Abstract
Repeated local administration of ethanol (EtOH) sensitized the posterior ventral tegmental area (pVTA) to the local dopamine (DA)-stimulating effects of EtOH. Chronic alcohol drinking increased nucleus accumbens (NAC) DA transmission and pVTA glutamate transmission in alcohol-preferring (P) rats. The objectives of the present study were to determine the effects of chronic alcohol drinking by P rats on the (a) sensitivity and response of the pVTA DA neurons to the DA-stimulating actions of EtOH, and (b) negative feedback control of DA (via D2 auto-receptors) and glutamate (via group II mGlu auto-receptors) release in the pVTA. EtOH (50 or 150 mg%) or the D2/3 receptor antagonist sulpiride (100 or 200 μM) was microinjected into the pVTA while DA was sampled with microdialysis in the NAC shell (NACsh). The mGluR2/3 antagonist LY341495 (1 or 10 μM) was perfused through the pVTA via reverse microdialysis and local extracellular glutamate and DA levels were measured. EtOH produced a more robust increase of NACsh DA in the ‘EtOH’ than ‘Water’ groups (e.g., 150 mg% EtOH: to ~ 210 vs 150% of baseline). In contrast, sulpiride increased DA release in the NACsh more in the ‘Water’ than ‘EtOH’ groups (e.g., 200 μM sulpiride: to ~ 190–240 vs 150–160% of baseline). LY341495 (at 10 μM) increased extracellular glutamate and DA levels in the ‘Water’ (to ~ 150–180% and 180–230% of baseline, respectively) but not the ‘EtOH’ groups. These results indicate that alcohol drinking enhanced the DA-stimulating effects of EtOH, and attenuated the functional activities of D2 auto-receptors and group II mGluRs within the pVTA.
Keywords: dopamine, ethanol, glutamate, LY341495, nucleus accumbens, sulpiride, ventral tegmental area
Introduction
The ventral tegmental area (VTA) dopamine (DA) system plays a central role in the rewarding and DA-stimulating actions of alcohol (Ding et al 2009b, Rodd et al 2004) and in regulating alcohol drinking (Hodge et al 1993, Rodd et al 2010). VTA DA neurons can undergo alterations in response to prior exposure to experimenter-administered ethanol (EtOH). Repeated i.p. injections of EtOH increased the responsiveness of DA neurons to EtOH excitation in VTA slice preparations from C57BL/6J mice (Brodie 2002). Repeated microinjections of EtOH into the posterior VTA (pVTA) of Wistar rats enhanced EtOH stimulation of local DA neurons projecting to the nucleus accumbens shell (NACsh, Ding et al 2009b). However, the effects of chronic voluntary alcohol drinking on the DA-stimulating actions of EtOH on pVTA DA neurons are unknown. Limited access to alcohol drinking in P rats increased the number of spontaneously active DA neurons within the VTA (Morzorati et al 2010). In addition, chronic alcohol drinking by P rats increased the sensitivity and response of the pVTA to the rewarding actions of EtOH (Rodd et al., 2005a). Furthermore, chronic alcohol drinking increased DA transmission in the NAC (Thielen et al 2004). Reduced D2 auto-receptor function was observed in the NAC following alcohol drinking (Thielen et al 2004), which may be a factor contributing to the observed increased DA transmission in the NAC. However, the effects of chronic alcohol drinking on D2 auto-receptor function within the pVTA has not been studied and may be a factor contributing to the enhanced DA-stimulating (Ding et al., 2009b) and rewarding (Rodd et al., 2005a) effects of EtOH observed following repeated or chronic alcohol administration, respectively.
Chronic alcohol exposure in Wistar or P rats increased glutamate transmission in the pVTA by increasing basal extracellular glutamate levels (Ding et al 2012, 2013) or enhancing excitatory synaptic activity on VTA DA neurons (Stuber et al 2008). The elevated basal extracellular glutamate levels appeared to be due in part to reduced glutamate clearance mediated by glutamate transporters (Ding et al, 2012, 2013). The VTA receives significant glutamate inputs from the medial prefrontal cortex and various sub-cortical regions (Geisler et al 2007, Vertes 2004). It is also possible that the increased glutamate transmission may be due to increased excitatory inputs from glutamate neurons in regions projecting to the pVTA and/or decreased group II mGlu auto-receptor function on glutamate terminals within the pVTA. The P rat has a defect in the Grm2 gene that prevents the expression of a functional gene (Zhou et al 2013). However, mGlu3 auto-receptors may still be present and compensate in part for the loss of mGlu2 receptors. Therefore, the functional activity of group II mGlu receptors within the pVTA was also examined.
This study tested the hypothesis that chronic alcohol drinking by P rats will increase the sensitivity and response of the pVTA to the DA-stimulating actions of EtOH, and decrease the functional activities of D2 and group II mGlu auto-receptors within the pVTA.
Methods and Materials
Animals
Adult female alcohol preferring P rats (weighing 225–250 g at the beginning of the experiments) were housed in pairs upon arrival in temperature- and humidity-controlled rooms maintained on a regular 12-hr light-dark cycle (light on at 7:00 a.m.) with food and water available ad libitum. After being acclimated for 2–3 weeks, rats were then individually housed during the alcohol drinking period. Female rats were used because they maintain their head size better than male rats for more accurate stereotaxic placements and have been used in several studies requiring accurate placements (Ding et al 2009b, Rodd et al 2005a). The estrous cycle was not monitored in the present study. However, counterbalanced experiments were conducted on different days so that any effect of a given phase of the estrous cycle would have been distributed across experimental conditions. All experimental procedures were conducted during the light phase as previously described (Ding et al 2009b, Engleman et al 2011, Thielen et al 2004). Since the P rats consume most of their EtOH during the dark phase, conducting microdialysis experiments during the light phase allows experiments to be conducted in the absence of EtOH without disrupting the daily EtOH intakes of the P rats. Protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were conducted in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Chemical agents
KCl, CaCl2, MgSO4 and NaC2H3O2 were purchased from Fisher Scientific (Fair Lawn, NJ, USA). NaCl, Na2HPO4·7H2O, MgCl2, d-glucose, sodium octyl-sulfate (SOS), ethylenediaminetetraacetic acid (EDTA), and acetonitrile were purchased from Sigma (St. Louis, MO, USA). KH2PO4 and NaHCO3 were purchased from Acros Organics (NJ, USA). O-phthalaldehyde powder and diluents for glutamate derivatization were purchased from Pickering Laboratory, Inc, CA. The salts used in the aCSF and mobile phase were all HPLC grade. EtOH (Decon Laboratories, Inc, King of Prussia, PA, USA), the dopamine D2/3 receptor antagonist (-)-sulpiride (St. Louis, MO, USA) and the mGlu2/3 receptor antagonist LY341495 (Tocris Bioscience, Minneapolis, MN, USA) were dissolved in the aCSF to desired concentrations.
EtOH drinking procedure
EtOH drinking followed the procedure as described previously (Ding et al 2013, Thielen et al 2004). The rats were randomly assigned to one of 2 groups (n = 5–9 / group). The ‘Water’ group received only water throughout. The ‘EtOH’ group received free access to 15% EtOH vs water for 8 weeks. For rats given EtOH, the positions of the EtOH and water bottles were randomly altered every week, and fluid intake was recorded to the nearest 0.1 g by weighing the water and EtOH bottles three times a week (Mondays, Wednesdays and Fridays). EtOH fluid intake measures were converted into grams of EtOH per kilogram of body weight (grams per kilogram per day). Body weights of the rats were recorded at the same time the EtOH and water bottles were weighed.
Stereotaxic surgery and probe insertion
Stereotaxic surgery was performed during the 7th week of EtOH drinking. Rats were stereotaxically implanted ipsilaterally with two guide cannulae (Plastics One, Inc., Roanoke, VA, USA) under 2% isoflurane inhalation anesthesia for microinjection-microdialysis experiments. The 18-gauge cannulae were used for microdialysis probes, and the 22-gauge cannulae were used for microinjection (Ding et al 2009b, 2011). One cannula was aimed above the pVTA (AP −5.6 mm, ML +2.1 mm, DV −9.0 mm), and the other above the NACsh (AP +1.7 mm, ML +2.3 mm, DV −8.5 mm, Paxinos & Watson 1998). For reverse microdialysis, one 18-gauge cannula was implanted in to the pVTA. The cannulae were implanted at a 10° angle to the vertical line. The study of pVTA is based on previous findings that the pVTA is the anatomical substrate supporting the reinforcing and stimulating effects of ethanol. For example, ethanol can be self-infused into the pVTA but not anterior VTA (Rodd et al 2005b, Rodd et al 2004). In addition, microinjection of ethanol into the pVTA but not the anterior VTA increased dopamine release in the NACsh (Ding et al 2009b). Rats were allowed to recover from surgery for at least 5 days prior to the microdialysis experiment. Approximately 16–18 hr before microdialysis, a loop-style probe (active membrane length 1.5 mm for NACsh and 1.0 mm for pVTA (inner diameter 200 μm, molecular weight cut-off: 13,000, Spectrum Laboratories, Inc, Rancho Dominguez, CA, USA) was inserted into the NACsh or pVTA, as previously described (Ding et al 2009b).
Microinjection-microdialysis procedure
These experiments examined the effects of chronic EtOH drinking on the response of pVTA DA neurons to EtOH and the functional activity of DA D2 auto-receptors within the pVTA. DA neurons in the pVTA mainly project to the shell sub-region of the NAC; such projections play an important role in mediating the reinforcing effects of ethanol (Ikemoto 2007). DA D2 receptors within the pVTA appear to function as inhibitory auto-receptors on local DA neurons projecting to the NACsh, as demonstrated by a previous study showing that blockade of pVTA D2 receptors increased DA release in the NACsh (Ding et al 2009a). Microinjection of EtOH into the pVTA was also shown to increase DA release in the NACsh (Ding et al 2009b).
On microdialysis days, EtOH bottles were removed at least 2–3 hours before the start of microdialysis. A previous study using the a similar EtOH drinking procedure did not detect measurable alcohol concentrations in brain microdialysis samples collected within approximately the same time period as the current experiments (Engleman et al 2011). The general procedure followed the one described previously (Ding et al 2009b, 2011). Briefly, rats were placed into microdialysis chambers and connected to a Harvard pump with PE20 tubing (inner diameter 0.38 mm, Becton Dickinson & Co., MD, USA). Microdialysis started with a 90-min wash-out period during which aCSF (140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4·7H2O, 1.0 mM MgCl2, pH 7.2–7.4) was perfused through probes. Then, four to five baseline samples were collected, which was followed by microinjections of either vehicle or the drug (EtOH: 50 or 150 mg%; or the D2/3 receptor antagonist sulpiride: 100 or 200 μM) for 10 min in the pVTA. The selection of ethanol concentrations was based on previous findings in P rats showing that 150 mg% ethanol was within the optimal concentrations for self-infusion into the pVTA, whereas 50 mg% ethanol was a subthreshold dose that was not self-infused into the pVTA (Rodd et al 2005b). Five samples were collected thereafter. All samples were collected from the NACsh at 20-min intervals at a flow rate of 1.0 μl/min into sample vials containing 3 μl of perchloric acid (0.1 N). Samples were frozen and stored at −80 °C before analysis for DA. Each animal received only one treatment.
Microinjections were conducted with an isolated pulse stimulator (A-M Systems, Inc, Carlsborg, WA), following the procedure described previously (Ding et al 2009b, 2011). Briefly, the A-M system was calibrated to inject a 100-nl solution in 5 seconds; a 15-sec timeout period followed each 5-sec injection. The injection-timeout cycle was repeated thirty times over a 10-min period. After the injections, the injector remained in place for one minute before being removed.
Reverse microdialysis
This experiment examined the effects of chronic alcohol drinking on the regulation of group II mGlu auto-receptors on local extracellular glutamate levels within the pVTA. Since our methods allow the measurements of both glutamate and DA in the same sample, we determined the extracellular levels of both transmitters. Reverse microdialysis allows for simultaneous drug delivery and sampling from the pVTA.
The general procedure followed the one described previously (Ding et al 2009a). Briefly, rats were placed into Plexiglas chambers and connected to a Harvard pump with PE20 tubing. Microdialysis aCSF was perfused through probes (2.0 μl/min). After a 90-min washout, three to four baseline samples were collected. Then, aCSF or LY341495 (1 or 10 μM) was perfused through the probe into the pVTA for 60 min before being switched back to aCSF. Samples were collected for another 40 mins following the termination of LY341495 perfusion. All samples were collected from the pVTA at 20-minute intervals into vials containing 5 μl of 0.1 N perchloric acid and divided into two parts, with one part for DA and the other for glutamate analysis. Each rat received only one treatment.
Sample analysis
DA was analyzed as described previously (Ding et al 2011). Briefly, samples were delivered onto an analytical column (BDS Hypersil C18, 3 μm, 100 mm × 1 mm, Thermo Fisher) with a mobile phase containing 50 mM phosphoric acid, 0.1 mM EDTA, 8 mM KCl, 100 mg/L OSA, and 10% MeOH, pH 6.0. DA was detected with a glassy-carbon electrode and an amperometric detector with the oxidation potential set at + 350 mV and sensitivity set at 100 pA/V. The signal then was analyzed with a ChromPerfect data station. The detection limit for DA was approximately100 pmol/l with a signal-to-noise ratio of 3:1.
Glutamate was analyzed as described previously (Ding et al 2013). Briefly, precolumn derivatization of glutamate with o-phthalaldehyde was performed using an ESA Model 542 autosampler. The mobile phase consisted of 25% methanol (v/v), 100 mM Na2HPO4.7H2O, pH 6.75 and was delivered by an ESA 582 solvent delivery system. Dialysis samples were injected onto a reversed-phase column (BDS Hypersil C18 Pioneer, 150 × 2 mm) and glutamate was separated and detected with a BAS LC-4C amperometric detector with the oxidation potential set at +550 mV and the sensitivity set at 0.2 μA. The output from the detector was sent to a ChromPerfect chromatography data analysis system. The concentration of glutamate was quantified by comparing peak area with an external standard curve.
Histology
At the end of each experiment, rats were euthanized with an overdose of CO2 inhalation, and 1% bromophenol blue was injected into the pVTA and perfused through microdialysis probes. Brains were removed quickly and frozen immediately on dry ice and stored at −80 °C. Sections (40 μm) were prepared on a cryostat microtome and stained with cresyl violet for verification of the placements of injection sites and probes with reference to the rat brain atlas of Paxinos & Watson (1998).
Statistical analysis
The baseline levels for extracellular DA and glutamate were averaged from three samples prior to the drug delivery and used for statistics. The time-course data were normalized by the averaged baseline and were expressed as percent of baseline. Repeated measures ANOVAs were performed on the time-course data. In addition, the values for area under the curve (AUC) for each group were calculated and analyzed with two-way ANOVA to determine the significant effects. Post-hoc Tukey’s b tests were performed following a significant main effect.
Results
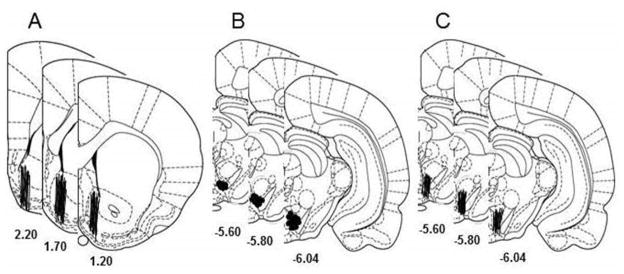
Fig. 1 shows the representative placements of probes and microinjection sites in the NACsh and pVTA in the microinjection-microdialysis studies (Fig. 1A&B) and of probes in the pVTA in the reverse microdialysis study (Fig. 1C). The pVTA is defined at the levels from 5.6 mm to 6.0 mm posterior to bregma (Ding et al 2009b, Rodd et al 2004). To be included, the probes should have at least 75% of the active membrane within the target area. Approximately 85% of animals fulfilled the criteria and were included in the analysis. Average EtOH intake levels in the ‘EtOH’ groups were 6.0 ± 0.4 g/kg/d before surgery, and were 5.4 ± 0.4 g/kg/d prior to microdialysis. These levels were consistent with levels previously reported in the P rat (Ding et al 2013, Thielen et al 2004). BECs were not measured in the current study. The P rat was shown to attain pharmacologically relevant BECs (60–90 mg%) with similar drinking procedures in previous studies (reviewed in McBride & Li 1998, Murphy et al 2002). Collapsed data show that average baseline DA levels in the NACsh were 0.7 ± 0.1 nM and 0.6 ± 0.1 nM for the ‘Water’ and ‘EtOH’ groups, respectively. Average baseline glutamate levels in the pVTA were 1.2 ± 0.2 μM and 1.5 ± 0.2 μM for the ‘Water’ and ‘EtOH’ groups, respectively. Average baseline DA levels in the pVTA were 0.5 ± 0.1 nM and 0.4 ± 0.1 nM in the ‘Water’ and ‘EtOH’ groups, respectively.
Figure 1.
Placements of microdialysis probes (lines) and microinjection sites (circles) in the nucleus accumbens shell (NACsh) and posterior ventral tegmental area (pVTA) in microinjection-microdialysis studies (A & B), and probes (lines) in the pVTA in the reverse microdialysis (C) study.
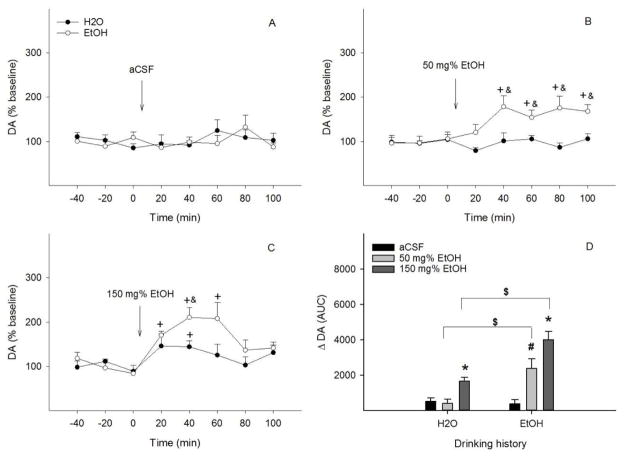
Figure 2 shows the effects of drinking histories on the DA-stimulating effects of aCSF or EtOH into the pVTA. Ethanol intakes were 7.6 ± 1.2, 5.0 ± 0.7, and 5.4 ± 1.0 g/kg/d for aCSF, 50 mg%, and 150 mg% ethanol group, respectively. ACSF did not significantly alter NACsh DA levels in either group (all F values <0.5, all p values > 0.74, Fig. 2A). Treatment with 50 mg% EtOH (Fig. 2B) showed significant effects of time (F 5, 9 = 6.5, p = 0.008), drinking history (F 1, 13 = 27.3, p < 0.001) and the interaction of time x drinking history (F 5, 9 = 4.1, p = 0.03). NACsh extracellular DA levels were not altered in the ‘Water’ group, but significantly increased to 160–180% of baseline during the 40–100 min period in the ‘EtOH’ group (p < 0.05). Microinjection of 150 mg% EtOH (Fig. 2C) demonstrated significant effects of time (F 5, 6 = 12.6, p = 0.004) and drinking history (F 1, 10 = 10.7, p = 0.008), but no significant effect of the interaction of time x drinking history (F 5, 6 = 2.5, p = 0.15). NACsh extracellular DA levels increased to approximately 150% of baseline during the first 40 min in the ‘Water’ group (p < 0.05), and increased to 170–210% of baseline during the first 60 min in the ‘EtOH’ group (p < 0.05). The increase was significantly greater in the ‘EtOH’ than ‘Water’ group (p < 0.05). AUC data (Fig. 2D) indicated significant effects of drinking history (F 1, 34 = 22.3, p < 0.001), challenge treatment (F 2, 34 = 20.6, p < 0.001), and the interaction of drinking history x challenge treatment (F 2, 34 = 6.9, p = 0.003). Both 50 and 150 mg% EtOH produced a greater increase of NACsh DA release in the ‘EtOH’ than the ‘Water’ group.
Figure 2.
Time-course effects of microinjection of aCSF (A), 50 mg% (B) or 150 mg% EtOH (C) into the posterior ventral tegmental area on dopamine release in the nucleus accumbens shell of EtOH or water drinking rats (n=5–8/group). + p < 0.05, significantly greater than baseline; & p < 0.05, significantly greater than levels in the ‘Water’ group. Panel D shows dopamine change ( DA) expressed as area-under-the-curve (AUC) data for each group. * p < 0.05, significantly greater than aCSF and 50 mg% EtOH groups in each drinking history; # p < 0.05, significantly greater than aCSF group in EtOH drinking history; $ p < 0.05, significantly different between water and EtOH drinking histories.
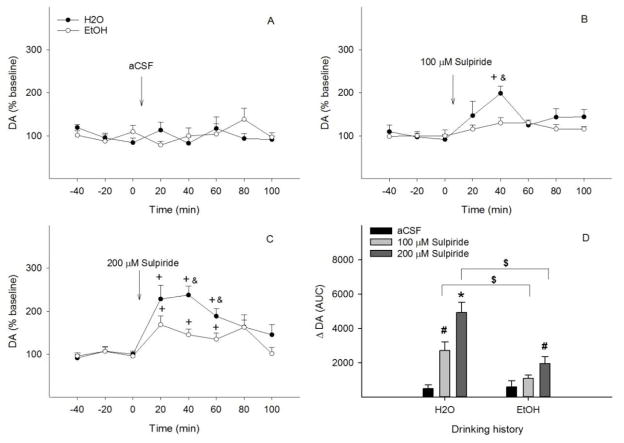
Figure 3 shows the effects of microinjection of aCSF or the D2/3 receptor antagonist sulpiride into the pVTA on NACsh extracellular DA levels in ‘Water’ and ‘EtOH’ groups. Ethanol intakes were 6.6 ± 1.4, 8.5 ± 1.2 and 6.2 ± 0.9 g/kg/d for the aCSF, 100 μM, and 200 μM sulpiride groups, respectively. Microinjection of aCSF into the pVTA did not alter NACsh extracellular DA levels in either group (all F values < 1.5, all p values > 0.35, Fig. 3A). Microinjection of 100 μM sulpiride (Fig. 3B) showed significant effects of time (F 5, 7 = 58.5, p < 0.001), drinking history (F 1, 11 = 7.7, p = 0.02) and the interaction of time x drinking history (F 5, 7 = 5.4, p = 0.02). 100 μM sulpiride significantly increased NACsh extracellular DA levels to approximately 200% of baseline (p < 0.05) in the ‘Water’ group, but had no effect in the ‘EtOH’ group (Fig. 3B). Significant effects of time (F 5, 10 = 12.7, p < 0.001), drinking history (F 1, 14 = 10.4, p = 0.006), and significant effect of the interaction of time x drinking history (F 5, 10 = 3.6, p = 0.04) were seen with microinjection of 200 μM sulpiride (Fig. 3C). NACsh extracellular DA levels increased to 190–240% of baseline during the first 60 min (p < 0.05) in the ‘Water’ group, but only increased to 150–160% of baseline during the first 60 min in the ‘EtOH’ group (p < 0.05), significantly lower than that in the ‘Water’ group (p < 0.05). AUC data (Fig. 3D) shows significant effects of drinking history (F 1, 35 = 16.5, p < 0.001), challenge treatment (F 2, 35 = 21.1, p < 0.001), and the interaction of drinking history x challenge treatment (F 2, 35 = 5.8, p = 0.007). Both 100 and 200 μM sulpiride induced greater increases of NACsh extracellular DA levels in the ‘Water’ than ‘EtOH’ group.
Figure 3.
Time-course effects of microinjection of aCSF (A), 100 μM (B) or 200 μM sulpiride (C) into the posterior ventral tegmental area on dopamine release in the nucleus accumbens shell of EtOH or water drinking rats (n=6–9/group). + p < 0.05, significantly greater than baseline; & p < 0.05, significantly greater than levels in the ‘EtOH’ group. Panel D shows dopamine change ( DA) expressed as area-under-the-curve (AUC) data for each group. * p < 0.05, significantly greater than aCSF and 100 /M sulpiride groups in water drinking condition; # p < 0.05, significantly greater than aCSF group in water or EtOH drinking condition; $ p < 0.05, significantly different between water and EtOH drinking conditions.
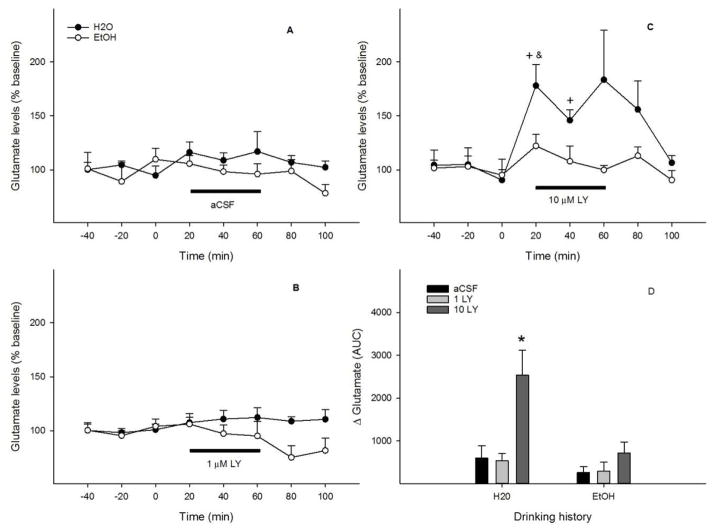
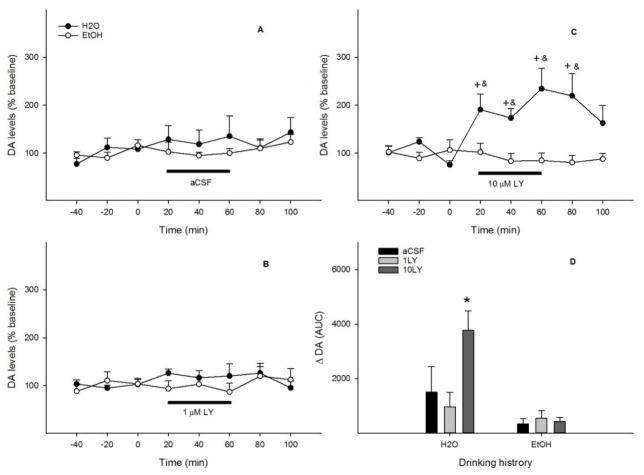
Reverse perfusion of the pVTA with aCSF or 1 μM LY341495 (Figs. 4A & B) had no effect on local extracellular glutamate levels with either drinking histories (all F values < 2.6, all p values > 0.15). Perfusion with 10 μM LY341495 (Fig. 4C) had significant effects of time (F 5, 10 = 4.6, p = 0.02) and drinking history (F 1, 14 = 10.4, p = 0.006), but no significant effect of the interaction of time x drinking history (F 5, 10 = 1.7, p = 0.23). Local extracellular glutamate levels increased to 150–180% of baseline during the first 40 min (p < 0.05) in the ‘Water’ group, but remained essentially unchanged in the ‘EtOH’ group. AUC data (Fig. 4D) revealed significant effects of drinking history (F 1, 34 = 8.8, p = 0.006), challenge treatment (F 2, 35 = 9.8, p < 0.001), and the interaction of drinking history x challenge treatment (F 2, 34 = 4.0, p = 0.03). 10 μM LY341495 produced a greater increase of local extracellular glutamate levels in the ‘Water’ group than the ‘EtOH’ group. Ethanol intakes were 5.3 ± 1.0, 5.3 ± 1.4 and 5.0 ± 0.6 g/kg/d for the aCSF, 1 μM, and 10 μM LY341495 groups, respectively.
Figure 4.
Time-course effects of perfusion of the posterior ventral tegmental area with aCSF (A), 1 μM (B) or 10 μM (C) LY341495 on local extracellular glutamate levels of EtOH or water drinking rats (n = 5–9/group). + p < 0.05, significantly greater than baseline; & p < 0.05, significantly greater than levels in the ‘EtOH’ group. Panel D shows glutamate change ( Glutamate) expressed as area-under-the-curve (AUC) data for each group. * p < 0.05, significantly greater than any other group.
Figure 5 shows the effects of reverse microdialysis of LY341495 in the pVTA on local extracellular DA levels in rats with different drinking histories. Perfusion with either aCSF (Fig. 5A) or 1 μM LY341495 (Fig. 5B) did not alter local extracellular DA levels (all F values < 1.5, all p values > 0.33). Perfusion with 10 μM LY341495 (Fig. 5C) showed significant effects of drinking history (F 1, 12 = 14.2, p = 0.003) and the interaction of time x drinking history (F 5, 8 = 4.4, p = 0.03), but no significant effect of time (F 5, 8 = 2.8, p = 0.09). Local extracellular DA levels increased to 180–230% of baseline during the first 80 min (p < 0.05) in the ‘Water’ group but not in the ‘EtOH’ group. The AUC analysis (Fig. 5D) revealed significant effects of drinking history (F 1, 30 = 14.6, p = 0.001), challenge treatment (F 2, 30 = 4.2, p = 0.03), and the interaction of drinking history x challenge treatment (F 2, 30 = 4.5, p = 0.02). 10 μM LY341495 produced a greater increase of local extracellular DA levels in the ‘Water’ than the ‘EtOH’ group.
Figure 5.
Time-course effects of perfusion of the posterior ventral tegmental area with aCSF (A), 1 μM (B) or 10 μM (C) LY341495 on local extracellular dopamine levels of EtOH or water drinking rats (n = 5–7/group). + p < 0.05, significantly greater than baseline; & p < 0.05, significantly greater than levels in the ‘EtOH’ group. Panel D shows dopamine change ( DA) expressed as area-under-the-curve (AUC) data for each group. * p < 0.05, significantly greater than any other group.
Discussion
The results from the current study indicate that intra-pVTA application of EtOH produced greater NACsh DA release in alcohol-drinking than water-drinking rats (Fig. 2), suggesting that alcohol drinking increased the sensitivity and responsiveness of pVTA DA neurons projecting to the NACsh to the DA-stimulating effects of EtOH. Chronic alcohol drinking by P rats attenuated evoked NACsh DA release by intra-pVTA application of the D2/3 receptor antagonist sulpiride (Fig. 3), suggesting a functional down-regulation of somatodendritic D2 auto-receptors within the pVTA. Alcohol drinking also reduced evoked pVTA glutamate release by local application of the mGluR2/3 antagonist LY341495 (Fig. 4), suggesting there was diminished inhibitory control by pre-synaptic group II mGluRs on glutamate release within the pVTA. In addition, alcohol drinking decreased evoked pVTA DA release by LY341495 (Fig. 5), suggesting a relationship between glutamate overflow and stimulation of somatodendritic DA release in the pVTA.
The current finding is consistent with down-regulation of presynaptic D2 auto-receptors within the NAC following chronic alcohol drinking by P rats (Engleman et al 2003, Thielen et al 2004), suggesting that down-regulation of D2 auto-receptor function may play an important role in maintaining alcohol drinking. However, this finding is contrary to an in vitro study on VTA slice preparations that reported enhanced auto-inhibition of VTA DA neurons following repeated i.p. EtOH injections in mice (Perra et al 2011). Several factors may contribute to the differences: (1) the study by Perra et al (2011) used C57 mice and repeated i.p. injections of EtOH over 7 days, which produced cycles of high blood alcohol levels and withdrawal, whereas the current study used P rats given 24-hr free-choice EtOH for 8 weeks and did not experience imposed cycles of EtOH exposure and withdrawal; (2) young male mice (3–4 weeks old) were used in the mouse study whereas adult female rats were used in the current study; and (3) the mice were killed for in vitro experiments during a period of withdrawal in the Perra et al study, whereas the current study was conducted to minimize withdrawal effects and was conducted in vivo.
It has been reported that the P rat lacks mGluR2 protein expression due to a stop codon within the coding region of the Grm2 gene (Zhou et al 2013). Given this, chronic alcohol drinking in the current study may have attenuated the function of mGluR3 within the pVTA of the P rat. Reduced presynaptic auto-inhibition may potentially lead to increased synaptic glutamate release. This notion is consistent with findings demonstrating that chronic alcohol drinking enhanced spontaneous glutamate release and basal extracellular glutamate concentrations within the VTA of Wistar (Stuber et al 2008) and P (Ding et al., 2013) rats, respectively. In addition to these changes, chronic alcohol drinking was shown to enhance excitatory synaptic strength onto VTA DA neurons by increasing the AMPAR/NMDAR-mediated currents (Stuber et al 2008). Taken together, these changes may lead to enhanced excitability of VTA DA neurons.
Mechanisms underlying EtOH-induced reduction of D2 auto-receptor and mGluR3 function are unknown. Possible mechanisms may include: (1) reduced receptor protein expression on cell membrane by reduced protein synthesis and trafficking, and/or increased protein degradation, (2) reduced receptor coupling to its downstream intracellular transduction cascade molecules, and/or (3) altered post-translational modification that may alter receptor function, e.g., phosphorylation and glycosylation. These will be important topics for future studies.
Ethanol at 150 mg% but not 50 mg% increased DA release to ~ 150% of baseline in alcohol naïve P rats (Fig. 2). The magnitude of DA change is in line with previous findings in Wistar rats where 200 mg% ethanol but not 100 mg% produced the same size of increase (~ 150% of baseline) of NACsh DA (Ding et al 2009b). In addition, chronic alcohol drinking could enhance the DA-stimulating effects of EtOH on pVTA DA neurons projecting to the NACsh (Fig. 2). These findings are consistent with a previous study showing that repeated local exposure of the pVTA to EtOH in Wistar rats increased the DA-stimulating effects of EtOH on pVTA DA neurons (Ding et al 2009b). The current findings are also consistent with findings that chronic alcohol drinking by P rats increased the sensitivity and response of the pVTA to the local rewarding actions of EtOH (Rodd et al 2005a). Furthermore, a recent human study reported that acute intravenous administration of ethanol increased DA in the ventral striatum of non-treatment-seeking alcoholics, whereas no change in ventral striatum DA was found in social drinkers, suggesting a sensitized DA response in alcoholics (Yoder et al 2016). However, a recent animal study reported that alcohol drinking in Wistar rats blunted NAC DA overflow evoked by systemic EtOH challenge (Feltmann et al 2016), which is in contrast to the current study. The differences of the current study vs the Feltmann et al study may be due to a combination of factors, including, but not limited to, different alcohol drinking conditions (24-hr continuous drinking of 15% ethanol for 8–10 weeks vs intermittent 24-hr drinking of 20% ethanol for 10 months), alcohol intake levels (~ 6 vs 4 g/kg/d), alcohol challenge administration (microinjection into the pVTA vs intraperitoneal injection), timing of the microdialysis to include or exclude withdrawal (~ 2–3 hr after the last possible drinking episode vs ~21 hr after the last drinking day), placement of the microdialysis probes (mostly in the NACsh vs no placement diagram provided), and/or strain of rats (P vs Wistar rats).
The mechanisms underlying the increased DA-stimulating effects of EtOH on pVTA DA neurons following chronic alcohol drinking observed in the current study are unknown, but may involve increased DA neuronal excitability and neurotransmission resulting from reduced D2 auto-receptor function in combination with increased glutamate transmission due to reduced mGluR3 auto-receptor function. This notion is consistent with findings that intermittent access to alcohol increased the number of spontaneously active DA neurons within the pVTA of P rats (Morzorati et al 2010) and facilitated the probability of burst firing in VTA DA neurons in Sprague-Dawley rats (Hopf et al 2007). This increased excitability may in turn contribute to increased response of these neurons to the DA-stimulating effects of EtOH. However, it should be noted that the current study does not exclude other possible mechanisms that may contribute to the alcohol-induced increase of local DA-stimulating effects of EtOH.
In summary, the current study demonstrated that chronic alcohol drinking produced sensitization of pVTA DA neurons to the local DA-stimulating effects of EtOH. These changes are accompanied by down-regulation of both somatodendritic D2 auto-receptor and presynaptic mGluR3 function within the pVTA, which may contribute to the altered response of these neurons to EtOH stimulation. In addition, functional down-regulation of D2 and group II mGlu auto-receptors has seen with other drugs of abuse (Calipari et al 2014, Moussawi & Kalivas 2010), suggesting that enhanced DA neurotransmission involving reduced D2 and group II mGlu auto-receptor function may constitute one of the common mechanisms underlying development of addictive behavior.
Highlights.
Neuroadaptations were examined in the posterior ventral tegmental area following long-term alcohol drinking
Chronic alcohol drinking increased the dopamine-stimulating effects of ethanol on dopamine neurons in the posterior ventral tegmental area
Chronic alcohol drinking down-regulated D2 auto-receptor function in the posterior ventral tegmental area
Chronic alcohol drinking reduced the functional activity of group II mGlu auto-receptors in the posterior ventral tegmental area
Acknowledgments
This study was supported, in part, by research grants AA07611 and AA012262. We thank Zhuhua Gu and Erin Larrabe for their technical help. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
Author contribution
ZMD, ZAR, and WJM were responsible for concept development and research design. CMI and ZMD participated in performing experiments and acquiring data. ZMD and WJM performed data analysis. ZMD and WJM contributed to the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–30. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, et al. Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology. 2014;39:1833–42. doi: 10.1038/npp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Katner SN, Rodd ZA, Truitt W, Hauser SR, et al. Repeated exposure of the posterior ventral tegmental area to nicotine increases the sensitivity of local dopamine neurons to the stimulating effects of ethanol. Alcohol. 2012;46:217–23. doi: 10.1016/j.alcohol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Liu W, Engleman EA, Rodd ZA, McBride WJ. Differential effects of dopamine D2 and GABAA receptor antagonists on dopamine neurons between the anterior and posterior ventral tegmental area of female Wistar rats. Pharmacol Biochem Behav. 2009a;92:404–12. doi: 10.1016/j.pbb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, et al. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology. 2011;216:245–55. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009b;33:1571–81. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Li T-K, Lumeng L, Murphy JM. Ethanol drinking experience attenuates (-)sulpiride-induced increase in extracellular dopamine levels in the nucleus accumbens of alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2003;27:424–31. doi: 10.1097/01.ALC.0000056618.57931.A5. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Keen EJ, Tilford SS, Thielen RJ, Morzorati SL. Ethanol drinking reduces extracellular dopamine levels in the posterior ventral tegmental area of nondependent alcohol-preferring rats. Alcohol. 2011;45:549–57. doi: 10.1016/j.alcohol.2011.02.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltmann K, Fredriksson I, Wirf M, Schilstrom B, Steensland P. The monoamine stabilizer (-)-OSU6162 counteracts downregulated dopamine output in the nucleus accumbens of long-term drinking Wistar rats. Addict Biol. 2016;21:438–49. doi: 10.1111/adb.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. Vental tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–75. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Marunde RL, Downey D. Limited access to ethanol increases the number of spontaneously active dopamine neurons in the posterior ventral tegmental area of nondependent P rats. Alcohol. 2010;44:257–64. doi: 10.1016/j.alcohol.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGluR2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–22. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academies Press; 2011. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D2 autoinhibition in the ventral tegemental area. Neuropsychopharmacology. 2011;36:993–1002. doi: 10.1038/npp.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, et al. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005a;29:358–66. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005b;30:330–38. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, et al. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol. 2010;44:245–55. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–57. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–20. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, et al. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–25. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, et al. Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend. 2016;160:163–9. doi: 10.1016/j.drugalcdep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, et al. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A. 2013;110:16963–68. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]