Abstract
Head and neck squamous cell carcinoma (HNSCC) is a frequently fatal disease due in large part to a high rate of second primary tumor (SPT) formation. The 4-nitroquinoline 1-oxide (4-NQO) mouse model of oral carcinogenesis provides a robust system in which to study chemopreventive agents in the context of chemically-induced HNSCC tumors. Signal transducer and activator of transcription 3 (STAT3) is a potent oncogene that is hyperactivated by tyrosine phosphorylation early in HNSCC carcinogenesis and is a rational therapeutic target. We recently reported that loss-of-function of the STAT3 phosphatase PTPRT promotes STAT3 activation in HNSCC tumors and pre-clinical models, and may serve as a predictive biomarker of response to STAT3 inhibitors, including the small molecule Stattic. We therefore investigated the hypothesis that PTPRT knockout (KO) mice would be more susceptible to 4-NQO-induced oral carcinogenesis and more sensitive to Stattic-mediated chemoprevention compared with wild-type (WT) mice. Herein we demonstrate that PTPRT WT and KO mice develop similar spectra of HNSCC disease severity upon 12-weeks of 4-NQO administration, with no apparent effect of PTPRT genotype on carcinogenesis or treatment outcome. Targeting of STAT3 with Stattic resulted in a chemopreventive effect against 4-NQO-induced oral cancer (P = 0.0402). While these results do not support a central role for PTPRT in 4-NQO-induced HNSCC carcinogenesis, further investigation of STAT3 as a chemoprevention target in this cancer is warranted.
Keywords: 4-nitroquinoline 1-oxide (4-NQO), PTPRT, phosphatase, Stattic, STAT3 inhibition
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a frequently fatal malignancy of the upper aerodigestive epithelium. While human papilloma virus (HPV) infection is an increasingly common etiologic factor in a subset of HNSCCs (1), cancers of the oral cavity in particular are infrequently associated with expression of viral genes. (2) Primary risk factors for development of HPV-negative HNSCC include exposure to environmental carcinogens, particularly tobacco and alcohol. These carcinogens contribute to the heterogeneous accumulation of genetic and epigenetic lesions throughout the oral mucosa, leading to “field cancerization” in which the entire tissue may be pre-cancerous and at elevated risk of malignant transformation. (3) Despite aggressive treatment with surgery, (chemo)radiation, and/or cetuximab, field cancerization likely contributes to second primary tumor (SPT) formation in ~4% of HNSCC patients annually, leading to a significant decrease in survival. (4) Strategies to inhibit tumorigenesis in the context of carcinogen-induced condemned oral mucosa are therefore under active investigation for prevention of SPT growth in HNSCC patients.
The 4-NQO (4-nitroquinoline 1-oxide) model of oral carcinogenesis enables investigation of the initiation and prevention of chemically-induced cancers of the oral epithelium in vivo. In this model, mice treated with 4-NQO develop invasive squamous cell carcinoma of the oral cavity with near 100% penetrance. (5) Importantly, these murine cancers share pathologic and biochemical features with tobacco-related human HNSCC, including epidermal growth factor receptor (EGFR) overexpression and downregulation of p16. (5, 6) Promising targets of interest in prior 4-NQO chemoprevention studies include cyclooxygenase-2 (COX-2) (7, 8) and EGFR (9). Targeting of COX-2 or EGFR, however, has not proven effective in clinical trials of patients with oral premalignancies due to toxicity of the agents used and the lack of predictive biomarkers (10–12). Development of alternate chemopreventive approaches that target distinct molecular pathways is therefore warranted.
Signal transducer and activator of transcription 3 (STAT3) is a proto-oncogenic transcription factor that is ubiquitously hyperactivated in HNSCC and other cancers, and represents a rational target for pharmacologic inhibition. (13) STAT3 is aberrantly activated by diverse mechanisms that culminate in constitutive phosphorylation and nuclear localization of active STAT3. While Gonçalves et al. recently reported that curcumin-induced chemoprevention in a 4-NQO rat model is associated with downregulation of STAT3 and other proteins (14), the chemopreventive effects of a direct STAT3 inhibitor has not been tested. We therefore investigated the efficacy of the small molecule STAT3 SH2 domain inhibitor Stattic (15) in this mouse model of oral carcinogenesis.
We recently reported that PTPRT (protein tyrosine phosphatase receptor type T) is frequently inactivated by somatic mutation or promoter hypermethylation in HNSCC and other cancers, leading to hyper-phosphorylation of STAT3, a PTPRT substrate. (16, 17) Together, these studies indicate that loss-of-function (LOF) of PTPRT contributes to constitutive STAT3 activation and may predict sensitivity to STAT3 inhibition. Additionally, Zhao et al. reported that total PTPRT LOF by germline knockout of the catalytic phosphatase domain dramatically sensitizes C57BL/6J mice to azoxymethane-induced colorectal carcinogenesis, indicating that PTPRT may protect against carcinogen-induced tumor formation. (18) In the present study, we utilized wild-type and PTPRT-null mice to evaluate the contribution of PTPRT to tumorigenesis in the 4-NQO mouse model or oral carcinogenesis. We further sought to determine the potential chemopreventive activity of targeting STAT3 with Stattic, and whether this activity might be impacted by PTPRT genotype.
Methods and Materials
Study Design and Statistics
Based upon our previous experience with the 4-NQO model (9), this study was designed to detect a reduction in dysplastic or cancerous lesions from 80% in the control group to 25% in the treatment group with 80% power at α = 0.025. This design required 12 mice per treatment group. At least 13 mice per group were included due to the potential for toxicity. Tumors were assigned a score from 0 to 6, reflecting increasing histologic severity from normal tissue (score = 0) to invasive SCC (score = 6). This disease severity was analyzed by a two way analysis of variance with interaction. Due to lack of interaction and negligible impact of mouse genotype, the data were combined across mouse types to test for differences in treatment effect using a two-tailed Wilcoxon test. pSTAT3 and total STAT3 were quantified by immunohistochemistry (IHC) and analyzed for effects of severity score, treatment group, and mouse genotype by linear regression.
Animals and Treatments
All animal manipulations were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. C57BL/6J mice lacking the intracellular catalytic domain of PTPRT (PTPRT KO) were obtained from Zhenghe Wang (Case Western Reserve University, Cleveland, OH) with the consent of the RIKEN BioResource Center (Japan). Age-matched wild-type (WT) C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice received 4-NQO-containing water as previously described (9) for 12 weeks. Briefly, 50 mg/mL 4-NQO (Sigma Aldrich, St. Louis, MO) stock solutions were prepared in DMSO and stored at −20°C. Weekly, 4-NQO stock was further diluted to 12.5 mg/mL in propylene glycol before addition of 2 mL 4-NQO solution to 250 mL fresh drinking water (100 μg/mL final concentration).
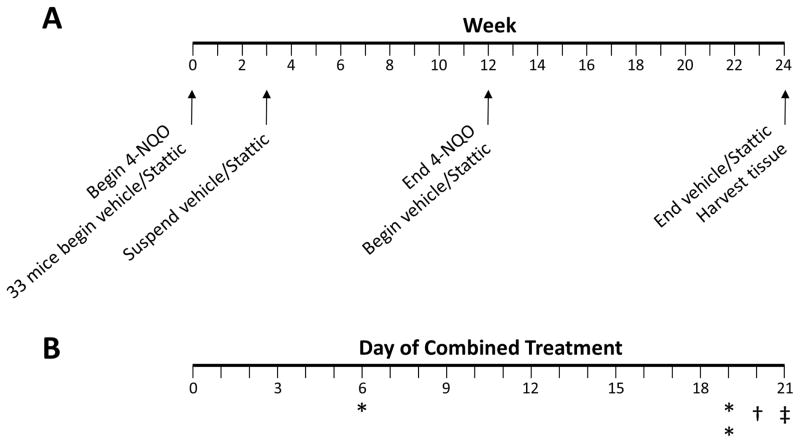
A timeline of experimental treatments is depicted in Figure 1A. At experiment initiation, a subset of mice were randomly assigned to vehicle (sterile PBS) or 50 mg/kg Stattic (Selleck Chemicals, Houston, TX) by oral gavage five times weekly. After three weeks, vehicle/Stattic treatment was suspended due to unforeseen toxicity when combined with 4-NQO, and 4-NQO administration was continued alone. After a subsequent round of births, a second group of age-matched mice received only 4-NQO. At the end of the 12-week 4-NQO administration period for each group, mice in the second 4-NQO round were randomized to receive vehicle or Stattic as above, while mice in the discontinued combined treatment group received the same treatment as previously (vehicle or Stattic). After an additional 12 weeks of vehicle or Stattic treatment, mice were sacrificed followed by excision of tongues which were immediately fixed in 10% formalin.
Figure 1. Timeline of 4-NQO and vehicle/Stattic administration and observed toxicities.
A) Overall experimental schema. B) Mice were found deceased on days 6 and 19 as indicated by an asterisk. † indicates one mouse found with poor body condition. ‡ indicates suspension of vehicle/Stattic treatment.
Histology
Tissue was processed and analyzed as previously described. (16) Mice with multiple lesions were categorized by the most severe lesion observed by hematoxylin and eosin (H&E) staining. Primary antibodies for IHC included anti-pSTAT3(Y705) and anti-STAT3 (Cell Signaling Technology, Danvers, MA). pSTAT3 IHC staining was quantified by nuclear v9 algorithm (Aperio, Sausalito, CA), and protein expression level represented by H score: % positive cells multiplied by staining intensity (1+, 2+, or 3+). Total STAT3 staining intensity was quantified by positive pixel count v9 algorithm (Aperio), and protein expression level is represented by staining intensity multiplied by the % positive area.
Results
Combined toxicity of 4-NQO and Stattic
We initially sought to determine the chemopreventive activity of Stattic when administered simultaneously with a chemical carcinogen. We therefore treated 33 mice with vehicle or 50 mg/kg Stattic by oral gavage five times weekly beginning concurrently with 4-NQO administration ad libitum in drinking water as depicted in Figure 1A. Several adverse events, including deaths, were observed within the first three weeks of combined treatment with 4-NQO and Stattic (Figure 1B). On day 6 post-treatment initiation, one WT mouse receiving Stattic died. An additional two WT mice receiving Stattic died on day 19. On day 20, one KO mouse was observed with a body condition score of 2, indicating poor health. (19) As all of the observed toxicities to this point occurred in mice receiving Stattic/4-NQO with no apparent toxicity in mice treated with vehicle/4-NQO, we suspended further vehicle or Stattic administration until completion of 4-NQO treatment at 12 weeks. After cessation of treatment, the mouse with poor body condition recovered rapidly, and no further toxicity was observed during the 12-week period. Table 1 outlines the number of surviving mice in each group that received 3 weeks of vehicle or Stattic at the beginning of the experiment and which were included for further analysis.
Table 1.
Number of mice that received vehicle or Stattic concurrently with 4-NQO for 3 weeks at initiation of the experiment. Parentheses indicate the number of mice that started treatment, while lead numbers indicate total surviving mice that were included in subsequent analyses.
| Wild-type | Knockout | Total | |
|---|---|---|---|
| Vehicle | 9 | 7 | 16 |
| Stattic | 5 (8) | 9 | 14 |
| Total | 14 (17) | 16 | 30 (33) |
4-NQO treatment induces diverse neoplasias
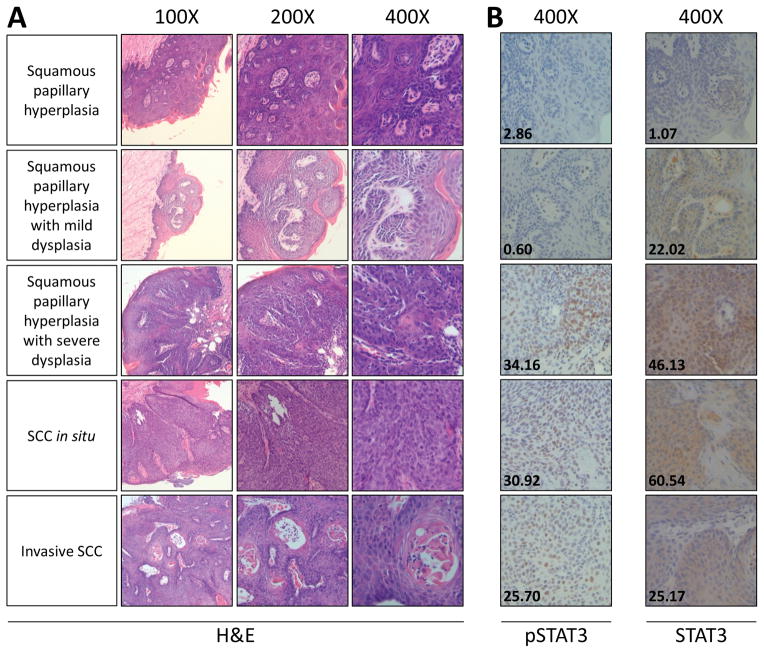
A second group of 28 age-matched mice received 12 weeks of 4-NQO (100 μg/mL) ad libitum followed by 12 weeks of Stattic (50 mg/kg) or vehicle control. At the end of this 24-week period, mice were sacrificed and tongues were harvested for analysis. The final number of mice per group is indicated in Table 2. A head and neck cancer pathologist (LW) blinded to the treatment groups analyzed each tongue and determined disease severity corresponding to no evidence of disease, squamous papillary hyperplasia, mild dysplasia, mild to moderate dysplasia, moderate to severe dysplasia, severe dysplasia, SCC in situ, or invasive SCC (representative images shown in Figure 2A). In WT mice that received vehicle (n = 13), every tongue displayed mild dysplasia or worse, with 7 mice (53.8%) displaying SCC of which 4 (30.8%) were invasive. This analysis indicates that our 4-NQO regimen produces dysplasia of diverse severity in C57BL/6J mice.
Table 2.
Total number of mice that were included for analysis. Parentheses indicate the number of mice that started treatment, while lead numbers indicate total surviving mice that were included in subsequent analyses.
| Wild-type | Knockout | Total | |
|---|---|---|---|
| Vehicle | 13 | 14 | 27 |
| Stattic | 13 (16) | 18 | 31 |
| Total | 26 (29) | 32 | 58 (61) |
Figure 2. Representative hematoxylin/eosin and immunohistochemical staining of mouse tongues after 24 weeks.
A) Tongue lesions and disease severity (left) were identified by a head and neck pathologist blinded to the treatment groups. B) Expression of the indicated proteins was analyzed and quantified by IHC (inset numbers).
PTPRT WT and KO mice respond similarly to Stattic-mediated 4-NQO chemoprevention
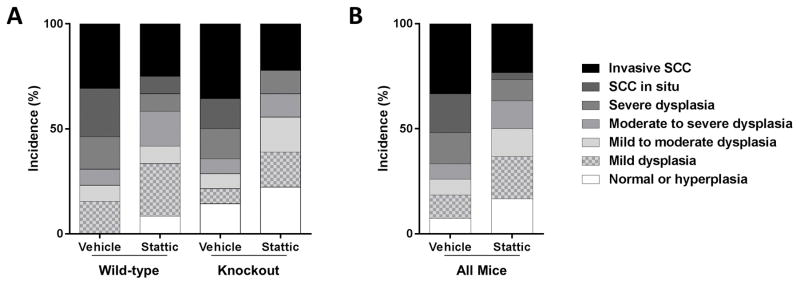
We first sought to test the hypothesis that PTPRT KO mice would be more susceptible than WT mice to 4-NQO-induced carcinogenesis, as well as more sensitive to Stattic-mediated chemoprevention. To that end, we began our analysis of the spectrum of dysplasias in WT and KO mice by crossing two types of mouse (WT or KO) with the two treatment groups (Stattic or vehicle) (Figure 3A). Table 2 indicates the total number of mice in each group included in this analysis. Using a rank transformation statistic to analyze our semi-quantitative ordered pathology scores, we detected no significant effect of mouse genotype (WT versus KO; P = 0.9985) and no significant interaction between mouse genotype and treatment (P = 1.0), indicating that PTPRT status was not a major determinant of susceptibility to tumor formation or sensitivity to Stattic-mediated chemoprevention in this model (Supplemental Figure S1).
Figure 3. Incidence of tongue dysplasias.
A) Wild-type and PTPRT knockout mice display similar patterns of histological response to Stattic chemoprevention (trend P = 0.9985). B) When all mice are considered together, regardless of genotype, effective chemoprevention is evident in Stattic-treated mice (P = 0.0402).
Stattic abrogates 4-NQO tumorigenesis
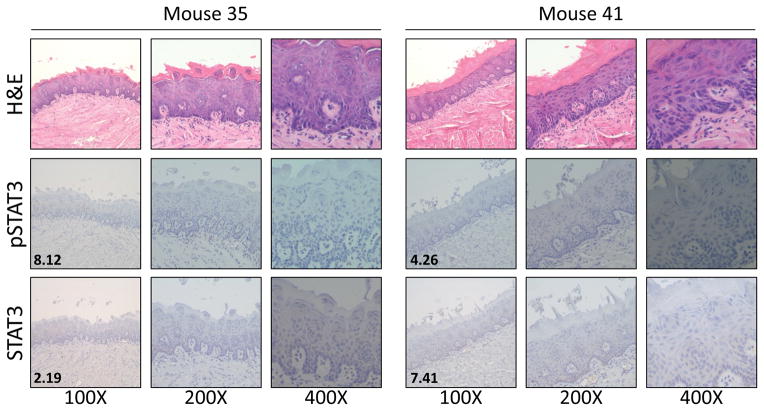
Because responses in WT and KO mice were statistically indistinguishable, we pooled all vehicle or Stattic-treated mice for further analysis regardless of PTPRT genotype. We observed effective chemoprevention in Stattic-treated mice (Wilcoxon P = .0402), with a reduced incidence of severe dysplasia and SCC, and an increased incidence of less advanced disease, resulting in a mean severity score of 2.7 compared to the vehicle mean of 4 (Figure 3B). While we observed no statistical effect of PTPRT loss in this model, it is interesting to note that the only two mice that remained disease-free were both PTPRT KO and treated with Stattic (Figure 4). These tongues also expressed low pSTAT3 and STAT3, suggesting on-target effects of Stattic in these mice.
Figure 4. Two PTPRT KO mice treated with 4-NQO and Stattic remain disease-free after 24 weeks.
Inset numbers represent IHC scores for the indicated proteins.
pSTAT3 and STAT3 expression in 4-NQO-induced tumors
Total STAT3 and nuclear pSTAT3 expression in mouse tongues were analyzed and quantitated by IHC (representative images shown in Figure 2B). pSTAT3 expression scores ranged from 0.60 to 41.0966 (mean = 13.02; median = 10.12), and STAT3 expression scores from 0.72 to 90.07 (mean = 25.08; median = 21.79). We observed no significant association between severity of disease/Stattic response and protein expression by IHC for STAT3 (P = 0.196) or pSTAT3 (P = 0.2081).
Discussion
HNSCC is the sixth most common cancer worldwide where nearly 700,000 HNSCCs are diagnosed each year according to World Health Organization estimates. HNSCC is particularly lethal following recurrence or growth of a second primary tumor, which occurs in ~4% of patients per year. (4) Development of chemopreventive strategies to inhibit secondary tumor growth would, therefore, be of wide clinical benefit leading to improved survival of HNSCC patients. Previous chemoprevention studies have evaluated natural products such as guggulipid and curcumin, as well as targeted small molecules such as erlotinib and celecoxib. (9, 14, 20) Despite some success in pre-clinical models, clinical trials have failed to demonstrate chemopreventive efficacy of celecoxib or erlotinib in patients with oral pre-malignant lesions, with no significant improvement in clinical response and unacceptable toxicities associated with chronic treatment. (10, 12) Thus, there remains an unmet clinical need for an effective chemopreventive strategy in HNSCC.
STAT3 is a persistently activated oncogenic transcription factor and rational therapeutic target in HNSCC. (21) We and others have demonstrated that STAT3 inhibition leads to robust tumor growth inhibition in established HNSCC models in vivo, as well as downregulation of STAT3 target gene expression in a Phase 0 window-of-opportunity clinical trial in HNSCC patients. (22, 23) Furthermore, STAT3 activation is an early event in HNSCC carcinogenesis, indicating that STAT3 inhibition prior to overt tumor establishment may be an effective prevention strategy, particularly in HNSCC patients who are at risk of developing SPTs. (24) We recently reported that loss-of-function of the STAT3 phosphatase PTPRT leads to overexpression of pSTAT3 in HNSCC models and may predict exquisite sensitivity to STAT3 inhibition. (16, 17) We therefore sought to evaluate susceptibility to chemical-induced oral carcinogenesis as well as sensitivity to STAT3 inhibitor-mediated chemoprevention in PTPRT WT or KO mice.
Previous chemoprevention studies have tested compounds that lead to inhibition of STAT3 signaling, including curcumin (14, 25) and erlotinib. (9) Erlotinib is a tyrosine kinase inhibitor that indirectly inhibits STAT3 via EGFR blockade, while curcumin inhibits multiple kinases upstream of STAT3. Importantly, a recent randomized clinical trial of erlotinib in 150 patients failed to demonstrate improved cancer-free survival, suggesting lack of chemopreventive activity. (12) Additionally, while curcumin has chemopreventive properties in the 4-NQO model, including STAT3 downregulation (14), it also targets over 100 other proteins which may be the primary mediator(s) of the observed effect. (26) To our knowledge, the chemopreventive activity of a direct and specific STAT3 inhibitor has not been reported in the 4-NQO model.
The present study was undertaken to evaluate the chemopreventive activity of Stattic, a direct STAT3 inhibitor, in WT or PTPRT KO mouse models of chemical-induced oral carcinogenesis. We initially treated a subset of mice with 4-NQO and concurrent Stattic, but ceased treatment due to unforeseen toxicity of the combination. Interestingly, similar toxicity was observed in a previous experiment combining 4-NQO with erlotinib or guggulipid in CBA/J mice, indicating that inhibition of the EGFR/STAT3 axis concurrently with 4-NQO administration may be particularly lethal and should be avoided in future studies. (9) We resumed Stattic treatment after the 12-week 4-NQO administration period for an additional 12 weeks before harvesting tissue for analysis. Mouse tongues displayed a range of histologies, from normal to invasive SCC. In contrast to a previous report demonstrating increased susceptibility to azoxymethane-induced colon tumors in PTPRT KO mice, we detected no significant difference in disease severity in WT versus PTPRT KO mice. (18) These differing results may reflect the reduced dynamic range of carcinogen sensitivity in C57BL/6J mice in oral versus colon carcinogenesis, where WT mice are resistant to azoxymethane but sensitive to 4-NQO. Alternatively, PTPRT may have divergent or pleiotropic roles in carcinogenesis of oral and colonic epithelium. The apparent lack of PTPRT contribution to 4-NQO-iduced HNSCC tumorigenesis is also notable given that PTPRT loss-of-function by mutation or promoter hypermethylation is frequently observed in established HNSCC tumors. (16, 17) Our present findings suggest that PTPRT loss may not be an early/initiating event and may be a common later event that instead contributes to tumor progression/maintenance. It may alternatively indicate that total PTPRT loss by knockout may not faithfully recapitulate the phenotype of mutation or promoter methylation. While we observed no statistical effect of PTPRT loss in this model, it is interesting to note that the only two mice that remained disease-free were both PTPRT KO and treated with Stattic. This suggests that there may be undefined complex biologic contexts that include PTPRT loss, which may predict an enhanced chemopreventive effect following STAT3 inhibition, particularly in genetically heterogeneous tumors caused by chemical carcinogen. Additional study in models of established HNSCC, including patient-derived xenografts harboring PTPRT alterations, may provide further evidence that PTPRT loss is predictive of response to STAT3 inhibition.
When considering all mice regardless of PTPRT genotype, we observed effective Stattic-mediated chemoprevention of 4-NQO-mediated oral carcinogenesis (Wilcoxon P = 0.0402), suggesting that targeting STAT3 may be efficacious in HNSCC prevention. The use of Stattic after carcinogen exposure may reflect inhibition of STAT3-mediated progression rather than tumor initiation. (27) While this study has indicated that PTPRT loss is unlikely to serve as a predictive biomarker for HNSCC chemoprevention, it ultimately supports further investigation of alternative putative predictive biomarkers of response to STAT3 inhibition in prevention and treatment of HNSCC. Further studies will also be necessary to define the impact of STAT3 inhibition on the tumor microenvironment in 4-NQO-induced oral carcinogenesis.
Supplementary Material
Acknowledgments
Financial Support: NIH/NIDCR F31DE024007 to N.D. Peyser; NIH/NCI R01CA077308 and NIH/NCI P50CA097190 to J.R. Grandis.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head & neck. 2013;35:747–55. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 2.Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral oncology. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Manjunath S, Sabharwal R, Kumar M, Sinha A, Gupta S, Sepolia S. Oral field cancerization: an update. Universal Research Journal of Dentistry. 2014;4:10. [Google Scholar]
- 4.León X, Quer M, Diez S, Orús C, López-Pousa A, Burgués J. Second neoplasm in patients with head and neck cancer. Head & neck. 1999;21:204–10. doi: 10.1002/(sici)1097-0347(199905)21:3<204::aid-hed4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Tang X-H, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clinical Cancer Research. 2004;10:301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 6.Nauta J, Roodenburg J, Nikkels P, Witjes M, Vermey A. Comparison of epithelial dysplasia—the 4NQO rat palate model and human oral mucosa. International journal of oral and maxillofacial surgery. 1995;24:53–8. doi: 10.1016/s0901-5027(05)80857-4. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Kitayama W, Denda A, Morisaki A, Kuniyasu H, Inoue M, et al. Suppressive effects of a selective cyclooxygenase-2 inhibitor, etodolac, on 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Experimental and Toxicologic Pathology. 2004;56:145–51. doi: 10.1016/j.etp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shiotani H, Denda A, Yamamoto K, Kitayama W, Endoh T, Sasaki Y, et al. Increased expression of cyclooxygenase-2 protein in 4-nitroquinoline-1-oxide-induced rat tongue carcinomas and chemopreventive efficacy of a specific inhibitor, nimesulide. Cancer research. 2001;61:1451–6. [PubMed] [Google Scholar]
- 9.Leeman-Neill RJ, Seethala RR, Singh SV, Freilino ML, Bednash JS, Thomas SM, et al. Inhibition of EGFR-STAT3 signaling with erlotinib prevents carcinogenesis in a chemically-induced mouse model of oral squamous cell carcinoma. Cancer Prevention Research. 2011;4:230–7. doi: 10.1158/1940-6207.CAPR-10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadimitrakopoulou VA, William WN, Dannenberg AJ, Lippman SM, Lee JJ, Ondrey FG, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clinical Cancer Research. 2008;14:2095–101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 11.William WN, Papadimitrakopoulou V, Lee JJ, Mao L, Lin H, Gillenwater AM, et al. Randomized placebo-controlled trial (RCT) of erlotinib for prevention of oral cancer (EPOC). ASCO Annual Meeting Proceedings; 2014. p. 6007. [Google Scholar]
- 12.William WN, Papadimitrakopoulou V, Lee JJ, Mao L, Cohen EE, Lin HY, et al. Erlotinib and the Risk of Oral Cancer: The Erlotinib Prevention of Oral Cancer (EPOC) Randomized Clinical Trial. JAMA oncology. 2015:1–8. doi: 10.1001/jamaoncol.2015.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyser ND, Grandis JR. Critical analysis of the potential for targeting STAT3 in human malignancy. OncoTargets and therapy. 2013;6:999. doi: 10.2147/OTT.S47903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Paiva Gonçalves V, Ortega AAC, Guimarães MR, Curylofo FA, Junior CR, Ribeiro DA, et al. Chemopreventive Activity of Systemically Administered Curcumin on Oral Cancer in the 4-Nitroquinoline 1-Oxide Model. Journal of cellular biochemistry. 2015;116:787–96. doi: 10.1002/jcb.25035. [DOI] [PubMed] [Google Scholar]
- 15.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chemistry & biology. 2006;13:1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Lui VWY, Peyser ND, Ng PK-S, Hritz J, Zeng Y, Lu Y, et al. Frequent mutation of receptor protein tyrosine phosphatases provides a mechanism for STAT3 hyperactivation in head and neck cancer. Proceedings of the National Academy of Sciences. 2014;111:1114–9. doi: 10.1073/pnas.1319551111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyser N, Freilino M, Wang L, Zeng Y, Li H, Johnson D, et al. Frequent promoter hypermethylation of PTPRT increases STAT3 activation and sensitivity to STAT3 inhibition in head and neck cancer. Oncogene. 2015 doi: 10.1038/onc.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhang X, Guda K, Lawrence E, Sun Q, Watanabe T, et al. Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T. Proceedings of the National Academy of Sciences. 2010;107:2592–7. doi: 10.1073/pnas.0914884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullman-Culleré MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Comparative Medicine. 1999;49:319–23. [PubMed] [Google Scholar]
- 20.Park K, Yang JH, Choi Y, Lee C, Kim SY, Byun Y. Chemoprevention of 4-NQO-induced oral carcinogenesis by co-administration of all-trans retinoic acid loaded microspheres and celecoxib. Journal of controlled release. 2005;104:167–79. doi: 10.1016/j.jconrel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Leeman RJ, Lui VWY, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. 2006 doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 22.Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer discovery. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: implications for cancer therapy. Oncogene. 2005;24:970–9. doi: 10.1038/sj.onc.1208316. [DOI] [PubMed] [Google Scholar]
- 24.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proceedings of the National Academy of Sciences. 2000;97:4227–32. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexandrow MG, Song LJ, Altiok S, Gray J, Haura EB, Kumar NB. Curcumin: A Novel Stat 3 Pathway Inhibitor for Chemoprevention of Lung Cancer. European Journal of Cancer Prevention. 2012;21:407. doi: 10.1097/CEJ.0b013e32834ef194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Beevers CS, Huang S. Targets of curcumin. Current drug targets. 2011;12:332. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. The Journal of clinical investigation. 2004;114:720–8. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.