Abstract
PURPOSE
To compare the diagnostic performance of the synthetic amino acid analogue positron emission tomography (PET) radiotracer anti-3-[18F]FACBC (fluciclovine) with computed tomography (CT) in the detection of recurrent prostate carcinoma.
METHODS
Retrospective analysis of 53 bone scan negative patients with suspected recurrent prostate carcinoma who underwent fluciclovine PET-CT and routine clinical CT within a 90-day interval. Correlation of imaging findings was made to histology and clinical follow-up. Positivity rates and diagnostic performance were calculated for fluciclovine PET-CT and CT.
RESULTS
41/53 (77.4%) fluciclovine versus 10/53 (18.9 %) CT examinations had positive findings for recurrent disease. Positivity rates were higher with fluciclovine than CT at all prostate-specific antigen (PSA) levels, PSA doubling time (DT) and original Gleason scores (GS). Fluciclovine identified 27/51 and 9/41 more true positive patients than CT in the prostate/bed and in the extraprostatic regions, respectively. Of the 43 index lesions used to prove positivity, 42/43 (97.7%) had histologic proof verification. In 51/53 patients who had sufficient follow-up to calculate diagnostic performance in the prostate/bed, fluciclovine PET-CT demonstrated 88.6% sensitivity, 56.3% specificity, 78.4% accuracy, 81.6% positive predictive value (PPV), and 69.2% negative predictive value (NPV) compared with values of 11.4%, 87.5%, 35.3%, 66.7% and 31.1%, respectively on CT. For 41/53 patients with sufficient follow-up to calculate diagnostic performance for extraprostatic disease, fluciclovine PET-CT demonstrated 46.2% sensitivity, 100% specificity, 65.9% accuracy, 100% PPV, and 51.7% NPV compared with 11.5%, 100%, 43.9%, 100% and 39.5%, respectively on CT.
CONCLUSION
Fluciclovine PET-CT detects more patients with recurrent prostate cancer than CT and can better delineate prostatic from extraprostatic recurrence.
Keywords: FACBC, fluciclovine, CT, PET, prostate cancer, diagnostic performance
INTRODUCTION
When prostate cancer recurs, differentiating prostatic from extraprostatic disease, typically metastasis to lymph nodes or bone, can be challenging. Yet, determining whether the recurrence is local, locoregional, or systemic influences the type of therapy offered [1–3].
While conventional imaging such as computed tomography (CT), bone scan and magnetic resonance imaging (MRI) can be useful in delineating local and distant prostate cancer recurrence, these modalities are also limited by sub-optimal diagnostic performance [1–8]. As a result, molecular imaging techniques are currently being explored for restaging of recurrent prostate cancer [2, 8, 9]. With the use of different radiotracers such as choline and now PSMA ligands, positron emission tomography (PET) holds promise in identifying recurrence earlier and more accurately than conventional imaging techniques [10–12].
Anti-1-amino-3-[18F] fluorocyclobutane-1-carboxylic acid (FACBC or fluciclovine) is an investigational amino acid based PET radiotracer which has been studied in the staging and restaging of prostate cancer [13–17]. The mechanism of uptake of fluciclovine is via transmembrane amino acid transporters, primarily ASCT2 and LAT1, which are reported to be upregulated in prostate cancer [18].
We have previously reported on a completed clinical trial in which the superior diagnostic performance of fluciclovine PET-CT versus 111In-capromab pendetide (ProstaScint) was demonstrated [14]. Yet, conventional CT is still more commonly employed for patients with biochemical (PSA) failure than 111In-capromab pendetide. In this analysis, we compared the performance of fluciclovine PET-CT with that of CT scan in the detection and restaging of recurrent prostate carcinoma in a subset of patients who had been part of this clinical trial and who also had undergone routine CT scanning as per standard of care for the detection of prostate cancer recurrence.
MATERIALS AND METHODS
Patient Selection and informed consent
After Emory University Institutional Review Board approval and informed consent, patients with suspected prostate cancer recurrence were recruited to receive fluciclovine PET-CT scans as part of a prospective parent clinical trial (NCT00562315) of fluciclovine PET-CT for detection of recurrent prostate cancer compared to conventional imaging with emphasis on 111In-capromab pendetide [14]. Inclusion criteria were: 1) an original diagnosis of localized prostate carcinoma with subsequent definitive treatment, 2) a suspicion of prostate cancer recurrence, based on the previous American Society for Radiation Oncology (ASTRO) criteria of 3 consecutive PSA increases and/or the more recent ASTRO/Phoenix criteria of PSA of at least nadir plus 2.0 ng/ml after radiotherapy or cryotherapy and/or >0.2 ng/ml after prostatectomy, and 3) a negative bone scan. The results of this clinical trial comparing the diagnostic performance of fluciclovine to 111In-capromab pendetide in recurrent prostate cancer have been previously published [14].
For the purpose of this retrospective analysis, out of the original cohort of patients in the parent trial, we analysed patients who had also undergone standard of care routine CT scans within 90 days of the fluciclovine scan.
Fluciclovine PET-CT and CT Imaging Protocols
Fluciclovine preparation and imaging acquisition protocols have been previously reported [14, 15, 19, 20]. The radiotracer was produced under US Food and Drug Administration investigational new drug application 72,437. No adverse events were reported among study participants.
Scanning was completed on a Discovery DLS or a 690 PET-CT scanner (GE Health-care, Milwaukee, Wisconsin). All patients fasted for 4 to 6 hours before the fluciclovine PET-CT scan. A CT without IV or oral contrast was obtained for anatomic localization and attenuation correction at approximately 100 mAs and 120 kVp. Average± SD (range) fluciclovine dose of 358±52.9 (163.9-469.9) MBq was then injected intravenously over 2 minutes. After a 3-minute delay for blood pool clearance, abdominopelvic PET-CT imaging was completed with 5 to 16 (early), 17 to 28 (delayed 1) and 29 to 40 (delayed 2) minute acquisitions.
Routine clinical CT at our facility was completed on a 16 slice GE-BS or a 64 slice GE-VCT CT scanner with multiple contiguous 5mm axial images from diaphragm to upper thigh for abdominopelvic CT and from lower abdomen to upper thigh for pelvic CT with 120 kVp and auto-adjusted mA with IV and oral contrast unless contraindicated. Contraindications for IV contrast at our facility included allergic history, elevated creatinine, and patient refusal of IV contrast. The choice of abdominopelvic vs pelvic CT was deferred to the ordering clinician. Scan results from outside facilities were included in analysis if diagnostic protocols were similar. Interval [average ±SD (range)] between fluciclovine and CT scans was 45.5±23.9 (0 – 88) days.
Image Interpretation
As part of the parent cohort, fluciclovine imaging was prospectively interpreted individually on a MIM-Vista workstation (MIM Software, Cleveland, Ohio) by two board certified nuclear medicine physicians blinded to other imaging and reference validation. Disagreement was resolved by consensus. Abnormal moderate (greater than marrow at L3) focal uptake deviating from expected bio-distribution and persisting from early to delayed images was interpreted as positive as previously reported [14, 15]. Anatomic findings on the CT portion of the PET-CT (e.g. lymph node size or shape) did not influence individual lesion interpretation. The fluciclovine scan interpretation obtained at the time of the parent trial was utilized in this subanalysis.
CT imaging had been interpreted as part of standard clinical workflow by a board certified radiologist without the knowledge of fluciclovine study results, but with ability to access all other patient imaging and clinical data. For the purpose of this retrospective analysis, data from clinical reports were utilized and it was assumed that outside facility reports were based on similar standard interpretative guidelines. CT scan images were not reinterpreted for the purpose of this study. All lesions reported as equivocal on CT were analysed as positive.
Reference Standard
As part of the completed parent prospective cohort, patients were followed up for up to 5 years and final consensus was achieved on the presence or absence of prostatic and extraprostatic disease by a multidisciplinary board comprised of 1 nuclear medicine physician, 2 urologists and 2 radiation oncologists, who utilized histologic results, imaging and clinical follow up to achieve consensus for the presence or absence of recurrent disease [14]. For this subset analysis, we utilized this consensus as the reference standard to calculate diagnostic performance of both CT and fluciclovine scan interpretations.
Briefly, and as previously reported in detail, consensus criteria were as follows [14]. In the prostate/bed, the standard of truth was histological sampling with transrectal ultrasound/biopsy. Absence of tissue to biopsy was deemed negative. If despite a negative biopsy, patient achieved PSA control (PSA < 0.2 ng/ml after prostatectomy or less than PSA nadir plus 2 ng/ml in non-prostatectomy) after salvage therapy to the prostate/bed alone, this situation was deemed positive for presence of prostate/bed disease.
For extraprostatic involvement, histological sampling via image guided needle biopsy, laparoscopic or open dissection was the primary verification standard for presence of disease with deliberate tracking of the index lesion to ensure concordance between positivity on the scan and site of biopsy. For bone only, concordant findings on 2 or more correlative imaging studies (MR, CT and/or subsequent bone scan) were accepted in lieu of histology. Similarly as with the prostate/bed, achieving durable PSA control after directed therapy only to the scan positive site was accepted as proof of disease in lieu of biopsy. Absence of extraprostatic disease was biochemically confirmed by achievement of durable PSA control after salvage therapy to prostate/bed only.
Spontaneous decline in PSA without therapy was interpreted as an overall absence of disease in both prostate and extraprostatic locations. Patients with inadequate follow up data to establish presence or absence of disease in the prostate and extraprostatic regions were excluded from the respective analysis of diagnostic performance [14, 15].
Statistical Analyses
On whole body analysis, positivity rates were calculated for fluciclovine and CT across PSA levels, PSA doubling time (DT) and original Gleason scores (GS) at diagnosis. According to the contemporary prostate cancer grading system, GS of 3+4=7 were classified as grade group 2 while GS of 4+3 =7 were classified as grade group 3 [21]. Statistical significance of difference in positivity rates of fluciclovine and CT across these measures were computed using McNemar test.
Measures of diagnostic performance for fluciclovine and CT as well as subset analysis of CT with contrast for recurrent prostatic/bed and extraprostatic disease detection were computed on a per patient basis. We used the exact binomial proportions to compute the 95% CI of each performance measure shown as (95% CI x, y) after each estimate. The statistical significance of the differences in sensitivity and specificity of fluciclovine and CT was determined using the McNemar test. In addition, the statistical significance of differences in the accuracy of both tests was evaluated using Chi-square test while the generalized score statistic method was used to determine the statistical significance of differences in PPV and NPV. A type I error rate of α = 0.05 was used. Analysis was done using Statistical Analysis Software (SAS Version 9.3 SAS Institute Inc. Cary, NC, USA) and Microsoft Excel 2010.
RESULTS
Demographics
Table 1 shows selected demographic characteristics of study participants. 53 patients met inclusion criteria for this retrospective sub-analysis with median (mean ± SD) PSA of 4.0(7.2±8.3) ng/ml. 7/53(13.2 %) had undergone prostatectomy, 5/53(9.4%) EBRT, 6/53(11.3%) brachytherapy, 4/53(7.5%) cryotherapy and 1/53(1.9%) hormone therapy while 30/53(56.6%) had combination of 2 or more treatment modalities.
Table 1.
Demographic characteristics of study participants
| Age at fluciclovine (years) | n=53 |
| -Mean ± SD | 67.57±8.03 |
| -Median (range) | 67.0(49–90) |
| -Q1,Q3 | 62,71 |
| PSA (ng/ml) | n=53 |
| -Mean ± SD | 7.2±8.3 |
| -Median (range) | 4.0(0.11–44.8) |
| -Q1,Q3 | 1.6,11.4 |
| PSA doubling Time (months) | n=49* |
| -Mean ± SD | 18.6±56.6 |
| -Median (range) | 7.7(−31.6–357.8) |
| -Q1,Q3 | 4.0,15.92 |
| PSA velocity (ng/ml/yr) | n=49* |
| -Mean ± SD | 4.5±8.7 |
| -Median (range) | 1.8(−7.3–43.4) |
| -Q1,Q3 | 0.2,5.8 |
| Fluciclovine Dose (mCi) | n=53 |
| -Mean ± SD | 9.7±1.4 |
| -Median (range) | 10.1(4.4–12.7) |
| -Q1,Q3 | 9.6,10.5 |
| Gleason Score Breakdown n (%) | n=49** |
| 3+4 (Grade Group 2) or less | 30/49 (61.2) |
| 4+3 (Grade Group 3) or greater | 19/49 (38.8) |
Note:
Sufficient data was not available to calculate PSA doubling time or velocity in 4 patients;
Gleason Score data was also not available in 4 patients
All CT scans were completed prior to fluciclovine PET-CT within a mean (± SD; range) time interval of 45.53(±23.94; 0-88) days. 39/53 CT scans were completed at our facility while 14/53 were from outside facilities. 36/53 CTs were pelvic scans while 17/53 were abdominopelvic. There were no cases in which disease was found on the fluciclovine study outside the clinical CT field of view. 30/53 CT scans were completed with IV contrast, and 23/53 had no IV contrast administered due to contraindications.
Reference Standard
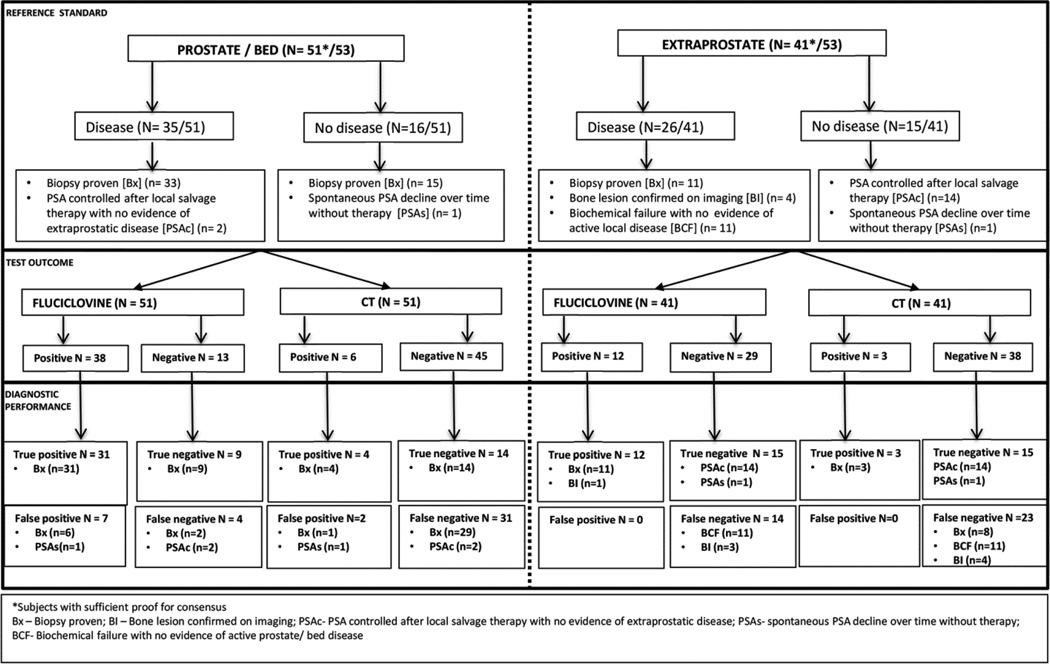
53/115 patients of the parent study had routine CT per standard of care within 90 days of the fluciclovine. There was sufficient follow-up data and histologic proof to determine the presence or absence of local prostatic disease recurrence in 51/53 patients and for extraprostatic disease in 41/53 patients (Fig. 1). 35/51 patients were determined to have disease in the prostate bed: 33/35 patients were biopsy proven while 2/35 patients had PSA control after local salvage therapy. 16/51 patients were determined to have no disease in the prostate bed: 15/16 patients were biopsy proven while 1 patient had spontaneous PSA decline over time without therapy.
Fig. 1.
Diagram of fluciclovine PET-CT and CT scan results with comparison to reference standard in both prostate/bed and extraprostatic regions.
26/41 patients were determined to have extraprostatic disease: 11/26 patients had histologic proof, 4/26 with a bone lesion confirmed on correlative imaging, and 11/26 patients that had biochemical failure with no evidence of active local disease were categorized conservatively to have occult extraprostatic disease. 15/41 patients were determined to have no extraprostatic disease: 14/15 patients had PSA control after local salvage therapy to the prostate/bed only while 1 patient (same as above) had spontaneous PSA decline despite having no therapy.
Positivity rates
On whole body basis, 41/53 (77.4 %) fluciclovine scans were positive. 26/41 (63.4%) were positive in the prostate/bed only, 3/41 (7.3%) in extraprostatic regions only and 12/41 (29.3%) in both the prostate/bed and extraprostatic regions. For CT, 10/53 (18.9 %) were reported as positive: 5/10 (50.0%) in the prostate/bed only, 4/10 (40.0 %) in extraprostatic regions only and 1/10 (10.0 %) in both the prostate/bed and extraprostatic regions.
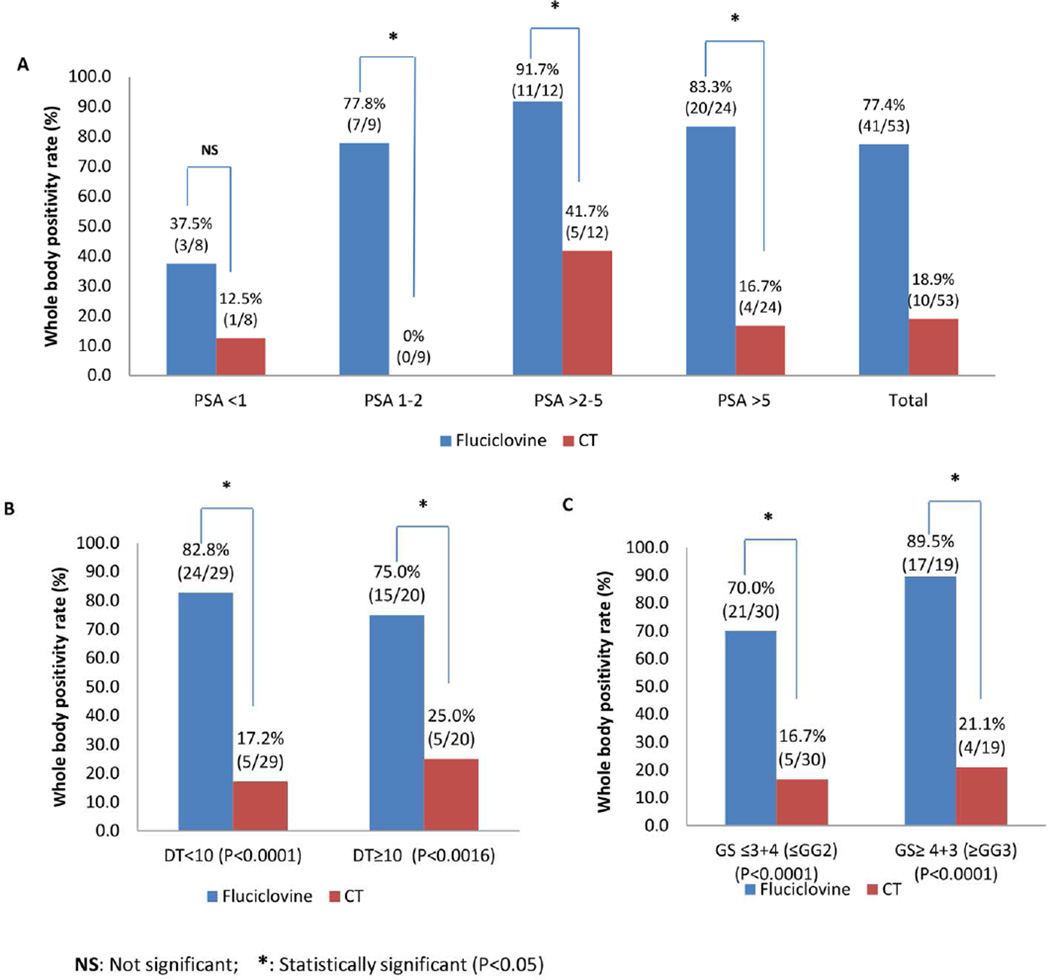
Positivity rates on both fluciclovine and CT scans increased as absolute PSA value increased (Fig. 2A). In addition, positivity on fluciclovine increased with shorter doubling time (DT) while the inverse occurred with CT (Fig. 2B). Significant increase in positivity was also observed with higher Gleason scores on fluciclovine but not with CT (2C). These positivity rates at absolute PSAs, DT and GS on fluciclovine are significantly different from that of CT except at PSA < 1ng/ml.
Fig. 2.
Whole body positivity rates in fluciclovine PET-CT and CT scans in relation to PSA (A), PSA doubling time (B) and Gleason Score (C).
Test outcome and diagnostic performance
Of the 43 index lesions used for true positivity on fluciclovine, 42/43(97.7%) had histologic proof. All lesions that were true positives on CT were also true positive on fluciclovine.
Prostate/bed
Of the 51 patients with adequate reference standard, on fluciclovine PET-CT scan, there were 31 true positives, 9 true negatives, 7 false positives, and 4 false negatives. For CT scan, there were 4 true positives, 14 true negatives, 2 false positives and 31 false negatives (Fig. 1). Of 33 patients with histologic proof of disease, fluciclovine detected 31 (93.9%) while CT detected 4 (12.1%).
For prostate/bed disease diagnostic performance, fluciclovine PET-CT had sensitivity of 88.6% (95% CI 72.3, 96.3), specificity of 56.3% (95% CI 30.6, 79.2), accuracy of 78.4% (95% CI 64.7, 88.7), PPV of 81.6% (95% CI 65.1, 91.7), and NPV of 69.2% (95% CI 38.9, 89.6). CT scans had sensitivity of 11.4% (95% CI 3.7, 27.7), specificity of 87.5% (95% CI 60.4, 97.8), accuracy of 35.3% (95% CI 22.4, 49.9), PPV of 66.7% (95% CI 24.1, 94.0), and NPV of 31.1% (95% CI 18.6, 46.8). Sensitivity, specificity, accuracy, PPV and NPV differed significantly between fluciclovine and CT (Table 2). Fig. 3 shows a biopsy proven prostate lesion.
Table 2.
Diagnostic performance of fluciclovine PET-CT vs. clinical CT
| Fluciclovine | CT | P value | |
|---|---|---|---|
| Prostate/bed (n=51/53) | |||
| True positives | 31 | 4 | - |
| True negatives | 9 | 14 | - |
| False positives | 7 | 2 | - |
| False negatives | 4 | 31 | - |
| %Sensitivity (95% CI) | 88.6 (72.3,96.3) | 11.4 (3.7,27.7) | <0.001* |
| %Specificity (95% CI) | 56.3 (30.6,79.2) | 87.5 (60.4,97.8) | <0.001* |
| %Accuracy (95% CI) | 78.4 (64.7–88.7) | 35.3 (22.4–49.9) | <0.001* |
| %PPV1 (95% CI) | 81.6 (65.1,91.7) | 66.7 (24.1,94.0) | <0.001* |
| %NPV2 (95% CI) | 69.2 (38.9,89.6) | 31.1 (18.6,46.8) | <0.001* |
| Extra prostate (n=41/53) | |||
| True positives | 12 | 3 | - |
| True negatives | 15 | 15 | - |
| False positives | 0 | 0 | - |
| False negatives | 14 | 23 | - |
| %Sensitivity (95% CI) | 46.2 (27.1,66.38) | 11.5 (3.0,31.3) | <0.001* |
| %Specificity (95% CI) | 100 (74.7,100) | 100.0 (74.7,100) | 0.32 |
| %Accuracy (95% CI) | 65.9 (49.4–79.9) | 43.9 (28.5–60.3) | 0.05 |
| %PPV1 (95% CI) | 100 (69.8,100) | 100.0 (31.0,100) | 0.30 |
| %NPV2 (95% CI) | 51.7 (32.9,70.1) | 39.5 (24.5,56.5) | <0.001* |
PPV= Positive predictive value;
NPV= Negative predictive value.
Statistically significant
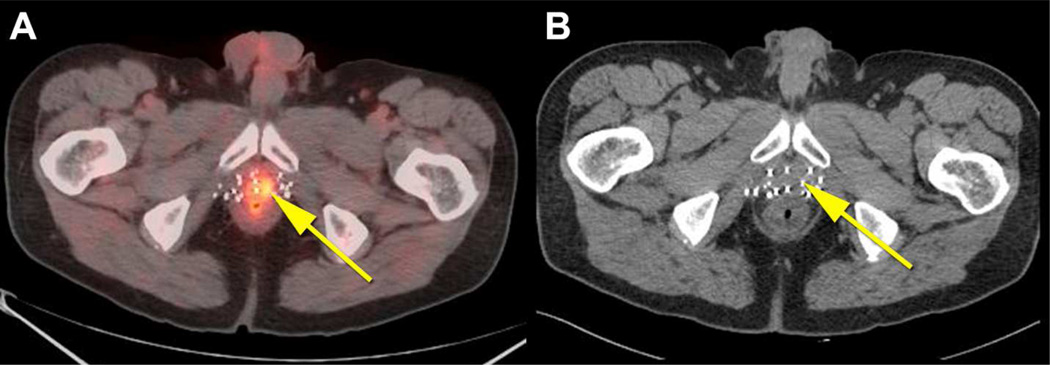
Fig. 3. True positive fluciclovine PET-CT and false negative CT in the prostate.
66 year old patient who had external beam radiation therapy and brachytherapy for prostate cancer with subsequent rise in PSA to 4.85ng/ml. Prostate was positive on biopsy (not shown). Co-registered fluciclovine PET-CT (A) shows abnormal uptake in the prostate meeting criteria for positivity (yellow arrow). CT scan (B) was negative in the prostate.
Extraprostatic
Of the 41 patients with adequate reference standard, on fluciclovine PET-CT scan, there were 12 true positives, 15 true negatives, 0 false positive, and 14 false negatives. CT scan had 3 true positives, 15 true negatives, 0 false positives and 23 false negatives (Fig. 1). Of the 12 true positive patients on fluciclovine, 10 had positive lymph nodes on biopsy, 1 had positive bone biopsy, while the remaining 1 had skeletal disease confirmed on correlative imaging. The 3 CT true positive patients were via index nodes confirmed histologically. Of the 11 patients with histologic proof of extraprostatic disease, fluciclovine detected 11(100%) while CT detected 3 (27.3%) patients. The short axis diameter of the smallest node considered positive on fluciclovine was 0.4cm while that of CT was 0.9cm. Fig. 4 shows a sub-centimetre lymph node with biopsy proven disease interpreted as positive on fluciclovine PET-CT but not on CT.
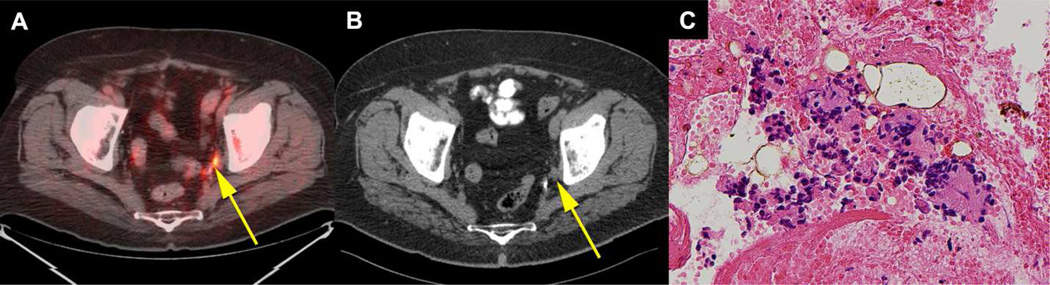
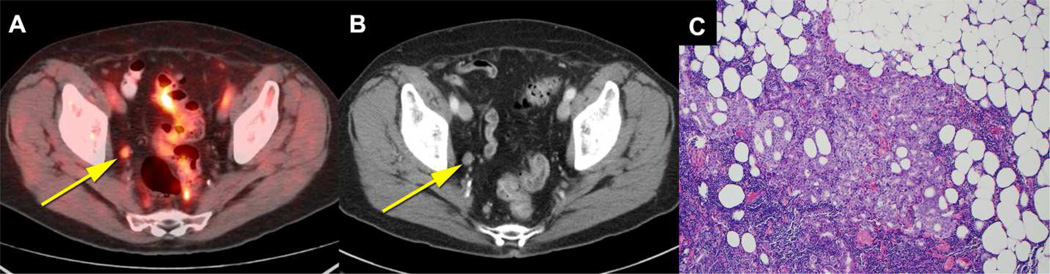
Fig. 4. True positive sub-centimetre lymph node on fluciclovine PET-CT and false negative on CT.
72 year old patient had prostatectomy, external beam radiation therapy and hormone therapy for prostate cancer with subsequent rise in PSA to1.78 ng/ml. 0.4cm × 0.6cm left obturator lymph node (yellow arrow) was positive on coregistered fluciclovine PET-CT (A) but negative on CT (B). Cytology specimen (400X) of the lymph node biopsy (C) demonstrates metastatic prostatic adenocarcinoma.
For extraprostatic disease diagnostic performance, fluciclovine PET-CT had sensitivity of 46.2% (95% CI 37.7, 68.8), specificity of 100% (95% CI 74.7, 100), accuracy of 65.9% (95% CI 49.4, 79.9), PPV of 100% (95% CI 69.8, 100) and NPV of 51.7% (95% CI 32.9, 70.1). CT had a sensitivity of 11.5% (95% CI 3.0, 31.3), specificity of 100.0% (95% CI 74.7, 100.0), accuracy of 43.9% (95% CI 28.5, 60.3), PPV of 100.0% (95% CI 31.0, 100.0) and NPV of 39.5% (95% CI 24.5, 56.5). Sensitivity and NPV differed significantly between fluciclovine and CT (Table 2). Fig. 5 shows biopsy proven nodal disease on both fluciclovine PET-CT and CT scans.
Fig. 5. True positive lymph node on both fluciclovine PET-CT and CT.
76 year old patient had external beam radiation therapy for prostate cancer with subsequent rise in PSA to 5.3ng/ml. Both co-registered fluciclovine PET-CT (A) and CT (B) were positive in a 1.0 cm round right obturator lymph node (yellow arrow). H&E stained section (100X) of the lymph node (C) demonstrates metastatic prostatic adenocarcinoma with extranodal extension.
Subanalysis of fluciclovine PET-CT versus CT with IV contrast
Since 30/53 of the clinical CT scans were performed with IV contrast, a subanalysis of these patients was done. Detailed results of diagnostic performance compared to fluciclovine are in Table 3. Compared to CT, fluciclovine had significantly higher sensitivity, accuracy, PPV and NPV for detection of prostate/bed disease as well as significantly higher sensitivity for detection of extraprostatic disease. Fluciclovine detected 11 more true positive prostate and 6 more extraprostatic lesions than CT with contrast further confirming the overall observation of better disease detection on fluciclovine compared to CT.
Table 3.
Diagnostic performance of fluciclovine PET-CT vs. clinical CT with contrast
| Fluciclovine | CT with contrast | P value | |
|---|---|---|---|
| Prostate/bed (n=29/30) | |||
| True positives | 14 | 3 | - |
| True negatives | 6 | 9 | - |
| False positives | 5 | 2 | - |
| False negatives | 4 | 15 | - |
| %Sensitivity (95% CI) | 77.8 (51.8–92.6) | 16.7 (4.4–42.3) | <0.001* |
| %Specificity (95% CI) | 54.6 (24.6,81.9) | 81.8 (47.8,96.8) | 0.17 |
| %Accuracy (95% CI) | 65.5 (45.7–82.1) | 17.2 (5.8–35.8) | <0.001* |
| %PPV1 (95% CI) | 73.7 (48.6,89.9) | 60.0 (17.0,92.7) | 0.01* |
| %NPV2 (95% CI) | 60.0 (27.4,86.3) | 37.5 (19.6,59.2) | 0.02* |
| Extra prostate (n=26/30) | |||
| True positives | 9 | 3 | - |
| True negatives | 10 | 10 | - |
| False positives | 0 | 0 | - |
| False negatives | 7 | 13 | - |
| %Sensitivity (95% CI) | 56.3 (30.6,79.3) | 18.8 (4.9,46.3) | 0.03* |
| %Specificity (95% CI) | 100 (65.5,100) | 100.0 (65.5,100) | 1 |
| %Accuracy (95% CI) | 73.1 (52.2–88.4) | 50.0 (29.9–70.1) | 0.09 |
| %PPV1 (95% CI) | 100 (62.9,100) | 100.0 (31.0,100) | 1 |
| %NPV2 (95% CI) | 58.8 (33.5,80.6) | 43.5 (23.9,65.1) | 0.3 |
PPV= Positive predictive value;
NPV= Negative predictive value.
- Statistically significant
DISCUSSION
We set out to compare the differences in whole body positivity rates between fluciclovine PET-CT and CT at varying absolute PSA levels, doubling times (DT), and Gleason Score (GS) as well as diagnostic performance in the detection of recurrent prostate carcinoma in prostatic and extraprostatic locations.
We found an overall positivity rate of 18.9% (10/53) on CT, but a higher positivity rate of 77.4% (41/53 patients) on fluciclovine PET-CT. This difference in positivity was sustained across absolute PSA levels, doubling times and Gleason scores. Fluciclovine PET-CT also had better overall performance than CT in the detection of both prostatic and extraprostatic disease. Fluciclovine PET-CT detected local prostatic disease in 31 patients compared to 4 patients detected by CT and also detected 12 patients with extraprostatic disease compared to 3 detected by CT. Although, both imaging modalities had similar PPV in the extraprostatic region, fluciclovine PET-CT detected more patients with disease than CT. Thus, fluciclovine PET-CT better differentiated prostatic from extraprostatic recurrence. One of the strengths of this study is that of the 43 index lesions (31 prostatic, 12 extraprostatic) used to establish the high PPV of fluciclovine, 97.7% had histologic proof.
Our findings are important since therapy for prostate cancer recurrence is informed by the location of recurrence either confined to the prostate/bed, pelvis, and/or extrapelvic regions [2, 22]. Conventional imaging plays an important role in this process [23, 24]. Yet, studies have shown low positivity rates with CT scan in the evaluation of patients with recurrent prostate cancer [5, 6]. While 18F-fluorodeoxyglucose (FDG) is currently the most commonly used PET radiotracer in cancer imaging, it is limited in the detection of prostate cancer [12,25–27]. Due to these limitations, molecular techniques using fluciclovine, choline, acetate, PSMA and other radiotracers are undergoing investigation for prostate cancer imaging [1, 12, 28–30].
Although PSA level is a critical factor in prostate cancer detection rate, PSA doubling time and Gleason Score also impact detection rate as reported with conventional and molecular imaging such as choline PET-CT [12, 25, 31, 32]. In our study, positivity rates with fluciclovine also correlated with increasing absolute PSA, shorter doubling time, and higher Gleason score. In a systemic review of 1000 patients, Choline PET demonstrated positivity rates of 31%, 43% and 81% % with fluciclovine comparing favourably, having positivity rates of 37.5%, 77.8% and 83.3-91.7 at PSA <1, 1-2, and >2 ng ml, respectively [33]. Despite reports showing an increase in disease detection on CT with shorter doubling time, a similar trend was not observed in this study possibly due to the overall low disease detection rate on CT and small sample size [34].
On a recent meta-analysis, choline has reported pooled sensitivity of 75.4% and specificity of 82% in the prostate bed and sensitivity of 100% and specificity of 81.8% for extraprostatic disease [11]. Though this reported diagnostic performance of choline seems to be superior to the diagnostic performance in this study for fluciclovine, a comparison between radiotracers using literature alone should be viewed with caution. Due to differences in study design, PSA kinetics and reference standards, the most reliable comparison is best done by a trial in which radiotracers are utilized within the same patient. Such study was recently reported by Nanni who noted significantly better performance of fluciclovine in comparison to 11C-Choline in the post-prostatectomy recurrence setting [35].
For detection of disease in the post-therapy prostate/bed and in extraprostatic locations, it is not surprising that a molecular technique such as fluciclovine PET will have greater sensitivity than CT. In this study, the sensitivity of 11.4% and 11.5% in the CT detection of recurrent tumour in prostatic/bed and extraprostatic regions respectively, is similar to that reported elsewhere in the literature [36]. Even though sensitivity was higher with CT in a subset of patients in which IV contrast was utilized, sensitivity was still significantly lower than on fluciclovine PET.
In the prostate/bed, specificity was higher with CT than fluciclovine PET-CT likely since significant abnormality must be present on CT in the prostate/bed to reach the threshold of suspicion. Yet, only 4 true positive prostate/beds were detected on CT compared with 31 true positives on fluciclovine. As previously reported, fluciclovine PET does have lower specificity in the prostate, likely due to the confounding effect of prostatic hypertrophy and inflammation [13–15, 37, 38].
For extraprostatic recurrence detection, CT had similarly high specificity and PPV to fluciclovine, likely due to larger nodal size threshold used to designate positivity on CT. Yet again, CT detected only 3 patients with extraprostatic disease compared to 12 patients with fluciclovine PET-CT which has the added advantage of metabolic activity detection. Hovels reported that specificity for diagnosis of nodal metastasis on CT decreases as the threshold size of lymph node positivity decreases but specificity remains high at lymph node size ≥ 1.5cm [26]. In our cohort, all nodes positive on CT were also positive on fluciclovine and small nodes considered benign on CT were detected as positive on imaging with fluciclovine.
The limitations of our study include that CT scans were performed an average of 45.53 days before the fluciclovine scans and although lesions may have grown in the interim to fluciclovine imaging, prostate cancer is typically indolent. While all 53 patients had fluciclovine PET-CT of the abdomen and pelvis, 36/53 CT scans were pelvic only. However, no disease detected on fluciclovine PET-CT had been outside the CT field of view on any patient. IV contrast could not be utilized on CT in 23 patients due to contraindications. Although this mirrors the limitations of routine clinical practice, it reduced the overall detection rate on CT. However, separate data analysis on CT with contrast did not change the overall conclusion of this study of greater sensitivity of fluciclovine compared with CT. Clinical interpretation of CT may have also benefited from access to other clinical imaging and data which were unavailable to the investigational fluciclovine interpretation. Despite detection of bone lesions with fluciclovine not seen on conventional imaging, conclusion regarding diagnostic performance of fluciclovine for bone metastasis is limited as a negative bone scan was an eligibility criterion for this study.
Furthermore, though the positivity rate of 37.5% for molecular imaging with fluciclovine in detecting prostatic disease recurrence at PSA < 1 ng/ml is an advancement over CT, continued improvement is required due to the trend of salvage radiotherapy with PSA<1ng/ml. Early reports have indicated that 68Ga PSMA PET imaging is promising in this regard and may also improve specificity in the prostate/bed over current radiotracers [39].
In conclusion, having earlier reported the superior diagnostic performance of fluciclovine PET compared to 111In–Capromab Pendetide in the restaging of prostate cancer, this sub-analysis of patients who had also undergone routine clinical CT demonstrates the superior performance of fluciclovine compared to CT [14, 15].
Acknowledgments
We acknowledge the significant contributions of Rianot Amzat, MD, Pooneh Taleghani, MD, Delicia Votaw, CNMT, Fenton G. Ingram, RT(R), CNMT, PET, Seraphinah Lawal, RT(R), CNMT, PET, Adam Brown, RT(N), CNMT, Ronald J. Crowe, RPh, BCNP, and the entire Cyclotron and synthesis team from the Emory Center for Systems Imaging, Beverly Hunter, RN, Michelle Faurot, BS, James R. Galt, PhD, John R. Votaw, PhD, our research nurse Leah-Madge Bellamy, RN, MSN and Pardeep Mittal, MD who provided professional and technical information on conventional imaging protocols.
Grant Sponsor: National Institutes of Health Grant (R01CA129356) and Georgia Cancer Coalition.
Funding: David Schuster, Oluwaseun Odewole, Funmilayo Tade, Oladunni Akin-Akintayo. Although not impacting this study, funding is or has been received from Blue Earth Diagnostics Ltd. and Nihon Medi-Physics Co., Ltd. through the Emory University Office of Sponsored Projects for other clinical trials using FACBC (fluciclovine).
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
Patents, Royalties and Licenses: Mark M. Goodman is entitled to a royalty derived from the sale of products related to the research described in this manuscript. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
References
- 1.Schiavina R, Brunocilla E, Borghesi M, Vagnoni V, Castellucci P, Nanni C, et al. Diagnostic imaging work-up for disease relapse after radical treatment for prostate cancer: how to differentiate local from systemic disease? The urologist point of view. Revista espanola de medicina nuclear e imagen molecular. 2013;32(5):310–323. doi: 10.1016/j.remn.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clinical oncology (Royal College of Radiologists (Great Britain)) 2010;22(1):46–55. doi: 10.1016/j.clon.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Boukaram C, Hannoun-Levi JM. Management of prostate cancer recurrence after definitive radiation therapy. Cancer treatment reviews. 2010;36(2):91–100. doi: 10.1016/j.ctrv.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Jager GJ, Ruijter E, Van de Kaa C, de la Rosette J, Oosterhof G, Thornbury JR, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR American journal of roentgenology. 1996;166(4):845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 5.Kramer S, Gorich J, Gottfried HW, Riska P, Aschoff AJ, Rilinger N, et al. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. The British journal of radiology. 1997;70(838):995–999. doi: 10.1259/bjr.70.838.9404201. [DOI] [PubMed] [Google Scholar]
- 6.Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61(3):607–611. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 7.Oyen RH, Van Poppel HP, Ameye FE, Van de Voorde WA, Baert AL, Baert LV. Lymph node staging of localized prostatic carcinoma with CT and CT-guided fine-needle aspiration biopsy: prospective study of 285 patients. Radiology. 1994;190(2):315–322. doi: 10.1148/radiology.190.2.8284375. [DOI] [PubMed] [Google Scholar]
- 8.Zukotynski K, Haider MA. Imaging in prostate carcinoma. Hematology/oncology clinics of North America. 2013;27(6):1163–1187. vii–viii. doi: 10.1016/j.hoc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. The Lancet Oncology. 2011;12(2):181–191. doi: 10.1016/S1470-2045(10)70103-0. [DOI] [PubMed] [Google Scholar]
- 10.Schiavina R, Ceci F, Borghesi M, Brunocilla E, Vagnoni V, Gacci M, et al. The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques? Current radiopharmaceuticals. 2013;6(2):92–95. doi: 10.2174/1874471011306020005. [DOI] [PubMed] [Google Scholar]
- 11.Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38(5):305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 12.Ceci F, Castellucci P, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. 11C-choline PET/CT detects the site of relapse in the majority of prostate cancer patients showing biochemical recurrence after EBRT. Eur J Nucl Med Mol Imaging. 2014;41(5):878–886. doi: 10.1007/s00259-013-2655-9. [DOI] [PubMed] [Google Scholar]
- 13.Odewole OA, Oyenuga OA, Tade F, Savir-Baruch B, Nieh PT, Master V, et al. Reproducibility and Reliability of Anti-3-[F]FACBC Uptake Measurements in Background Structures and Malignant Lesions on Follow-Up PET-CT in Prostate Carcinoma: an Exploratory Analysis. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2014 doi: 10.1007/s11307-014-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. The Journal of urology. 2014;191(5):1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster DM, Savir-Baruch B, Nieh PT, Master VA, Halkar RK, Rossi PJ, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259(3):852–861. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey B, Mena E, Aras O, Garvey B, Grant K, Choyke PL. Functional and molecular imaging: applications for diagnosis and staging of localised prostate cancer. Clinical oncology (Royal College of Radiologists (Great Britain)) 2013;25(8):451–460. doi: 10.1016/j.clon.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kairemo K, Rasulova N, Partanen K, Joensuu T. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. BioMed research international. 2014;2014:305182. doi: 10.1155/2014/305182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okudaira H, Oka S, Ono M, Nakanishi T, Schuster DM, Kobayashi M, et al. Accumulation of trans-1-amino-3-[(18)F]fluorocyclobutanecarboxylic acid in prostate cancer due to androgen-induced expression of amino acid transporters. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2014;16(6):756–764. doi: 10.1007/s11307-014-0756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConathy J, Yu W, Jarkas N, Seo W, Schuster DM, Goodman MM. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Medicinal research reviews. 2012;32(4):868–905. doi: 10.1002/med.20250. [DOI] [PubMed] [Google Scholar]
- 20.McConathy J, Voll RJ, Yu W, Crowe RJ, Goodman MM. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine. 2003;58(6):657–666. doi: 10.1016/s0969-8043(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 21.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talab SS, Preston MA, Elmi A, Tabatabaei S. Prostate cancer imaging: what the urologist wants to know. Radiologic clinics of North America. 2012;50(6):1015–1041. doi: 10.1016/j.rcl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. The Journal of urology. 2008;179(3):906–910. doi: 10.1016/j.juro.2007.10.059. discussion 10. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson JK, Oesterling JE. Patient evaluation if prostate-specific antigen becomes elevated following radical prostatectomy or radiation therapy. The Urologic clinics of North America. 1994;21(4):677–685. [PubMed] [Google Scholar]
- 25.Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level<0.5 ng/mL. Clin Nucl Med. 2013;38(9):342–345. doi: 10.1097/RLU.0b013e31829af913. [DOI] [PubMed] [Google Scholar]
- 26.Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Zaorsky NG, Raj GV, Trabulsi EJ, Lin J, Den RB. The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Seminars in oncology. 2013;40(3):322–336. doi: 10.1053/j.seminoncol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Bouchelouche K, Turkbey B, Choyke P, Capala J. Imaging prostate cancer: an update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep. 2010;11(3):180–190. doi: 10.1007/s11934-010-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44(4):549–555. [PubMed] [Google Scholar]
- 30.Avanesov M, Karul M, Derlin T. [(68)Ga-PSMA as a new tracer for evaluation of prostate cancer: comparison between PET-CT and PET-MRI in biochemical recurrence] . Radiologe. 2015;55(2):89–91. doi: 10.1007/s00117-014-2792-6. [DOI] [PubMed] [Google Scholar]
- 31.Treglia G, Ceriani L, Sadeghi R, Giovacchini G, Giovanella L. Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med. 2014;52(5):725–733. doi: 10.1515/cclm-2013-0675. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Zhao Y, Li X, Sun P, Wang M, Wang R, et al. Imaging primary prostate cancer with 11C-Choline PET/CT: relation to tumour stage, Gleason score and biomarkers of biologic aggressiveness. Radiol Oncol. 2012;46(3):179–188. doi: 10.2478/v10019-012-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cimitan M, Evangelista L, Hodolic M, Mariani G, Baseric T, Bodanza V, et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1,000 patients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(2):209–215. doi: 10.2967/jnumed.114.141887. [DOI] [PubMed] [Google Scholar]
- 34.Okotie OT, Aronson WJ, Wieder JA, Liao Y, Dorey F, De KJ, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. The Journal of urology. 2004;171(6 Pt 1):2260–2264. doi: 10.1097/01.ju.0000127734.01845.99. [DOI] [PubMed] [Google Scholar]
- 35.Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin Nucl Med. 2015;40(8):e386–e391. doi: 10.1097/RLU.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone PA, Tarman GJ, Riffenburgh R, Rohde DC, Puckett ML, Kane CJ. Yield of imaging and scintigraphy assessing biochemical failure in prostate cancer patients. Urologic oncology. 1997;3(4):108–112. doi: 10.1016/s1078-1439(98)00007-6. [DOI] [PubMed] [Google Scholar]
- 37.Schuster DM, Taleghani PA, Nieh PT, Master VA, Amzat R, Savir-Baruch B, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F]-fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3(1):85–96. [PMC free article] [PubMed] [Google Scholar]
- 38.Turkbey B, Mena E, Shih J, Pinto PA, Merino MJ, Lindenberg ML, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270(3):849–56. doi: 10.1148/radiol.13130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(5):668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]