Abstract
Both genetics and physical activity (PA) contribute to bone mineral density (BMD), but it is unknown if the benefits of physical activity on childhood bone accretion depend on genetic risk. We therefore aimed to determine if PA influenced the effect of bone fragility genetic variants on BMD in childhood. Our sample comprised U.S. children of European ancestry enrolled in the Bone Mineral Density in Childhood Study (N=918, 5–19 years, and 52.4% female). We used a questionnaire to estimate hours per day spent in total, high, and low impact PA. We calculated a BMD genetic score (% BMD lowering alleles) using adult genome-wide association study (GWAS)-implicated BMD variants. We used dual energy X-ray absorptiometry to estimate femoral neck, total hip, and spine areal-BMD and total body less head (TBLH) bone mineral content (BMC) Z-scores. The BMD genetic score was negatively associated with each bone Z-score (e.g. TBLH-BMC: estimate=−0.03, P=1.3×10−6). Total PA was positively associated with bone Z-scores; these associations were driven by time spent in high impact PA (e.g. TBLH-BMC: estimate=0.05, P=4.0×10−10); and were observed even for children with lower than average bone Z-scores. We found no evidence of PA-adult genetic score interactions (P-interaction >0.05) at any skeletal site, and there was no evidence of PA-genetic score-Tanner stage interactions at any skeletal site (P-interaction >0.05). However, exploratory analyses at the individual variant level revealed that PA statistically interacted with rs2887571 (ERC1/WNT5B) to influence TBLH-BMC in males (P-interaction=7.1×10−5), where PA was associated with higher TBLH-BMC Z-score among the BMD lowering allele carriers (rs2887571 AA homozygotes: estimate=0.08 [95% CI: 0.06, 0.11], P=2.7×10−9). In conclusion, the beneficial effect of PA on bone, especially high impact PA, applies to the average child and those genetically predisposed to lower adult BMD (based on GWAS-implicated BMD variants). Independent replication of our exploratory individual variant findings is warranted.
Keywords: Physical activity, exercise, genetic, bone mineral density, children
Introduction
In the U.S., 10.3% of adults over 50 years of age have osteoporosis(1), with the prevalence highest among postmenopausal women (15.4%)(1). Enhancing bone accretion to maximize peak bone mass in early life could help reduce the risk of osteoporosis later in adulthood(2). Physical activity, especially high impact physical activity, is beneficial for bone accretion(3,4), yet benefits may vary across puberty stage(5). Most importantly, it is unknown whether physical activity equally benefits bone accretion in all children, particularly those who present with lower than average bone mineral density (BMD) and those who are genetically predisposed to adult bone fragility.
Physical activity-gene interaction studies to date have predominantly been performed in adults, used cross-sectional study designs, and investigated candidate genes thought to have a role in skeletal health(6–13). Only two cross-sectional pediatric studies, involving Finnish and Chinese females, have investigated if physical activity associations with BMD are influenced by candidate gene variants(14,15). More recently, genome-wide association studies (GWAS) identified loci that are robustly associated with BMD in adults(16–20). We previously demonstrated that a proportion of the adult GWAS implicated BMD loci were associated with BMD in children(21,22), with some associations sex and/or puberty stage dependent. It is unknown if physical activity interacts with variation at these same loci to influence BMD in childhood.
We first tested if physical activity and overall BMD genetic risk score were associated with areal bone mineral density (aBMD) and bone mineral content (BMC) in children. Our primary aim was to determine whether physical activity interacted with BMD genetic risk score to influence aBMD and BMC. We further investigated whether physical activity, BMD genetic risk score and maturation interacted to influence aBMD and BMC. We also performed exploratory analyses with each individual single nucleotide polymorphism (SNP) comprising the BMD genetic score.
Materials and Methods
Participants
The Bone Mineral Density in Childhood Study (BMDCS) established norms for aBMD and BMC in healthy U.S. children aged 5–19 years(23). Participants aged 6–18 years were recruited in 2002–2003, and annual follow-ups occurred until 2008–2009 at five sites (Los Angeles, CA; Cincinnati, OH; Omaha, NE; Philadelphia, PA; and New York, NY)(23). The study was extended to include 5 and 19 years olds beginning in 2006–2007; these younger and older participants completed annual follow-up until 2008–2009. Inclusion and exclusion criteria are listed in Supplemental Table 1. Blood or saliva was collected at the final visit, from which DNA was extracted. For the present study each participant’s population ancestry was determined using whole genome genotyping data by principal components analysis and admixture(24). Participants of European ancestry were included in this analysis (N=918 [a total of 452 non-European ancestry participants were not included in the analyses]), as the tag SNPs being investigated have only been validated in European populations(16–20). In 2010, a cross-sectional sample of 486 European ancestry children aged 5–18 years were enrolled at two sites(Cincinnati, OH and Omaha, NE) to serve as the “replication cohort” to help validate findings in the original “discovery cohort”; the same inclusion and exclusion criteria were used for the replication cohort (Supplemental Table 1). Written informed consent was obtained for participants ≥18 years; participants <18 years provided assent along with consent from a parent or guardian. The Institutional Review Boards at each site approved the BMDCS study protocol.
Genotyping
DNA samples were SNP genotyped genome-wide at the Children’s Hospital of Philadelphia’s Center for Applied Genomics using high-throughput technology: IlluminaInfinium™ II OMNI Express plus ExomeBeadChip (Illumina, San Diego)(25). We investigated 67 SNPs near known adult bone mass loci(16,18,20,26). SNPs were modeled under an additive model. The BMD genotype risk score was calculated as a percentage of the number of “lowering BMD risk alleles” carried.
Physical Activity
Physical activity was estimated using the modified Slemenda questionnaire that included activities related to sports, leisure and activities of daily living(27). This questionnaire has been used in previous studies and indirect validity has been demonstrated through associations with bone phenotypes(4,27). Total number of hours in the past week spent in each physical activity was queried. Times spent in high impact physical activities were summed to generate the high impact physical activity variable (weight bearing physical activities with a ground reaction force greater than 2× body weight involving sprinting, turning or jumping actions [Supplemental Table 2]). Times spent in low impact physical activities were summed to generate the low impact physical activity variable(non-weight-bearing physical activities or weight-bearing physical activities with a ground reaction force of 1–2× body weight [Supplemental Table 2]). Trained research assistants checked the questionnaires for completion and accuracy.
Bone Mass and Content Outcomes
Dual energy X-ray absorptiometry (DXA) scans of the lumbar spine, proximal femur and total body were obtained using Hologic, Inc. (Bedford, MA) bone densitometers (QDR4500A, QDR4500W, Delphi A and Apex models). Scans were analyzed at the University of California, San Francisco’s DXA Core Laboratory using Hologic software (Discovery 12.3 at baseline and Apex 2.1 at post-baseline study visits using the “compare” feature). Spine, total hip and femoral neck aBMD, and total body less head (TBLH) BMC values were adjusted based on the cross-calibration of DXA devices and longitudinal calibration stability. TBLH BMC (rather than aBMD) was used because it is the preferred measure of bone status for the total body when adjusted for body size(28). aBMD and BMC Z-scores were calculated using the BMDCS reference values to account for known increases and sex differences in aBMD/BMC during growth(23). aBMD and BMC-Z were adjusted for height-for-age Z-scores to minimize potential confounding by skeletal size(29).
Covariates
Height (m) and weight (kg) were measured(23), and body mass index (BMI) Z-scores calculated using U.S. standards(30). Pubertal stage was assessed by physicians or nurses with expertise in pediatric endocrinology. Participants were categorized as pre-pubertal, pubertal and post-pubertal (Tanner stages I, II to IV, and V, respectively)(31). Dietary calcium intakes (g/d) were assessed using a semi-quantitative food frequency questionnaire (Block Dietary Data Systems, Berkeley, CA)(32), which included forty-five food and beverage items. Trained research assistants checked the questionnaires for completion and accuracy.
Statistical Analysis
The number of study visits per participant ranged from 1 to 7. Analyses were performed separately by sex, and for both sexes combined (opposite sex siblings were included in the BMDCS; for the sex combined analyses we removed the older sibling [N=113]). Sex stratified analyses were done because bone accretion timing and rates differ for males and females; males achieve higher peak bone mass(33); and bone fragility in later life is more common among females(1). Furthermore, our past genetic studies using BMDCS data demonstrate evidence of sex differences(21,22,34). An overview of our analyses is provided in Supplemental Table 3.
We first investigated if physical activity and genetic risk score were independently associated with bone Z-scores using mixed-effects linear regression, with subject-level random intercepts to account for the correlation between repeated measures. The Huber-White approach was used to construct robust standard errors using thevce (robust) option in Stata. The model included the time-invariant genetic risk score and time-variant physical activity variables. One set of models tested total physical activity; another set combined low and high impact physical activity variables in the same models. All models were adjusted for the time-variant covariates: age, BMI Z-score, dietary calcium and puberty stage. We also used longitudinal quantile regression to investigate if the physical activity variables were associated with bone Z-scores for children with below average and above average bone Z-scores(35,36), adjusting for the same covariates. For these analyses, P<0.05 was used to determine associations between physical activity and bone Z-scores and genetic risk score and bone Z-scores.
We then tested for total physical activity interactions with genetic risk score, using mixed-effects linear regression, as well as three-way interactions between total physical activity, puberty stage and genetic risk score (the lower order two-way interactions were included). The latter three-way interactions were to determine if physical activity and pubertal stage modified any genetic associations, as physical activity may be particularly important for bone accrual before full maturation is attained(5). For interaction analyses involving the genetic risk score, P<0.05 was used to determine if there were statistical significant interactions.
We may not be powered to identify physical activity interaction with the individual variants that comprise the genetic risk score, so we consider these analyses to be exploratory. For these exploratory interaction analyses involving individual variants, we applied the Benjamini and Hochberg false discovery rate approach to account for the 67 independent loci investigated. Total physical activity was used first in the interaction analyses. If we observed statistical evidence of an interaction that survived correction for multiple testing, we repeated the analysis using high and low impact physical activity. For interaction P-values that survived correction for multiple testing, we tested our findings both in the replication cohort and in the discovery and replication cohorts combined. We used Stata 12.0 (StataCorp LP, College Station, TX) to conduct all analyses. Importantly, because we modeled bone Z-scores negative estimates indicate that a predictor is associated with lower bone mass expected for age and sex. Positive estimates indicate that a predictor is associated with higher bone mass expected for age and sex. Therefore, estimates not statistically different from zero indicate that a predictor is associated with maintenance of bone mass expected for age and sex.
Results
The discovery cohort comprised 918 participants of European descent (female, N=481; Table 1). The males averaged 1.8 hours per day of total physical activity (71% reporting ≥1 hr/d), and the females averaged 1.5 hours per day of total physical activity(62% reporting ≥1 hr/d). The proportions of time spent in high impact physical activity and low impact physical activity were 42% and 58% in males, respectively; and 35% and 65% in females, respectively. Similar physical activity patterns were observed in the cross-sectional replication cohort comprising 486 participants (female, N=245; Supplementary Table 4).
Table 1.
Descriptive characteristics of the discovery cohort sample
| Baseline | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | |
|---|---|---|---|---|---|---|---|
| Total Sample, N | 918 | 823 | 791 | 657 | 656 | 605 | 514 |
| Original Cohort, N | 692 | 692 | 672 | 657 | 656 | 605 | 514 |
| Extension Cohort, N | 226 | 131 | 119 | ||||
| Female, N (%) | 481 (52.4) | 429 (52.1) | 408 (51.6) | 341 (51.9) | 344 (52.4) | 311 (51.4) | 274 (53.3) |
| Age, mean (SD), year | 11.1 (4.4) | 11.1 (3.5) | 12.0 (3.3) | 13.7 (3.1) | 14.7 (3.0) | 15.3 (2.7) | 15.6 (2.3) |
| Height, mean (SD), m | 1.4 (0.2) | 1.4 (0.2) | 1.5 (0.2) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.7 (0.1) |
| Weight, mean (SD), kg | 41.0 (18.6) | 41.1 (16.7) | 44.4 (16.7) | 51.8 (16.1) | 55.2 (15.4) | 57.9 (14.5) | 59.5 (12.6) |
| BMI, mean (SD), Z-score | 0.29 (0.8) | 0.26 (0.9) | 0.27 (0.9) | 0.25 (0.9) | 0.24 (0.9) | 0.24 (0.9) | 0.24 (0.9) |
| Dietary Calcium, mean (SD), g/d | 0.96 (0.5) | 0.94 (0.5) | 0.93 (0.5) | 0.91 (0.5) | 0.91 (0.6) | 0.88 (0.5) | 0.84 (0.5) |
| Puberty Stage, N (%) | |||||||
| Tanner Stage I | 451 (49.1) | 399 (48.5) | 326 (41.2) | 145 (22.1) | 99 (15.1) | 68 (11.2) | 106 (20.6) |
| Tanner Stages II–IV | 248 (27.0) | 227 (27.6) | 219 (27.7) | 209 (31.8) | 201 (30.6) | 178 (29.4) | 92 (17.9) |
| Tanner Stage V | 219 (23.9) | 197 (23.9) | 246 (31.1) | 303 (46.1) | 356 (54.3) | 359 (59.3) | 316 (61.5) |
| Total physical activity, mean (SD), hr/d | 1.64 (1.32) | 1.53 (1.22) | 1.79 (1.30) | 1.82 (1.15) | 1.81 (1.22) | 1.84 (1.26) | 1.63 (1.25) |
| - Male | 1.83 (1.45) | 1.73 (1.32) | 1.90 (1.41) | 1.89 (1.17) | 1.98 (1.32) | 2.02 (1.38) | 1.82 (1.43) |
| - Female | 1.47 (1.15) | 1.35 (1.09) | 1.69 (1.17) | 1.76 (1.14) | 1.65 (1.11) | 1.67 (1.11) | 1.47 (1.05) |
| ≥1 h/d total physical activity, % | 66.8 | 60.9 | 72.2 | 76.3 | 71.5 | 74.6 | 65.2 |
| - Male | 71.6 | 66.2 | 76.8 | 77.9 | 74.0 | 79.9 | 68.3 |
| - Female | 62.4 | 55.9 | 67.9 | 74.8 | 69.2 | 69.5 | 62.4 |
| High impact physical activity, mean (SD), % | 38.6 (29.9) | 40.3 (30.7) | 51.4 (28.2) | 50.9 (29.2) | 49.3 (29.1) | 47.0 (28.5) | 46.5 (31.4) |
| - Male | 42.4 (29.6) | 43.2 (29.7) | 53.2 (27.5) | 51.5 (29.2) | 51.4 (28.7) | 49.3 (27.4) | 46.3 (31.4) |
| - Female | 35.1 (29.9) | 37.6 (31.4) | 49.8 (28.8) | 50.4 (29.2) | 47.3 929.3) | 44.7 (29.4) | 46.6 (31.5) |
| Low Impact physical activity, mean (SD), % | 61.4 (29.9) | 59.7 (30.7) | 48.6 (28.2) | 49.1 (29.2) | 50.7 (29.1) | 53.0 (28.5) | 53.5 (31.4) |
| - Male | 57.6 (29.6) | 56.8 (29.7) | 46.8 (27.5) | 48.5 (29.2) | 48.6 (28.7) | 50.7 (27.4) | 53.7 (31.4) |
| - Female | 64.9 (29.9) | 62.4 (31.4) | 50.2 (28.8) | 49.6 (29.2) | 52.7 (29.3) | 55.3 (29.4) | 53.4 (31.5) |
Total physical activity was positively associated with average bone Z-scores (Table 2). This association was driven entirely by time spent in high impact physical activity; time spent in low impact physical activity was not associated with higher or lower bone Z-scores (Table 2). For example, each hour per day spent in high impact physical activity associated with 0.05 higher TBLH-BMC Z-score in males and females (95% confidence interval: 0.03, 0.06; P=4.0×10−10). The genetic risk score (% BMD lowering alleles) was associated with lower bone Z-scores (Table 2). For example, each 1% increase in the genetic risk score associated with 0.03 lower TBLH-BMC Z-score in males and females (95% confidence interval: −0.04, −0.02; P=1.3×10−6).
Table 2.
Physical activity and genetic risk score associations with bone Z-scores
| Males (N=428) | Females (N=476) | Males & Females (N=795) | ||||||
|---|---|---|---|---|---|---|---|---|
| Site | Model | Exposurea | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value |
| SpineaBMD | 1 | Genetic risk score | −0.02 (−0.04, −0.003) | 0.024 | −0.05 (−0.07, −0.03) | 4.6×10−8 | −0.04 (−0.05, −0.02) | 3.4×10−7 |
| 1 | Total Physical Activity | 0.02 (0.01, 0.04) | 0.006 | 0.01 (−0.003, 0.03) | 0.104 | 0.02 (0.01, 0.03) | 0.002 | |
| 2 | High Impact Physical Activity | 0.06 (0.03, 0.08) | 8.0×10−6 | 0.04 (0.01, 0.06) | 0.006 | 0.04 (0.02, 0.06) | 6.6×10−5 | |
| 2 | Low Impact Physical Activity | −0.01 (−0.03, 0.01) | 0.374 | −0.00 (−0.03, 0.02) | 0.698 | 0.00 (−0.02, 0.02) | 0.913 | |
| Total HipaBMD | 1 | Genetic risk score | −0.03 (−0.05, −0.01) | 0.007 | −0.06 (−0.08, −0.04) | 2.7×10−9 | −0.04(−0.06, −0.03) | 1.0×10−7 |
| 1 | Total Physical Activity | 0.02 (0.01, 0.04) | 0.002 | 0.01 (−0.002, 0.02) | 0.086 | 0.02 (0.01, 0.03) | 2.4×10−4 | |
| 2 | High Impact Physical Activity | 0.06 (0.04, 0.09) | 8.0×10−7 | 0.04 (0.02, 0.06) | 7.0×10−5 | 0.05 (0.03, 0.06) | 5.4×10−8 | |
| 2 | Low Impact Physical Activity | −0.01 (−0.03, 0.01) | 0.313 | −0.01 (−0.03, 0.005) | 0.148 | −0.00 (−0.02, 0.01) | 0.599 | |
| Femoral NeckaBMD | 1 | Genetic risk score | −0.03 (−0.05, −0.02) | 5.6×10−4 | −0.06 (−0.08, −0.04) | 7.4×10−9 | −0.04 (−0.06, −0.03) | 5.7×10−9 |
| 1 | Total Physical Activity | 0.03 (0.01, 0.04) | 3.3×10−4 | 0.01 (−0.01, 0.03) | 0.227 | 0.02 (0.01, 0.04) | 1.6×10−4 | |
| 2 | High Impact Physical Activity | 0.08 (0.05, 0.10) | 5.1×10−8 | 0.05 (0.03, 0.08) | 2.9×10−5 | 0.06 (0.04, 0.08) | 2.6×10−9 | |
| 2 | Low Impact Physical Activity | −0.01 (−0.03, 0.01) | 0.466 | −0.02 (−0.05, 0.003) | 0.083 | −0.01 (−0.03, 0.01) | 0.330 | |
| TBLH-BMC | 1 | Genetic risk score | −0.01 (−0.03, 0.002) | 0.094 | −0.04 (−0.05, −0.03) | 3.1×10−9 | −0.03 (−0.04, −0.02) | 1.3×10−6 |
| 1 | Total Physical Activity | 0.02 (0.01, 0.03) | 0.001 | 0.01 (−0.005, 0.02) | 0.192 | 0.02 (0.01, 0.03) | 3.3×10−4 | |
| 2 | High Impact Physical Activity | 0.06 (0.04, 0.08) | 5.5×10−8 | 0.04 (0.02, 0.06) | 6.8×10−6 | 0.05 (0.03, 0.06) | 4.0×10−10 | |
| 2 | Low Impact Physical Activity | −0.01 (−0.03, 0.002) | 0.202 | −0.02 (−0.04, 0.004) | 0.107 | −0.01 (−0.02, 0.01) | 0.219 | |
The estimates for the physical activity variables represent the change in bone Z-scores per additional hour per day spent physically active. The estimates for the genetic risk score represent the change in bone Z-score per additional 1% increase in the genetic risk score.
Genetic risk score, percentage of the number of lowering bone mineral density alleles carried at 63 loci identified in an adult genome wide association study(20).
High Impact Physical Activity, hours per day spent in weight bearing physical activity with a ground reaction force >2× body weight.
Low Impact Physical Activity, hours per day spent in non-weight bearing physical activity or weight bearing physical activity with a ground reaction force 1–2× body weight. All analyses involved participants in the longitudinal discovery cohort using linear mixed models; adjusted for age, puberty stage, body mass index Z-score, and dietary calcium intake (gender was included as a covariate in the male and females combined analyses with older sibling was removed). In model 1, total physical activity and genetic risk score were included with the covariates. In model 2, low and high impact physical activity, as well as genetic risk score, were included with the covariates. Abbreviations: TBLH-BMC, total body less head bone mineral content.
The total physical activity associations, driven by high impact physical activity, were evident even for children with below average bone Z-scores who may be at genetic risk for future bone fragility; these findings were derived from the quantile regression models (Figure 1 and Supplemental Figures 1 and 2). Further support that physical activity benefits the skeleton of all children, we found no statistical evidence that physical activity interacted with genetic risk scoreto influence bone Z-scores. Also, there was no evidence that physical activity associations differed by genetic risk score and pubertal stages to influence bone Z-scores (i.e. no physical activity-genetic risk score-maturation statistical interaction). Physical activity associations with each bone Z-score by genetic score tertiles are illustrated in Figure 2 to show the comparable physical activity associations across genetic susceptibility bone fragility groups.
Figure 1.
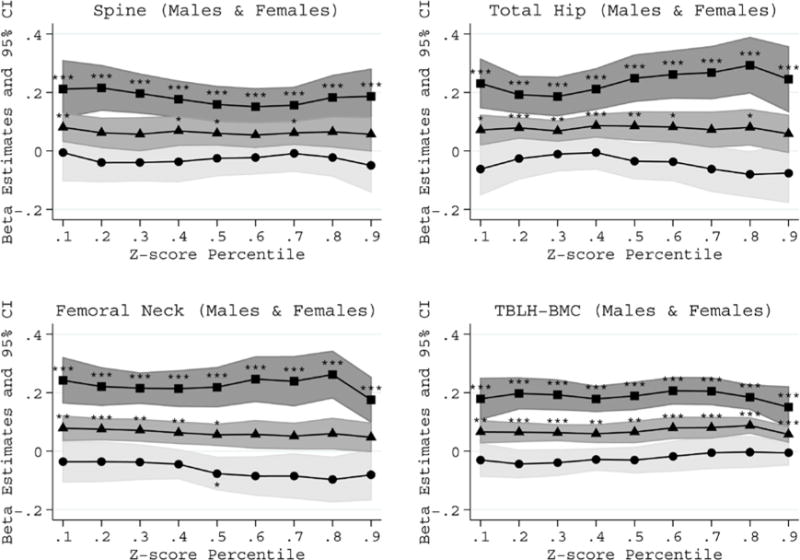
Physical activity associations with bone Z-score percentiles in males and females combined. The associations for total (triangles), high impact (squares) and low impact (circles) physical activity are presented. The percentiles were modeled using longitudinal quantile regression to represent below average (<50th percentile), average (50th percentile) and above average (>50th percentile) bone Z-scores. The models were puberty stage, body mass index Z-score and genetic risk score. The percentile specific estimates and 95% confidence intervals represent the change in Z-score for each 1-hour per day increase in physical activity. The estimates statistically different from zero are indicated: *P≤0.01, **P≤0.001 and ***P≤1.0×10−4.
Figure 2.
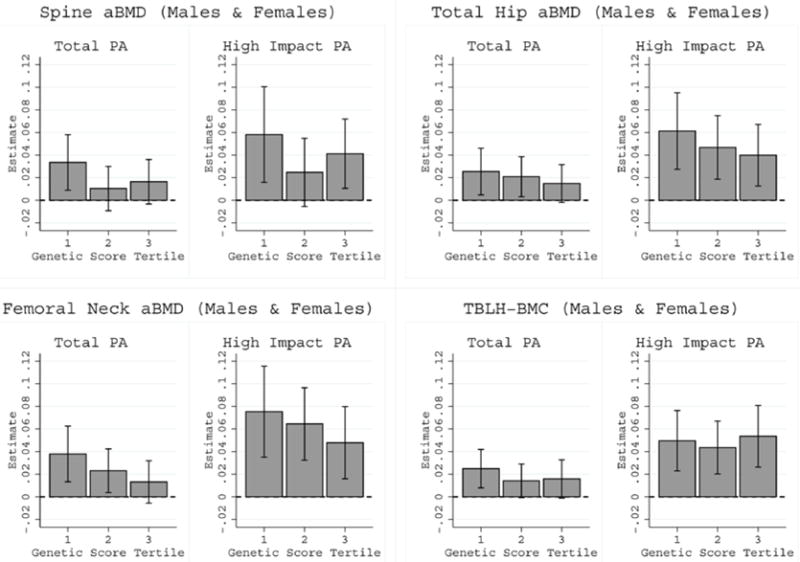
Comparable total and high impact physical activity associations with bone Z-scores by BMD genetic risk score tertiles in males and females combined. All physical activity-genetic score interaction P-values >0.05.
We next performed exploratory analyses that investigated physical activity interactions involving that the individual variants used to calculate the genetic risk score. A significant interaction between total physical activity and one variant was observed that passed correction for multiple testing. Specifically, total physical activity statistically interacted with rs2887571 (ERC1/WNT5B) to influence TBLH-BMC (Table 3). This interaction survived correction for multiple testing in males (P=7.1×10−5), was nominally significant in females (P=0.027), and was significant in males and females combined (P=6.7×10−4). Overall, among rs2887571 risk (BMC lowering) A allele homozygotes, total physical activity was positively associated with TBLH-BMC Z-score (estimate=0.03, P=8.2×10−6) and this was driven by high impact physical activity (Supplemental Table 6 and Supplemental Figure 3). However, this exploratory finding did not reach statistical significance in the cross-sectional replication cohort (Table 3). Note, using a more conservative multiple correction approach to account for the 4 skeletal sites, and for conducting analyses for both sexes combined and separately for males and females (P-interaction < 6.2×10−5 [0.05/804]), the total physical activity interaction with rs2887571 remains only in males. No other physical activity interactions with the individual variants passed correction for multiple testing. However, a number of nominally significant total physical activity-SNP interactions (P<0.05) were observed and these variants are listed in Supplemental Table 5.
Table 3.
Total physical activity interactions with a SNP near the ERC1/WNT5B locus and TBLH-BMC
| GG | AG | AA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Nearest Gene | Risk Allelea | Sex | Site | Cohort | P-interaction | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value |
| rs2887571 | ERC1/WNT5B | A | M | TB | Discovery (N=444) | 7.1×10−5 | −0.02 (−0.04, 0.002) | 0.069 | 0.01 (−0.005, 0.02) | 0.212 | 0.04 (0.02, 0.05) | 1.2×10−6 |
| Replication (N=241) | 0.999 | 0.02 (−0.22, 0.26) | 0.867 | 0.02 (−0.10, 0.14) | 0.734 | 0.02 (−0.10, 0.14) | 0.734 | |||||
| Both (N=685) | 6.0×10−5 | −0.02 (−0.04, 0.001) | 0.066 | 0.01 (−0.004, 0.02) | 0.204 | 0.04 (0.02, 0.05) | 8.1×10−7 | |||||
| rs2887571 | ERC1/WNT5B | A | F | TB | Discovery (N=485) | 0.027 | −0.03 (−0.08, 0.01) | 0.094 | −0.01 (−0.03, 0.01) | 0.409 | 0.02 (0.002, 0.03) | 0.029 |
| Replication (N=244) | 0.463 | −0.08 (−0.2, 0.09) | 0.505 | −0.02 (−0.10, 0.06) | 0.689 | 0.04 (−0.06, 0.14) | 0.571 | |||||
| Both (N=729) | 0.031 | −0.03 (−0.07, 0.01) | 0.104 | −0.01 (−0.03, 0.01) | 0.434 | 0.02 (0.002, 0.03) | 0.030 | |||||
| rs2887571 | ERC1/WNT5B | A | M+F | TB | Discovery (N=816) | 6.7×10−4 | −0.02 (−0.04, 0.004) | 0.109 | 0.01 (−0.005, 0.02) | 0.296 | 0.03 (0.02, 0.04) | 8.8×10−6 |
| Replication (N=484) | 0.853 | −0.003 (−0.17, 0.16) | 0.975 | 0.01 (−0.07, 0.09) | 0.858 | 0.02 (−0.06, 0.09) | 0.691 | |||||
| Both (N=1300) | 9.1×10−4 | −0.01 (−0.03, 0.004) | 0.132 | 0.01 (−0.004, 0.02) | 0.255 | 0.03 (0.01, 0.04) | 8.2×10−6 | |||||
The estimates represent the change in bone Z-scores per additional hour per day spent physically active, by rs2887571 genotype.
Risk allele was associated with lower bone mineral density in a genome-wide association study of adults(20).
Discovery: Analyses involving participants in the longitudinal discovery cohort using linear mixed models; Replication: Analyses involving participants in the cross-sectional replication cohort using linear regression; Both: Analyses involving participants in the discovery and replication cohorts using linear mixed models; all models were adjusted for age, puberty stage, body mass index Z-score, and dietary calcium intake (gender was included as a covariate in the male and females combined analyses; older sibling was removed for the male and female combined analyses). P-interactions in bold survive correction for multiple testing for 67 independent loci. Abbreviations: F, female; M, male; SNP, single nucleotide polymorphism; TB, total body less head bone mineral content.
We also conducted exploratory analyses that tested for statistical interactions between total physical activity, each SNP and puberty stage in order to determine whether associations between physical activity and bone Z-scores for a given variant were different according to puberty stage. Interactions involving two SNPs were statistically significant for total hip and femoral neck Z-scores in females: rs6959212 (STARD3NL) and rs9303521 (CRHR1) (Supplementary Table 7 and Supplementary Figures 4 and 5). However, these interaction P-values were not statistically significant in the cross-sectional replication cohort (Supplementary Table 7). Furthermore, using a more conservative multiple correction approach to account for the 4 skeletal sites, and for conducting analyses for both sexes combined and separately for males and females (P-interaction < 6.2×10−5 [0.05/804]), the interactions involving these two variants would not be considered statistically significant.
Discussion
Longitudinal studies(37), including BMDCS(4), and randomized controlled trials(3) have demonstrated the importance of physical activity to promote bone accretion and development of peak bone mass. Our longitudinal study is the first to investigate differential effects of physical activity across the spectrum of pediatric bone Z-scores, as well as physical activity-gene interactions on childhood bone Z-scores using adult GWAS-implicated bone density loci. We found that total physical activity, driven by high impact physical activity, was positively associated with bone Z-scores, and genetic risk score was negatively associated with childhood bone Z-scores. Interestingly, the physical activity benefits included children with lower than average bone Z-scores, as well as children genetically predisposed to lower adult BMD (i.e. no physical activity-genetic risk score interactions). Furthermore, in our exploratory analyses we observed a statistical interaction between total physical activity and a SNP near the ERC1/WNT5B locus. Importantly, total physical activity and high impact physical activity associations with TBLH-BMC were beneficial for children carrying the BMD lowering risk allele at this locus. Therefore, U.S. children genetically predisposed to lower adult BMD as defined by GWAS-implicated variants should aim to meet the physical activity guidelines and increase their high impact physical activity to enhance or maintain age-appropriate bone accretion(38). This conclusion is reinforced with our other exploratory observations in females that some total physical activity-gene interactions varied across puberty stage in females, but high impact physical activity was associated with maintenance or higher bone Z-scores regardless of genetics or puberty stage.
Previous related studies adopted cross-sectional study designs, investigated candidate bone density genes primarily in adult populations, and found little to no evidence that physical activity interacted with these loci to influence aBMD in adults(6,9–13). Importantly, these candidate loci have not (yet) been associated with aBMD in GWAS(20), except for common variation near LRP5 in adult populations(16,20). LRP5 is a co-receptor in the WNT signaling pathway, which is critical for mechanosensation(39). Kiel et al. found physical activity interactions with SNPs near the LRP5 locus for spine aBMD in adult males(8). We did not replicate this finding in children. However, in our exploratory analyses we observed nominal evidence of statistical interactions with other WNT signaling loci (WNT5B, WNT16, AXIN1, SOST and STARD3NL). Our most robust total physical activity interaction involved the WNT5B locus and TBLH-BMC. These associations are biologically plausible because of the role of the WNT signaling pathway in mechanosensitivity of the skeleton, as supported by mechanical loading studies of animals with Lrp5 and Sost mutations(39,40).
We previously reported significant sex differences involving some of the 67 GWAS implicated adult BMD loci with childhood bone outcomes(21) and that the genetic risk score effect was stronger and more consistent in females(22). Here we further observed in our exploratory analyses that some physical activity interactions were sex-specific. For example, among females we observed statistically significant interactions involving total physical activity, puberty stage and SNPs near the STARD3NL (WNT signaling pathway) and CRHR1 loci with regard to hip aBMD Z-scores. The observed sex and pubertal stage specific interactions could be due to male and female preferences for types of physical activities along with changes in physical activity levels from early to late maturation(41). Indeed, females spent less time in high impact physical activity compared to males. Variation in sex hormones contributing to bone accretion could also affect the sex and pubertal stage specific associations(5), as could sex and pubertal stage differences in lean mass(42). Improving our understanding of these sex differences and physical activity effects on bone is in line with current NIH priorities(43).
The medical and public health fields have an important role to play in promoting physical activity guidelines for Americans(38,44). Based on our findings, future physical activity guidelines should consider the proportion of high versus low impact physical activity for bone health. Indeed, for children spending 1 hour per day in total physical activity, we estimate that bone Z-scores would be 0.06 greater if 100% were dedicated to high impact physical activity versus 100% low impact physical activity. In cumulative terms, a female between ages 10 and 16 years could move from the 5th to the 10th percentile, without changing her total physical activity, but increasing her proportion of high impact physical activity. Furthermore, with advances in precision medicine, genetic predisposition data will one-day be routinely used in pediatric practice. We tested if children genetically predisposed to bone fragility in adulthood, based on GWAS-implicated BMD variants, responded to physical activity exposure. Based on our results, pediatricians at this time can recommend high impact physical activity to aid life-long bone health, in generally healthy children, even if they carry a large proportion of GWAS-implicated BMD lowering alleles. However, this recommendation should be continuously assessed as our understanding of the genetic regulation of the developing skeleton advances; our study findings can only be interpreted in the context of GWAS-implicated BMD variant; future studies could identify genetic variants that do impact the responsiveness of the pediatric skeleton to physical activity exposure.
Our study has several strengths. Our longitudinal study design spanned early to late maturation. We used rigorously acquired aBMD and BMC-Z measures from multiple skeletal sites and applied quantile regression to investigate physical activity associations for children with below average bone Z-scores. Our study also has some limitations. The GWAS-implicated variants only partly explain the variance in adult BMD (≈6%)(20), and our study cannot rule out that other genetic variants could influence how the pediatric skeleton responds to physical activity exposure. Our physical activity questionnaire allowed for estimation of high and low impact physical activity. While accelerometry offers a more objective approach to estimating physical activity, it is important to note that accelerometry is unable to accurately estimate physical activity while performing certain non-weight bearing activities (e.g. cycling and swimming). Nonetheless, it will be important to replicate our findings using other physical activity methods, including accelerometry. Other factors, such as calcium intake, may modulate the effect of physical activity on bone accretion(45). In addition, we did not have vitamin D data to test if insufficiency states affected our results. Our findings can only be generalized to U.S. children of European descent. We only included participants of European ancestry due to the fact that the BMD lowering alleles we include in our study have only been established to date to yield associations in populations of European ancestry and may well not tag the underlying causative variants among other ethnicities(20). As such, it will be important to conduct this research in other ancestry groups using optimal tag-SNPs for each ethnicity in future studies. Follow-up studies using other modalities to estimate additional bone phenotypes are needed. Finally, our replication cohort included cross-sectional data; repeated measures would have conferred additional statistical power.
In conclusion, the benefits of physical activity on bone accretion apply to children with below average aBMD/BMC and to those who carry a large proportion of GWAS-implicated BMD lowering alleles. Children should be encouraged to increase their total physical activity and dedicate a greater proportion of their time to high impact physical activity to enhance or maintain age appropriate bone accretion.
Supplementary Material
Acknowledgments
The study was funded by R01 HD58886; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contracts (N01-HD-1-3228, -3329, -3330, -3331, -3332, -3333); and the CTSA program Grant 8 UL1 TR000077. Jonathan Mitchell was supported by K01 HL123612. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We appreciate the dedication of the study participants and their families, and the support of Dr. Karen Winer, Scientific Director of the Bone Mineral Density in Childhood Study.
Study conception and design: JM, SG and BZ. Acquisition of data: SG, BZ, HK, JL, VG, SO and JS. Data analysis: JM and AC. Interpretation of data: JM, AC, OE, SM, SR, HK, JL, VG, SO, JS, AK, BS and SG. Drafting manuscript: JM. Revising manuscript content: AC, OE, SM, SR, HK, JL, VG, SO, JS and AK. Approving final version of manuscript: JM, AC, OE, SM, SR, HK, JL, VG, SO, JS, AK, BS and SG. JM takes full responsibility for the integrity of the data analysis.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi:[10.1002/jbmr.2872]
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golden NH, Abrams SA. Optimizing bone health in children and adolescents. Pediatrics. 2014;134(4):e1229–43. doi: 10.1542/peds.2014-2173. [DOI] [PubMed] [Google Scholar]
- 3.Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res. 2014;29(2):467–78. doi: 10.1002/jbmr.2036. [DOI] [PubMed] [Google Scholar]
- 4.Lappe JM, Watson P, Gilsanz V, et al. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J Bone Miner Res. 2015;30(1):156–64. doi: 10.1002/jbmr.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. Br J Sports Med. 2002;36(4):250–7. doi: 10.1136/bjsm.36.4.250. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentil P, de Lima Lins TC, Lima RM, et al. Vitamin-d-receptor genotypes and bone-mineral density in postmenopausal women: interaction with physical activity. J Aging Phys Act. 2009;17(1):31–45. doi: 10.1123/japa.17.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Lorentzon M, Eriksson AL, Nilsson S, Mellstrom D, Ohlsson C. Association between physical activity and BMD in young men is modulated by catechol-O-methyltransferase (COMT) genotype: the GOOD study. J Bone Miner Res. 2007;22(8):1165–72. doi: 10.1359/jbmr.070416. [DOI] [PubMed] [Google Scholar]
- 8.Kiel DP, Ferrari SL, Cupples LA, et al. Genetic variation at the low-density lipoprotein receptor-related protein 5 (LRP5) locus modulates Wnt signaling and the relationship of physical activity with bone mineral density in men. Bone. 2007;40(3):587–96. doi: 10.1016/j.bone.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari SL, Karasik D, Liu J, et al. Interactions of interleukin-6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the Framingham Osteoporosis Study. J Bone Miner Res. 2004;19(4):552–9. doi: 10.1359/JBMR.040103. [DOI] [PubMed] [Google Scholar]
- 10.Blanchet C, Giguere Y, Prud’homme D, Dumont M, Rousseau F, Dodin S. Association of physical activity and bone: influence of vitamin D receptor genotype. Med Sci Sports Exerc. 2002;34(1):24–31. doi: 10.1097/00005768-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa I, Kitagawa Y, Nagaya T, Tokudome S. Interplay of physical activity and vitamin D receptor gene polymorphism on bone mineral density. J Epidemiol. 2001;11(5):229–32. doi: 10.2188/jea.11.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salamone LM, Glynn NW, Black DM, et al. Determinants of premenopausal bone mineral density: the interplay of genetic and lifestyle factors. J Bone Miner Res. 1996;11(10):1557–65. doi: 10.1002/jbmr.5650111024. [DOI] [PubMed] [Google Scholar]
- 13.Jarvinen TL, Jarvinen TA, Sievanen H, et al. Vitamin D receptor alleles and bone’s response to physical activity. Calcif Tissue Int. 1998;62(5):413–7. doi: 10.1007/s002239900453. [DOI] [PubMed] [Google Scholar]
- 14.Li X, He GP, Zhang B, Chen YM, Su YX. Interactions of interleukin-6 gene polymorphisms with calcium intake and physical activity on bone mass in pre-menarche Chinese girls. Osteoporos Int. 2008;19(11):1629–37. doi: 10.1007/s00198-008-0613-3. [DOI] [PubMed] [Google Scholar]
- 15.Suuriniemi M, Mahonen A, Kovanen V, et al. Association between exercise and pubertal BMD is modulated by estrogen receptor alpha genotype. J Bone Miner Res. 2004;19(11):1758–65. doi: 10.1359/JBMR.040918. [DOI] [PubMed] [Google Scholar]
- 16.Richards JB, Rivadeneira F, Inouye M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–12. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, et al. New sequence variants associated with bone mineral density. Nat Genet. 2009;41(1):15–7. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 18.Rivadeneira F, Styrkarsdottir U, Estrada K, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan EL, Danoy P, Kemp JP, et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 2011;7(4):e1001372. doi: 10.1371/journal.pgen.1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JA, Chesi A, Elci O, et al. Genetics of Bone Mass in Childhood and Adolescence: Effects of Sex and Maturation Interactions. J Bone Miner Res. 2015;30(9):1676–83. doi: 10.1002/jbmr.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell JA, Chesi A, Elci O, et al. Genetic Risk Scores Implicated in Adult Bone Fragility Associate With Pediatric Bone Density. J Bone Miner Res. 2016;31(4):789–95. doi: 10.1002/jbmr.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakonarson H, Grant SF, Bradfield JP, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448(7153):591–4. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 26.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, et al. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358(22):2355–65. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- 27.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC., Jr Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6(11):1227–33. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 28.Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–73. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 31.Zachmann M, Prader A, Kind HP, Hafliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29(1):61–72. [PubMed] [Google Scholar]
- 32.Ollberding NJ, Gilsanz V, Lappe JM, et al. Reproducibility and intermethod reliability of a calcium food frequency questionnaire for use in Hispanic, non-Hispanic Black, and non-Hispanic White youth. J Acad Nutr Diet. 2015;115(4):519–27 e2. doi: 10.1016/j.jand.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. In: Peadiatric bone biology and diseases. Glorieux F, Pettifor J, Juppner H, editors. San Diego: Academic Press; 2003. [Google Scholar]
- 34.Chesi A, Mitchell JA, Kalkwarf HJ, et al. A trans-ethnic genome-wide association study identifies gender-specific loci influencing pediatric aBMD and BMC at the distal radius. Hum Mol Genet. 2015;24(17):5053–9. doi: 10.1093/hmg/ddv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyerlein A. Quantile regression-opportunities and challenges from a user’s perspective. Am J Epidemiol. 2014;180(3):330–1. doi: 10.1093/aje/kwu178. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Pere A, Koenker R, He X. Quantile regression methods for reference growth charts. Stat Med. 2006;25(8):1369–82. doi: 10.1002/sim.2271. [DOI] [PubMed] [Google Scholar]
- 37.Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM. Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med. 2014;48(13):1032–6. doi: 10.1136/bjsports-2014-093574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Services UDoHaH, editor. Services UDoHaH. 2008 Physical Activity Guidelines for Americans. 2008. [Google Scholar]
- 39.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 40.Morse A, McDonald M, Kelly N, et al. Mechanical Load Increases in Bone Formation via a Sclerostin-Independent Pathway. J Bone Miner Res. 2014;29(11):2456–67. doi: 10.1002/jbmr.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nader PR, Bradley RH, Houts RM, McRitchie SL, O’Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300(3):295–305. doi: 10.1001/jama.300.3.295. [DOI] [PubMed] [Google Scholar]
- 42.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98(1):49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr. 2013;167(7):595–6. doi: 10.1001/jamapediatrics.2013.2144. [DOI] [PubMed] [Google Scholar]
- 45.Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res. 2003;18(5):885–92. doi: 10.1359/jbmr.2003.18.5.885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


