Abstract
Objective
To assess the ability of a novel imaging system designed for intraoperative detection of residual cancer in tumor beds to distinguish neoplastic from normal tissue in dogs undergoing resection of soft tissue sarcoma and mast cell tumor.
Study design
Non-randomized prospective clinical trial.
Animals
12 dogs with soft tissue sarcoma and 7 dogs with mast cell tumor.
Methods
A fluorescent imaging agent that is activated by proteases in vivo was administered to the dogs 4-6 or 24-26 hours prior to tumor resection. During surgery, a handheld imaging device was used to measure fluorescence intensity within the cancerous portion of the resected specimen and determine an intensity threshold for subsequent identification of cancer. Selected areas within the resected specimen and tumor bed were then imaged, and biopsies (n=101) were obtained from areas that did or did not have a fluorescence intensity exceeding the threshold. Results of intraoperative fluorescence and histology were compared.
Results
The imaging system correctly distinguished cancer from normal tissue in 93/101 biopsies (92%). Using histology as the reference, the sensitivity and specificity of the imaging system for identification of cancer in biopsies were 92% and 92%, respectively. There were 10/19 (53%) dogs which exhibited transient facial erythema soon after injection of the imaging agent which responded to but not consistently prevented by intravenous diphenhydramine.
Conclusion
A fluorescence-based imaging system designed for intraoperative use can distinguish canine STS and MCT tissue from normal tissue with a high degree of accuracy. The system has potential to assist surgeons in assessing the adequacy of tumor resections during surgery, potentially reducing the risk of local tumor recurrence. Although responsive to antihistamines, the risk of hypersensitivity needs to be considered in light of the potential benefits of this imaging system in dogs.
The primary mode of therapy for many canine solid tumors, such as soft tissue sarcoma (STS) and mast cell tumor (MCT), is surgical excision of gross, visible tumor with a wide margin of surrounding normal tissue.1 Following surgery, the surgical margins of the resected tissue are evaluated microscopically by a pathologist. Incomplete histologic margins are associated with local tumor recurrence,2-10 and are often considered to be an indication for resection of the surgical scar and tumor bed, or adjuvant radiation therapy. A comprehensive histologic assessment of surgical margins requires several days to complete and therefore, cannot be used as an intraoperative guide. In addition, the association between margin analyzes and local recurrence is imperfect.2-10 Thus, some animals are likely to receive unnecessary additional therapy, incurring unnecessary morbidity and cost of care. Technologies for intraoperative assessment of the tumor bed for residual cancer cells have the potential to assist the surgeon in determining whether adequate tissue has been removed, as well as aid in the selection of candidates for adjuvant therapy. Currently available methods for intraoperative margin assessment include ultrasound,11 radiofrequency spectroscopy,12 and frozen section histology.13 Each method has limitations. Intraoperative ultrasound has little capacity for magnification and is not likely to detect small quantities of residual cancer. Radiofrequency spectroscopy is technically complex and may be difficult to adapt to the live operative setting.12 The ability to have frozen sections analyzed is limited in veterinary medicine and intraoperative time constraints restrict the number of areas from the tumor bed that can be sampled and examined.
The wide field-of-view, handheld imaging device has been developed to allow real-time examination of the entire tumor bed and is used in conjunction with intravenous far red or near infrared fluorescent imaging agents.14 The imaging device has a spatial resolution of approximately 16 μm and a field of view of approximately 4 cm2. Proteases upregulated in cancer cleave the peptide backbone of the fluorescent imaging agent in vivo, thereby activating the fluorescent molecules and enabling their fluorescence to be measured after excitation by an appropriate wavelength of light. The imaging agent must be injected several hours prior to the planned resection to allow preferential distribution to the tumor and proteolytic activation to occur.14 Proteases, such as cathepsin, are overexpressed in murine STS and other tumors,14,15 and can activate the imaging agents causing cancer tissue to fluoresce with greater intensity than normal tissue. Cathepsin expression varies with normal tissue type, and imaging agents that are cleaved by cathepsins may not be appropriate for tumors located in tissues with high cathepsin concentrations, such as the liver.14 Several cathepsin proteases are known to be overexpressed in canine sarcomas.16 The imaging device combined with commercially available imaging agents administered 24 hours before surgery has been used to image tumor beds of genetically engineered mice undergoing marginal and wide resections of sarcomas.14,15,17 Microscopic quantities of residual cancer could be detected in tumor beds consisting of skeletal muscle and the presence of residual fluorescence corresponded to local recurrence. Resection of fluorescent tissue improved local control.14,18 A pilot clinical trial assessing the imaging system in 9 dogs with naturally occurring STS or MCT that received a commercially available imaging agent 24 hours before surgery is also reported.16 There were no adverse clinical or laboratory effects observed after intravenous administration of the imaging agent, and fluorescence was apparent in all resected tumors. In that study, 1 dog with MCT had an area of residual fluorescence during intraoperative assessment of the tumor bed which corresponded to a focal area of tumor extension to the margin of excision. No tumor beds in the other dogs showed residual fluorescence, although 1 resected tumor had incomplete surgical margins on microscopic examination.
The imaging agents investigated to date are intended for research purposes and have not been approved for human clinical trials. They consist of large macromolecules that were injected approximately 24 hours before surgery in order to generate the tumor to normal tissue fluorescence ratio.19 A smaller macromolecule, LUM015 (Lumicell, Inc, Wellesley, MA) that produces far red fluorescence and more rapidly accumulates in cancer cells has been developed and potentially allows for relatively short intervals between injection and surgery. Rapid distribution and activation of the agent to tissues allows surgery to be performed several hours after injection, rather than a 24 hour delay, which improves clinical workflow. LUM015 has been tested in a Phase I clinical trial of ex vivo imaging in people with soft tissue sarcomas and breast cancer (NCT01626066). Based on the safety profile of LUM015 and the promising initial ex vivo imaging results in this first-in-human clinical trial, a clinical trial of intraoperative imaging is underway to evaluate the efficacy of LUM015 and the imaging device to detect residual cancer during breast cancer surgery (NCT02438358).
The primary objective of this clinical trial in dogs was to use the imaging device in combination with LUM015 and assess the accuracy of the imaging system in distinguishing cancerous from normal tissue in dogs undergoing resections of STS and MCT. These tumors were chosen because they are frequently removed in practice and because their overall prognosis is often largely dependent on the completeness of surgical excision. Secondary objectives were to assess the dogs for clinically-apparent adverse effects following injection, to determine whether the excised tumors exhibited fluorescence after preoperative (4-6 and 24-26 hours) injection of LUM015 and to determine whether neoplastic-to-normal-tissue fluorescence intensity ratios were adequate to allow residual cancer to be detected in tumor beds consisting of skeletal muscle, fat, and other connective tissues.
Methods and Materials
Nineteen client-owned dogs with STS (n=12) or MCT (n=7) were enrolled in the clinical trial between August 2012 and February 2015. All procedures were approved by the Tufts University Clinical Studies Review Committee (Protocol # 033-11) or the Duke University Institutional Animal Care and Use Committee (Protocol # A213-10-08). All tumors were confirmed by histology or cytology as STS or MCT prior to surgery. Because the trial was not designed to guide treatment or to otherwise benefit dogs that were enrolled, and because the imaging agent had not been used in dogs prior to the trial, owners were offered a financial incentive to participate. Owners signed a consent form prior to enrolling their dogs in the study.
LUM015 was administered over 2-3 minutes (1.0-2.0 mg/kg intravenously [IV]), either 4-6 hours (10 dogs) or 24-26 hours (9 dogs) prior to surgery. Dogs were randomly assigned to receive the agent at 4-6 hours (6 STS, 4 MCT) or 24-26 hours (6 STS, 3 MCT). Dogs were continuously observed for adverse reactions during the first 30 minutes after administration, and at hourly intervals thereafter.
All tumors were removed with the objective of complete excision. When possible, lateral margins included 2 cm of normal tissue, and deep margins included a fascial plane or 2 cm of normal tissue. The excised tumor with its surrounding soft tissue was removed from the operative field and placed on an adjacent table. An incision was made that partially bisected the resection specimen. An assistant used the imaging device (Fig 1) to obtain fluorescence intensity data from a central area of the cut surface of the tumor (cancer tissue). The imaging device was connected to a computer which displayed images of the tissue being examined on a monitor. Images were analyzed using Image J freeware (http://imagej.nih.gov). The imaging data from the exposed cancerous tissue was used to determine the cancer detection threshold. In subsequent imaging, the cancer detection threshold declared areas cancerous when any pixel with fluorescence intensity greater than 80% of the minimum fluorescence intensity was observed in the tumor. The imaging device was then covered with a sterile sleeve, passed to the sterile field, and used to image the tumor beds. Regions of the imaged tissue containing pixels with fluorescence intensity that exceeded the cancer detection threshold were displayed in red (Fig 2). The entire tumor bed was imaged in 5 dogs, but to limit anesthesia time devoted to imaging, the remaining dogs had imaging only for several randomly selected fields of view within each tumor bed. After the tumor beds were imaged, the imaging device was passed back to the assistant for additional imaging of randomly selected grossly normal and grossly cancerous regions within the resected specimens.
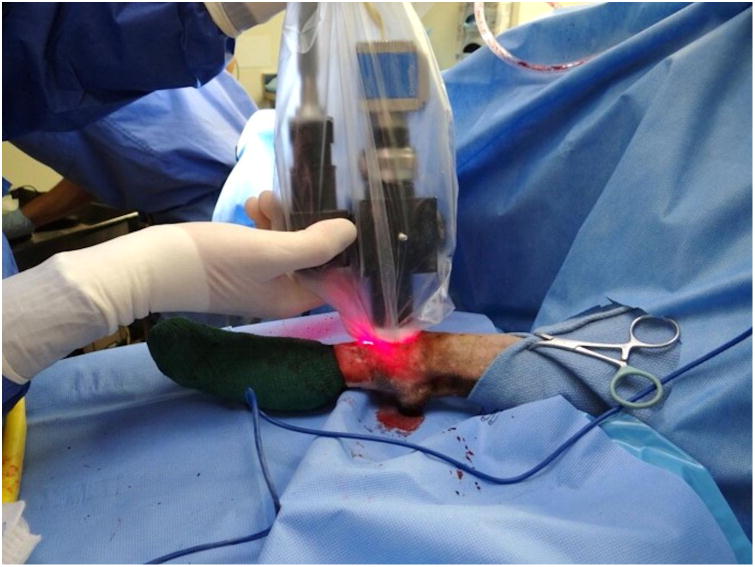
Figure 1.

(A) Optical layout of the imaging device. Light enters the device through a fiber bundle and is reflected through a filter, producing excitation light with a wavelength of 649 nm. This light is reflected onto the tumor bed by a dichroic mirror, exciting the fluorescent molecules. The emitted light has a wavelength of 670 nm and returns through a series of lenses and a filter to a charge-coupled device (CCD) that maps the fluorescence of clusters of cancer cells. (B) The imaging device prior to application of the sterile sleeve. (C) The imaging device in use in a dog.
Figure 2.

Fluorescence image displayed on the computer monitor. The red area corresponds to fluorescence intensity exceeding the cancer detection threshold set at 80% of the minimum fluorescence intensity observed in the resected tumor.
To determine neoplastic-to-normal tissue fluorescence intensity ratios for each dog, and to determine the accuracy of the imaging system in distinguishing cancerous from normal tissue, small incisional biopsies were obtained from multiple locations within selected fields of view after measuring the fluorescence intensities within the fields. Biopsies were approximately 0.5 cm3 in size, and were obtained using a scalpel blade. All tissue with a fluorescence intensity exceeding the cancer detection threshold that was discovered in the tumor bed or within the grossly normal tissue in the resected specimen was biopsied. A total of 101 biopsies were collected. Nineteen biopsies came from the grossly cancerous central portion of the excised tumors used to determine the cancer detection threshold. Thirty biopsies came from other grossly cancerous (n=11) or grossly normal (n=19) regions within the resected specimens. Fifty-two biopsies came from grossly cancerous (n=3) or grossly normal (n=49) regions within the tumor beds. In total, 39 biopsies came from fields of view with a fluorescence intensity exceeding the cancer detection threshold, and 62 biopsies came from fields of view with a fluorescence intensity that did not exceed the threshold. Because the study was designed to investigate the accuracy of the imaging system, not to guide the tumor resections, areas of the tumor bed with a fluorescence intensity exceeding the threshold were not widely excised. All biopsies were submitted for routine histologic examination, and 1-3 sections were prepared from each biopsy. Pathologists were unaware of whether biopsies were obtained from grossly cancerous or grossly normal tissue, the in vivo orientation of the biopsy relative to surrounding tissue, and the fluorescence intensity of the biopsy tissue. MCT was considered to be present in biopsies if clusters of mast cells were identified.
Neoplastic-to-normal tissue fluorescence intensity ratios for each dog were calculated using the average of 3 to 4 intensity readings from the cancerous portion of the resected specimen and the average of 3 to 4 intensity readings from normal tissue within the resected specimen or tumor bed. Fluorescence intensity ratios in dogs receiving LUM015 4-6 hours prior to surgery were compared to ratios for dogs receiving the agent 24-26 hours prior to surgery using a Mann-Whitney U test. Statistical analyses were performed using SYSTAT 13 (Systat Software, Inc., San Jose, CA). P <.05 was considered significant.
Results
The preoperative diagnoses of all tumors was confirmed on final histologic examination (Table 1). Tumor locations were distal limb (7), proximal limb (5), abdomen (4) and thorax (3). Tumor size ranged from 1.0 cm × 2.0 cm × 3.0 cm to 7.5 cm × 8.5 cm × 5.0 cm.
Table 1. Frequency of tumor types and grades for 19 dogs with soft tissue sarcoma (STS) or mast cell tumor (MCT).
| Tumor Type | n | High Grade | Moderate Grade | Low Grade |
|---|---|---|---|---|
| STS - All | 12 | 2 | 4 | 6 |
| Unspecified | 6 | 2 | 2 | 2 |
| MPNST* | 5 | 0 | 2 | 3 |
| Myxosarcoma | 1 | 0 | 0 | 1 |
| MCT | 7 | 1 | 2 | 4 |
MPNST malignant peripheral nerve sheath tumor
No single dog breed was represented more than twice. There were 10 spayed female, 1 intact female, and 8 neutered male dogs. Mean age at surgery was 9 years (range 4-13). Mean body weight was 23.6 kg (range 4.7-42.3 kg).
Two of the first 4 dogs enrolled in the study developed facial erythema and pruritus within 2-5 minutes of agent administration. Diphenhydramine (1.0 mg/kg intramuscularly [IM]) was administered and the cutaneous reaction resolved within 5 minutes. The subsequent 15 dogs were pretreated with diphenhydramine at 1.0 mg/kg IM at 5 minutes prior to agent administration. Eight of these dogs developed the cutaneous reaction, but the reaction resolved within 5 minutes following additional administration of diphenhydramine. No other clinically apparent drug reactions were observed.
Eighteen of the 19 tumors were resected with adequate wide margins, resulting in tumor beds that appeared grossly to contain only normal tissue to the surgeon. One resection involved planned cytoreduction in a dog with MCT, in which grossly visible cancer on the plantar surfaces of the metacarpal bones could not be completely excised.
All midline cut surfaces of the resected tumors demonstrated marked fluorescence when imaged. The mean (standard deviation) neoplastic-to-normal tissue fluorescence intensity ratio was 8.4 (9.6). Fluorescence intensity ratios for dogs receiving LUM015 24-26 hours prior to surgery (mean 10.3, standard deviation 6.5) were significantly higher than ratios for dogs receiving the agent 4-6 hours before surgery (mean 8.1, standard deviation 11.9, P=.047).
The 3 biopsies which had a false negative imaging result were collected from grossly normal regions of the resected specimens of 3 different dogs, 2 with MCT (1 injected 4 hours preoperative and 1 injected 24 hours preoperative) and 1 with STS (injected 24 hours preoperative) (Tables 2 and 3). Histologic examination of each sample demonstrated small foci of cancerous tissue intermixed with normal tissue.
Table 2. Frequency of histologic findings and corresponding imaging result (correct vs. incorrect) for 101 biopsies taken from 19 dogs with soft tissue sarcoma or mast cell tumor.
| Histopathology findings | n | Imaging (correct vs. incorrect) |
|---|---|---|
| Cancerous tissue | 33 | 33 vs. 0 |
| Cancerous and normal tissue intermixed | 4 | 1 vs. 3 (false negatives) |
| Normal skeletal muscle | 19 | 19 vs. 0 |
| Normal fat | 15 | 11 vs. 4 (false positives) |
| Unspecified normal tissue | 11 | 11 vs. 0 |
| Normal collagenous tissue | 6 | 5 vs. 1 (false positive) |
| Normal fat and collagenous tissue intermixed | 7 | 7 vs. 0 |
| Normal skin | 5 | 5 vs. 0 |
| Hemorrhage | 1 | 1 vs. 0 |
Table 3.
Corresponding histologic findings, gross tissue appearance and imaging results for 101 biopsies from 19 dogs with soft tissue sarcoma and mast cell tumor.
| Biopsies from resected specimen (n=49) | |||
|---|---|---|---|
| Histopathology findings | Gross tissue | Imaging | Imaging conclusion |
| Normal tissue 17 | Normal 17 Cancerous 0 |
Normal 16 Cancerous 1 |
True negative 16 False positive 1 |
| Cancerous tissue 32 | Cancerous 29 Normal 3 |
Cancerous 29 Normal 3 |
True positive 29 False negative 3 |
| Biopsies from tumor bed (n=52) | |||
| Normal tissue 47 | Normal 47 Cancerous tissue 0 |
Normal tissue 43 Cancerous tissue 4 |
True negative 43 False positive 4 |
| Cancerous tissue 5 | Cancerous 3 Normal 2 |
Cancerous tissue 5 Normal tissue 0 |
True positive 5 False negative 0 |
| All biopsies (n=101) | |||
| Normal tissue 64 | Normal 64 Cancerous tissue 0 |
Normal tissue 59 Cancerous tissue 5 |
True negative 59 False positive 5 |
| Cancerous tissue 37 | Normal tissue 5 Cancerous tissue 32 |
Normal tissue 3 Cancerous tissue 34 |
True positive 34 False negative 3 |
One of the 5 biopsies that gave false positive imaging results came from a grossly normal region in the resected specimen of a dog with STS (injected 24 hours preoperative). The histologic examination revealed normal fat but no evidence of cancer. The remaining 4 biopsies with false positive imaging results came from tumor beds. Two of these came from the tumor bed of a dog with MCT (injected 24 hours preoperative) which both showed normal fat without evidence of cancer on histologic examination. The 2 other biopsies were from dogs with STS (injected 4 hours preoperative) for which 1 biopsy contained normal fat and the other biopsy contained collagenous tissue without evidence of cancer.
Small areas of residual fluorescence were detected in 3 additional tumor beds, and histologic examination of each of these areas revealed cancer. One was a bed containing a small region of grossly visible residual MCT on the plantar surface of the metacarpal bones that could not be completely excised. Each of the 3 biopsies from this region contained MCT on histologic examination. This dog was injected 4 hours preoperative. The second was the tumor bed of a dog with a high grade MCT in which an area of residual fluorescence was identified that was confirmed as MCT on histologic examination. This dog was injected 24 hours preoperative. The third was the tumor bed of a dog with a high grade STS, and the area of residual fluorescence was confirmed as STS on histologic examination. This dog was injected 4 hours preoperative. In the latter 2 cases, the fluorescent tissue was not grossly or palpably distinguishable from the surrounding tissue.
Using histology results as the reference, the overall accuracy of the imaging system in distinguishing cancerous from normal tissue in all 101 biopsies was 92%. The sensitivity and specificity for identification of cancer in biopsies were 92% and 92%, respectively. The positive and negative predictive values were 87% and 95%, respectively.
Discussion
This study provides encouraging preliminary evidence that in dogs undergoing resection of STS and MCT, this intraoperative imaging system can accurately distinguish cancerous from normal tissue and can detect small areas of residual cancer in tumor beds that have a grossly normal appearance. It is possible that the system will prove applicable to a wide range of animal cancers occurring in various anatomic sites, providing that the cancer in question overexpresses proteases, such as cathepsins, relative to the surrounding normal tissue.
The transient facial erythema and pruritus observed in 10 dogs was not observed in a previous study in dogs, although a different imaging agent was used.16 Dogs are uniquely sensitive to certain injectable agents including liposomes and imaging agents that contain reactive substituent groups, and the reaction observed was likely a hypersensitivity reaction mediated by complement activation and or histamine release from circulating mast cells (Personal communication, Gad SC, Gad Consulting Services, Cary, NC, April 2012). Hypersensitivity reactions may be accompanied by a transient hypotensive response, and although blood pressures were not measured while the reaction was occurring, no dogs exhibited clinical signs of hypotension. The facial erythema resolved within 5 minutes in all dogs, suggesting that if hypotension does occur, it is unlikely to be a complicating factor during anesthesia several hours later. The reaction may not occur with administration of imaging agents with different chemical structures, and is unlikely to be observed in other species (Personal communication, Gad SC, Gad Consulting Services, Cary, NC, April 2012). Studies with LUM015 in mice, rats, rabbits and people did not show the hypersensitivity reaction observed in the dogs in this study. The cutaneous reaction in the dogs in this study was responsive to diphenhydramine administration, although administration of diphenhydramine before LUM015 did not consistently prevent the reaction. Corticosteroid administration prior to injection of LUM015 and other imaging agents may also aid in preventing hypersensitivity reactions. It is possible that in the event that LUM015 is administered a second time, for example prior to surgery for locally recurrent tumors, the hypersensitivity reaction may occur with higher frequency or potency.
The imaging system described here relies on in vivo proteolytic activation of the imaging agent and its preferential accumulation within cancer cells as compared to normal cells which requires the agent to be administered several hours before surgery. In the present study, acceptable neoplastic-to-normal tissue fluorescence intensity ratios were observed when LUM015 was injected either 4-6 or 24-26 hours prior to surgery, suggesting that following injection, the window during which the surgery may be performed is wide, making the system adaptable to the logistics of hospital scheduling. It is possible that the range of acceptable preoperative time points for injection is broader than the range tested here, and identification of a range of time points that produces optimal fluorescence intensity ratios would require additional study. The observation that ratios among dogs receiving LUM015 24-26 hours before surgery were significantly higher than ratios for dogs receiving the agent 4-6 hours before surgery suggests that cancerous tissue can be successfully imaged when the agent is administered more than 24-26 hours preoperative. Fluorescence ratios were acceptable regardless of whether normal tissues consisted of skeletal muscle, fat, collagenous tissue or skin, indicating the system is useful for detecting residual MCT or STS in tumor beds composed of these tissues. The investigators have successfully imaged a limited number of epithelial tumors in dogs, and an optical imaging system has been used to image ovarian cancer in women.20
While a high degree of concordance was observed between intraoperative imaging and histology of corresponding biopsies, occasional false negatives and false positives were observed. In clinical practice, both errors could have important consequences for the animal. False negative imaging results could result in local tumor recurrence and false positive imaging results could cause the surgeon to remove additional normal tissue or pursue adjuvant treatment unnecessarily. Minimizing risk of tumor recurrence is likely to be the highest priority in most clinical situations. To reduce this risk, a tumor detection level that is below the minimum level of fluorescence intensity observed in the cancerous portion of the resected specimen can be selected to reduce the risk of false negatives, as was done in the present study. The threshold chosen here of 80% of the minimum fluorescence intensity of the excised tumor was based on previous experience with the system in a mouse model of soft tissue sarcoma.14 All 3 biopsies with false negative imaging results contained small foci of cancer intermixed with normal tissue. These samples may have come from areas in the resected specimen or tumor bed containing foci of cancer that did not fluoresce above the tumor detection threshold, or that were obscured by a thin layer of overlying normal tissue, reducing the intensity of the fluorescence signal. Lack of fluorescence above the tumor detection threshold could result from selection of an excessively high threshold, failure of the cancer focus to adequately accumulate or activate the imaging agent, or an interval between administration of the agent and surgery that allowed the fluorescence of cancer foci to fall below the detection threshold. In the present study, there were only a few false negatives and false positives observed for each of the injection intervals chosen so no conclusion can be drawn on the effect of time interval on discordant imaging results. The depth at which cancer can be detected by fluorescence-based imaging systems is a complex relationship between the size and depth of the deposit, the optical properties of any overlying tissue, the cancer-to-normal tissue fluorescence ratio, tissue concentrations of the imaging agent, and characteristics of the imaging device.21 The depth of fluorescence detection of this imaging device was investigated in mouse STS tissue samples harvested 6 hours after administration of LUM015 and placed under tissue phantoms with thicknesses of 0, 2, 4, 6, 8, and 10 mm.22 Fluorescence above background levels could be detected underneath the 10 mm phantom, and tumor fluorescence was at least twice that of background fluorescence at a depth of 8 mm.22 Thus, imaging systems similar to the one used in the present study should reliably detect residual cancer cells near the surface of the tumor bed but may have more limited sensitivity for cancer cells beyond the surface.14,21
There are several possible causes of the false positive imaging results observed in the study. The surgeon may have obtained biopsies that failed to capture nearby nests of residual cancer cells. The imaging device used here has a field of view of approximately 4 cm2, but this prototype device does not have a mechanism for directing the surgeon to a precise location within that field. Such a mechanism is currently under development. Residual cancer cells may have been present in the biopsies, but were missed when the tissue was sectioned. The interval between injection of the imaging agent and surgery may have been too short, causing incomplete clearance of the agent from normal tissues. The cancer detection threshold selected may have been too low, causing highly fluorescent normal tissue to be considered cancerous. Finally, it is conceivable that some normal tissues may exhibit high levels of auto-fluorescence, and some tissues (eg. liver) have naturally high levels of cathepsin proteases.23
It is important to recognize that while the present study demonstrated that both false negative and false positive imaging results can occur, the study design did not allow conclusions about the likely frequency of these results in clinical practice. The 101 biopsies included multiple samples from the same dog and were not independent experimental units. Thus, these biopsies may have some co-dependence which could bias the assessment of accuracy. To limit the amount of anesthesia time devoted to collecting fluorescence intensity data from individual fields of view and obtaining corresponding biopsies, entire tumor beds were not imaged, and tumor beds were not exhaustively examined with histologic examination. In clinical practice, tumor beds can be scanned by continuously moving the imaging device systematically over the bed, stopping to obtain intensity data only when the image on the monitor indicates that a field of view contains tissue with a fluorescence intensity exceeding the threshold. It is anticipated that approximately 5-10 minutes will be required to scan a 20 cm × 20 cm tumor bed that does not contain residual fluorescence.
This was a small, initial clinical trial designed primarily to determine whether the fluorescence-based imaging system can accurately distinguish STS and MCT tissue from normal tissue in dogs injected with LUM015. While results were encouraging, the study had several important limitations in addition to those identified above. The system investigated here is still under development and the prototype imaging device and the tumor detection algorithm could undergo modification before the system becomes commercially available. Doses of the imaging agent and intervals between administration and surgery have yet to be optimized. Most importantly, because the majority of the tumors in this study were widely excised, it is possible that a relatively small proportion of our dogs had residual cancer in their tumor beds, limiting our ability to test the system's sensitivity to small foci of residual cancer. While it was encouraging that the imaging system identified 2 small foci of residual cancer in tumor beds that appeared to be grossly normal, in general, results of imaging closely paralleled the surgeon's impressions based on gross inspection of the tissue. Future research ideally would consist of larger, well-controlled prospective trials that enroll dogs with large tumors that are difficult to resect. The entire surgical fields would ideally be imaged with all foci of residual fluorescence excised and undergo histologic examination. Followup to monitor dogs for local tumor recurrence would be important.
Figure 3.

Distributions of fluorescence intensity in 3 regions of a resected specimen and corresponding tumor bed. The orange curve represents the distribution of fluorescence intensity obtained during calibration of the imaging device using the central cancerous portion of the excised specimen. The red bar indicates the cancer detection threshold for subsequent imaging, set at 80% of the minimum tumor fluorescence intensity observed during calibration. The green curve represents the distribution of fluorescence intensity in an area of normal tissue in the surgical field. The purple curve represents a region of the tumor bed with fluorescence intensity exceeding the cancer detection threshold, possibly indicating residual cancer cells.
Acknowledgments
The project was supported by Grant Number U43CA165024 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Presented, in part, at the ACVS Surgery Summit, San Diego, CA, October, 2014.
Disclosure: Two authors (DGK, JMF) certify that they have received or may receive payments or benefits from Lumicell, Inc. (Wellesley, MA, USA) during the study period of an amount of $10,000 to $100,000. Lumicell, Inc. sells commercial in vivo imaging systems. One author (DGK) certifies that he is a member of the scientific advisory board for Lumicell, Inc. One author (JMF) certifies that he is an employee of Lumicell, Inc.
References
- 1.Ehrhart N. Soft-tissue sarcomas in dogs: a review. J Am Anim Hosp Assoc. 2005;41:241–246. doi: 10.5326/0410241. [DOI] [PubMed] [Google Scholar]
- 2.Kuntz CA, Dernell WS, Powers BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996) J Am Vet Med Assoc. 1997;211:1147–1151. [PubMed] [Google Scholar]
- 3.Séguin B, Besancon MF, McCallan JL, et al. Recurrence rate, clinical outcome, and cellular proliferation indices as prognostic indicators after incomplete surgical excision of cutaneous grade II mast cell tumors: 28 dogs (1994-2002) J Vet Intern Med. 2006;20:933–940. doi: 10.1892/0891-6640(2006)20[933:rrcoac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Stefanello D, Morello E, Roccabianca P, et al. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996-2006) Vet Surg. 2008;37:461–465. doi: 10.1111/j.1532-950X.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 5.Weisse C, Shofer FS, Sorenmo K. Recurrence rates and sites for grade II canine cutaneous mast cell tumors following complete surgical excision. J Am Anim Hosp Assoc. 2002;38:71–73. doi: 10.5326/0380071. [DOI] [PubMed] [Google Scholar]
- 6.Scarpa F, Sabattini S, Marconato L, et al. Use of histologic margin evaluation to predict recurrence of cutaneous malignant tumors in dogs and cats after surgical excision. J Am Vet Med Assoc. 2012;240:1181–1187. doi: 10.2460/javma.240.10.1181. [DOI] [PubMed] [Google Scholar]
- 7.Dennis MM, McSporran KD, Bacon NJ, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol. 2011;48:73–84. doi: 10.1177/0300985810388820. [DOI] [PubMed] [Google Scholar]
- 8.McSporran KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet Pathol. 2009;46:928–933. doi: 10.1354/vp.08-VP-0277-M-FL. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki K, Yamagami T, Nomua K, et al. Prognostic significance of surgical margin, Ki-67 and cyclin D1 protein expression in grade II canine cutaneous mast cell tumor. J Vet Med Sci. 2007;69:1117–1121. doi: 10.1292/jvms.69.1117. [DOI] [PubMed] [Google Scholar]
- 10.Schultheiss PC, Gardiner D, Rao S, et al. Association of histologic tumor characteristics and size of surgical margins with clinical outcome after surgical removal of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2011;238:1464–1469. doi: 10.2460/javma.238.11.1464. [DOI] [PubMed] [Google Scholar]
- 11.Haid A, Knauer M, Dunzinger S, et al. Intra-operative sonography: a valuable aid during breast-conserving surgery for occult breast cancer. Ann Surg Oncol. 2007;14:3090–3101. doi: 10.1245/s10434-007-9490-9. [DOI] [PubMed] [Google Scholar]
- 12.Allweis TM, Kaufman Z, Lelcuk S, et al. A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg. 2008;196:483–489. doi: 10.1016/j.amjsurg.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Olson TP, Harter J, Munoz A, et al. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol. 2007;14:2953–2960. doi: 10.1245/s10434-007-9437-1. [DOI] [PubMed] [Google Scholar]
- 14.Mito JK, Ferrer JM, Brigman BE, et al. Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging. Cancer. 2012;118:5320–5330. doi: 10.1002/cncr.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirsch DG, Dinulescu DM, Miller JB, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 16.Eward WC, Mito JK, Eward CA, et al. A novel imaging system permits real-time in vivo tumor bed assessment after resection of naturally occurring sarcomas in dogs. Clin Orthop Relat Res. 2013;471:834–842. doi: 10.1007/s11999-012-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mito JK, Riedel RF, Dodd L, et al. Cross species genomic analysis identifies a mouse model as undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma. PLoS One. 2009;4:e8075. doi: 10.1371/journal.pone.0008075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuneo KC, Mito JK, Brigman BE, et al. Effects of radiation therapy on the detection of microscopic residual cancer in the surgical bed using a protease-activated fluorescent probe in a primary soft tissue sarcoma model. IJROBP. 2011;81:S729–S730. [Google Scholar]
- 19.Weissleder R, Tung CH, Mahmood U, et al. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nature Biotechnology. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 20.Van Dam GM, Themelis G, Crane LOMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nature Medicine. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 21.Thurber GM, Figueiredo JL, Weissleder R. Detection limits of intraoperative near infrared imaging for tumor resection. J Surg Onc. 2010;102:758–764. doi: 10.1002/jso.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarides AL, Whitley MJ, Strasfeld DB, et al. A fluorescence-guided laser ablation system for removal of residual cancer in a mouse model of soft tissue sarcoma. Theranostics. 2016;6:155–166. doi: 10.7150/thno.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moin K, Day NA, Sameni M, et al. Human tumour cathepsin B. Comparison with normal liver cathepsin B. Biochem. J. 1992;285:427–432. doi: 10.1042/bj2850427. [DOI] [PMC free article] [PubMed] [Google Scholar]