 0.015 | −0.09 | (−0.55, 0.37) | 0.704 | 0.236 |
| rs6542457 (C) | 0.07 | EN1 | Total hip | 0.11 | (−0.02, 0.24) | 0.112 | 0.12 | (−0.06, 0.29) | 0.203 | 0.03 | (−0.14, 0.21) | 0.703 | 0.733 |
| rs6542457 (C) | 0.07 | EN1 | Fem neck | 0.11 | (−0.02, 0.24) | 0.084 | 0.17 | (−0.01, 0.36) | 0.067 | −0.01 | (−0.19, 0.16) | 0.879 | 0.321 |
| rs6542457 (C) | 0.07 | EN1 | Spine | 0.08 | (−0.05, 0.20) | 0.222 | 0.22 | (0.04, 0.39) | 0.014 | −0.18 | (−0.3, −0.01) | 0.042 | 0.013 |
| rs6542457 (C) | 0.07 | EN1 | Radius | 0.13 | (0.01, 0.24) | 0.027 | 0.20 | (0.05, 0.36) | 0.011 | 0.06 | (−0.08, 0.21) | 0.409 | 0.205 |
| rs6542457 (C) | 0.07 | EN1 | TBLH | 0.04 | (−0.05, 0.14) | 0.365 | 0.08 | (−0.05, 0.21) | 0.208 | −0.03 | (−0.16, 0.10) | 0.624 | 0.340 |
| rs55983207 (C) | 0.06 | EN1 | Total hip | 0.12 | (−0.04, 0.28) | 0.143 | 0.10 | (−0.11, 0.31) | 0.332 | 0.14 | (−0.08, 0.37) | 0.218 | 0.883 |
| rs55983207 (C) | 0.06 | EN1 | Fem neck | 0.12 | (−0.03, 0.27) | 0.125 | 0.12 | (−0.08, 0.32) | 0.235 | 0.13 | (−0.07, 0.34) | 0.209 | 0.957 |
| rs55983207 (C) | 0.06 | EN1 | Spine | 0.15 | (−0.00, 0.30) | 0.055 | 0.05 | (−0.14, 0.24) | 0.596 | 0.24 | (0.03, 0.44) | 0.025 | 0.202 |
| rs55983207 (C) | 0.06 | EN1 | Radius | 0.02 | (−0.14, 0.17) | 0.831 | 0.00 | (−0.23, 0.23) | 0.999 | 0.04 | (−0.16, 0.24) | 0.696 | 0.689 |
| rs55983207 (C) | 0.06 | EN1 | TBLH | 0.08 | (−0.03, 0.20) | 0.145 | 0.05 | (−0.10, 0.19) | 0.549 | 0.11 | (−0.04, 0.26) | 0.157 | 0.838 |
| rs11024028 (G) | 0.19 | SOX6 | Total hip | 0.12 | (0.03, 0.22) | 0.009 | 0.08 | (−0.04, 0.21) | 0.180 | 0.16 | (0.03, 0.28) | 0.014 | 0.289 |
| rs11024028 (G) | 0.19 | SOX6 | Fem neck | 0.13 | (0.04, 0.22) | 0.003 | 0.11 | (−0.02, 0.23) | 0.089 | 0.13 | (0.01, 0.25) | 0.031 | 0.723 |
| rs11024028 (G) | 0.19 | SOX6 | Spine | 0.10 | (0.00, 0.19) | 0.044 | 0.00 | (−0.12, 0.11) | 0.967 | 0.19 | (0.06, 0.31) | 0.004 | 0.059 |
| rs11024028 (G) | 0.19 | SOX6 | Radius | 0.02 | (−0.06, 0.11) | 0.616 | 0.04 | (−0.07, 0.15) | 0.438 | −0.02 | (−0.15, 0.10) | 0.691 | 0.207 |
| rs11024028 (G) | 0.19 | SOX6 | TBLH | 0.09 | (0.02, 0.16) | 0.007 | 0.06 | (−0.03, 0.14) | 0.170 | 0.11 | (0.01, 0.20) | 0.024 | 0.337 |
SNP=single nucleotide polymorphism; EAF=effect allele frequency; CI=confidence interval; Fem neck=femoral neck; TBLH=total body less head.
Beta coefficients, 95% CIs, and p values are from linear mixed-effects models adjusted for age, Tanner stage, body mass index Z-score, physical activity, dietary calcium (and sex in the sex combined analyses). The p values in bold survived correction for multiple testing of the 5 SNPs. The participants had repeated bone Z-score measures with up to 7 annual study visits.
Opposite-sex siblings are included in the Bone Mineral Density in Childhood Study (BMDCS); the older siblings were removed for the sex combined analyses (n = 113).
Of the three other SNPs near EN1, the T allele of rs188303909 was associated with higher female total hip aBMD (beta = 0.66, p = 0.005) (Table 1). Interestingly, this association with total hip aBMD in females increased in strength with increasing chronological age and puberty status (Fig. 1).
Fig. 1.
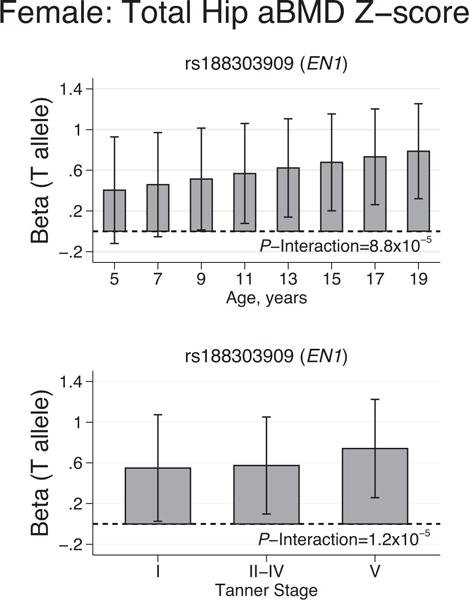
Rare variant rs188303909 near EN1 and associations with female total hip BMD Z-scores by age and Tanner stage. The T allele of rs188303909 associated with higher total hip aBMD Z-score; this association was stronger among the older and more mature females. The beta coefficients and standard errors are extracted from linear mixed-effects models with age × rs188303909 or Tanner stage × rs188303909 interaction terms. All models were adjusted for sex, BMI Z-score, physical activity, and dietary calcium.
Finally, we tested for associations with the common variant near SOX6. In the sex-combined analyses, the G allele of rs11024028 associated with higher total hip aBMD, femoral neck aBMD, and TBLH-BMC (Table 1). Furthermore, this variant was associated with higher spine aBMD among males (Table 1). These associations did not change as a function of maturation.
Discussion
Adult bone fragility has a strong genetic component that necessitates investigation to identify mechanistically focused strategies for the prevention and treatment of osteoporosis and fracture. Rare variants near EN1 associate with adult aBMD, and in the context of all established genetic variants, the effect sizes are the largest identified to date.(2,3) However, it was not known from the original study if the same locus operated in the pediatric setting. We report for the first time to our knowledge associations between rare variants near EN1 and pediatric aBMD. Importantly, the associations are directionally consistent with those reported in adults,(3) and in fact the effect sizes are even higher in magnitude. These data further validate the findings of the initial study and demonstrate that these rare variants act early in life.
Osteoporosis is more common among women, and there are well-established sex differences known to influence bone accretion and peak bone mass.(1,10) We have reported sex differences in the context of associations between adult GWAS-implicated aBMD loci and pediatric bone Z-scores in the BMDCS.(4–6) It is, therefore, possible that the genetic regulation of skeletal development differs for males and females and could set the scene for greater osteoporosis risk among women in later life. Indeed, our sex-stratified analyses revealed that the rare variants near EN1 associated with pediatric aBMD in females only. Sex differences in fat and lean mass—resulting in differences in circulating myokines, adipokines, and mechanical loads upon the skeleton—could affect how the same genetic variants regulate the male and female skeletons. Similarly, differences in circulating sex hormones and behaviors (diet and physical activity) could also explain why the same genetic variants discordantly regulate the male and female skeletons. In contrast to the sex differences we observed, Zheng and colleagues reported similar associations with the rare variants near EN1 in adult males and females.(3) This suggests that the EN1 sex differences could be a pediatric phenomenon during bone accretion. However, we note that our male-specific results are based on 8 heterozygote participants with a total of 40 observations across 7 study visits, so we may have lower power to detect strong effects in that sex. As such, we cannot definitively conclude that rare variants near EN1 only associate with female pediatric aBMD. Future studies should take into account our observations and test for possible sex differences regarding the effects of EN1 on bone outcomes in adults and children, and explicitly describe the sex and test for EN1 sex differences in functional studies using mice.(3,11)
The SOX6 locus is one of the established GWAS-implicated adult aBMD loci.(2) However, the common variant rs11024028, near SOX6, identified by Zheng and colleagues is independent from the GWAS reported variant near SOX6 (rs7108738).(2,3) Interestingly, this latter GWAS-implicated variant was not associated with bone Z-scores in the BMDCS,(5) but in the present study we did observe evidence of associations between rs11024028 and bone Z-scores. This provides further evidence that adult identified variants (rare and common) operate in the pediatric setting to influence aBMD.
Our study has strengths and limitations. The BMDCS participants were extensively followed for up to 7 years, and a large number of children and adolescents were phenotyped to estimate aBMD at multiple skeletal sites. However, our results are only generalizable to US children and adolescents of European ancestry. In addition to rare variants near EN1 and common variation near SOX6, Zheng and colleagues also identified a rare variant near WNT16 (rs148771817) that was associated with adult aBMD;(3) however, in the BMDCS, only one participant was homozygous for this rare variant near WNT16 and no participants were heterozygous; therefore, we were not able to statistically test for associations with this rare variant. We, and others, have shown that common variants near WNT16 are strongly associated with pediatric aBMD,(4,5,12) and it will be of interest to test for associations with rs148771817 in larger pediatric samples in the future. We did not use the most conservative approach to correct for multiple testing; however, the primary finding involving rs11692564 remains using the most conservative correction for multiple testing (0.05/50). Finally, our bone Z-score phenotypes were estimated using DXA; testing for associations between the rare variants near EN1 with bone phenotypes estimated from peripheral quantitative computed tomography (p-QCT) are warranted.
In summary, we have replicated associations, with strong effect sizes, between rare EN1 variants and aBMD in children and adolescents and thereby have extended the original adult findings to a pediatric cohort while demonstrating sex differences (which need to be independently replicated). We also replicated, and contextualized, the association between a common variant near SOX6 and pediatric aBMD. This represents a major advance in the musculoskeletal field because little is known about the genetic determinants of bone accretion during childhood. Together with those reported by Zheng and colleagues, our findings indicate that rare genetic variants near EN1 strongly associate with aBMD across the life span, especially in females, and point to an early-life genetic mechanism for the risk of osteoporosis and fracture in later life.
Supplementary Material
Acknowledgments
The study was funded by R01 HD58886; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contracts (N01-HD-1-3228, -3329, -3330, -3331, -3332, -3333); and the CTSA program Grant 8 UL1 TR000077. JAM is supported by K01 HL123612. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We appreciate the dedication of the study participants and their families, and the support of Dr Karen Winer, Scientific Director of the Bone Mineral Density in Childhood Study.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Study conception and design: JAM, AC, SFAG, and BSZ. Acquisition of data: SFAG, BSZ, HJK, JML, VG, SEO, and JAS. Data analysis: JAM and AC. Interpretation of data: JAM, AC, SEM, SMR, DLC, HJK, JML, VG, SEO, JAS, AK, BSZ, and SFAG. Drafting manuscript: JAM. Revising manuscript content: AC, SEM, SMR, DLC, HJK, JML, VG, SEO, JAS, and AK. Approving final version of manuscript: JAM, AC, SEM, SMR, DLC, HJK, JML, VG, SEO, JAS, AK, BSZ, and SFAG. JAM takes full responsibility for the integrity of the data analysis.
References
- 1.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng HF, Forgetta V, Hsu YH, et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–7. doi: 10.1038/nature14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesi A, Mitchell JA, Kalkwarf HJ, et al. A trans-ethnic genome-wide association study identifies gender-specific loci influencing pediatric aBMD and BMC at the distal radius. Hum Mol Genet. 2015;24(17):5053–9. doi: 10.1093/hmg/ddv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell JA, Chesi A, Elci O, et al. Genetics of bone mass in childhood and adolescence: effects of sex and maturation interactions. J Bone Miner Res. 2015;30(9):1676–83. doi: 10.1002/jbmr.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell JA, Chesi A, Elci O, et al. Genetic risk scores implicated in adult bone fragility associate with pediatric bone density. J Bone Miner Res. doi: 10.1002/jbmr.2744. Epub 2015 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14(10):1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 11.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina-Gomez C, Kemp JP, Estrada K, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8(7):e1002718. doi: 10.1371/journal.pgen.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


