Abstract
Objectives
The purpose of this analysis was to evaluate sex differences in the rate of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) accrual in adults. Secondary analyses examined differences in the rate of VAT and SAT accrual in pre-, peri-, and post-menopausal women.
Subjects/Methods
Participants were 472 (60% female) non-Hispanic whites, aged 18-84 years at baseline in whom abdominal VAT and SAT were assessed using multiple-image magnetic resonance imaging at two time points, with an average follow-up of 7.3 ± 2.6 years. Linear regression models were used to examine the effects of sex, baseline age and their interaction on rate of change per year in body composition measures (ΔBMI, ΔVAT, and ΔVAT/SAT ratio (ΔVSR)) independent of baseline body composition measures, visit year, income, marital status, physical activity, smoking and alcohol intake. Secondary analyses examined differences in rate of fat change by menopausal status (pre, peri, post).
Results
Levels of BMI, VAT, and VSR all increased over the 7 year period on average (p<.001); however, the change in BMI (mean ΔBMI = +0.5%) was far smaller than for VAT (mean ΔVAT= +6.8%), SAT (mean ΔSAT = +2.4%), and VSR (mean ΔVSR = +3.6%). ΔBMI, ΔVAT, and ΔSAT decreased linearly with age in both sexes (p<0.01), such that older individuals had lower rates of BMI, VAT, and SAT gain, and this deceleration in BMI, VAT, and SAT accrual was greater in men than women (p for interaction <0.05). ΔVSR did not vary with age in either sex, but remained higher in men than women throughout adulthood. There were no differences in rate of weight or fat gain by menopausal status after adjustment for age.
Conclusions
Men and women continue to accrue abdominal adiposity with age, but the rate of weight and fat gain decreases over time, particularly in men.
Introduction
Abdominal obesity is an established risk factor for metabolic and cardiovascular diseases.1 Excess abdominal visceral adipose tissue (VAT), in particular, is associated with an increased risk of metabolic syndrome, diabetes, cardiovascular disease, hypertension and all-cause mortality,2-7 independent of body mass index (BMI). Furthermore, the association between VAT and adverse health outcomes appears to be stronger in women than in men.8, 9 Women generally have less VAT, greater subcutaneous adipose tissue (SAT), and a smaller VAT to SAT ratio (VSR) then men, thus presenting a more favorable risk profile.10 However, VAT disproportionately increases with age in both men and women,11-16 thereby increasing the aforementioned health risks.
Studies have reported increases in VAT in women across the menopausal transition,17-22 suggesting that this is a sensitive period for women during which their adiposity profile becomes more similar to that of men. However, few studies have directly examined sex differences in VAT and SAT accrual across adulthood. Limited evidence suggests that the sexual dimorphism of age-related changes in fat distribution shrinks over time, with older women experiencing accelerated gains in VAT in comparison to younger women or similar-aged men, in concert with menopausal changes.12, 23
Kotani et al., examined age-related differences in VAT and SAT in a cross-sectional sample of 162 Japanese men and women aged 10-79 years.12 VAT accumulated slowly in women across pre-menopausal age groups, but VAT was 2.6 times greater in post-menopausal women as compared to pre-menopausal women, mimicking the strong age-related association seen in men. A more recent study by van der Leeuw and colleagues also examined VAT and SAT across advancing age groups in pre- and post-menopausal women and similar-aged men from the Netherlands.23 Contrary to the findings by Kotani et al., this cross-sectional study reported that 10-year differences in VAT were smaller in post-menopausal women as compared to pre-menopausal women, and the authors concluded that menopause was not associated with accelerated VAT gains. However, this study also found that post-menopausal women had greater 10-year differences in VAT gain compared to similar-aged men, supporting the notion that the sexual dimorphism of age-related changed diminishes over time.
A major limitation of the existing literature examining sex differences in VAT accrual is the reliance on cross-sectional data, which may not provide an accurate picture of age-related changes for individuals due to the potential for large cohort effects. A direct test of sex differences in the rate of abdominal adiposity accrual requires longitudinal VAT data which have been largely lacking. While sex differences in abdominal adiposity distribution and accrual represent a normal aspect of human biology, they have major implications for differences in the risk of chronic disease between men and women.24 Given the current limitations in the exiting literature, and different cardiometabolic risks associated with VAT in men and women, there is a need for longitudinal studies to examine sex differences in the natural progression of abdominal obesity alterations over time.
Furthermore, it is important to compare changes in abdominal obesity with concurrent changes in BMI. Increases in abdominal adiposity with aging may occur independent of changes in body weight, as individuals lose lean body mass while experiencing gains in adipose tissue.15 Therefore, BMI alone is likely failing to capture important adiposity changes with age. Growing evidence suggests that VAT and its alterations over time may be a better marker of cardiovascular and metabolic disease risk than BMI.25, 26 However, few longitudinal studies have compared and contrasted changes in weight or BMI against corresponding changes in VAT or SAT in both men and women.
We address these important gaps in the literature by studying a large sample of men and women with BMI, VAT and SAT measures at two time points, across a wide age-range. The purpose of this analysis was to evaluate, using one of the largest datasets of longitudinal VAT and SAT data, sex differences in the rate of VAT and SAT accrual over adulthood. We also examined change in BMI over time to determine how this measure compared to changes in VAT and SAT. Given that existing evidence suggests the menopausal transition may influence changes in body fat composition and distribution, a secondary analysis examined differences in the rate of VAT and SAT accrual in pre-, peri- and post-menopausal women. We hypothesized that the rate of VAT gain is greater in women than men, particularly in women after the menopausal transition, and that changes in BMI will not capture changes in adiposity distribution.
Subjects and Methods
Study population
Participants in this study included those who had taken part in the Fels Longitudinal Study and ancillary studies conducted at the Lifespan Health Research Center (LHRC) at Wright State University (Dayton, OH) from 2001-2014. The Fels Longitudinal Study is the longest running longitudinal study of growth, maturation and body composition. Magnetic resonance imaging (MRI) data collection at the LHRC first took place from 2001-2007 and included 804 participants as part of an ancillary study. In 2010-2014, MRI data collection became a regular part of the Fels Longitudinal Study protocol under a separate funding mechanism. Participants in the Fels Longitudinal Study follow specific visit schedules depending on age and sex; for participants aged 18 years of age and older, visits occur every 2 to 5 years. Due to funding limitations and the need to adhere to the Fels Longitudinal Study visit schedule, only 453 of the original MRI participants were scheduled for additional MRI data collection. Seventy-nine other participants who did not have an initial MRI measurement were seen twice from 2010 to 2015. This resulted in a total of 532 non-Hispanic white participants aged 18-84 years who had at least two abdominal MRI measurements. Of the 532 participants with at least two MRI visits, 60 participants with reported chronic disease at baseline, including type 2 diabetes or cardiovascular disease (myocardial infarction, angina, arteriosclerosis, atherosclerosis, ischemia, congestive heart failure, valvular heart disease, peripheral artery disease, stroke, or transient ischemic attack) were excluded, resulting in a final sample size of 472 participants (189 males, 283 females) for the present study. Participants were screened before each visit to verify they were free of any contraindications for MRI. All participants provided written informed consent to participate in the study. The study was approved by the Institutional Review Board at Wright State University and the University of Minnesota.
Magnetic resonance imaging
Abdominal MRI was completed at the Good Samaritan Hospital Greater Dayton MRI Consortium and Kettering Health Network, using the same protocol described previously.27, 28 Briefly, images were obtained every 1 cm from the 9th thoracic vertebra (T9) to the first sacral vertebra (S1). A single trained observer segmented axial images onto VAT and SAT areas (cm2) using SLICE-O-MATIC image analysis software (version 4.2; Tomo-vision Inc, Montreal, Canada). VAT and SAT areas were summed across all 24 images to obtain VAT and SAT volumes, which were then multiplied by the density of adipose tissue (0.9255g/cm3) to obtain total VAT mass (kg) and total SAT mass (kg). VAT mass was divided by SAT mass to obtain the VSR. Annualized rates of change were calculated as the difference of baseline and follow-up body composition measures divided by the time between baseline and follow-up in years (ΔBMI, ΔVAT, ΔSAT and ΔVSR). Percent change per year was calculated by dividing change variables by the corresponding baseline body composition measure and multiplying by 100 (%ΔBMI, %ΔVAT, %ΔSAT, and %ΔVSR).
Menopausal Status
Menopausal status was determined by questionnaire. Peri-menopause was defined as having missed 3 consecutive menstrual periods, while menopause was determined as missing more than 12 consecutive months of missed periods or physician diagnosed menopause or having bilateral oophorectomy/hysterectomy.
Anthropometric measures
All anthropometric variables followed a protocol specified by Lohman et al.29 Participants were asked to wear light clothing during all exam measures. Weight was measured to the nearest 0.01 kg using a digital scale. Height was measured to the nearest to the nearest 0.01 cm using a digital stadiometer. Waist circumference was measured to the nearest centimeter, immediately superior to the left iliac crest. Hip circumference was measured at the greater trochanters.
Covariates
For these analyses, information on study covariates was taken from the baseline visit. Sociodemographic variables included baseline age, sex, marital status (modeled in 3 groups: single; married or living with partner; separated, divorced or widowed), gross family income (categorized as <$35 000/year, $35 000-<$75 000, or ≥$75 000/year), and education (highest attained level of completed education, modeled in 4 groups: less than or equal to high school, some college, college graduate, or graduate degree). Self-reported health behaviors at baseline include current smoking status (yes or no), current alcohol use (yes or no), and current physical activity as measured by the Baecke sport activity index.30
Statistical Analyses
Analyses were conducted using SAS® (SAS Institute Inc., Cary, NC), version 9.4. All continuous variables were examined for skewness and kurtosis. There were no outliers identified for any of the baseline variables (defined as ±3 SD from mean), but because baseline BMI, VAT, SAT and VSR exhibited positive skewness and kurtosis these variables were transformed using the natural logarithm. Rate of change and percent change variables exhibited kurtosis, but natural logarithm, square root, and inverse square root transformations did not substantially alter the distributions. After removal of outliers (N=6-12 depending on variable, not dependent on age or sex), kurtosis was resolved and raw values were used for subsequent analyses.
Differences in baseline characteristics and rate of change in body composition measures between men and women were examined using t-tests or chi-square tests, as appropriate. Differences in body composition measures from baseline to follow-up were examined using paired t-tests (all p-values from two-sided tests). Linear regression was used to examine trends across age groups (18 to <30 years, 30 to <45 years, 45 to <60 years, and ≥60 years) for baseline and rate of change in body composition measures. Linear regression was also used to examine the effects of sex, age and their interaction on ΔBMI, ΔVAT, ΔSAT and ΔVSR each. Baseline characteristics associated with the rate of fat change in bivariate analyses were then added to the model, including: baseline body composition measures, income, marital status, physical activity, smoking and drinking. The covariates were not significant and did not improve the proportion of the variance explained in the tested models, with the exception of baseline BMI in the ΔBMI model and baseline SAT in the ΔSAT model; therefore only these two covariates were included in final analyses. Visit year was added to the model to account for possible period effects. We did not adjust for height in analyses as this variable remained constant within individuals from baseline to follow-up. Secondary analyses used linear regression to examine the effect of baseline menopausal status (pre, peri and post) on rate of change in body composition measures while controlling for baseline age. Given that longitudinal analyses have shown a decrease in BMI after age 70,31 we conducted exploratory analyses to examine whether study findings were altered after excluding participants aged 70 years or older at baseline (n=37). Overall findings did not change, and therefore we present data using the entire sample.
Results
Participant Characteristics
Baseline participant characteristics are shown in Table 1. Mean age at baseline was 45 years with an average follow up time of 7.3 years ± 2.6 (range 1.8-13.4). Approximately 44% of the subjects were normal weight (38% men, 48% women), 32% overweight (41% men, 26% women) and 24% obese (21% men, 27% women). At baseline, 46% of women were pre-menopausal, 17% peri-menopausal and 37% postmenopausal. Men had a significantly larger waist circumference, VAT, VSR and physical activity score and a smaller hip circumference and SAT mass compared to women. Baseline BMI, VAT, SAT and VSR increased across advancing age groups (18 to <30, 30 to <45, 45 to <60, and ≥60 years, see Table 2).
Table 1. Baseline participant characteristics (N=472).
| Men (n=189) | Women (n=283) | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Range | Mean (SD) | Range | p-value1 | |
| Age, years | 44.59 (16.58) | 18.00, 82.52 | 45.46 (15.01) | 18.10, 84.12 | 0.555 |
| ΔAge, years | 7.01 (2.40) | 1.83, 12.64 | 7.49 (2.69) | 1.83, 13.38 | 0.049 |
| BMI (kg/m2) | 26.76 (4.68) | 18.31, 46.63 | 26.94 (5.97) | 15.87, 55.08 | 0.943 |
| Hip circumference (cm) | 104.21 (8.44) | 87.05, 129.25 | 107.36 (12.13) | 81.90-167.05 | 0.002 |
| Waist circumference (cm) | 98.87 (13.74) | 72.10, 139.50 | 92.61 (13.45) | 67.33, 140.40 | <0.001 |
| VAT (kg) | 3.00 (1.95) | 0.20, 9.67 | 1.57 (1.08) | 0.15, 5.65 | <0.001 |
| SAT (kg) | 3.66 (2.28) | 0.59, 14.62 | 4.73 (2.86 ) | 0.93, 20.45 | <0.001 |
| VSR | 0.82 (0.39) | 0.25, 2.73 | 0.33 (0.13) | 0.07, 0.81 | <0.001 |
| Physical Activity Score2 | 2.45 (0.72) | 1.00, 4.50 | 2.16 (0.65) | 1.00, 4.50 | <0.001 |
|
| |||||
| N | % | N | % | p-value3 | |
|
| |||||
| Education | 0.799 | ||||
| ≤HS graduate or GED | 42 | 22.22 | 62 | 21.91 | |
| Some college | 62 | 32.80 | 103 | 36.40 | |
| College graduate | 53 | 28.04 | 69 | 24.38 | |
| Graduate degree | 30 | 15.87 | 44 | 15.55 | |
| Missing | 2 | 1.06 | 5 | 1.77 | |
| Annual Household Income | 0.349 | ||||
| <$35,000 | 53 | 28.04 | 97 | 34.28 | |
| $35,000-74,999 | 63 | 33.33 | 89 | 31.45 | |
| ≥$75,000 | 73 | 38.62 | 97 | 32.28 | |
| Marital Status | 0.013 | ||||
| Single | 51 | 26.98 | 52 | 18.37 | |
| Married or living with partner | 110 | 58.2 | 174 | 61.48 | |
| Separated, divorced or widowed | 11 | 5.82 | 34 | 12.01 | |
| Missing | 17 | 8.99 | 23 | 8.13 | |
| Current smoker | 36 | 19.05 | 46 | 16.25 | 0.433 |
| Current drinker | 138 | 73.02 | 197 | 69.61 | 0.374 |
| Menopausal status | |||||
| Pre-menopausal | - | - | 128 | 45.55 | |
| Peri-menopausal | - | - | 48 | 17.08 | |
| Post-menopausal | - | - | 105 | 37.37 | |
Abbreviations: BMI = body mass index; VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; VSR = visceral to subcutaneous adipose tissue ratio
P-value for sex difference from independent-sample t-test
Physical activity measured by the Baecke sport activity index
P-value for sex difference from chi-square test
Table 2. Baseline and rate of change per year in body composition measures by age category.
| Mean ± SD (Range) | p-value1 | ||||
|---|---|---|---|---|---|
|
| |||||
| Baseline | 18 to <30 years (N=105) | 30 to <45 years (N=107) | 45 to <60 years (N=175) | ≥60 years (N=85) | |
| BMI (kg/m2) | 25.04 ± 5.81 | 26.80 ± 5.08 | 27.7 ± 5.88 | 27.49 ± 4.08 | <0.001 |
| (16.60, 47.03) | (17.47, 43.87) | (15.87, 55.08) | (20.47, 43.02) | ||
| VAT (kg) | 1.18 ± 1.02 | 1.99 ± 1.62 | 2.45 ± 1.61 | 2.90 ± 1.80 | <0.001 |
| (0.20, 5.63) | (0.15, 7.79) | (0.18, 7.04) | (0.58, 9.67) | ||
| SAT (kg) | 3.67 ± 3.15 | 4.29 ± 2.59 | 4.70 ± 2.77 | 4.28 ± 1.77 | 0.028 |
| (0.59, 18.00) | (0.62, 13.40) | (0.90, 20.45) | (1.47, 10.29) | ||
| VSR | 0.36 ± 0.19 | 0.46 ± 0.27 | 0.57 ± 0.39 | 0.72 ± 0.44 | <0.001 |
| (0.09, 0.96) | (0.11, 1.38) | (0.07, 2.73) | (0.19, 2.01) | ||
|
| |||||
| Rate of Change in Original Units | |||||
|
| |||||
| ΔBMI (kg/m2/year) | 0.26 ± 0.44 | 0.20 ± 0.35 | 0.08 ± 0.32 | 0.02 ± 0.29 | <0.001 |
| (-1.06, 1.53) | (-0.78, 1.43) | (-0.98, 1.20) | (-0.77, 1.06) | ||
| ΔVAT (kg/year) | 0.11 ± 0.14 | 0.11 ± 0.13 | 0.10 ± 0.10 | 0.06 ± 0.10 | 0.015 |
| (-0.37, 0.52) | (-0.29, 0.51) | (-0.25, 0.52) | (-0.19, 0.26) | ||
| ΔSAT (kg/year) | 0.14 ± 0.22 | 0.08 ± 0.18 | 0.05 ± 0.16 | 0.00 ± 0.14 | <0.001 |
| (-0.70, 0.82) | (-0.68, 0.52) | (-0.48, 0.53) | (-0.61, 0.42) | ||
| ΔVSR (/year) | 0.02 ± 0.02 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.416 |
| (-0.03, 0.08) | (-0.04, 0.07) | (-0.04, 0.07) | (-0.03, 0.08) | ||
Abbreviations: BMI = body mass index; VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; VSR = visceral to subcutaneous adipose tissue ratio
p-value for linear trend across age categories
Compared to those who completed only one MRI measurement from 2001-2007 (n=351), those who had multiple MRI visits from 2001-2015 were older at baseline (mean ages 41.8 and 45.1 years, respectively, p=0.006) and had higher levels of education (66% and 78% completed some college, respectively, p=0.003). Among the participants with multiple MRI visits, those with chronic disease at baseline who were excluded from analyses were older and had higher adiposity levels (all p<0.04).
Comparison of Changes in Adiposity during Adulthood
As seen in Table 2, there was an inverse linear trend across advancing age groups for ΔBMI, ΔVAT, and ΔSAT (p<.001). There was no difference by age group for ΔVSR. Mean changes per year in body composition measures from baseline to follow-up by sex are shown in Table 3. All body composition measures significantly increased over time for both men and women (p<.001). Men had significantly greater ΔVAT and ΔVSR as compared to women. There were no sex differences for ΔBMI or ΔSAT. Because the absolute increases in body composition measures per year were relatively small, we also examined percent change to illustrate differences by outcome. On average, participants had a relative increase in BMI of 0.5% per year (%ΔBMI), compared with a 6.8% increase in VAT, 2.4% increase in SAT, and 3.6% increase in VSR. Percent change in body composition measures did not significantly differ between men and women.
Table 3. Rate of change per year in body composition measures by sex*.
| n | Mean ± SD (Range) | p-value1 | |||
|---|---|---|---|---|---|
|
| |||||
| Rate of Change in Original Units | Total | Men | Women | ||
| ΔBMI (kg/m2/year) | 466 | 0.13 ± 0.36 | 0.15 ± 0.34 | 0.12 ± 0.37 | 0.520 |
| (-1.06, 1.53) | (-0.77, 1.53) | (-1.06, 1.43) | |||
| ΔVAT (kg/year) | 468 | 0.10 ± 0.12 | 0.13 ± 0.15 | 0.07 ± 0.09 | <0.001 |
| (-0.37, 0.52) | (-0.37, 0.52) | (-0.29, 0.48) | |||
| ΔSAT (kg/year) | 464 | 0.07 ± 0.18 | 0.08 ± 0.18 | 0.06 ± 0.18 | 0.161 |
| (-0.70, 0.82) | (-0.70, 0.82) | (-0.68, 0.53) | |||
| ΔVSR (/year) | 464 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.01 | <0.001 |
| (-0.04, 0.08) | (-0.04, 0.08) | (-0.04, 0.05) | |||
|
| |||||
| Rate of Change as a Percent of Baseline | |||||
|
| |||||
| %ΔBMI (/year) | 464 | 0.53 ± 1.31 | 0.57 ± 1.31 | 0.50 ± 1.32 | 0.538 |
| (-3.55, 5.31) | (-2.22, 5.31) | (-3.55, 5.13) | |||
| %ΔVAT (/year) | 460 | 6.83 ± 8.50 | 7.15 ± 9.28 | 6.62 ± 7.96 | 0.526 |
| (-17.63, 38.99) | (-17.63, 38.17) | (-13.33, 38.99) | |||
| %ΔSAT (/year) | 460 | 2.44 ± 5.17 | 3.04 ± 5.61 | 2.06 ± 4.83 | 0.056 |
| (-15.90, 21.57) | (-9.13, 21.57) | (-15.90, 21.50) | |||
| %ΔVSR (/year) | 460 | 3.55 ± 3.90 | 3.15 ± 3.35 | 3.81 ± 4.21 | 0.064 |
| (-9.14, 16.46) | (-9.14, 11.92) | (-8.91, 16.43) | |||
Abbreviations: BMI = body mass index; VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; VSR = visceral to subcutaneous adipose tissue ratio
All body compositions changes were significantly greater than zero (p<.001)
p-value for sex difference from independent-sample t-test
Sex and Age Differences in Adiposity Change
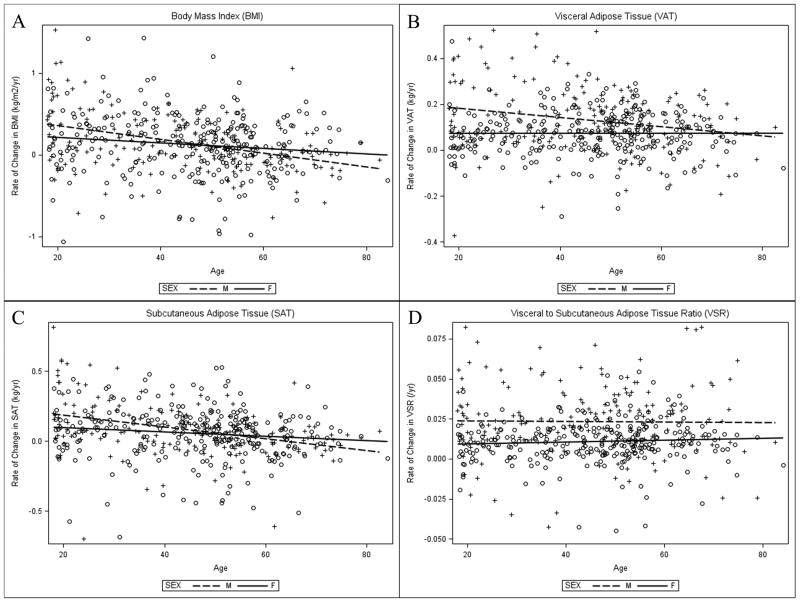
The main effect of sex was significant for ΔVAT (β = 0.06, p<.001) and ΔVSR (β = 0.01, p<.001) only, with men experiencing greater changes in VAT and VSR than women (see Table 4). The main effect of age was significant for ΔBMI (β = -0.05, p<.001), ΔVAT (β = -0.01, p=.004) and ΔSAT (β = -0.02, p<.001), with men and women experiencing slower rates of fat change with increased age. The main effect of age on ΔVSR was not significant. There was a significant interaction between sex and age for ΔBMI (β = -0.08, p=0.019), ΔVAT (β = -0.03, p=0.004) and ΔSAT (β = -0.04, p=.030). With age, men experienced a greater decrease in the rate of BMI, VAT and SAT accrual as compared to women, as seen in Figure 1. Of note, there does not appear to be a curvilinear relationship or age-threshold after which changes in adiposity accelerate or change.
Table 4. Associations between rate of change per year in body composition measures and sex and baseline age.
| Dependent Variable | Independent Variables* | Model F (p-value) |
Beta (SE) |
p-value | Model R2 |
|---|---|---|---|---|---|
| ΔBMI (kg/m2/yr) | 8.34 | 0.083 | |||
| (<0.001) | |||||
| Sex | 0.018 | 0.593 | |||
| (0.033) | |||||
| Age | -0.045 | <0.001 | |||
| (0.022) | |||||
| Sex* Age | -0.077 | 0.019 | |||
| (0.033) | |||||
| ΔVAT (kg/yr) | 11.18 (<0.001) | 0.088 | |||
| Sex | 0.059 (0.011) | <0.001 | |||
| Age | -0.001 (0.007) | 0.004 | |||
| Sex* Age | -0.031 (0.011) | 0.004 | |||
| ΔSAT (kg/yr) | 10.14 | 0.100 | |||
| (<0.001) | |||||
| Sex | 0.008 (0.017) | 0.635 | |||
| Age | -0.019 | <0.001 | |||
| (0.011) | |||||
| Sex* Age | -0.036 | 0.030 | |||
| (0.017) | |||||
| ΔVSR(/yr) | 16.66 | 0.126 | |||
| (<0.001) | |||||
| Sex | 0.013 | <0.001 | |||
| (0.002) | |||||
| Age | 0.001 | 0.855 | |||
| (0.001) | |||||
| Sex* Age | -0.001 | 0.488 | |||
| (0.002) |
Abbreviations: BMI = body mass index; VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; VSR = visceral to subcutaneous adipose tissue ratio
Tested covariates included: baseline body composition measures, income, marital status, physical activity, smoking, and drinking status. None of the variables improved the R2 of the models and were therefore removed from final analyses, with the exception of baseline BMI in the ΔBMI model and baseline SAT in the ΔSAT model. All models controlled for baseline visit year.
Figure 1.
Rate of change per year in body composition measures by sex and age Scatter plots of baseline age and rate of change per year in body composition measures, stratified by sex: (A) body mass index; (B) visceral adipose tissue; (C) subcutaneous adipose tissue; (D) visceral to subcutaneous adipose tissue ratio. Dashed lines and plus symbols (+) represent males, solid lines and open circles represent females. There was a significant interaction of age and sex for rate of change per year in body mass index, visceral adipose tissue, and subcutaneous adipose tissue (panels A, B, and C).
Associations of Body Composition Measures and Menopausal status
In unadjusted models, menopausal status was associated with ΔBMI (t(276) = 1.75, p=0.048) and ΔSAT (t(273) = 2.09, p=0.013), with a progressive decrease in rate of adiposity gain from pre- to peri- to post-menopause. However, after controlling for age, the associations were attenuated. Menopausal status was not associated with ΔVAT or ΔVSR in unadjusted or age-adjusted models (see Table 5).
Table 5. Associations between rate of change per year in body composition measures and menopausal status and baseline age.
| β Regression Coefficients (SE) | p-value* | |||
|---|---|---|---|---|
|
| ||||
| Pre-menopausal | Peri-menopausal | Post-menopausal | ||
| ΔBMI (kg/m2/yr) | ||||
| Model 1 | Ref | -0.098 (0.063) | -0.116 (0.049) | 0.048 |
| Model 2 | Ref | -0.079 (0.079) | -0.087 (0.090) | 0.557 |
| ΔVAT (kg/yr) | ||||
| Model 1 | Ref | -0.002 (0.016) | -0.007 (0.013) | 0.844 |
| Model 2 | Ref | -0.012 (0.020) | -0.023 (0.023) | 0.593 |
| ΔSAT (kg/yr) | ||||
| Model 1 | Ref | -0.027 (0.031) | -0.072 (0.024) | 0.013 |
| Model 2 | Ref | -0.041 (0.039) | -0.095 (0.045) | 0.099 |
| ΔVSR (/yr) | ||||
| Model 1 | Ref | -0.001 (0.002) | -0.001 (0.002) | 0.843 |
| Model 2 | Ref | -0.003 (0.003) | -0.004 (0.003) | 0.424 |
p-value for difference in rate of change in body composition measures by menopausal status
Model 1: unadjusted
Model 2: adjusted for age
Discussion
To our knowledge, this is the first longitudinal study with serial measures of VAT and SAT to focus on sex differences in the rate of abdominal adiposity change during adulthood. We found that while both men and women experience increases in BMI, VAT and SAT with age, the rates of accrual diminishes with age, particularly among men. We also found that menopausal status was not associated with the rate of VAT or SAT gain in women.
The annual percent gain in both VAT (6.8%) and SAT (2.4%) far exceeded the observed percent increase in BMI (0.5%), thus demonstrating that BMI is insensitive to changes in abdominal adiposity over time. Shah et al., also observed a small percent change in weight (0.3%) over a median 3.3 years of follow-up as compared to concomitant changes in VAT (7.1%) or SAT (5.8%) in an ethnically diverse sample of men and women aged 54-75 years from the Multi-Ethnic Study of Atherosclerosis.25
We found that men experienced significantly greater ΔVAT and ΔVSR between the two measurement visits as compared with women. Several cross-sectional studies have also reported that the age-related increase in VAT is greater in males than in females.12, 14, 32 Our findings extend the existing literature by using serial measures to examine the rate of change in body composition measures. When we examined the relative increase in percent fat (%ΔVAT and %ΔVSR), there were no differences between men and women. However, evidence suggests that the absolute quantity of VAT has a stronger association with cardiovascular disease risk factors than relative amounts.22 Therefore, the finding that men gain absolutely more visceral fat than women has important clinical implications due to the known associations between VAT and cardiometabolic risk factors.2-7
While men and women both experienced increases in VAT, SAT and VSR over the follow-up period, we found that the rate of fat gain decreased with age across participants. Interestingly, men experienced greater decreases in the rate of VAT and SAT accumulation over time, compared to women. In other words, we found that men are slowing down in their rate of fat gain to meet the rate of gain observed in women, while women maintain a more consistent (albeit decreasing) rate of fat change during adulthood. These finding are contrary to the highly cited work of Kotani et al., who reported that the increase in VAT accelerated across age groups in postmenopausal women compared to premenopausal women, mirroring VAT gains seen in men.12 The aforementioned study differs from ours in that it had a cross-sectional study design, relatively small sample size which excluded normal weight individuals, and was limited to Asian participants who have been shown to carry more abdominal fat than Whites.33 Furthermore, the acceleration of VAT gain found in their study, as compared to the deceleration found in our study, may be the result of their study reporting relative fat volume expressed as a percentage of a participant's total fat volume. Two more recent cross-sectional studies examining VAT changes across men and women from a wide age spectrum also report decelerations in VAT gains across advancing age groups.23, 32
In secondary analyses we found no difference in ΔBMI, ΔVAT, ΔSAT or ΔVSR between pre-, peri- and post-menopausal women, after controlling for age. Several other studies have also reported that the postmenopausal increase in VAT was not a result of menopause per se, but rather caused by the effect of age, increased adiposity generally, or reduced physical activity.34-38 Multiple cross-sectional20, 39, 40 and longitudinal studies17-19, 41, 42 have reported an increase in central adiposity across the menopausal transition, but most of these studies examined average levels of abdominal fat, rather than rates of fat change, as done in this study. Our study suggests that menopause is not a particularly sensitive period for adiposity accrual in women. Increases in VAT as well as SAT gradually decline with age in both men and women, with no differences in rates by menopausal status.
While it is not clear why rate of fat gain decreases with age, gonadal hormones may in part explain why men and women experience different trajectories in rate of fat change with age. It is well established that both estrogen and testosterone play a role in directing fat distribution in both sexes;12, 20, 43-45 however, the literature examining hormones and fat distribution have focused primarily on absolute levels of VAT or SAT, and little is known about the role of hormones in rate of fat change. Future studies should explore the biological explanations for these gender differences. Faster decline in the rate of adiposity accrual with age in men may be part of the faster overall rate of biological senescence observed in men than women.46
These findings have important implications for cardiovascular disease risk. Although prior work has emphasized the greater absolute levels of abdominal adiposity in older adults, the rate of change in VAT is actually lower in older men and women as compared to younger men and women. Therefore, in order to maximize risk reduction there is a need for intervention in younger adults to minimize the rate of VAT gain. Exercise interventions have shown to be effective at reducing VAT mass in both men and women, independent of fat loss.47-49 This is a promising approach to limiting VAT gain in both younger and older adults that should be further explored.
Our study has several strengths and limitations that should be noted. Our findings extend the existing literature by examining adiposity changes in men and women across a wide age range using serial VAT and SAT data. Additionally, we had a relatively large sample size and used multiple-slice MRI images to quantify VAT and SAT volume over the entire abdominal region. Prior work commonly used single-slice computerized tomography (CT) scans or MRI images between the 4th and 5th lumbar vertebrae, but growing evidence suggests this single slice method is not ideal and does not account for sex differences in VAT deposition.50 Finally, the average length of follow-up in this study was longer than other studies in the literature with serial VAT measures.17, 19, 25, 51
A limitation of the present study is that the sample was limited to non-Hispanic white men and women, which restricts the generalizability of findings to other races and ethnicities. Evidence suggests there are racial/ethnic differences in body fat distribution,15, 22, 33 therefore there is a need for future studies with diverse samples of men and women to examine changes in abdominal adiposity over time. Additionally, as a result of complexities in the study design, there was variation in length of follow-up. However, to account for this variation we examined annualized rates of change in our analyses. Due to the wide age range in our sample, it is possible that cohort effects may in part explain the age-trends observed in rate of adiposity accrual. Specifically, we observed a greater rate of abdominal fat deposition in younger individuals as compared to older individuals; however, when these younger individuals age they may not exhibit the same trends as observed in the older individuals in this study. A longer length of follow-up would be necessary to fully disentangle true aging effects from cohort effects.
It is also important to note that our models explained a small proportion of the variance in adiposity accrual. We attempted to control for factors that may influence adiposity accrual in our analyses, including baseline body composition measures, physical activity, smoking and alcohol consumption status, as well as income and marital status. However, these covariates failed to improve the proportion of the variance explained in adiposity accrual. It is possible this is in part due to measurement error. For example, physical activity was assessed using a self-reported questionnaire and is therefore subject to recall and social desirability bias. We also assessed whether participants were smokers or drinkers, but did not account for smoking frequency or the quantity of alcohol intake, therefore providing an incomplete picture of smoking and drinking behaviors. We did not have data on dietary intake, and unmeasured genetic or hormonal factors may explain additional variance in adiposity accrual. Future research is needed to further examine the predictors of abdominal adiposity accrual.
In summary, men and women continue to accrue abdominal adiposity with age, but the rate of adiposity gain decreases over time, with men experiencing steeper declines in the rate of fat gain as compared to women. Therefore, our findings suggest that the sexual dimorphism in fat distribution does indeed become smaller with age. However, this is because men begin to experience fat gains more similar to women, rather than women experiencing accelerated fat gains with age. Future work will examine sex differences in the cardiometabolic impact of changing VAT accrual during adulthood, to identify sensitive periods of the life course when interventions are most likely to improve health.
Acknowledgments
This work was supported by grants R01HD012252, R01DK064870, and R01DK064391 from the National Institutes of Health. KMW was supported in part by research training grant T32 HL007779 from the National Heart, Lung and Blood Institute. The authors thank the investigators, the staff, and the participants of the Fels Longitudinal study for their valuable contributions.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 2.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14(2):336–41. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23(4):465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med. 2004;140(12):992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19(5):457–66. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284(6):E1065–71. doi: 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- 7.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 8.Abraham TM, Pedley A, Massaro JM, Hoffman U, Fox CS. Association Between Visceral and Subcutaneous Adipose Depots and Incident Cardiovascular Disease Risk Factors. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gast KB, den Heijer M, Smit JW, Widya RL, Lamb HJ, de Roos A, et al. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis. 2015;241(2):547–54. doi: 10.1016/j.atherosclerosis.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 11.Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44(6):739–46. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- 12.Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18(4):207–2. [PubMed] [Google Scholar]
- 13.Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. 2005;81(6):1330–4. doi: 10.1093/ajcn/81.6.1330. [DOI] [PubMed] [Google Scholar]
- 14.Machann J, Thamer C, Schnoedt B, Stefan N, Stumvoll M, Haring HU, et al. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. MAGMA. 2005;18(3):128–37. doi: 10.1007/s10334-005-0104-x. [DOI] [PubMed] [Google Scholar]
- 15.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339–48. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Lara-Castro C, Weinsier RL, Hunter GR, Desmond R. Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10(9):868–74. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- 17.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32(6):949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulnour J, Doucet E, Brochu M, Lavoie JM, Strychar I, Rabasa-Lhoret R, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause. 2012;19(7):760–7. doi: 10.1097/gme.0b013e318240f6f3. [DOI] [PubMed] [Google Scholar]
- 19.Ho SC, Wu S, Chan SG, Sham A. Menopausal transition and changes of body composition: a prospective study in Chinese perimenopausal women. Int J Obes (Lond) 2010;34(8):1265–74. doi: 10.1038/ijo.2010.33. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010;18(3):604–10. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthrie JR, Dennerstein L, Taffe JR, Ebeling PR, Randolph JF, Burger HG, et al. Central abdominal fat and endogenous hormones during the menopausal transition. Fertil Steril. 2003;79(6):1335–40. doi: 10.1016/s0015-0282(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 22.Kanaley JA, Giannopoulou I, Tillapaugh-Fay G, Nappi JS, Ploutz-Snyder LL. Racial differences in subcutaneous and visceral fat distribution in postmenopausal black and white women. Metabolism. 2003;52(2):186–91. doi: 10.1053/meta.2003.50024. [DOI] [PubMed] [Google Scholar]
- 23.van der Leeuw J, Wassink AM, van der Graaf Y, Westerveld HE, Visseren FL, Second Manifestations of ADSG Age-related differences in abdominal fat distribution in premenopausal and postmenopausal women with cardiovascular disease. Menopause. 2013;20(4):409–17. doi: 10.1097/gme.0b013e31827212a5. [DOI] [PubMed] [Google Scholar]
- 24.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21(3):415–30. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7(12):1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012;13(Suppl 2):6–13. doi: 10.1111/j.1467-789X.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 27.Demerath EW, Ritter KJ, Couch WA, Rogers NL, Moreno GM, Choh A, et al. Validity of a new automated software program for visceral adipose tissue estimation. Int J Obes (Lond) 2007;31(2):285–91. doi: 10.1038/sj.ijo.0803409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demerath EW, Shen W, Lee M, Choh AC, Czerwinski SA, Siervogel RM, et al. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85(2):362–8. doi: 10.1093/ajcn/85.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 30.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 31.Droyvold WB, Nilsen TI, Kruger O, Holmen TL, Krokstad S, Midthjell K, et al. Change in height, weight and body mass index: Longitudinal data from the HUNT Study in Norway. Int J Obes (Lond) 2006;30(6):935–9. doi: 10.1038/sj.ijo.0803178. [DOI] [PubMed] [Google Scholar]
- 32.Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim U, Ernst T, Buchthal SD, Latch M, Albright CL, Wilkens LR, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1:e6. doi: 10.1038/nutd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douchi T, Yonehara Y, Kawamura Y, Kuwahata A, Kuwahata T, Iwamoto I. Difference in segmental lean and fat mass components between pre- and postmenopausal women. Menopause. 2007;14(5):875–8. doi: 10.1097/GME.0b013e318032b2f9. [DOI] [PubMed] [Google Scholar]
- 35.Pansini F, Cervellati C, Guariento A, Stacchini MA, Castaldini C, Bernardi A, et al. Oxidative stress, body fat composition, and endocrine status in pre- and postmenopausal women. Menopause. 2008;15(1):112–8. doi: 10.1097/gme.0b013e318068b285. [DOI] [PubMed] [Google Scholar]
- 36.Cervellati C, Pansini FS, Bonaccorsi G, Pascale G, Bagni B, Castaldini C, et al. Body mass index is a major determinant of abdominal fat accumulation in pre-, peri- and post-menopausal women. Gynecol Endocrinol. 2009;25(6):413–7. doi: 10.1080/09513590902770123. [DOI] [PubMed] [Google Scholar]
- 37.Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, et al. Abdominal fat distribution in pre- and postmenopausal women: The impact of physical activity, age, and menopausal status. Metabolism. 2001;50(8):976–82. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- 38.Demerath EW, Rogers NL, Reed D, Lee M, Choh AC, Siervogel RM, et al. Significant associations of age, menopausal status and lifestyle factors with visceral adiposity in African-American and European-American women. Ann Hum Biol. 2011;38(3):247–56. doi: 10.3109/03014460.2010.524893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;24(2):226–31. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 40.Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, et al. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89(7):3425–30. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 41.Franklin RM, Ploutz-Snyder L, Kanaley JA. Longitudinal changes in abdominal fat distribution with menopause. Metabolism. 2009;58(3):311–5. doi: 10.1016/j.metabol.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32(2):257–62. doi: 10.2337/dc08-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mongraw-Chaffin ML, Anderson CA, Allison MA, Ouyang P, Szklo M, Vaidya D, et al. Association between sex hormones and adiposity: qualitative differences in women and men in the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015;100(4):E596–600. doi: 10.1210/jc.2014-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab. 2007;92(7):2696–705. doi: 10.1210/jc.2006-1847. [DOI] [PubMed] [Google Scholar]
- 45.Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39(9):897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 46.Seifarth JE, McGowan CL, Milne KJ. Sex and life expectancy. Gend Med. 2012;9(6):390–401. doi: 10.1016/j.genm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Ross R, Janiszewski PM. Is weight loss the optimal target for obesity-related cardiovascular disease risk reduction? Can J Cardiol. 2008;24(Suppl D):25D–31D. doi: 10.1016/s0828-282x(08)71046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 49.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12(5):789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 50.Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88(5):1263–71. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janssen I, Powell LH, Jasielec MS, Kazlauskaite R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity (Silver Spring) 2015;23(2):488–94. doi: 10.1002/oby.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]