Abstract
A growing body of work has demonstrated that cancer metastasis is not a random spontaneous event; rather it is the culmination of a cascade of priming steps through which a sub-population of the tumor cells acquires invasive traits while readying a permissive environment, termed the pre-metastatic niche, in which distant metastases can occur. Signals from the primary tumor mobilize and adapt immune cells as well as directly communicate with distant niche cells to induce a broad spectrum of adaptations in target organs, including the induction of angiogenesis, inflammation, extracellular matrix remodeling, and metabolic reprogramming. Together these interactions facilitate the formation of a pre-metastatic niche composed of a variable mix of resident and recruited immune cells, endothelial cells, and stromal cells connected through a complex signaling network that we are only beginning to understand. Here we summarize the latest findings on how cancer induces and guides the formation of this pre-metastatic niche as well as potential prognostic markers and therapeutic targets that may lead to a better understanding and effective treatment of metastatic disease.
Introduction
Metastasis, the spread of cancer cells from a primary tumor to other organs, is the leading cause of mortality in cancer patients. This is partially due to the limited therapeutic options and the short time window that would allow a successful treatment of clinically detectable metastases. Therefore, there is a great and urgent need to elucidate metastasis-driving molecular and cellular events before and during early stages of metastatic colonization, which may guide development of therapies to prevent or eradicate metastases before they reach an incurable stage. Recent evidences highlight the important role of a pre-metastatic niche, initially proposed and proven by David Lyden et al. (1, 2), in cancer’s preparation for metastasis. A pre-metastatic niche is free of cancer cells, but has captured cancer-associated properties that are permissive and sometimes even supportive for cancer cells originating from a foreign tissue to grow. These earliest, non-cancerous pathological changes in a tumor-free organ have the unique potential to serve as prognostic biomarkers and therapeutic targets in the prevention and treatment of metastasis. An overview of the metastatic niche as whole has been covered in several important reviews on related topics (3–8), including the excellent articles from Lyden’s group on pre-metastatic niche (9, 10). Here we focus on the most recent findings that improve our understanding of the pre-metastatic niche and provide rationales for the development of therapies against cancer metastasis.
The Pre-metastatic Niche Model
The long known “seed and soil” hypothesis for metastasis by Steven Paget (11) has been complemented and refreshed by modern cancer research. In the basic framework, migratory tumor cells (the “seeds”) leave the primary tumor through intravasation, disseminate throughout the body via the circulation, and eventually engraft in a distant organ that provides an appropriate microenvironment (the “soil”). Recent studies indicate that dissemination of cancer cells from the primary site occurs during early cancer stages but is not sufficient for metastasis (9, 12, 13), emphasizing the essential role of a conducive niche in the target distant organ. The concept of a pre-metastatic niche was first proposed by Lyden and Rafii et al. in 2005 after the discovery that bone marrow (BM)-derived hematopoietic progenitor cells that are VEGFR1+ are recruited to future metastatic sites before the tumor cells arrive, where the BM-derived cells promote the chemoattraction and attachment of disseminated cancer cells through mechanisms including the SDF-1/CXCR4 axis (1). Subsequent studies reveal that in a pre-metastatic niche, various types of cells together determine the fate of disseminated cancer cells in multiple aspects, including their extravasation, survival, colonization and aggressive growth. Adaptation of a pre-metastatic niche prior to the arrival of tumor cells has been recognized as an important means for cancer to facilitate metastasis (6, 10, 14–20). It is worth noting that the pre-metastatic niche model may co-operate with other models depicting different steps and modes of metastasis (3), such as the tumor self-seeding model proposed by Joan Massague et al. (21) (Figure 1).
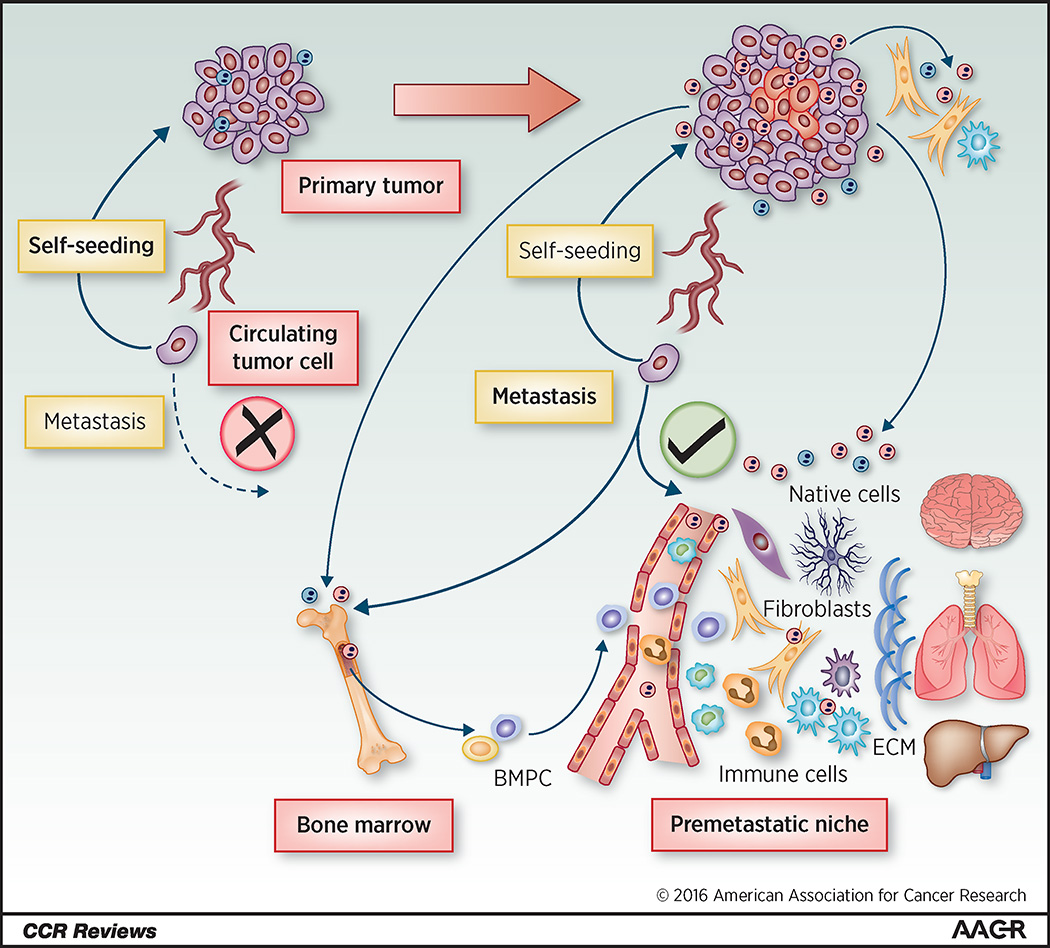
Figure 1.
Tumor-directed formation of a pre-metastatic niche. Recent studies including those discussed in this review suggest that EVs and other factors secreted by the primary tumor initiate and direct the formation of a pre-metastatic niche. At an early tumor stage (left), circulating tumor cells (CTCs) can be detected, but are not capable of metastatic colonization due to insufficient invasive traits and/or lack of a permissive metastatic niche. Most CTCs will die, but some may travel back to the primary tumor to evolve a more aggressive phenotype according to the tumor self-seeding model. As the tumor grows and progresses (right), tumor cells experience additional genetic, epigenetic, or environmental alterations, including metabolic stresses (e.g., hypoxia), and secrete a variety of EV-associated and other factors. These tumor-secreted factors, upon release into the circulation, may cause a broad spectrum of systemic effects, including the induction of angiogenesis, inflammation, ECM remodeling, and metabolic reprogramming at a pre-metastatic site. All types of resident cells in a pre-metastatic organ can be affected directly or indirectly in this process. New types of non-cancerous cells, such as the bone marrow progenitor cells (BMPCs), are recruited and often reprogrammed to form the pre-metastatic niche. Metastatic colonization will succeed once CTCs have acquired sufficient intrinsic potential and a conducive pre-metastatic niche has been established. Combinatorial therapies targeting both cancer cells and factors driving the formation of pre-metastatic niche may hold promise for the prevention and treatment of metastasis.
Traits of a Pre-metastatic Niche
Recruitment of bone marrow-derived cells (BMDCs)
BMDCs can be mobilized into the circulation and thereby participate in the establishment of a primary or (pre-)metastatic tumor microenvironment as a non-resident cellular component. Factors secreted by primary tumor cells can activate resident fibroblast-like stromal cells at a pre-metastatic site, resulting in an increased production of the extracellular matrix (ECM) component fibronectin, which enables the adhesion and clustering of migratory BMDCs that express the fibronectin receptor VLA-4 (integrin α4β1), as well as genes related to their mobilization, including MMP9 and Id3, in the pre-metastatic niche (1). This leads to the expression of SDF-1 in the pre-metastatic niche resulting in the recruitment of CXCR4+ cancer cells. The SDF-1/CXCR4 chemokine axis also participates in the homing of BMDCs. A recent paper shows that extracellular matrix metalloproteinase inducer (EMMPRIN) in cancer cells can induce the expression and secretion of several factors such as SDF-1 and VEGF that mediate BMDC recruitment to the lungs and liver (22). For primary tumors with STAT3 activation, BMDC recruitment can be partially mediated by tumor-secreted factors that are induced by STAT3 signaling, such as IL-6 and IL-10 (23). These secreted factors lead to a widespread STAT3 activation in pre-metastatic lungs, activate fibroblasts to produce fibronectin, and induce the formation of clusters of CD11b+ myeloid-derived suppressor cells (MDSCs) in the lungs, resulting in enhanced metastatic growth. MDSCs may also be recruited to the pre-metastatic lung through hypoxia-induced secreted factors such as MCP-1 from the primary tumor (24) and the induction of the inflammatory proteins S100A8 and S100A9 in endothelial and myeloid cells (10). CCL9 is induced through TGF-β signaling in myeloid cells in the pre-metastatic lungs, whereas it enhances tumor cell survival and promotes metastasis (25). Another recent study has found that lysyl oxidase-like 2 (LOXL2) and the bHLH transcription factor E47, which function together to induce EMT, also contribute to the recruitment of BMDCs to pre-metastatic lungs through transcriptional regulation of fibronectin and cytokines including GM-CSF (26). TNFα, TGFβ, and VEGF-A secreted by the primary tumor can induce the expression of S100A8 and S100A9 in pre-metastatic lung endothelial cells which act as potent chemoattractants for Mac-1+ myeloid cells and cancer cells through SAA3-induces TLR4 signaling (17, 18).
The heterogeneity of immune cells
It is becoming increasingly apparent that the immune constituents of the pre-metastatic niche are profoundly different between model systems, even within the same type of cancer, with some model systems showing the recruitment of one major cell type whereas others indicating a larger cross-talking network of cells. In the MMTV-polyoma middle T antigen mouse mammary tumor model, neutrophils are found to be the primary immune cells recruited to the pre-metastatic lungs, although these cells have a low frequency in the primary tumor microenvironment (27). However this may be due to the timing of the experiments as a recent study has shown that immune cells arrive at the pre-metastatic lung in three separate waves, and some of these cells are only transiently present in the tumors (28). The first wave of immune cells consists of neutrophils and peaks at 15–30 minutes after tumor cell injection. The second wave is primarily composed of conventional monocytes and peaks at 4 hrs after tumor cell injection. Lastly non-alveolar macrophages, patrolling monocytes and DCs arrive at the pre-metastatic niche which peaks at 6–24 hrs. In mice bearing the primary mammary tumors, infiltration of lung tissues by CD11b+Ly6G+ neutrophils starts before cancer cells can be detected in the lungs, and further increases during the metastatic stage. Compared to neutrophils from healthy lungs, tumor-mobilized lung neutrophils are also mature but exhibit minor differences in gene expression. Pre-metastatic lung neutrophils secrete leukotrienes, which enhance the tumorigenic and metastatic potentials of primary tumor cells (27). Another study shows that mammary tumors induce a systemic expansion and polarization of neutrophils through IL-1β-activated, IL-17-producing γδ T cells in a G-CSF-dependent manner. These neutrophils may help to establish a pre-metastatic niche, where they suppress CD8+ T cell activation to facilitate metastasis (29).
Monocytes/macrophages also contribute to the establishment of a pre-metastatic niche. Palmitoylated surface antigens on breast cancer secreted exosomes induce NFκB signaling in macrophages at a pre-metastatic site through activating TLR2, stimulating macrophages to secrete pro-inflammatory factors such as IL-6 to promote cancer cell growth (30). Other macrophage secreted factors such as granulin can indirectly support cancer cell growth through the activation of fibroblasts to generate a more permissive niche (31). Macrophages may also co-migrate with cancer cells, inducing the expression of Mena in the cancer cells, and the direct interaction between perivascular macrophages, endothelial cells, and Mena-overexpressing tumor cells is significantly correlated with metastatic disease in ER+/HER2− breast cancer (32). In the pre-metastatic lymph nodes of a lung tumor model, COX-2 and SDF-1 are induced in dendritic cells (DCs), which further increase lymphangiogenesis and the recruitment of Tregs, suggesting a role of DCs and prostaglandin E2 (PGE2) in the establishment of a pre-metastatic niche (33). However DCs can also inhibit metastasis by engulfing tumor-secreted vesicles termed cytoplasts and traveling to the mediastinal lymph node to activate ovalbumin-specific CD8+ T cells. Depletion of DCs has been shown to increase metastasis (28), highlighting the complexity of the immune components of the pre-metastatic niche. Similarly, as a part of cancer immunosurveillance, non-classical “patrolling” CX3CR1highCD14dimCD16+ monocytes are enriched in the microvasculature of the lungs where they inhibit tumor cell adhesion to the vasculature and promote natural killer cell recruitment and activation to reduce lung metastases. In response to tumor challenge, lung endothelial cells increase the expression of CX3CL1, which attracts the patrolling monocytes expressing CX3CR1 (34). Other myeloid cells contributing to a pre-metastatic niche include platelets and granulocytes. Platelets form aggregates with circulating tumor cells, which reprogram them to secrete CXCL5 and CXCL7 to recruit granulocytes, forming an early metastatic niche for subsequent metastatic progression (35).
CD8+ T cells are capable of constraining myeloid cell accumulation in pre-metastatic lymph nodes by inducing myeloid cell apoptosis. Metastatic melanoma patients had decreased CD8+ T cell infiltration and increased STAT3 in lymph node myeloid cells, suggesting that metastatic tumors may inhibit CD8+ T cell expansion or homing to the pre-metastatic sites (36). Another study shows that complement C5a receptor (C5aR) facilitates metastasis by suppressing CD4+ and CD8+ T cells in the lungs and livers. This immunosuppression is mediated by recruitment of MDSCs, regulation of their TGF-β and IL-10 production, and generation of Treg cells (37). Immunosuppression in the pre-metastatic niche may also be mediated by factors such as MCP-1 that are secreted from the primary tumor in response to hypoxia resulting in the recruitment of MDSCs and immature natural killer (NK) cells, which have reduced cytotoxic activity, to the pre-metastatic lung (24). The tissue-resident alveolar macrophages, which are accumulated in the pre-metastatic lungs through C5aR-mediated proliferation instead of recruitment from the circulation, promote cancer metastasis to the lungs by shifting the T cell population from Th1 towards Th2 to suppress their antitumor activity (38). S100A4 also increases primary tumor growth as well as metastasis by reducing the Th1/Th2 ratio in the lungs in a mammary tumor model (39).
Reprogramming of stromal cells
The formation of a pre-metastatic niche not only involves the recruitment of foreign cells such as immune cells, but also the re-programming of the resident stromal cells to facilitate metastatic growth. Normal lung fibroblasts express miR-30 family members to restrain MMPs, such as MMP9, to stabilize the lung vasculature (40). Cancer cells reprogram fibroblasts to decrease their expression of miR-30 family members resulting in enhanced MMP activity, vascular permeability, and metastasis. Factors secreted from the primary tumor induce the expression of αSMA in pre-metastatic fibroblasts, activating them to induce extracellular matrix remodeling through secretion of fibronectin, LOX, and LOXL2 thereby generating a more permissive microenvironment for metastasis (1, 41). The induction of senescence in osteoblasts in the bone increases their secretion of factors such as IL6 to promote osteoclastogenesis resulting in increased metastases (42). Pre-metastatic immune cells may also facilitate the re-programming of stromal cells. Granulin secreted by CD11b+F4/80+Ly6GnegCCR2+ metastasis-associated macrophages induces the expression of αSMA in pre-metastatic hepatic stellate cells and induces their secretion of ECM remodeling proteins such as periostin to enhance metastatic growth (31). This relationship is reciprocal as fibrocytes can secrete CCL2, CCL5, and MMP9 to induce the recruitment of Ly-6C+, Ly-6Glow monocytes into the pre-metastatic lung to promote metastasis (43). In some instances cancer may also co-migrate with stromal cells such as fibroblasts, which enhance the viability of cancer cells at the pre-metastatic site (44).
Alterations in the extracellular matrix (ECM)
Alterations in the pre-metastatic ECM are among the first steps in the formation of the pre-metastatic niche. Factors secreted by the primary tumor including exosomes can induce the accumulation of fibronectin in the pre-metastatic niche through several mechanisms including secretion from the primary tumor and reprogramming of fibroblasts (1, 45). Pre-metastatic niche fibronectin can activate dormant metastatic cancer cells and mediate the recruitment of immune cells and metastatic cancer cells. Binding of VEGFR1+ BMDCs to fibronectin induces α4β1 integrin signaling resulting in increased MMP9 expression, enhancing the recruitment of BMDCs and cancer metastasis (1). MMP9 expression in lung endothelial cells and macrophages increases metastasis, enhances lung vascular permeability, and recruits BMDCs and monocytes (1, 10, 40, 43). Hypoxia in the primary tumor induces the secretion of fibronectin and lysl oxidase (LOX) leading to their accumulation in the pre-metastatic niche (14). LOX co-localizes with fibronectin-rich regions to recruit CD11b+ myeloid cells and c-Kit+ myeloid progenitor cells to the lungs. LOX-mediated collagen cross-linking increases the MMP2 activity in the recruited myeloid cells. MMP2 enhances myeloid cell invasion and mediates collage IV degradation, releasing collagen IV peptides into the blood where they act as chemoattractants to generate a positive feedback loop for the recruitment of myeloid cells to the pre-metastatic niche. Activated fibroblasts in the pre-metastatic niche, often generated as a result of fibrosis, have increased expression and excretion of LOX and to a lesser extent LOXL2 resulting in increased collagen deposition and ECM stiffening, promoting metastatic cancer cell survival and cancer and immune cell engraftment (41, 46). Hypoxia also induces the secretion of exosomes containing LOXL2 on their outer surface, promoting collagen cross-linking (45). Cancer cells may also secrete factors such as osteopontin to facilitate the recruitment of granulin expressing immune cells to the pre-metastatic niche, resulting in increased expression of ECM components and ECM remodeling factors (46).
Alterations in the vasculature
Blood vessels in a pre-metastatic niche directly control the arrest and extravasation of circulating cancer cells, and are critical targets for tumor-derived adaptations in preparation for metastasis. Tumor-secreted extracellular vesicles (EVs), including exosomes, systemically transfer tumor-derived regulators of the vascular endothelial barriers. Metastatic breast cancer cells, by secreting EV-encapsulated miR-105, downregulate tight junctions in endothelial cells and induce systemic vascular leakiness to promote metastasis (20). EVs secreted by brain-metastatic breast cancer cells contain miR-181c, which promotes the destruction of blood brain barrier (BBB) through modulation of actin dynamics to facilitate brain metastases (47). In addition to EV-mediated mechanisms, pre-metastatic lungs express higher levels of Angiopoietin-2, MMP3, and MMP10, which possibly result from cancer-secreted TGF-β1 and TNF-α, and synergistically induce vascular permeability and the extravasation of circulating cancer cells (48). Another group also found that VEGF secreted by breast cancer cells induces Angiopoietin-2 expression in brain microvascular endothelial cells, leading to impaired tight junction structures and increased BBB permeability (49). EMMPRIN expression induces the expression and secretion of SDF-1 and VEGF to induce BMDC-mediated angiogenesis (22). Cancer-secreted VEGF also recruits BMDCs to pre-metastatic lungs to increase inflammation, angiogenesis, and metastasis through inducing PGE2 production in endothelial cells (50). The peripheral blood plasma and bone marrow plasma from breast cancer patients increase transendothelial migration of breast cancer cells, which may involve systemic factors as well as factors in a pre-metastatic bone niche. Peripheral blood was only able to increase the migration of non-metastatic cancer cells, suggesting that it acts through a mechanism that has already been acquired by metastatic cells (51). VEGFR1 expression in benign lymph nodes predicts recurrence of prostate cancer, however the VEGFR1-targeting drug axitinib fails to reduce lymph node VEGFR1 highlighting the need for better targets (52). In addition, CCL2 secreted by the primary tumor enhances CCL2 and CCR2 expression in lung endothelial cells and leukocytes resulting in enhanced vascular permeability in the lungs through a S100A8-TLR4 mediated pathway (15). Healthy lung fibroblasts express miR-30 family members to inhibit the expression of MMPs, including MMP9, through targeting Skp2 resulting in stabilization of lung vasculature and inhibition of metastasis. Distant tumors are able to decrease the expression of miR-30 family members in pre-metastatic lung fibroblasts, resulting in vascular destabilization and increased metastasis (40).
The acquisition of epithelial-to-mesenchymal transition (EMT) is an important step in the development of invasive and metastatic traits in the primary tumor, and also results in the secretion of factors that facilitate angiogenesis. EVs secreted by cancer cells that have undergone partial or full EMT have greater enrichment of factors such as Rac1, tissue factor, and ECM remodeling proteins which can promote endothelial cell proliferation and tube formation (53–55). Furthermore EVs secreted by mesenchymal-like breast and ovarian cancer cells carry angiogenic molecules to activate endothelial cells through Akt phosphorylation. Activated endothelial cells, in turn, increase their secretion of vesicles to induce EMT in epithelial cancer cells and promote metastasis (56). Given these findings, the acquisition of EMT in the primary tumor may lead to the release of exosomes that can enhance vascular permeability in the pre-metastatic niche to facilitate cancer and immune cell engraftment. However, further work must be done to demonstrate that these EMT-induced exosomes exert an effect outside of the primary tumor.
Lymphatic endothelial cells within pre-metastatic lungs and lymph nodes express CCL5 in response to IL-6 secreted by breast cancer, directing cancer cell dissemination into these tissues. Mice treated with breast tumor-conditioned medium show enhanced angiogenesis and lymphangiogenesis in the lymph nodes, as well as enhanced lymphangiogenesis with unchanged angiogenesis in the primary tumors and lungs (57, 58). These results highlight a role of the tissue-residing lymphatic vessels, in addition to blood vessels, in the establishment of a pre-metastatic vascular niche.
Metabolic reprogramming of native cells
In a niche, which in ecology refers to the interactive position of a species in an ecosystem, the competition between different species for limited resources is one of the driving factors for dynamic population changes. When metastatic cancer cells arrive at a distant site, they must compete with the resident niche cells for the nutrients to establish a metastatic colony. Breast cancer cells can secrete EV-encapsulated miR-122, which can be taken up by niche cells such as lung fibroblasts and astrocytes to decrease the glucose consumption in these cells by targeting pyruvate kinase (19). This increases the availability of glucose for cancer cells, thus increasing their proliferation and survival to enhance metastasis. Another study shows that colorectal cancer cells, by secreting creatine kinase, convert liver-produced creatine into phosphocreatine that is subsequently taken up to fuel cancer cells during liver metastasis (59). These recent findings demonstrate an active role of non-cancerous cells at a pre-metastatic site in rebalancing the metabolic needs between cancer and niche cells in response to cancer’s exploitation of nutrients.
Metabolic stresses, such as hypoxia, are important drivers of tumor progression and also contribute to pre-metastatic niche formation. HIF-1α stabilization under hypoxia induces cancer cells to secrete factors such as MCP-1, G-CSF, TNF-α, VEGF, TIMP-1, and MMP-9, which promote the recruitment of CD11b+/Ly6Cmed/Ly6G+ MDSCs as well as CD3−/NK1.1+/CD11blow/CD27low immature NK cells with reduced cytotoxicity to the pre-metastatic lungs and enhance metastasis (24). Hypoxic breast cancer cells secrete lysyl oxidase (LOX), which leads to pre-metastatic osteolytic lesions and promotes bone metastases through NFATc1-driven osteoclastogenesis independent of RANK ligand (60). For hepatocellular carcinomas, hypoxia and TGF-β induce LOXL2 in the primary tumor and in patient sera, thereby increasing tissue stiffness and promoting cancer cell adhesion and metastasis (61). As an indirect mechanism, hypoxia-induced expression of carbonic anhydrase IX in breast cancer cells leads to the secretion of G-CSF, which mobilizes granulocytic MDSCs to pre-metastatic lungs and promotes metastasis (62). The effects of other types of stresses in the primary tumors, such as nutrient deprivation, on the establishment of a pre-metastatic niche are yet to be identified.
Tumor-Derived Formation of a Pre-metastatic Niche
Tumor-secreted extracellular vesicles (including exosomes)
EVs are released into the extracellular environment by many cell types including cancer cells. These membrane-encapsulated structures can transfer a variety of cellular materials including RNA, DNA, and proteins between adjacent or distant cells (upon systemic delivery via the circulation) (63–66). Many recent studies on EVs focus on exosomes, a subset of EVs that are 30–100 nm with an endocytic origin. Cancer cells have been noted for their enhanced secretion of exosomes with altered contents in comparison to their non-cancerous counterparts, and as a result cancer-specific serum exosome miRNAs and proteins have been proposed as biomarkers for cancer (67–71). Recent studies indicate that exosomes contain fibronectin on their external surface which facilitated interaction with target cells through heparin sulfate (72). As the accumulation of fibronectin in the pre-metastatic niche is one of the earliest stages of pre-metastatic niche formation, exosomes may be the earliest drivers of pre-metastatic niche formation. Metastatic cancer cells secrete exosomes from their leading edge to promote adhesion and enhance directional trafficking (73). Whether this occurs in vivo remains to be seen. Cancer-secreted EVs can be internalized by other cell types in the primary tumor microenvironment and pre-/metastatic niches. Cargos loaded in these EVs, which to a certain extent reflect the molecular alterations in cancer cells, can be transferred to recipient niche cells to exert profound effects (74–76). Recent EV tracing studies have indicated that melanoma-derived EVs primarily travel to the tumor draining lymph nodes where they are taken up by a protective barrier of subcapsular sinus CD169+CD11b+SSClow macrophages (77). This protective barrier can be compromised by tumor progression or by anti-cancer treatments allowing tumor EVs to interact with B cells in the tumor draining lymph nodes, promoting tumor progression. On the other hand, non-cancerous cells in a cancer-hosting niche also secreted EVs to influence cancer behaviors (78, 79). A recent study indicates that cancer-secreted exosomes arriving to a pre-metastatic niche follow the tropism of their parent cells, and that this organotropism in exosome homing is partially determined by the exosomal integrin profile. Mice pre-treated with lung-tropic exosomes can shift the metastatic preference of bone-tropic cells to the lungs (80). Some recently reported EV-mediated mechanisms that can contribute to the complex intercellular communications at a pre-metastatic niche are summarized in Table 1.
Table 1.
Recently reported EV-mediated adaptations in a pre-metastatic niche
| Effector cells | Target cells | EV cargos | Effects | References |
|---|---|---|---|---|
| glioblastoma cells |
brain endothelial cells; glioma cells |
mRNA (including EGFRvIII), miRNA, angiogenic proteins |
stimulate angiogenesis and glioma cell proliferation |
Skog et al. (64) |
| melanoma cells | BM progenitors | MET, TYRP2, VLA-4, HSP70, an HSP90 isoform |
induce vascular leakiness; educate BMDCs to be pro- angiogenic |
Peinado et al. (82) |
| multiple types of cancer cells |
endothelial cells | miR-9 | promote endothelial cell migration and tumor angiogenesis |
Zhuang et al. (89) |
| breast cancer cells |
endothelial cells | miR-105 | induce vascular leakiness |
Zhou et al. (20) |
| brain-metastatic breast cancer cells |
brain endothelial cells |
miR-181c | destroy BBB to promote brain metastases |
Tominaga et al. (47) |
| breast cancer cells; ovarian cancer cells |
macrophages; monocytes; dendritic cells |
palmitoylated proteins; ? |
induce NFκB- and STAT3-target cytokines |
Chow et al. (30) Bretz et al. (90) |
| lung cancer cells |
immune cells | miR-21 and miR-29a |
trigger a TLR-mediated pro-metastatic inflammatory response |
Fabbri et al. (91) |
| melanoma cells | sentinel lymph nodes |
? | induce angiogenic pathways, ECM modification, and cancer cell recruitment |
Hood et al. (92) |
| multiple types of cancer cells |
MDSCs | Hsp72 | induce STAT3- dependent immunosuppression |
Chalmin et al. (93) |
| pancreatic cancer cells |
Kupffer cells | MIF | induce TGF-β secretion and fibronectin production by hepatic stellate cells |
Costa-Silva et al. (45) |
| lung and pancreatic cancer cells |
myoblasts | miR-21 | Promote muscle cell death and cachexia |
He et al. (94) |
| multiple types of cancer cells |
stromal fibroblasts |
TGF-β | promote differentiation into myofibroblasts |
Webber et al. (95) |
| breast cancer cells |
lung fibroblasts, astrocytes |
miR-122 | suppress glucose metabolism |
Fong et al. (19) |
| prostate cancer cells |
prostate fibroblasts |
miR-100, miR- 21 etc. |
Increase MMP and RANKL expression and fibroblast migration |
Sanchez et al. (96) |
| Pancreatic cancer cells |
lung fibroblasts, lymph node cells, BM cells, endothelial cells |
? (require other soluble factors) |
reprogram gene expression to promote metastasis |
Jung et al. (97) |
| metastatic breast cancer cells |
non-metastatic breast cancer cells |
miR-200 | promote EMT and metastasis |
Le et al. (98) |
| stromal fibroblast |
breast cancer cells |
Cd81 | mobilize autocrine Wnt-PCP signaling to drive metastasis |
Luga et al. (78) |
| astrocytes | breast cancer cells |
PTEN-targeting miRNAs |
downregulate PTEN to promote brain metastasis |
Zhang et al. (79) |
Non-vesicular tumor-secreted factors
Tumor-secreted factors such as G-CSF, OPN, SDF-1, TNF-α, TGF-β, VEGF-A, and PIGF have long been known to influence a metastatic niche through inducing inflammation, remodeling ECM, altering niche cells, and recruiting immune cells (8, 10, 29, 50). A thorough list of these factors and their effects on pre-metastatic niche has been summarized (8). More recent work has shown that factors secreted by hypoxic tumor cells, including LOX, LOXL2, and G-CSF, direct a pro-metastatic niche reprogramming (60–62). LOXL2 has also been shown to collaborate with E47 to induce EMT and increase the secretion of GM-CSF to recruit BMDCs to pre-metastatic lungs (26). VEGF, another hypoxia-induced factor, has been known to play an important role in cancer growth and metastasis through the induction of angiogenesis in the primary tumor. Tumor-secreted VEGF also increases angiogenesis and recruits MDSCs to the pre-metastatic lungs through the induction of COX-2 and its downstream target PGE2 in pulmonary endothelial cells (50). In addition, VEGF secreted by metastatic cancer cells can disrupt BBB by inducing Angiopoietin-2 in brain microvascular endothelial cells (49). Together these studies suggest that the formation of a pre-metastatic niche may begin as soon as the primary tumor grows large enough for the formation of hypoxic regions and systemic dissemination of hypoxia-induced factors.
Clinical Implications
Many cancer-associated circulating exosomal markers, including those listed in Table 1, have shown promise as a non-invasive means of assessing the metastatic potential of the primary tumor. Serum levels of miR-105 and miR-122 have been shown to be prognostic indicators for metastasis in early-stage breast cancer patients (20, 81). Exosomal miR-181c has been shown to be increased in patients with brain metastases, however it is unknown whether it is increased at a pre-metastatic phase (47). Exosomal MET, pMET, TYRP2, VLA-4, and HSP70 have shown a remarkable prognostic value in melanoma patients (82), whereas exosomal levels of ITGβ4 and ITGαv in breast and pancreatic cancer patients at a pre-metastatic stage are respectively associated with organotropic metastases to the lungs and liver (80).
One of the challenges of studying exosomes in vivo is that the exosomes circulating in the blood originate from multiple cell types including both normal and cancer cells. Glypican-1 (GPC1) has been proposed as a marker of cancer-derived exosomes and has shown promise in the early detection of pancreatic cancer (67). GPC1 outclasses the current clinical standard carbohydrate antigen 19-9 ELISA in discriminating benign pancreatic disease and healthy individuals from patients with pancreatic cancer precursor lesions. While exosome collection and screening does require more time and procedures than standard serum screens, the exosomal markers offer greater specificity and sensitivity in comparison to unfractionated serum (67).
The enhanced permeability and retention effect (EPR) describes the retention of large (>40~50 kDa) macromolecules within the tumor due to its abnormal vasculature. It is unclear whether the EPR effect applies to exosomes. Studies have shown that exosome-sized nanoparticles exhibit the EPR effect (83), but the notable increase of cancer-derived exosomes in patient blood indicates that the primary tumor is able to release a substantial number of exosomes which are not being substantially retained by the tumor. Although established metastases demonstrate the EPR effect (84), it is not yet known whether the vasculature in the pre-metastatic niche may become transformed enough to display this effect before the arrival of cancer cells. This needs to be further elucidated as a potential mechanism that may influence subsequent tissue uptake of exosomes as well as the delivery and retention of therapeutic agents in a pre-metastatic niche.
Factors and pathways driving the tumor-directed reprogramming of normal niche cells during the establishment of a pre-metastatic niche are potential therapeutic targets for the prevention and early treatment of metastases. Trebananib targeting Ang-1/2 has been incapable of extending the life of cancer patients receiving chemotherapy, except for ovarian cancer (85–87). However, recent studies suggest that the drug may be used to target brain metastasis. As discussed earlier, cancer cells can induce Ang-2 in brain endothelial cells through the secretion of VEGF; inhibition of Ang-2 with trebananib reduces tumor-induced BBB disruption in mice (49). Further work needs to be done to determine whether trebananib may increase the survival of brain metastatic patients.
Promising results have been seen in pre-clinical models with LOX inhibition and bisphosphonate zoledronic acid in decreasing osteolytic lesions and bone metastasis (60), and with the Alox5 inhibitor zileuton in decreasing leukotriene-promoted lung metastasis (27). Several studies have proposed therapies that inhibit the recruitment of immune cells to the pre-metastatic niche, including targeting CXCR2 to decrease platelet-mediated granulocyte recruitment (35), C5aR to decrease Treg recruitment (37), and COX-2 to decrease DC recruitment (33). Recent evidence however suggests that caution should be used regarding therapies that inhibit the release of immune cells into the circulation (88). CCL2 inhibition is found to reduce metastasis by inhibiting the mobilization of monocytes from BM, however termination of CCL2 inhibition leads to a larger release of monocytes into the blood as well as increased angiogenesis and cancer cell proliferation in the lungs, resulting in reduced survival compared to untreated mice (88). Therapies targeting the mobilization of immune cells may need to be given for prolonged time and combined with other therapies that would overcome the adverse effects. This also suggests that the tumor microenvironments (including pre-metastatic niches) and other organs harboring tumor-promoting cells (such as the BM) undergo dynamic remodeling in response to targeted therapies, which may result in unpredictable clinical outcome and need to be carefully evaluated in pre-clinical models. Nevertheless, further characterization of the causes and phenotypes of pre-metastatic niches would reveal additional markers with diagnostic and prognostic values, and guide the development of new therapies to simultaneously target cancer cells and the pre-metastatic niche to control cancer metastasis.
Acknowledgments
The authors apologize to the authors of those excellent studies they are not able to include in this review due to the space limit.
Grant Support
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01CA166020 (to S.E. Wang) and R01CA163586 (to S.E. Wang), California Breast Cancer Research Program grant 20IB-0118 (S.E. Wang), and Breast Cancer Research Foundation-AACR grant 12-60-26-WANG (S.E. Wang).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan RN, Rafii S, Lyden D. Preparing the "soil": the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 6.Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 8.McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature cell biology. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 12.Alix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013–5021. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- 13.Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratsuka S, Ishibashi S, Tomita T, Watanabe A, Akashi-Takamura S, Murakami M, et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun. 2013;4:1853. doi: 10.1038/ncomms2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011;108:3725–3730. doi: 10.1073/pnas.1100446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 18.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 19.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Gou X, Kong DK, Wang X, Wang J, Chen Z, et al. EMMPRIN regulates tumor growth and metastasis by recruiting bone marrow-derived cells through paracrine signaling of SDF-1 and VEGF. Oncotarget. 2015;6:32575–32585. doi: 10.18632/oncotarget.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, et al. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 25.Yan HH, Jiang J, Pang Y, Achyut BR, Lizardo M, Liang X, et al. CCL9 induced by TGFbeta signaling in myeloid cells enhances tumor cell survival in the premetastatic organ. Cancer Res. 2015;75:5283–5298. doi: 10.1158/0008-5472.CAN-15-2282-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canesin G, Cuevas EP, Santos V, Lopez-Menendez C, Moreno-Bueno G, Huang Y, et al. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene. 2015;34:951–964. doi: 10.1038/onc.2014.23. [DOI] [PubMed] [Google Scholar]
- 27.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531:513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohan TE, Xue X, Lin HM, D'Alfonso TM, Ginter PS, Oktay MH, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, Hosono K, et al. Prostanoid induces premetastatic niche in regional lymph nodes. J Clin Invest. 2014;124:4882–4894. doi: 10.1172/JCI73530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111:E3053–E3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Zhang C, Li W, Deng J, Herrmann A, Priceman SJ, et al. CD8+ T-cell immunosurveillance constrains lymphoid premetastatic myeloid cell accumulation. Eur J Immunol. 2015;45:71–81. doi: 10.1002/eji.201444467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vadrevu SK, Chintala NK, Sharma SK, Sharma P, Cleveland C, Riediger L, et al. Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 2014;74:3454–3465. doi: 10.1158/0008-5472.CAN-14-0157. [DOI] [PubMed] [Google Scholar]
- 38.Sharma SK, Chintala NK, Vadrevu SK, Patel J, Karbowniczek M, Markiewski MM. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194:5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 39.Grum-Schwensen B, Klingelhofer J, Beck M, Bonefeld CM, Hamerlik P, Guldberg P, et al. S100A4-neutralizing antibody suppresses spontaneous tumor progression, pre-metastatic niche formation and alters T-cell polarization balance. BMC Cancer. 2015;15:44. doi: 10.1186/s12885-015-1034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi F, He T, Jia L, Song N, Guo L, Ma X, et al. The miR-30 family inhibits pulmonary vascular hyperpermeability in the premetastatic phase by direct targeting of Skp2. Clin Cancer Res. 2015;21:3071–3080. doi: 10.1158/1078-0432.CCR-14-2785. [DOI] [PubMed] [Google Scholar]
- 41.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer research. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo X, Fu Y, Loza AJ, Murali B, Leahy KM, Ruhland MK, et al. Stromal-initiated changes in the bone promote metastatic niche development. Cell Rep. 2016;14:82–92. doi: 10.1016/j.celrep.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Deventer HW, Palmieri DA, Wu QP, McCook EC, Serody JS. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J Immunol. 2013;190:4861–4867. doi: 10.4049/jimmunol.1202857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. The Journal of cell biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69:7529–7537. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- 49.Avraham HK, Jiang S, Fu Y, Nakshatri H, Ovadia H, Avraham S. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol. 2014;232:369–381. doi: 10.1002/path.4304. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Jiang M, Zhao Q, Li S, Peng Y, Zhang P, et al. Vascular endothelial growth factor plays a critical role in the formation of the pre-metastatic niche via prostaglandin E2. Oncol Rep. 2014;32:2477–2484. doi: 10.3892/or.2014.3516. [DOI] [PubMed] [Google Scholar]
- 51.Martinez LM, Vallone VB, Labovsky V, Choi H, Hofer EL, Feldman L, et al. Changes in the peripheral blood and bone marrow from untreated advanced breast cancer patients that are associated with the establishment of bone metastases. Clin Exp Metastasis. 2014;31:213–232. doi: 10.1007/s10585-013-9622-5. [DOI] [PubMed] [Google Scholar]
- 52.Pal SK, Vuong W, Zhang W, Deng J, Liu X, Carmichael C, et al. Clinical and translational assessment of VEGFR1 as a mediator of the premetastatic niche in high-risk localized prostate cancer. Mol Cancer Ther. 2015;14:2896–2900. doi: 10.1158/1535-7163.MCT-15-0367. [DOI] [PubMed] [Google Scholar]
- 53.Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287:43565–43572. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gopal SK, Greening DW, Hanssen EG, Zhu HJ, Simpson RJ, Mathias RA. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget. 2016 Feb 22; doi: 10.18632/oncotarget.7573. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA, et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics. 2013;12:2148–2159. doi: 10.1074/mcp.M112.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasquier J, Thawadi HA, Ghiabi P, Abu-Kaoud N, Maleki M, Guerrouahen BS, et al. Microparticles mediated cross-talk between tumoral and endothelial cells promote the constitution of a pro-metastatic vascular niche through Arf6 up regulation. Cancer Microenviron. 2014;7:41–59. doi: 10.1007/s12307-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun. 2014;5:4715. doi: 10.1038/ncomms5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee E, Pandey NB, Popel AS. Pre-treatment of mice with tumor-conditioned media accelerates metastasis to lymph nodes and lungs: a new spontaneous breast cancer metastasis model. Clin Exp Metastasis. 2014;31:67–79. doi: 10.1007/s10585-013-9610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393–406. doi: 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cox TR, Rumney RM, Schoof EM, Perryman L, Hoye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Wong CC, Tse AP, Huang YP, Zhu YT, Chiu DK, Lai RK, et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60:1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]
- 62.Chafe SC, Lou Y, Sceneay J, Vallejo M, Hamilton MJ, McDonald PC, et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res. 2015;75:996–1008. doi: 10.1158/0008-5472.CAN-14-3000. [DOI] [PubMed] [Google Scholar]
- 63.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 64.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 66.Redzic JS, Balaj L, van der Vos KE, Breakefield XO. Extracellular RNA mediates and marks cancer progression. Semin Cancer Biol. 2014;28:14–23. doi: 10.1016/j.semcancer.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 69.Duijvesz D, Luider T, Bangma CH, Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur Urol. 2011;59:823–831. doi: 10.1016/j.eururo.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 70.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PloS One. 2014;9:e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem. 2016;291:1652–1663. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nature communications. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thuma F, Zoller M. Outsmart tumor exosomes to steal the cancer initiating cell its niche. Semin Cancer Biol. 2014;28:39–50. doi: 10.1016/j.semcancer.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells. Biomed Res Int. 2014;2014:179486. doi: 10.1155/2014/179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aleckovic M, Kang Y. Regulation of cancer metastasis by cell-free miRNAs. Biochim Biophys Acta. 2015;1855:24–42. doi: 10.1016/j.bbcan.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352:242–246. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu X, Somlo G, Yu Y, Palomares MR, Li AX, Zhou W, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med. 2012;10:42. doi: 10.1186/1479-5876-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342–347. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 84.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae DS, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:799–808. doi: 10.1016/S1470-2045(14)70244-X. [DOI] [PubMed] [Google Scholar]
- 86.Dieras V, Wildiers H, Jassem J, Dirix LY, Guastalla JP, Bono P, et al. Trebananib (AMG 386) plus weekly paclitaxel with or without bevacizumab as first-line therapy for HER2-negative locally recurrent or metastatic breast cancer: a phase 2 randomized study. Breast. 2015;24:182–190. doi: 10.1016/j.breast.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Peeters M, Strickland AH, Lichinitser M, Suresh AV, Manikhas G, Shapiro J, et al. A randomised, double-blind, placebo-controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. Br J Cancer. 2013;108:503–511. doi: 10.1038/bjc.2012.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 89.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 93.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci U S A. 2014;111:4525–4529. doi: 10.1073/pnas.1402714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 96.Sanchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7:3993–4008. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124:5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]