Abstract
Newborn screening (NBS) is intended to identify congenital conditions prior to the onset of symptoms in order to provide early intervention that leads to improved outcomes. NBS is a public health success, providing reduction in mortality and improved developmental outcomes for screened conditions.. However, it is less clear to what extent newborn screening achieves the long-term goals relating to improved health, growth, development and function. We propose a framework for assessing outcomes for the health and well-being of children identified through NBS programs. The framework proposed here, and this manuscript, were approved for publication by the Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC). This framework can be applied to each screened condition within the Recommended Uniform Screening Panel (RUSP), recognizing that the data elements and measures will vary by condition. As an example, we applied the framework to sickle cell disease and phenylketonuria (PKU), two diverse conditions with different outcome measures and potential sources of data. Widespread and consistent application of this framework across state NBS and child health systems is envisioned as useful to standardize approaches to assessment of outcomes and for continuous improvement of the NBS and child health systems.
Keywords: newborn screening, long-term follow-up, outcomes, quality improvement
Introduction
The purpose of public health newborn screening (NBS) is to identify children with specific congenital disorders prior to the onset of symptoms and, through prompt initiation of monitoring and treatment, to assure best possible health outcome for affected individuals (1). Steps taken by state-based NBS programs in the United States (U.S.) include sample collection and delivery; analysis and result reporting; referral for diagnostic confirmation; and assurance of the initiation of specialty care and services. A broader NBS system exists which variably engages primary and specialty clinics and diverse stakeholders such as families and community-based groups to provide prompt intervention and long-term management and to obtain services to support the health, growth, development and function of children with special health care needs(2, 3). The extent to which these multiple entities provide comprehensive services is variable and often limited by a paucity of resources, by divided or unclear responsibilities, competing priorities or incomplete clarity about child health quality measures and care (3–5).
Useful monitoring to optimize the outcomes of public health programs requires the selection of appropriate targets. Stakeholders for the NBS and child health systems need to identify appropriate targets and then select measures to track progress towards these outcomes, with an emphasis on achieving optimal child health and development. The NBS and child health systems will then need to engage in continuous, system-wide quality improvement to make this progress. The aim of this paper is to present a framework for defining specific health outcomes and processes to appropriately assess overall health and well-being of children identified through NBS. To illustrate application of the framework, we tested its use for two distinct screened conditions included in the Recommended Uniform Screening Panel (RUSP), sickle cell disease and phenylketonuria (PKU). Each condition has different impact measures. The types and sources of data will vary by condition, providing an opportunity to test the framework’s application across conditions to assess child health and other outcomes in the U.S.; other nations would have other data sources, e.g. centralized national registries.
Developing the framework
The U.S. Department of Health and Human Services Discretionary Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders of Newborns and Children (ACHDNC) charged its Follow-Up and Treatment Subcommittee (FUTR) to consider how to assess whether the NBS system is meeting its goals by developing a systematic approach to answer that question across the screened conditions. The FUTR identified necessary outcome measure categories (6). Members of the FUTR, in consultation with other stakeholders (see authorship and ACHDNC membership http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/about/index.html), identified potential data elements and data sources to create a draft framework. The framework presented here can be used to identify the appropriate goals, measures and data sources to assess successful outcomes for each condition. The framework proposed here, and this manuscript, were approved for publication by the ACHDNC.
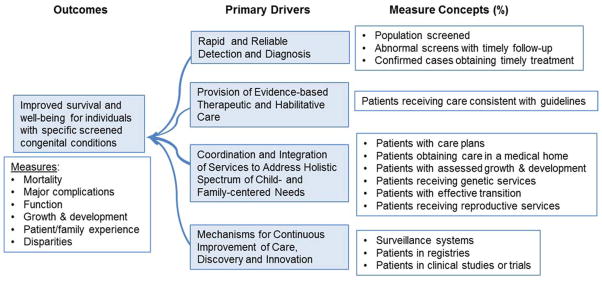
The framework is compatible with a driver-diagram model (Figure) (7). The model identifies the [FIGURE] primary drivers, i.e. system components that contribute directly to outcomes. The driver diagram model focuses efforts on the specific key outcomes of NBS, and the necessary processes at a conceptual level to achieve these outcomes, making it a useful tool for future efforts in quality assessment and improvement. The driver diagram is informed by the attributes and overarching questions that define long-term follow-up for newborn screening (1, 6). For the current application, optimal physical, developmental and social outcomes for children with NBS conditions are the specific aims, with the primary drivers identified as the factors or system components needed to achieve that aim.
Figure.
The driver diagram establishes the elements and primary goals needed to attain optimal outcomes for children diagnosed through public health newborn screening.
Consistent with the work of Kemper et al. (2008) and Hinton et al. (2011), the four primary drivers proposed are: 1) rapid and reliable detection and diagnosis, 2) provision of evidence-based therapeutic and habilitative care, 3) coordination and integration of services to address holistic spectrum of child and family centered needs and a mechanism for continuous improvement of care, and 4) discovery and innovation. The driver diagram points to the domains of measurement. The aim is reflected by outcome measures, which in this case ought to: a) assess the traditional metrics of mortality and morbidity, proxy measures such as preventable health care utilization (hospitalizations, emergency department visits), measures of harm associated with treatment, developmental and social-emotional outcomes, and family experience of care; and b) identify potential disparities in these outcomes among individuals or groups.
The framework
The framework was designed as a table identifying key measures to assess NBS outcomes, with general categories shared by all RUSP conditions populated by specific condition-specific elements. Within each category for each condition, specific measures will need to be defined so as to assess the number and proportion of children diagnosed by NBS with regular health assessment and screening and the reduction of complications associated with each condition (Table 1, columns 2–4)(8). Finally, potential key attributes of a NBS and long-term follow-up system addressed by each aim and goal are proposed (Table 1, column 5).
TABLE 1.
A framework for defining and assessing the goals of newborn screening, by screened condition. Here, sickle cell disease is used as an example, with potential goals and measures populating the table. Data sources may vary by condition.
| CATEGORICAL UNIVERSAL AIM (All Conditions) | CONDITION SPECIFIC GOAL (SCD as an example) | POTENTIAL MEASURE (SCD as an example) | POTENTIAL DATA SOURCES (SCD as an example) | KEY ATTRIBUTE (SCD as an example) |
|---|---|---|---|---|
| Rapid and Reliable Detection and Diagnosis | ||||
| Condition detected by NBS | Universal detection | #/of HbSS, HbSC, HbS-beta thalassemia detected at birth | State NBS programs, RuSH | Universal screening performed |
| Condition confirmed and diagnosed | Prompt confirmation with definitive diagnosis | % infants with confirmed diagnoses before 2 months of age - Condition sub-type confirmed (e.g., HbSS, HbSC, other variants) |
State NBS programs, RuSH | Diagnosis through universal screening |
| Provision of Evidence-based Care | ||||
| Prevention of major disease-related mortality and morbidities | Prevention of disease-related mortality | Number and age of childhood deaths | State mortality data, RuSH | Evidence-based treatment |
| - Early initiation of PCN prophylaxis - Continuous prescription of PCN prophylaxis |
▪ #/% infants prescribed PCN by 3 months of age▪ #/% children younger than age 5 continuously prescribed PCN | NBS programs, RuSH HRSA-supported surveys |
Evidence-based treatment | |
| Appropriate immunizations related to loss of splenic function | ▪ #/% children completing pneumococcal immunizations by age 3 ▪ #/% children completing meningococcal immunizations by certain age ▪ #/% children ages 1–21 receiving annual immunization for influenza ▪ #/% children with significant pneumococcal infection |
State and local vaccine databases, RuSH, HRSA-supported surveys Medical claims database |
Evidence-based treatment | |
| ▪ Prevention of stroke ▪ Prevention of acute and chronic disease- related pulmonary disease |
▪ #/% children with strokes ▪ #/% children with acute chest syndrome, pulmonary hypertension, chronic hypoxemia or other |
Medical claims database Clinical database |
Evidence-based treatment | |
| ▪ Stroke risk initial assessment ▪ Stroke risk annual assessment |
▪ #/% children at age 2 who have had TCD | RuSH HRSA-supported surveys |
Evidence-based treatment | |
| ▪ Pulmonary initial assessment ▪ Pulmonary annual assessment |
▪ #/% children age X and older who have had annual TCD ▪ #/% children at age 5 who have had PFTs, O2 saturation ▪ #/% children age X and older who have had annual PFTs, O2 saturation |
Medical claims database | ||
| Prevention of iron overload | #/% children on chronic transfusion therapy assessed for iron burden annually | Medical claims database Clinical database |
||
| - Initiation of disease modifying therapy* (hydroxyurea, chronic transfusion, transplant) - Continuation of disease modifying Rx* |
- #/% children age 2 currently on a disease-modifying therapy - #/% children age 10 currently on a disease- modifying therapy |
RuSH HRSA-supported surveys; Insurance database, patient registries (consumer-driven, medical care and private sector) |
Evidence-based treatment | |
| Use of disease modifying therapies* (hydroxyurea, chronic transfusion, transplant) when transitioning to adolescent and adult care | % children at age 16 on disease-modifying therapy | RuSH HRSA-supported surveys Insurance database, registries (see above) |
Evidence-based treatment | |
| Growth and development | Growth | Weight, BMI: - 1-year-olds - 5-year-olds |
NBS program follow-up Clinical database |
Evidence-based treatment |
| Educational/functional performance (Grade level for age, employment status) | ▪ Grade level X at age X ▪ High school diploma or GED by age 25* - #/% employed or in school (5 year assessments) |
Public health Department of Education databases | Evidence-based treatment | |
| Coordination and Integration of Services | ||||
| Patient-centered engagement and satisfaction | Family experiences family-centered care | ▪ Rating of experience of care; - Rating of involvement in decision making #/% of patients with care plan incorporating patient/family goals |
HRSA-NICHQ Surveys CAHPS RuSH |
Care coordination through medical home |
| Primary care provider | Primary care provider informed by state | #/% diagnosed infants with a primary care provider identified by age 1 month - At least 1 visit documented by age 2 months |
NBS programs, HRSA-supported surveys | Care coordination through medical home |
| Regular primary care | #/% infants receiving regular care by primary care provider during the 1st year of life | Medical claims database Vaccine database |
Care coordination through medical home | |
| Specialty care provider | Specialist informed by state | #/% diagnosed infants with specialty medical care identified by age 1 month - At least 1 specialist visit documented by age 3 months |
NBS programs, HRSA-supported surveys | Care coordination through medical home |
| Regular specialty care | #/% infants receiving care by specialty provider during the 1st year of life | Medical claims database | Care coordination through medical home | |
| Genetic services | Counseling and SC gene assessment for hemoglobinopathies provided by genetic or hemoglobinopathy counselor | #/% families receiving genetic counseling by a certified counselor | HRSA-supported surveys | Care coordination through medical home |
| Trait assessment and counseling for parents* | #/% of families identified with one or more children with SC trait in which parents know their trait status | RuSH HRSA-supported surveys |
Care coordination through medical home | |
| Genetic services for the population of at- risk adolescents | #/% of adolescents in the community who know their trait status and have received anticipatory care | HRSA-supported surveys | Care coordination through medical home | |
| Other Community Resources | Family put in contact with necessary community resources | #/% families receiving information about community resources | HRSA-supported surveys | Care coordination through medical home |
| Continuous Improvement of Care, Discovery and Innovation | ||||
| Patients enrolled in registries | #/% of patients enrolled in condition registries | To be determined | New knowledge discovery | |
| Patients enrolled in clinical studies or trials | #/% of patients enrolled in clinical studies or trials | To be determined | New knowledge discovery | |
| Demonstrated improvements in care | # critical care processes with statistically meaningful improvements in past 12 months | Clinical records | Continuous improvement | |
| Demonstrated improvements in outcomes | # outcomes showing statistically meaningful improvements in past 24 months | Clinical, educational and public health records | Continuous improvement | |
Health People 2020 Goal (http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=4)
Abbreviations:
BMI: Body Mass Index
CAHPS: Consumer Assessment of Healthcare Providers and Systems
DHPR: Dihydropteridine Reductase
GED: General Education Development
Hb SC: Hemoglobin SC (trait)
Hb SS: Hemoglobin SS (Sickle cell anemia)
HRSA: Health Resources and Services Administration
NBS: newborn screening
NICHQ: National Institute for Children’s Health Quality
O2: oxygen
OFC: occipitofrontal circumference
PCN: penicillin
PFT: pulmonary function test
RBC: red blood cell
RuSH: Registry and Surveillance for Hemoglobinopathies
Rx: prescription
TCD: Trans-cranial Doppler
Primary Outcomes of Care
The goals of comprehensive care are both the prevention of major disease-related mortality and morbidity and the attainment of optimal health. While specific complications and interventions vary among NBS conditions, each will have specific surrogate measures that can be used to demonstrate success. In the case of sickle cell disease (Table 1), to illustrate how the framework can be used, we populated the table with potential indicators of the former include the number of children with the specific complications of stroke, acute chest syndrome or other lung disease, or iron overload from transfusion therapy. The percentage of children demonstrating normal growth and development, are examples of indicators of the attainment of optimum health. These health measures are only suggestions to illustrate potential utility of the framework as applied to specific screened conditions. Final selection of actual health measures are beyond the scope of this paper.
Detection and Diagnosis
Measures of detection and diagnosis would typically include timely detection of the condition by newborn screening and the use of appropriate confirmatory testing for diagnosis. In the case of sickle cell disease, timely detection involves analysis of hemoglobins (e.g., electrophoresis) or DNA testing, which should be completed so that prophylactic penicillin is started by 2 months of age (9). This approach has significantly reduced infant mortality (10). Each of the other NBS conditions has comparable interventions to justify the pre-symptomatic detection at birth. For some conditions, tracking infants who are diagnosed through newborn screening, yet are clinically unaffected, will be necessary and require an additional data element.
Linkage to Services and Care
The immediate benefit of NBS is the timely initiation of evidence-based interventions. In our framework example, one such intervention would include the provision of prophylactic penicillin for infants with sickle cell disease. Linkages to services and care extend beyond a single intervention to include access to primary and subspecialty services and other resources to achieve primary outcomes of care. Many youth need special accommodation in school, and families typically benefit from linkage with other families and community-based organizations. Family-centered engagement and satisfaction with the experience of care are other important measures of whether a patient and family are connected with an integrated and inclusive health care system.
Provision of Evidence-Based Care
Measures of the provision of evidence-based care would assess the timing of appropriate treatment and the consistency of treatment with evidence-based recommendations, including screening for preventable complications. Each NBS condition has a specific set of essential interventions and services that is associated with reduction in morbidity and/or mortality. For example, metrics reflecting essential elements of evidence-based care for individuals with sickle cell disease should capture treatment with penicillin, appropriate and timely immunizations, screening for stroke risk with transcranial Doppler ultrasound, monitoring of growth and development, and receipt of coordinated care in a medical home with the capacity to manage acute disease-related complications. Another metric of provision of evidence-based care should track use of disease-modifying therapies such as hydroxyurea therapy, which is known to positively affect the course of sickle cell disease (11, 12).
Mechanism for continuous improvement of care, discovery and innovation
Monitoring is a key public health function; measurement is also an essential element of high performing health care systems. Measures related to this driver could include whether a registry or other surveillance system is in place, the proportion of children with a condition in that registry, the timeliness and accuracy of that information, and the extent to which the data are used for quality improvement and discovery.
Applying the framework to other conditions
The FUTR explored the suitability of the framework for other conditions by applying it to PKU (Table 2). PKU was selected because it was the first NBS condition to be screened for in the United States and it would therefore be of interest to consider availability of data sources for outcomes. In addition, PKU has a very different clinical presentation and different outcomes of interest from sickle cell disease, with less emphasis on health outcomes and more on developmental outcomes. As a result of the exercise in applying the framework to PKU, the headings and other elements of the table were refined. The framework proved to be applicable for PKU, especially with a data source specifically designed for inborn errors of metabolism, the Inborn Error of Metabolism Information System (IBEM-IS) (13).
TABLE 2.
A framework for defining and assessing the goals of NBS, by screened condition. Here, phenylketonuria (PKU) is used as an example. Data sources will vary by condition.
| CATEGORICAL UNIVERSAL AIM, (All Conditions) |
CONDITION SPECIFIC GOAL (PKU as an example) |
POTENTIAL MEASURE (PKU as an example) |
POTENTIAL Data Sources (PKU as an example) |
Key ATTRIBUTE (PKU as an example) |
|---|---|---|---|---|
| Rapid and Reliable Detection and Diagnosis | ||||
| Condition detected by NBS | Universal detection | Newborn screening performed -Date first newborn screen collected -Phe on first screen -Tyr on first screen |
State programs NewSTEPS IBEM-IS | Universal screening |
| Condition confirmed and diagnosed | Accurate diagnosis | Diagnostic labs performed: -Blood spot -phenylalanine -Plasma amino acid profile -Plasma tyrosine -Urine organic acids -Blood spot tyrosine -Plasma phenylalanine -RBC (DHPR) activity -Urine pterins |
IBEM-IS NewSTEPs |
Universal diagnosis through screening |
| Rapid initiation of treatment | -Days of age from birth until intervention for this condition -Days of age from birth until first seen by subspecialist -Days of age at initiation of phenylalanine restriction -Days of age (after dietary phenylalanine restriction was initiated) at time first phenylalanine level was documented below 360 μmol/L (6 mg/dL) |
NewSTEPS IBEM-IS Medication-specific registries |
Universal diagnosis through screening | |
| Provision of Evidence-based Care | ||||
| Growth and development | Maintenance of normal growth | Length, weight, OFC, BMI at visits | Clinics IBEM-IS |
Evidence-based treatment |
| Maintenance of normal development | Full scale IQ obtained | IBEM-IS Medication-specific registries Parent-report registry |
Evidence-based treatment | |
| Continuing developmental assessment | -Developmental assessment at visits, who did and how – collects information if formal neuropsychometrics done -Assessment of developmental/intellectual, executive functioning, behavioral/emotional and adaptive skills and if referral made -Early Intervention Services: occupational therapy, physical therapy, speech- language therapy -Educational evaluation–type of special education services (Individualized Educational Program or 504 plan and nature of disabilities) |
IBEM-IS | Evidence-based treatment | |
| Educational/functional performance (Grade level for age, special services, employment status) | -Grade level X at age X -High school diploma or GED by age 25* - employed or in school |
Public health Department of Education databases IBEM-IS |
Evidence-based treatment | |
| Maintenance of normal nutritional status | -dietary intake -nutrient analysis, - nutrition counseling/ education -What medical food is prescribed? -How much? -Who pays? -How much phenylalanine prescribed? |
Clinics IBEM-IS |
Evidence-based treatment | |
| Genotype-based facilitation of pharmacologic management | Genotype | Clinics IBEM-IS |
Evidence-based treatment | |
| Other therapies | Optimization of management | Use of sapropterin Payment source for medications |
IBEM-IS, Medication-specific registries | Evidence based treatment |
| Coordination and Integration of Services | ||||
| Primary care provider | Patients need a medical home | Patient has primary provider (name) Distance to primary provider Receiving preventive care Immunizations Sick visits since last visit Had anesthesia? Had surgery? Complications of surgery? |
IBEM-IS Public Health LTFU |
Care coordination through a medical home |
| Metabolic care provider | Patients need access to appropriate specialty care | -Patient has had an outpatient metabolic visit -Date of last outpatient metabolic visit -Other provider seen at metabolic care visits, -Missed visits |
IBEM-IS | Care coordination through a medical home |
| Other specialty services/providers | Patients need access to appropriate specialty care | Other specialty providers seen since last visit | IBEM-IS | Care coordination through a medical home |
| Genetic counseling services | Patients should have genetic counseling | Genetic counseling provided Provider of genetic counseling |
IBEM-IS | Care coordination through a medical home |
| Other community resources | Patients require access to community resources | Receiving services (specifies what services) | IBEM-IS | Care coordination through a medical home |
| Patient-centered engagement and satisfaction | Access to care | -Locations of services -Distances to services -Type of service (e.g., telemedicine, in person) |
IBEM-IS | Care-coordination |
| Patient has coverage | Patients need insurance coverage | [Captures coverage type, list of options: unknown, commercial/private, military, none, self-pay, state program (non-Medicaid/Medicare), state/federal program (Medicaid/Medicare),other] | IBEM-IS | Care coordination through a medical home |
| Pregnancy | Prevention of maternal PKU syndrome | [special data collection tool used for pregnancy progress] | IBEM-IS | Evidence based treatment |
| Continuous Improvement of Care, Discovery and Innovation | ||||
| Engagement in research | Patient enrolled in study Which study? |
IBEM-IS | New knowledge generation | |
Health People 2020 Goal (http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=4)
Abbreviations:
BMI: Body Mass Index
DHPR: Dihydropteridine Reductase
IBEM-IS: Inborn Errors of Metabolism – Information System
IQ: Intelligence Quotient
NBS: newborn screening
NewSTEPS: Newborn Screening Technical Assistance and Evaluation Program
OFC: occipitofrontal circumference
RBC: red blood cell
LTFU: long-term follow-up
Moving from concept to implementation
Identifying potential data sources
State-based data sources
Most state NBS programs incorporate some form of data collection system to monitor screening test performance and short-term outcomes for newborns screened. Quality improvement measures for short-term follow-up were previously developed and monitored through the Health Resources and Services Administration (HRSA)-funded National Newborn Screening Information System (NNSIS) (14). These measures are being redesigned and updated with the recently developed system known as NewSTEPs (Newborn Screening Technical assistance and Evaluation Program).
Other primary state data sources include birth and death files, Medicaid records, hospital discharge and emergency room admission data, files from Early Intervention programs, birth defect surveillance, immunization databases, and education records. Ideally, states would have cross-state data sharing agreements to capture information on babies born in one jurisdiction but whose treatment occurs in a neighboring state and the use of linked measurement systems across states and across conditions could enable continuous improvement of NBS system performance. The increasing sophistication of health information technology can provide the data and data linkages necessary to populate many of these measures, and reduce the cost and enhance the timeliness of data collection.
Clinical and public health data sources
Some states collect annual reports from clinicians or specialty care centers on all or selected disorders diagnosed through NBS. Activities of the Maternal and Child Health Bureau of HRSA support collection of NBS and follow-up information by funded grantees (13, 15–19), and CDC has piloted a project leveraging existing population-based surveillance, such as birth defects, for long-term follow-up (20). NIH funds the Newborn Screening Translational Research Network (NBSTRN) that has developed the Longitudinal Pediatric Data Resource (LPDR)(21) in conjunction with data collection efforts funded by HRSA(13, 22) for clinical data collection, sharing, management and analysis for conditions identified as part of newborn screening. For sickle cell disease, one example is federally sponsored Registry and Surveillance System for Hemoglobinopathies (RuSH) that focused on linkage of multiple data sources to lay the groundwork for a population-level surveillance system for newborns and individuals diagnosed with sickle cell disease in seven states (23). Other data sources will depend upon the conditions and types of data under consideration.
As successful as these projects are, however, they point to the limitations of voluntary, project-based or localized efforts compared to a consistent national approach. None of the sickle cell efforts, for example, consistently and effectively link state systems with clinical systems, nor do they bring together information from the full spectrum of stakeholders (e.g., patient/family reported data). Coalescence of federally-support activities focused on a specific condition, in this case sickle cell disease, offers an opportunity to address questions within the framework. As always, interoperability across electronic data systems is challenging, but is expected to ultimately provide effective data management (24–26).
Consumer sources
There is no mechanism in place to measure the vital role community-based organizations can play with regard to education for families during the process following diagnosis; nor that of the community-based links between the families and the medical centers that support the successful management of a child with a screened condition and education for families regarding genetic services. Voluntary registries generated and maintained by disease advocacy groups already exist for some of the screened conditions, e.g., cystic fibrosis, as well as those registries maintained by treatment manufacturers, e.g. Genzyme’s Pompe Registry. These data sources may provide a backbone of data elements and data sources for direct consumer input for patient-centered outcomes, an approach that is likely to grow over time (27–29).
Challenges
Data sources
The implementation of this framework faces a number of impediments. It is likely there will be considerable variability in the availability of data regarding outcomes for individuals with different NBS conditions, depending on data types, sources and proximity from initial diagnosis. However, utilization of a uniform framework to consider key issues and outcomes in a standardized way provides the opportunity to identify gaps and elements in common, enhancing the potential for shared approaches and resources across conditions. Without complete data, essential elements of the framework will be missing and the similarities and differences in outcomes observed for NBS conditions across states cannot be determined.
Other limitations involve the data sources themselves. Case definitions and primary case identifiers are inconsistent, identical variables may have different formats, data sources may not include the same variables, missing elements may not be coded uniformly, and the time needed to retrieve and match data elements vary. Potential means to address data variation include use of health information technology (HIT) standards such as those developed by HL7 (Health Level Seven)(30) and use of LOINC (Logical Identifier Names and Codes)(31) coding to address field formatting, labeling, and value coding to facilitate comparison among databases to identify changes, and automatic data-mining to cut down on people-time. The NBSTRN LPDR provides an example of a database using standardized core and condition-specific data field definitions for clinical follow-up of children identified with NBS conditions. Most challenging, the duration of tracking by NBS or other Maternal and Child Health - programs may need to be expanded, or modified to accommodate data collection and the successful capture of some of the health and education outcome measures.
Defining reliable and accessible indicators for each condition is a challenge, given the variability of phenotypes. Models exist for multi-state standardization of care and data elements, and for data gathering and care quality improvement (13, 20, 21). Given the considerable limitations discussed above, short-term expectations for data collection and tracking may need to be tiered first into universal and less detailed analyses. Subsequent aspirational goals could be set for access to shared, more complete databases.
Some of the data could be gleaned from state databases by linking a child’s diagnosis to hospital admissions, emergency room use, prescription medications, and deaths. These databases may be sufficiently granular to assess key items such as regular preventive care, growth and development, and educational achievement. For immunization, all but one state, as well as five cities and nine U.S. territories maintain databases of pediatric immunization (32). Education databases could provide valuable information about graduation rates, but access to these data has its own challenges.
Resources Needed
Limited public health resources limit expansion and linking of the databases. Linking between clinical and public health databases has occurred to variable extents, as already mentioned, but additional resources to maintain and expand these efforts are required. Costs for data collection, integration and application to the framework must be acknowledged. These costs would be overlaid on an already strained public health system, but existing data collection efforts could be leveraged to minimize program costs (20). Collection of clinical information without reimbursement is problematic.
Access to data
Privacy considerations for children identified by NBS generally have not been a challenge to programs, as these children are covered by state and federal regulations for data oversight. Nonetheless, as governmental and clinical databases share data, these efforts may accentuate concerns about privacy for members of the public. De-identification and merged data are the bedrocks for managing privacy for personal records. As data collection, integration and utilization expand for public heath purposes, those involved must be responsible for continued assurance of the proper use of data (33). Moreover, responsible parties must continue to communicate public health goals and the regulated methods to attain those goals, while vigorously attending to appropriate oversight and privacy concerns.
Next steps and a call to action
Determining condition-specific aims, goals and measures for the outcomes framework
To reach the goals of NBS, the treatments and expected outcomes for each condition should be identified by experts and consumers and vetted by public health professionals who have direct responsibility for oversight. In many cases, the short-term goals have already been developed by state NBS programs in collaboration with clinical specialty sites, though there may be variation among state programs. Demonstrating the utility of an outcomes framework for sickle cell disease and for PKU permits application of the framework to other screened conditions for which guidelines or standards of care have been more difficult to establish. The current framework lacks specific measures for “knowledge generation,” as such measures are generally not defined for specific conditions. Traditional measures of “knowledge generation” such as counts of peer reviewed publications or grants based on specific data systems may be a reasonable first approximation. Nonetheless, this category is importance for capturing progress in generating knowledge.
Expanding the framework to a systems-level monitoring and improvement tool
Fundamentally, it will be vital that a shared systems-level framework is accepted by the key stakeholders for NBS and children’s health. This NBS framework is aligned with the National Quality Strategy (34) and with electronic health records’ meaningful use goals, which include improving quality of care and safety, engaging patients and families in care, improving care coordination, improving population and public health, reducing disparities and making care more affordable. The framework must also align with current goals and efforts of state NBS and other public health programs, as well as those of clinicians, consumers and other partners.
Supporting the effort entailed in collecting the standardized data necessary to populate this framework and expanding to measure systems levels outcomes will require political will and capital. Political will necessitates demonstrating a compelling rationale for a comprehensive performance framework for the NBS system. The Institute of Medicine declared that the purpose of the health care system is the continuous improvement of the health of the people of the United States (35, 36). Similarly, the goal of the NBS system is to improve children’s health and development. With a systems-level framework in place, society can evaluate the extent to which implemented standards translate to uniform and improved outcomes. Of utmost importance is the understanding that measurement systems, such as the outcomes framework, in isolation are difficult to support. They are not compelling enough to provoke political will. But measurements systems in the larger context of improving outcomes for children are easier to justify politically, and need to be linked to the use of data to improve overall system quality.
Key measures need to match data collection and reporting efforts between clinical and public health programs. Through use of the driver-diagram model (7), the systems-level framework should permit assessment to determine where goals are not being met, allowing stakeholders to use the data to re-orient the roles and responsibilities of those charged with carrying out the various components of NBS, including long-term follow-up. Such intervention must be timely and on-going to assure success in ensuring accessible, quality health care and to integrate research for best practices in collaboration with providers and families to achieve quality improvement and across health systems (36). The proposed framework will allow comparison of strategies and suggest options for data collection improvement across states and clinical centers. Moreover, in examining differences in outcomes within conditions, states and clinicians can learn more about the characteristics of public health, clinical, and community practices associated with better measured outcomes.
Although the challenges in moving from an outcomes framework to systems-level performance monitoring and quality improvement are substantial, the benefits in terms of health outcomes are likely to outweigh these challenges. The expanded use of electronic health records, new technologies allowing data exchange and increased capacity for direct patient and family entry all should make this task more feasible. We recommend moving forward by implementing use of the outcomes framework for a limited set of NBS conditions and, as learning occurs, expanding the approach over time.
Summary
This paper presents an outcomes framework for organizing measures for condition-specific health outcomes, and an approach to identifying sources and challenges to populating those measures, and was approved for publication by the ACHDNC. The framework will be useful for any condition, allowing for customization of condition-specific and program-specific outcomes. It is a tool for evaluation of whether necessary data exist, or whether there are gaps indicating the need for additional data collection. This framework, built on a driver diagram, also provides a vision for a comprehensive approach to monitor and continuously improve the NBS system as opportunities arise for better outcomes through new measures and improved treatments in the U.S. and could be used by other nation’s NBS programs. Such an approach and system is necessary if the NBS system is to achieve its overall goal of maximizing health outcomes for children identified through public health newborn screening programs.
Significance.
Successful interventions for newborn screening conditions have been a driving force for public health newborn screening for over fifty years. Organizing interventions and outcome measures into a standard framework to systematically assess outcomes has not yet come into practice. This paper presents a customizable outcomes framework for organizing measures for newborn screening condition-specific health outcomes, and an approach to identifying sources and challenges to populating those measures.
Footnotes
The views expressed herein are solely those of the author(s) and do not necessarily reflect the views of the Secretary of the United States Department of Health and Human Services, or of the individual members of the Secretary’s Discretionary Advisory Committee on Heritable Disorders in Newborns and Children, nor do they necessarily represent the official position of the authors’ respective agencies or organizations. The authors have no commercial interest that might pose or create a conflict of interest with the information presented in this manuscript.
References
- 1.Kemper AR, Boyle CA, Aceves J, et al. Long-term follow-up after diagnosis resulting from newborn screening: Statement of the US Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. [Editorial] Genetics in Medicine. 2008 Apr;10(4):259–61. doi: 10.1097/GIM.0b013e31816b64f9. [DOI] [PubMed] [Google Scholar]
- 2.Pass KA, Lane PA, Fernhoff PM, et al. US newborn screening system guidelines II: follow-up of children, diagnosis, management, and evaluation. Statement of the Council of Regional Networks for Genetic Services (CORN) J Pediatr. 2000;137(4 Suppl):S1–46. doi: 10.1067/mpd.2000.109437. [DOI] [PubMed] [Google Scholar]
- 3.Therrell BL, Johnson A, Williams D. Status of newborn screening programs in the United States. Pediatrics. 2006;117(5 Pt 2):S212–52. doi: 10.1542/peds.2005-2633C. [DOI] [PubMed] [Google Scholar]
- 4.Hoff TP, Hoyt AMPH, Therrell BP, et al. Exploring barriers to long-term follow-up in newborn screening programs. [Article] Genetics in Medicine. 2006 Sep;8(9):563–70. doi: 10.1097/01.gim.0000237790.54074.3d. [DOI] [PubMed] [Google Scholar]
- 5.Therrell BL, Williams D, Johnson K, et al. Financing newborn screening: sources, issues, and future considerations. Journal of public health management and practice: JPHMP. 2007;13(2):207–13. doi: 10.1097/00124784-200703000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Hinton CF, Feuchtbaum L, Kus CA, et al. What questions should newborn screening long-term follow-up be able to answer? A statement of the US Secretary for Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children. Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13(10):861–5. doi: 10.1097/GIM.0b013e3182209f09. [DOI] [PubMed] [Google Scholar]
- 7.Svoronos T, Mate KS. Evaluating large-scale health programmes at a district level in resource-limited countries. Bulletin of the World Health Organization. 2011;89(11):831–7. doi: 10.2471/blt.11.088138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langley GJ. The improvement guide: a practical approach to enhancing organizational performance. San Francisco, CA: Jossey-Bass Publishers; 1996. [Google Scholar]
- 9.Section on Hematology/Oncology, Committee on Genetics. Health Supervision for Children with Sickle Cell Disease. Pediatrics. 2002;109(3):526–35. doi: 10.1542/peds.109.3.526. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Mortality among children with sickle cell disease identified by newborn screening during 1990–1994--California, Illinois, and New York. MMWR Morbidity and mortality weekly report. 1998;47(9):169–72. [PubMed] [Google Scholar]
- 11.Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22):4304–10. doi: 10.1182/blood-2012-03-419879. quiz 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, DC: [Accessed April 21 2014]. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=26. [Google Scholar]
- 13.Berry SA, Jurek AM, Anderson C, et al. The inborn errors of metabolism information system: A project of the Region 4 Genetics Collaborative Priority 2 Workgroup. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S215–9. doi: 10.1097/GIM.0b013e3181fe5d23. [DOI] [PubMed] [Google Scholar]
- 14.Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12(4):236–45. doi: 10.1002/mrdd.20124. [DOI] [PubMed] [Google Scholar]
- 15.Sahai I, Eaton RB, Hale JE, et al. Long-term follow-up to ensure quality care of individuals diagnosed with newborn screening conditions: early experience in New England. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S220–7. doi: 10.1097/GIM.0b013e3181fe5d37. [DOI] [PubMed] [Google Scholar]
- 16.Singh RH, Hinman AR. Newborn dried bloodspot screening: long-term follow-up activities and information system requirements. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S261–6. doi: 10.1097/GIM.0b013e3181fe5f6c. [DOI] [PubMed] [Google Scholar]
- 17.Feuchtbaum L, Dowray S, Lorey F. The context and approach for the California newborn screening short- and long-term follow-up data system: preliminary findings. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S242–50. doi: 10.1097/GIM.0b013e3181fe5d66. [DOI] [PubMed] [Google Scholar]
- 18.Kronn D, Mofidi S, Braverman N, et al. Diagnostic guidelines for newborns who screen positive in newborn screening. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S251–5. doi: 10.1097/GIM.0b013e3181fe5d8b. [DOI] [PubMed] [Google Scholar]
- 19.Wright EL, Van Hove JL, Thomas J, et al. Mountain States Genetics Regional Collaborative Center’s Metabolic Newborn Screening Long-term Follow-up Study: a collaborative multi-site approach to newborn screening outcomes research. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S228–41. doi: 10.1097/GIM.0b013e3181fe5d50. [DOI] [PubMed] [Google Scholar]
- 20.Hinton CF, Mai CT, Nabukera SK, et al. Developing a public health-tracking system for follow-up of newborn screening metabolic conditions: a four-state pilot project structure and initial findings. Genetics in medicine: official journal of the American College of Medical Genetics. 2013 doi: 10.1038/gim.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newborn Screening Translational Research Network (NBSTRN) Longitudinal Pediatric Data Resource (LPDR) American College of Medical Genetics and Genomics; Bethesda, MD: [Accessed April 16 2014]. https://www.nbstrn.org/research-tools/longitudinal-pediatric-data-resource. [Google Scholar]
- 22.Berry SA, Lloyd-Puryear MA, Watson MS. Long-term follow-up of newborn screening patients. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(12 Suppl):S267–8. doi: 10.1097/GIM.0b013e3181fea476. [DOI] [PubMed] [Google Scholar]
- 23.Hoots WK. The registry and surveillance in hemoglobinopathies: improving the lives of individuals with hemoglobinopathies. American journal of preventive medicine. 2010;38(4 Suppl):S510–1. doi: 10.1016/j.amepre.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Abhyankar S, Lloyd-Puryear MA, Goodwin R, et al. Standardizing newborn screening results for health information exchange. AMIA... Annual Symposium proceedings / AMIA Symposium AMIA Symposium; 2010; pp. 1–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Zuckerman AE. The role of health information technology in quality improvement in pediatrics. Pediatric clinics of North America. 2009;56(4):965–73. doi: 10.1016/j.pcl.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Office of the National Coordinator for Health Information Technology. Connecting health and care for the nation: a shared interoperability roadmap version 1.0. 2015. [Google Scholar]
- 27.McLaughlin SE, Diener-West M, Indurkhya A, et al. Improving Transition From Pediatric to Adult Cystic Fibrosis Care: Lessons From a National Survey of Current Practices. Pediatrics. 2008;121(5):e1160–6. doi: 10.1542/peds.2007-2217. [DOI] [PubMed] [Google Scholar]
- 28.Prasad SA, Main E, Dodd ME. Finding consensus on the physiotherapy management of asymptomatic infants with cystic fibrosis. Pediatric Pulmonology. 2008;43(3):236–44. doi: 10.1002/ppul.20741. [DOI] [PubMed] [Google Scholar]
- 29.Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: Design, methodology, and early findings from the Pompe Registry. Molecular Genetics and Metabolism. 2011;103(1):1–11. doi: 10.1016/j.ymgme.2011.02.004. http://dx.doi.org/10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Dolin RH, Alschuler L, Boyer S, et al. HL7 Clinical Document Architecture, Release 2. Journal of the American Medical Informatics Association: JAMIA. 2006;13(1):30–9. doi: 10.1197/jamia.M1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald CJ, Huff SM, Suico JG, et al. LOINC, a Universal Standard for Identifying Laboratory Observations: A 5-Year Update. Clinical Chemistry. 2003;49(4):624–33. doi: 10.1373/49.4.624. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. Immunization Information Systems (IIS) Atlanta, GA: [Accessed April 14 2014]. http://www.cdc.gov/vaccines/programs/iis/contacts-registry-staff.html. [Google Scholar]
- 33.Botkin JR, Lewis MH, Watson MS, et al. Parental permission for pilot newborn screening research: guidelines from the NBSTRN. Pediatrics. 2014;133(2):e410–7. doi: 10.1542/peds.2013-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agency for Healthcare Quality and Reasearch. The National Quality Strategy: Report to Congress. Rockville, MD: 2011. [Google Scholar]
- 35.Institute of Medicine. Crossing the Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 36.Olsen LA, Aisner D, McGinnis JM, editors. The Learning Healthcare System: Workshop Summary. Washington DC: National Academy of Sciences; 2007. [PubMed] [Google Scholar]