Abstract
Depression and psychostimulant abuse are common comorbidities among humans with immunodeficiency virus (HIV) disease. The HIV regulatory protein TAT is one of multiple HIV-related proteins associated with HIV-induced neurotoxicity. TAT-induced dysfunction of dopamine and serotonin systems in corticolimbic brain areas may result in impaired reward function, thus, contributing to depressive symptoms and psychostimulant abuse. Transgenic mice with doxycycline-induced TAT protein expression in the brain (TAT+, TAT− control) show neuropathology resembling brain abnormalities in HIV+ humans. We evaluated brain reward function in response to TAT expression, nicotine and methamphetamine administration in TAT+ and TAT− mice using the intracranial self-stimulation procedure. We evaluated the brain dopamine and serotonin systems with high-performance liquid chromatography. The effects of TAT expression on delay-dependent working memory in TAT+ and TAT− mice using the operant delayed nonmatch-to-position task were also assessed. During doxycycline administration, reward thresholds were elevated by 20% in TAT+ mice compared with TAT− mice. After the termination of doxycycline treatment, thresholds of TAT+ mice remained significantly higher than those of TAT− mice and this was associated with changes in mesolimbic serotonin and dopamine levels. TAT+ mice showed a greater methamphetamine-induced threshold lowering compared with TAT− mice. TAT expression did not alter delay-dependent working memory. These results indicate that TAT expression in mice leads to reward deficits, a core symptom of depression, and a greater sensitivity to methamphetamine-induced reward enhancement. Our findings suggest that the TAT protein may contribute to increased depressive-like symptoms and continued methamphetamine use in HIV-positive individuals.
Keywords: anhedonia, methamphetamine, cognition, dopamine, serotonin
1. Introduction
Compromised reward and cognitive function after human immunodeficiency virus (HIV) infection may contribute to comorbid drug dependence and depressive symptoms. Impairments in the response to pleasure (i.e., reward deficits or anhedonia) represent a key feature of depression (Pizzagalli et al., 2005, Der-Avakian et al., 2014), and may explain increased rates of major depression/mood disorders after HIV infection and methamphetamine dependence (Kesby et al., 2015, Panee et al., 2015). In HIV-infected subjects, depressed moods, methamphetamine and nicotine dependence have all been associated with decreased likelihood to initiate highly active antiretroviral therapy (HAART) (Tegger et al., 2008, King et al., 2012). Moreover, depressive symptoms predict greater functional decline and cognitive complaints in patients (Sadek et al., 2007). Understanding the relationship between depressive and cognitive symptoms, drug dependence and HIV infection is critical to implementing effective therapeutic strategies.
Both HIV infection and drugs of abuse, including methamphetamine, target subcortical brain structures (Volkow et al., 2001, Gorry et al., 2003, Wang et al., 2012) and dopaminergic systems in particular, which are critically involved in reward processes (Koob and Volkow, 2010). Therefore, impairments in dopaminergic transmission may be one of the mechanisms underlying reward deficits in people with HIV (Kaul and Lipton, 2006, Ferris et al., 2008). Moreover, damage to corticolimbic brain regions such as the basal ganglia, hippocampus, and cerebral cortex (Gorry et al., 2003) are also implicated in HIV-associated cognitive deficits (Heaton et al., 1995, Heaton et al., 2011). The substantial overlap between the associated damage to these brain regions and neurotransmitter systems after HIV infection suggests that reward function may be central to depressive symptoms, drug dependence and cognitive function.
In HIV-infected subjects on antiretroviral treatment, cognitive and depressive symptoms are still prevalent (Heaton et al., 2011), suggesting factors other than viral load may be involved. For example, we have previously shown that expression of HIV-associated gp120 protein increases the sensitivity to methamphetamine and exacerbates cognitive impairments in mice (Kesby et al., 2014, Kesby et al., 2015). The viral TAT protein has also been implicated in HIV-induced neuropathology given its central role in the pathogenesis of HIV infection (for review, (Li et al., 2009)). Inducible transgenic mice that express the viral TAT protein in the brain, under the glial fibrillary acidic protein (GFAP) promoter, provide a useful in vivo model to study the temporal impact of TAT protein in brain function. TAT-expressing mice show neuropathology similar to that observed in HIV-infected humans including apoptosis, astrocytosis, neurodegeneration of the cortex, degeneration of dendrites and inflammation (Kim et al., 2003). Recent imaging studies have revealed reduced gray matter density and cerebral fractional anisotropy abnormalities in multiple brain areas in TAT-expressing mice (Carey et al., 2013, Carey et al., 2015). TAT protein induces dysfunction of dopaminergic neurotransmission in corticolimbic brain circuits (Ferris et al., 2009b, Zhu et al., 2009, Midde et al., 2012, Theodore et al., 2012) that are involved in reward function (Koob and Volkow, 2010). However, it is not known if TAT-induced alterations in dopaminergic function in corticolimbic circuits result in changes in reward processes.
The goal of the present study was to determine the acute and persistent impact of TAT expression on brain reward function (specifically, reward deficits or anhedonia) and neurochemistry. Furthermore, whether TAT-induced impairments in reward function can predict impairments in working memory, a commonly observed deficit in HIV-infected patients (Heaton et al., 2004), was also determined. The use of a doxycycline-inducible transgenic mouse allowed for the assessment of both acute and prolonged effects of TAT expression in the adult mice. Brain reward function in response to TAT expression and acute psychostimulant administration (nicotine and methamphetamine), was assessed in the intracranial self-stimulation (ICSS) procedure (Barnes et al., 2014). The ICSS procedure is particularly sensitive to alterations in limbic dopaminergic projections critical to the motivational aspects of anhedonia (Der-Avakian and Markou, 2012). In addition, dopaminergic and serotonergic function was assessed in the striatum at two time points after the doxycycline regimen by high-performance liquid chromatography (HPLC). Considering that subjects with HIV exhibit deficits in working memory (Heaton et al., 2011), delay-dependent working memory was assessed in TAT-expressing mice using a spatial delayed nonmatch-to-position task (Woolley and Ballard, 2005).
2. Materials and methods
2.1. Animals
For the ICSS study, a total of 30 male mice, with 16 mice containing either the GFAP-null alleles or the TAT protein transgene (TAT−) and 14 mice containing both the GFAP-null alleles and TAT protein transgene (TAT+) were used. An additional cohort of mice (n=6 TAT− and n=6 TAT+ mice) were used to assess neurotransmitter function 3-days after completion of the doxycycline regimen. This time point ensured that TAT protein expression was still evident (Paris et al., 2014c) and differences in neurotransmitter content were not influenced by the acute effects of doxycycline treatment (i.e., the half-life of doxycycline in mice is approximately 170 min (Bocker et al., 1981)). For the DNMTP study, a total of 29 male mice (n=15 TAT− and n=14 TAT+ mice) were used. Inducible TAT transgenic mouse colonies on a C57BL/6J background were obtained by generation of two separate transgenic lines Teton-GFAP mice and TRE-Tat86 mice, and then cross-breeding of these two lines of transgenic mice as previously described (Kim et al., 2003). The mice were housed in a humidity- and temperature-controlled animal facility on a 12 h/12 h reverse light/dark cycle (lights off at 7:00 AM). Mice used for the DNMTP study were housed in groups of 2–4 and food deprived to 85% free-feeding weight, but had ad libitum access to water. Mice used for the ICSS study were housed individually after surgery with ad libitum access to food and water. Behavioral testing was conducted during the dark phase of the light/dark cycle. All of the experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
2.2. Intracranial self-stimulation
The ICSS procedure was conducted as previously described (Stoker et al., 2008, Der-Avakian et al., 2014), detailed methods can be found in the Supplementary Materials. At 4 months of age, mice were anesthetized and a stainless steel bipolar electrode (0.20 mm diameter; 6 mm length; Plastics One, Roanoke, VA, USA) was implanted into the medial forebrain bundle using the following coordinates: anterior/posterior, +1.6 mm; medial/lateral, −1.0 mm; dorsal/ventral, −5.3 mm; from flat skull (Paxinos and Franklin, 2001). The mice were allowed seven post-surgery recovery days prior to commencing training.
ICSS training and testing were conducted in eight Plexiglas operant chambers (30.5×24×27 cm; Med Associates, St. Albans, VT, USA). Mice were tested in the current-threshold procedure. A test session consisted of four alternating series of descending and ascending current intensities, starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the intensity changed by 4–5 μA steps between blocks of trials. The initial stimulus intensity was set approximately 30–40 μA above the baseline current-threshold for each animal. Each test session provided several dependent variables: threshold, response latency and timeout responses. The threshold value of each series was defined as the midpoint in microamperes between the current intensity level at which the animal made two or more positive responses out of the three stimulus presentations and the level at which the animal made less than two positive responses. The animal’s estimated reward threshold for each test session was the mean of the four series’ thresholds. The response latency was defined as the average time in seconds that elapsed between the delivery of the electrical stimulus and the turning of the wheel manipulandum for all of the trials that led to a positive response. Timeout responses were defined as the total number of responses that occurred during the intertrial interval for a test session. All mice were successfully trained in the ICSS task confirming correct electrode placement (Stoker and Markou, 2011).
2.3. ICSS Experimental Timeline
Mice underwent stereotaxic surgery and were subsequently trained to perform in the ICSS procedure until stable baseline reward threshold levels were achieved after at least 15 days of testing. Subsequently, all mice were treated with a doxycycline hyclate (DOX; Sigma, St. Louis, MO, USA) regimen consisting of 100 mg/kg, intraperitoneally (i.p.), once a day for 7 days. This regimen was based on the previously demonstrated efficacy of TAT induction at this dose of DOX (Carey et al., 2012). Only mice containing both the GFAP-null alleles and TAT protein transgene (TAT+) generate TAT protein after DOX administration. To assess the acute effects of DOX, mice were tested 2–3 h after the first DOX administration. Subsequently, mice were tested daily in the ICSS procedure in the morning (08:00–11:00h) and given the remaining six DOX injections in the evening (17:00h). After the final DOX administration, mice were tested daily for 14 days. Then, using a within subject Latin square design, dose-response function for acute nicotine (12 days) followed by acute methamphetamine (19 days) were assessed. Acute subcutaneous nicotine (nicotine hydrogen tartrate; Sigma) injections at doses of 0.075, 0.15 and 0.3 mg/kg base were given 5 min before testing, three days apart. Acute methamphetamine (methamphetamine hydrochloride; Sigma) injections (i.p.) at doses of 0.25, 0.5 and 1 mg/kg were given 20 min before testing, four days apart. The highest dose of methamphetamine (2 mg/kg) was given last, after administration of the all the other doses. Mice were euthanized three days after the final acute methamphetamine administration and the caudate putamen (CPu) and nucleus accumbens (Acb) dissected from the contralateral hemisphere to the electrode placement. Certain data from mice were excluded from the final analyses (see Supplementary Materials).
2.4 High-performance liquid chromatography of neurotransmitters
Brain neurochemistry was analyzed using a HPLC system with electrochemical detection as previously described (Kesby et al., 2009, Kesby et al., 2016), see Supplementary Materials for details. Catecholamine and indoleamine levels in the CPu and Acb were assessed for acute effects at three days and persistent effects at 40 days after the completion of the doxycycline regimen. Data was processed with Dionex Chromeleon software (v7.2, Thermo Scientific, CA, USA).
2.5. Delayed nonmatch-to-position (DNMTP) testing
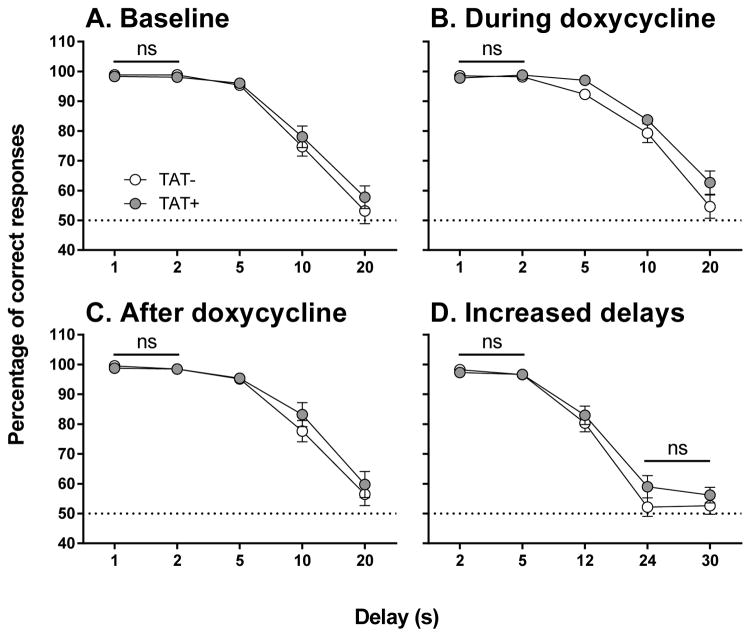
The DNMTP task was established in the laboratory based on published literature (Woolley and Ballard, 2005), see Supplementary Materials for details. Briefly, DNMTP training and testing were conducted in twelve Plexiglas operant chambers (model ENV-307A, Med Associates Inc., St. Albans, VT, USA), each enclosed in a sound-attenuating cubicle. The nonmatch-to-position rule consisted of sample and choice stages during 60 trials in each daily session. During the sample stage, a single lever was presented and the stimulus light above the active lever was illuminated. The mouse was required to press the sample lever, which immediately retracted, and to nose poke into the food magazine to begin the choice stage. During the choice stage, both levers were presented and associated stimulus lights illuminated. Pressing the opposite lever to that previously presented at the sample stage resulted in the delivery of a single food reward (20 mg Sucrose pellet, TestDiet, IN, USA). If the mouse pressed the other lever it was recorded as an incorrect response and was unrewarded. An incorrect response or failure to respond to either the sample or choice levers during the 10 s limited hold (i.e., an omission) resulted in a time out period (house lights on) of 5 s. The delay periods for DNMTP testing were 1, 2, 5, 10, 20 s. Delay intervals were presented in a pseudorandom manner forcing mice to continuously nosepoke during the delay period in order to avoid mediating behaviour. Correct responses were calculated as a percentage of the total trials (not including omissions) across all seven days. All other measures including the latencies to respond and collect reward were averaged to provide a single value of each performance variable for each mouse.
After the acquisition of stable baseline performance (>80% correct responses on the two lowest delays over 3 days), mice were administered 7 daily DOX injections (see section 2.3) in the evening (17:00h) and tested daily in the morning (08:00–10:00 h) during DOX administration and for 7 days after the final DOX injection. At this stage, the delays were increased to 2, 5, 12, 24, 30 s (DNMTP2) for 4 days. Behavioral measures included percentage correct responses for the five delay time periods during each phase of testing (baseline, during DOX administration, post-DOX administration and the final DNMTP2 testing). Number of omissions was calculated as the total number of missed trials during the session, i.e. no response to the lever or food magazine during the delay period. Latency measures included the amount of time (ms) the animals took to respond to the sample lever, choice levers (separated for correct and incorrect responses) and to collect the food reward.
2.6. Statistical analyses
All analyses were performed with IBM SPSS Statistics 20 (Armonk, NY, USA). Data were analyzed using analysis of variance (ANOVA), with TAT as the between-subject factor. Repeated-measures ANOVAs were used when within-subject factors were present. When appropriate, post hoc comparisons were performed using Least Significant Difference (LSD) analyses. ICSS threshold data were expressed as the percentage of baseline thresholds during DOX administration and the post-DOX period, and as a percentage of saline treatment in experiments with acute nicotine and methamphetamine challenges. To determine if there were any effects of the nicotine and methamphetamine dosing order on reward thresholds, additional analyses were conducted with Dosing Group (4 groups used for the Latin square design) included as a between-subjects factor. ICSS timeout responses were expressed as the difference from baseline or saline responses as appropriate. Given the high variance in total amount, all neurochemicals were converted to a percentage of the TAT− mean and analysed accordingly. Data on DNMTP trials completed were analyzed using an ANOVA with Block (baseline week, DOX week (days immediately after DOX injections) and post-DOX week) as the repeated measure. The percentage of correct responses was calculated from all trials included in each seven- or four-day period (7-day: baseline, DOX and post-DOX; 4-day: DNMTP2), and analyzed with Block as the repeated measure. Mean latencies were analyzed using an ANOVA with Block (baseline, DOX, post-DOX and DNMTP2) as the repeated measure. Results are expressed as mean ± SEM. Differences were considered statistically significant at p<0.05.
3. Results
3.1. ICSS testing during doxycycline-induced TAT expression
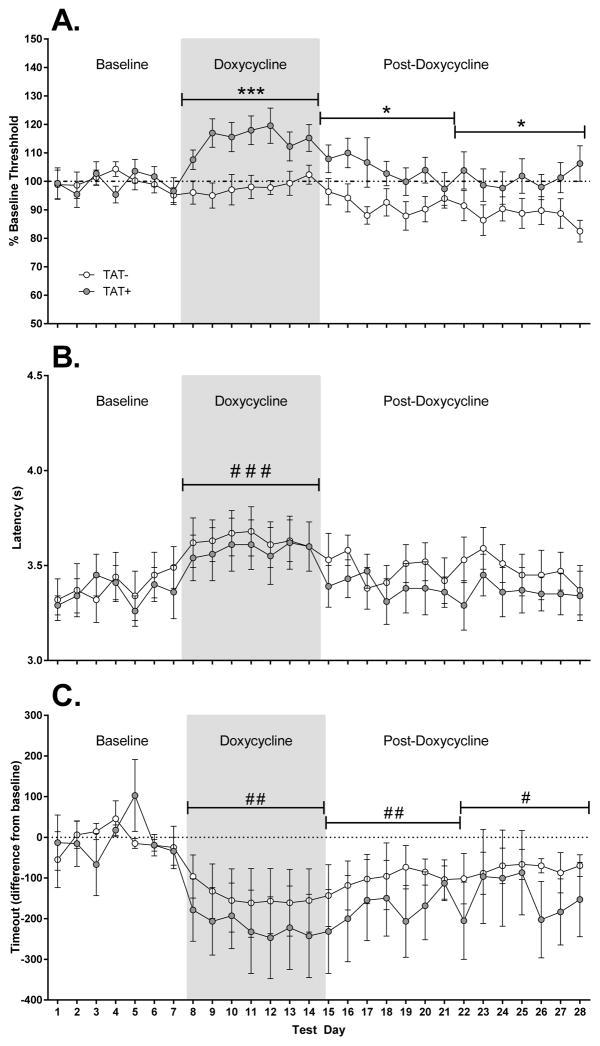
ICSS data were analyzed as four blocks of 7 days, consisting of the baseline, DOX (days immediately after DOX injections) and two post-DOX weeks (Figure 1). For reward thresholds (Figure 1A), there was a significant main effect of Block (F3,81=8.0, p<0.001) with reward thresholds significantly higher during DOX compared with thresholds during the baseline or post-DOX periods (p<0.01). There was also significant main effect of TAT (F1,27=9.1, p<0.01) and a significant interaction of Block x TAT (F3,81=4.6, p<0.01). TAT+ mice showed significantly higher reward thresholds than TAT− mice during DOX administration (p<0.001) and during the first (p<0.05) and second (p<0.05) post-DOX weeks. For latencies (Figure 1B), there was a significant main effect of Block (F3,81=10.0, p<0.001) with the latency to respond significantly higher during DOX compared with the baseline or post-DOX periods (p<0.001). For timeout responses (Figure 1C), there was a significant main effect of Block (F3,81=8.5, p<0.001) with the number of timeout responses lower during DOX and post-DOX periods than at baseline (p<0.05). Timeout responses were also significantly lower during DOX compared with the first (p<0.05) and second (p<0.05) post-DOX weeks. There were no differences between TAT− and TAT+ for reward thresholds (TAT−: 155.6±10.5%; TAT+: 144.6±14.2%), response latencies (TAT−: 4.07±0.11 s; TAT+: 3.72±0.17 s) or timeout responses (TAT−: −184.3±85.4 responses; TAT+: −279.7±100.3 responses) after acute DOX administration (2 h prior to testing, day 1).
Figure 1. Effects of TAT protein expression on reward thresholds, latency to respond and timeout responses.
TAT protein expression significantly elevated reward thresholds in TAT+ mice (A). This effect was most evident during doxycycline administration. The latency to respond was increased during doxycycline administration but no differences between TAT− and TAT+ mice were observed (B). Timeout responses decreased during doxycycline administration (C) and remained lower than baseline testing for the two weeks following the doxycycline regimen.
* p < 0.05, *** p < 0.001 significant difference between TAT− and TAT+ mice.
#p < 0.05, ## p < 0.01, ### p < 0.001 significant difference when compared to baseline.
3.2. Effects of acute nicotine on brain reward function
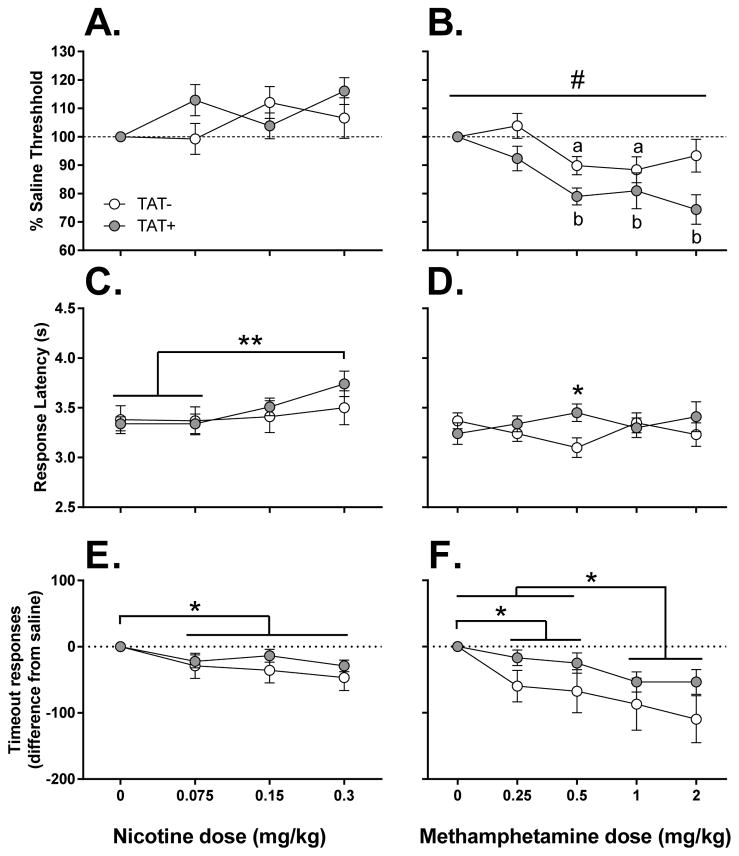
Acute nicotine administration did not alter reward thresholds (Figure 2A) in either TAT− or TAT+ mice with no significant effect of Dose or TAT detected. However, there was a significant main effect of Dose on the latency to respond (F3,75=6.2, p<0.01). The highest nicotine dose, 0.3 mg/kg, significantly increased response latency compared to saline (p<0.01) and 0.075 mg/kg (p<0.01)(Figure 2C). There was also a significant main effect of Dose on the number of timeout responses (F3,75=5.4, p<0.01) with all doses of nicotine decreasing timeout responses compared with saline treatment (p<0.05)(Figure 2E). nalysis of the dosing order on reward thresholds revealed no significant effects of Dosing Group or interaction of Dosing Group x TAT.
Figure 2. Effects of TAT protein expression after acute nicotine and methamphetamine on reward thresholds, latency to respond and timeout responses.
Acute nicotine failed to significantly alter reward thresholds (A) but dose-dependently increased response latencies (C) and decreased timeout responses (E). TAT protein expression potentiated methamphetamine-induced reward enhancement (B) as indicated by significantly lower reward thresholds in TAT+ mice compared with TAT− mice after methamphetamine. TAT+ mice showed a greater response latency than TAT− mice after 0.5 mg/kg methamphetamine (D). Methamphetamine also significantly decreased timeout responses in all mice (F).
* p < 0.05, *** p < 0.001.
#p < 0.05 significant main effect of TAT.
ap < 0.05 compared to saline response in TAT− mice.
bp < 0.05 compared to saline response in TAT+ mice.
3.3. The effects of acute methamphetamine on brain reward function
Acute methamphetamine administration lowered reward thresholds (Figure 2B) with a significant main effect of Dose present (F4,88=10.0, p<0.001). A significant main effect of TAT (F1,22=6.5, p<0.05) was detected with TAT+ mice showing a greater reward threshold lowering after methamphetamine than TAT− mice. Compared with saline treated mice, TAT− mice showed significantly threshold lowering after 0.5 and 1 mg/kg of methamphetamine (p<0.05); whereas TAT+ mice showed significantly threshold lowering after 0.5, 1 and 2 mg/kg of methamphetamine (p<0.05). A significant interaction of Dose x TAT (F4,88=3.2, p<0.05) was detected for the response latency after methamphetamine (Figure 2D) with a greater response latency after 0.5 mg/kg methamphetamine observed in TAT+ mice compared to TAT− mice (p<0.05). For timeout responses (Figure 2F), there was a significant main effect of Dose (F4,88=9.9, p<0.001) with all doses of methamphetamine decreasing the number of timeout responses compared with saline (p<0.05). Furthermore, a stepwise effect was also observed with the higher doses of methamphetamine (1 and 2 mg/kg) reducing timeout responses more than the lower doses (0.25 and 0.5 mg/kg; p<0.05). Analysis of the dosing order on reward thresholds revealed no significant effects of Dosing Group or interaction of Dosing Group x TAT. Similarly, there were no significant effects of the nicotine Dosing Group for the response to methamphetamine.
3.4. Dopamine and serotonin levels
3.4.1. Effects of acute TAT expression on neurochemistry (3 days after doxycycline regimen)
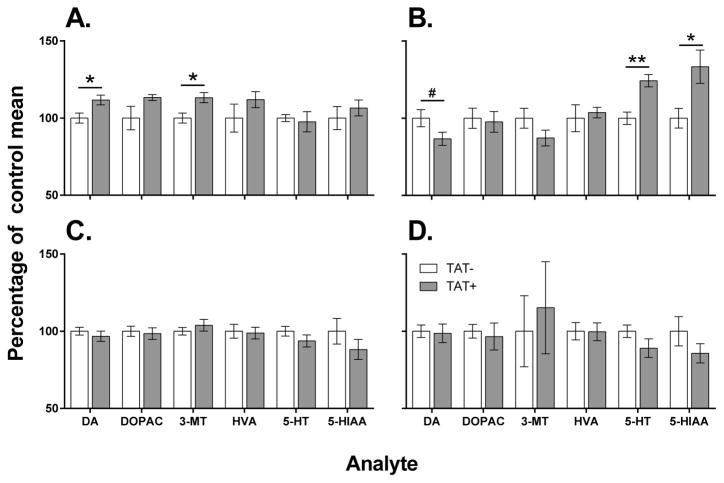
In the CPu (Figure 3A), TAT+ mice had increased levels of dopamine (F1,10=6.9, p<0.05) and 3-MT (F1,10=8.1, p<0.05) compared to TAT− mice. In the Acb (Figure 3B), TAT+ mice had increased levels of serotonin (F1,10=18.6, p<0.01) and 5-HIAA (F1,10=7.1, p<0.05) compared to TAT− mice. There was also a trend for decreased Acb levels of dopamine (F1,10=3.6, p<0.1) in TAT+ mice compared to TAT− mice
Figure 3. Acute and persistent effects of TAT protein expression on dopaminergic and serotonergic function.
Acute TAT expression (3 days after doxycycline treatment) increased dopamine (DA) and 3-methoxytyramine (3-MT) levels in the caudate putamen of TAT+ mice compared with TAT− mice (A). In the nucleus accumbens (B), acute TAT expression increased levels of serotonin (5-HT) and 5-hydroxy-indoleacetic acid (5-HIAA) in TAT+ mice compared with TAT− mice. There was also a trend toward decreased dopamine levels in the nucleus accumbens of TAT+ mice compared with TAT− mice. There were no persistent effects of prior TAT expression (40 days after doxycycline treatment) in the caudate putamen (C) or nucleus accumbens (D) of TAT+ mice compared with TAT− mice. DOPAC, dihydroxyphenylacetic acid; HVA, homovanillic acid.
* p < 0.05, ** p < 0.01, # p < 0. 1.
3.4.2. Persistent effects of TAT expression on neurochemistry (40 days after doxycycline regimen)
Prior TAT expression had no significant effect of dopaminergic or serotonergic function in either the CPu (Figure 3C) or Acb (Figure 3D).
3.5. DNMTP training and trials completed
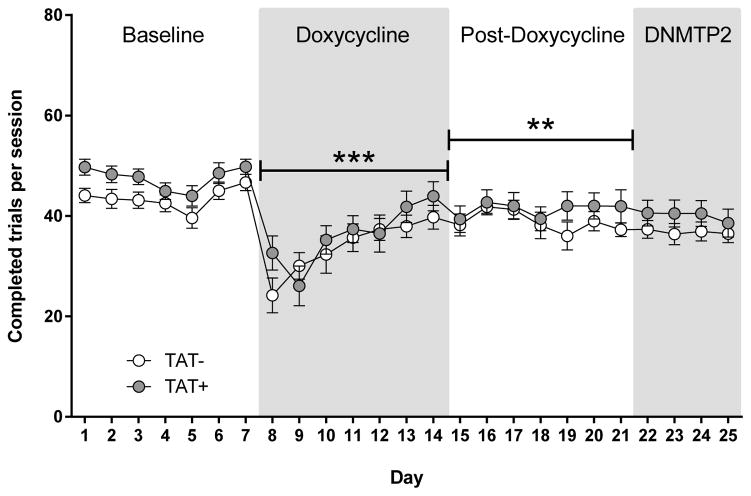
There were no significant differences between TAT− and TAT+ mice on the number of days to reach criterion in each training stage (Figure S1). Data on DNMTP trials completed were analyzed as three blocks of 7 days, consisting of the baseline, DOX (days immediately after DOX injections) and the post-DOX week (Figure 4). There were significant main effects of Block (F2,54=39.8, p<0.001) and Day (F6,162=7.7, p<0.001), and a significant interaction of Block x Day (F12,324=7.6, p<0.001) on the trials completed. All the blocks were significantly different from each other (p<0.01) with the greatest number of trials completed during the baseline period and the lowest number of trials completed during DOX administration. The effect of DOX was most severe initially, with mice gradually increasing the trials completed across the days of testing. There were no significant differences between TAT− and TAT+ mice on trials completed during DNMTP2 testing with increased delays.
Figure 4. Effects of TAT protein expression on trials completed during delayed nonmatch-to-position testing.
Doxycycline administration significantly decreased the trials completed in all mice. This was most evident during the doxycycline regimen but also persisted during the following week of testing. No differences between TAT− and TAT+ mice were observed at any stage of testing.
** p < 0.01, *** p < 0.001 when compared to baseline.
3.6. DNMTP testing
The percentage of correct responses was calculated from all trials over seven days for the baseline, DOX and post-DOX periods, or four days for the DNMTP2 period. There were no differences between TAT− and TAT+ mice at any stage of testing (Figure 5). There was a significant main effect of Delay during baseline (F4,108=154.6, p<0.001), DOX (F4,108=141.3, p<0.001), post-DOX (F4,108=109.2, p<0.001) and DNMTP2 (F4,108=197.6, p<0.001). Performance decreased with delays greater than 2 s with each increase in delay further impairing performance during baseline, DOX and post-DOX (p<0.01). Mice tended to respond at chance levels (50% correct responses) at delays of 24 s or greater as demonstrated by a plateau in performance at 24 and 30 s delays in the DNMTP2 testing.
Figure 5. Effects of TAT protein expression on delay-dependent memory prior to TAT expression.
Delay-dependent memory during the baseline period (A), during doxycycline administration (B), after doxycycline administration (C) and during subsequent testing with increased delays (D; DNMTP2). There were no differences between TAT− and TAT+ mice at any stage of testing. Within each panel all delays are significantly different from one another (p < 0.05) except for comparisons highlighted as not significant (ns).
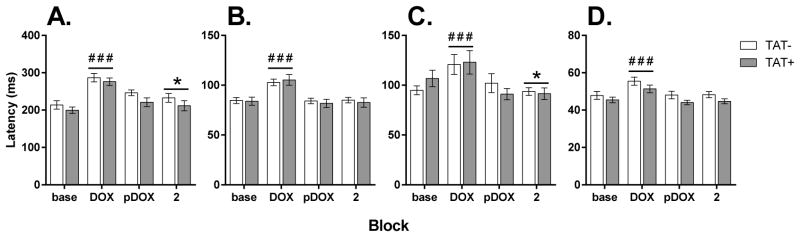
Mean latencies were analyzed as four blocks (baseline, DOX, post-DOX and DNMTP2). There was a significant effect of Block for all response latencies (Figure 6) including the lever response during the sample stage (F3,81=57.9, p<0.001), correct responses during the choice stage (F3,81=81.3, p<0.001), incorrect responses during the choice stage (F3,81=11.7, p<0.001) and reward collection (F3,81=40.7, p<0.001). In all cases, latencies were significantly greater during DOX administration compared with all other periods of testing (p<0.01). For both sample lever responses and incorrect responses, latencies were also significantly different during DNMTP2 testing than during baseline (p<0.05). There were no differences between TAT+ and TAT− mice in response latencies.
Figure 6. Effects of TAT protein expression on latencies during delayed nonmatch-to-position testing.
The latency to press the sample lever (A, left), press the correct choice lever (B, middle left), press the incorrect choice lever (C, middle right) and to collect the reward (D, right) in TAT− and TAT+ mice. No significant differences between TAT− and TAT+ mice were observed in any measure or at any stage of testing. Doxycycline (DOX) administration increased the latencies for all measures when compared to baseline testing (base) but these differences did not persist post-DOX (pDOX). Increasing the delays during DNMTP2 (2) increased the latency to press the sample lever and decreased the latency to press the incorrect choice lever when compared to baseline testing.
##p < 0.01, ### p < 0.001 when compared to all other blocks.
* p < 0.05 compared to baseline testing.
4. Discussion
The present study demonstrated that acute TAT protein expression led to reward deficits or anhedonia as demonstrated by elevated reward thresholds in the ICSS task. Furthermore, increased serotonergic function and decreased dopaminergic function were observed in the Acb of TAT+ mice at a time point associated with TAT-induced anhedonia. In addition, prior TAT exposure led to an increased sensitivity to methamphetamine-induced reward enhancement but did not affect nicotine-induced alterations in brain reward function. TAT expression did not affect delay-dependent working memory in the DNMTP task suggesting that alterations in brain reward function do not predict cognitive impairments, at least for this cognitive construct.
Anhedonia reflects decreased interest or pleasure in rewarding stimuli and is a trait feature of major depressive disorder (Pizzagalli et al., 2005, Der-Avakian et al., 2014). Little is known about the effects of HIV-related proteins including TAT on depression-like behaviour in animal models, which remains an unmet need in HIV-related basic science research (Barreto et al., 2014). In the present study, we demonstrated that TAT protein expression led to a significant elevation in reward thresholds reflecting anhedonia. Consistent with our findings, intracerebroventricular administration of TAT protein in rats resulted in depression-like behavior in the forced swim test and sucrose-preference test (Lawson et al., 2011). Female HIV-1 transgenic rats have also been shown to exhibit a range of depression-like behaviors (learned helplessness) and social anxiety (Nemeth et al., 2014). In addition, TAT expression alters other affective behaviors, such as anxiety-like behavior assessed in a variety of behavioral procedures (Paris et al., 2014b, Paris et al., 2014c, Hahn et al., 2015). Our findings demonstrate that expression of the TAT protein alone is sufficient to induce anhedonia and may contribute to comorbid depression in HIV-infected subjects.
The motivational aspects of reward, which the ICSS procedure is most sensitive to, are thought to be driven primarily by dopaminergic projections from the ventral tegmental area to the Acb (Der-Avakian and Markou, 2012). For example, electrical self-stimulation increases dopamine release in the Acb (Owesson-White et al., 2008, Beyene et al., 2010). Psychostimulant administration potentiates stimulated dopamine release (Hernandez et al., 2012) resulting in reward enhancement, that is lowering of reward thresholds. In contrast, anhedonia is reflected by ICSS threshold elevations and is associated with decreased dopamine transmission in the Acb (Carlezon et al., 2006). Consistent with this premise, assessment of neurotransmission 3 days after the final doxycycline treatment, when TAT protein expression (Paris et al., 2014c) and anhedonia (see Figure 1) is evident, revealed a trend for decreased dopamine levels in the Acb of TAT+ mice. In agreement with our data, TAT infusions into the Acb impair dopamine transporter function (Ferris et al., 2009a) and decrease stimulated dopamine release (Ferris et al., 2009b). Consistent with the effects of TAT in animal models and the high rate of comorbid depression, HIV-infected subjects have significantly reduced dopamine transporter density in the striatum (Chang et al., 2008) and decreased dopamine levels in cerebrospinal fluid (Berger et al., 1994). Thus, the suppressing effects of TAT protein on dopamine function in mesolimbic pathways may mediate the observed anhedonia in the present study and in HIV-infected subjects. The concomitant increase in dopamine and dopamine release (3-MT represents a proxy marker for dopamine release) in the CPu 3-days after doxycycline administration is somewhat perplexing. In vitro studies have demonstrated decreased dopamine transporter function and uptake after TAT protein exposure (Zhu et al., 2009, Midde et al., 2012) which may partially explain increased levels of 3-MT. Increased dopamine levels may represent a compensatory response in striatonigral projections. Importantly, changes in Acb or CPu dopaminergic function were no longer present 11 (Kesby et al., 2016) or 40 days (see Figure 3C) after doxycycline treatment indicating no persistent effects of TAT expression on baseline levels of dopamine.
The observed increases in serotonergic function in the Acb of TAT+ mice may also contribute to TAT-induced anhedonia. Consistent with our findings, systemic treatments that increase serotonin levels have been shown to elevate reward thresholds in rats (Harrison and Markou, 2001). Thus, increased serotonergic transmission exerts an inhibitory influence on reward processes leading to anhedonia. Together, these findings are contradictory to clinical observations that drugs that enhance serotonin neurotransmission, serotonin reuptake inhibitors, are considered the first line of treatment for depressed HIV+ subjects (Arseniou et al., 2014). However, the response to antidepressant treatment in depression is complicated and aspects of plasticity-mediated brain changes may be more relevant than discrete actions on serotonergic function (Manji et al., 2001). Furthermore, altered serotonin synthesis has been identified as a potential mediator of neuroinflammation mediated depression (Dantzer et al., 2008). Intracerebroventricular TAT administration in mice is associated with increased inflammatory mediators (Lawson et al., 2011), and some inflammatory mediators can increase serotonergic function (Dunn et al., 2005). Thus, inflammation-associated increases in serotonergic transmission may mediate TAT-induced anhedonia in the present study.
A host of molecular pathways have been identified through which the effects of TAT and methamphetamine either overlap or synergize (Mediouni et al., 2015). However, little is known about the combined effects of TAT and methamphetamine on behavioral outcomes. Our findings suggest that previous TAT exposure increases the sensitivity to methamphetamine-induced reward enhancement. Importantly, this effect was observed nearly four weeks after the final doxycycline treatment, assuring that the TAT protein was not present during the acute challenges (Paris et al., 2014c). It is important to consider that TAT-induced differences after acute methamphetamine challenge were only observed for reward thresholds and not timeout responses or the latency to respond. Thus, it is unlikely that alterations in impulsivity-like disinhibition of responding (Amitai et al., 2009) or indirect effects on motor function contributed to the present results. Similar reward alterations have also been observed by others during acute TAT expression. For example, acute TAT expression increased the conditioned rewarding effects of both ethanol and cocaine in the conditioned place preference test (Paris et al., 2014a, McLaughlin et al., 2015). Furthermore, TAT infusions into the Acb increased locomotor activation induced by acute cocaine (Harrod et al., 2008). We have previously found enhancement of the rewarding properties of methamphetamine independent of changes in the reward or motivation for a natural food reinforcer in gp120 expressing mice (Kesby et al., 2014). Infusions of TAT or gp120 impair dopamine function in the rat striatum (Bansal et al., 2000) which may be mediated by similar mechanisms (Wallace et al., 2006). Taken together, these data indicate that HIV-associated proteins, such as TAT or gp120, may enhance the rewarding properties of methamphetamine, and other drugs of abuse, by impairing dopaminergic function in mesolimbic circuitry. Our findings suggest that the use of TAT vaccines, currently being tested in clinical trials (Loret et al., 2016), if administered early enough may prevent alterations in brain reward function, and decrease the rewarding properties of methamphetamine or other drugs of abuse in the HIV+ population.
Prior TAT exposure did not alter the response to the effects of nicotine on reward function suggesting that TAT-induced increases in reward sensitivities to drugs of abuse are not ubiquitous. Unlike others (Fowler et al., 2013), we did not observe nicotine-induced reward enhancement in control TAT− mice. However, our findings do not preclude that other methods may reveal TAT-induced alterations in sensitivity to the rewarding effects of nicotine or the potential for alterations in the response to nicotine during acute TAT protein expression. Recent work demonstrated decreased nicotine-induced locomotor sensitization in HIV-1 transgenic rats (Midde et al., 2011) and after infusions of TAT into the ventral tegmental area in rats (Zhu et al., 2015). These findings suggest that the TAT protein may reduce sensitivity to nicotine. It has been demonstrated that nicotine-based reward enhancement is not dependent on direct effects on the dopamine transporter, unlike methamphetamine (Gerasimov et al., 2000). TAT protein has been shown to directly alter dopamine transporter function (Zhu et al., 2009, Midde et al., 2012) which may explain the methamphetamine-specific effects on reward enhancement observed in the present study. Thus, TAT protein appears to interact differently with nicotine compared to methamphetamine, perhaps due to their differing mechanisms in activating the dopamine system.
TAT expression did not impair delay-dependent working memory in the present study, although working memory deficits are common in HIV-infected individuals (Heaton et al., 1995, Heaton et al., 2011). Anhedonia and motivational deficits, observed in the ICSS procedure during acute TAT expression, can impair behavioral performance in reward-based cognitive tasks. However, in the DNMTP task, behavioral measures reflective of motivational state, including the total trials completed, rewards gained, latencies to respond and to collect the reward, were not significantly different between TAT− and TAT+ mice. In contrast to our findings, spatial memory impairments have been observed after TAT exposure in mice (Carey et al., 2012) but this may be due to learning impairments induced by concomitant TAT protein expression rather than specific memory impairments (Kesby et al., 2016). Thus, the TAT protein may not contribute to delay-dependent spatial working memory in HIV-infected individuals although it may contribute to other forms of memory encoding and retrieval. Alternatively, a longer period of TAT protein exposure (via doxycycline containing food or water) may lead to more robust neuropathology and thus impact working memory. If this is the case, TAT vaccines (Loret et al., 2016) may prevent aspects of cognitive decline in HIV+ individuals. Our results suggest that short-term TAT protein exposure is insufficient to induce delay-dependent working memory impairments but sufficient to induce persisting alterations to the rewarding properties of methamphetamine.
5. Conclusions
Our findings in mice suggest that TAT protein expression is sufficient to induce anhedonia and lead to a long-lasting increase in sensitivity to the rewarding effects of methamphetamine. However, TAT-mediated effects on reward function do not predict impairments in delay-dependent working memory and, therefore, do not represent an impairment in general brain function. Our findings suggest that TAT expression may contribute to comorbid depression in treated HIV-infected subjects, even those with adequate viral suppression. Furthermore, impairments in reward function may increase the risk of developing methamphetamine dependence in HIV-infected subjects. The DOX-inducible, TAT-expressing mouse represents a valuable tool to investigate neurobiological mechanisms underlying comorbid depression and methamphetamine dependence after HIV infection.
Supplementary Material
Highlights.
TAT protein expression in the brain produced an anhedonia-like phenotype.
TAT protein expression altered mesolimbic dopamine and serotonin levels.
Prior TAT expression increases the rewarding effects of methamphetamine.
Prior TAT expression does not impair delay-dependent working memory.
TAT expression may contribute to comorbid depression in HIV+ subjects.
TAT expression may exacerbate methamphetamine dependence in HIV+ subjects.
Acknowledgments
This work was supported by an NIH/NIDA grant (DA033849 to SS), the Translational Methamphetamine AIDS Research Center (P50DA026306) and the Interdisciplinary Research Fellowship in NeuroAIDS (R25MH081482 to JPK). AM has received contract research support from Forest Laboratories and Astra-Zeneca and consulting fees from AbbVie during the past 2 years. AM and SS have a patent on metabotropic glutamate compound use for the treatment of nicotine dependence, unrelated to the present research. JPK, AM and SS have no competing financial interests in relation to the work described.
Footnotes
Author contributions
JPK, AM and SS were responsible for the study concept and design. JPK was responsible for the acquisition of the animal data, data analysis and manuscript drafting. SS and AM provided critical revision of the manuscript for important intellectual content. All of the authors critically reviewed the content and approved the final version for publication.
Conflict of Interest Statement
AM has received contract research support from Forest Laboratories and Astra-Zeneca and consulting fees from AbbVie during the past 2 years. AM and SS have a patent on metabotropic glutamate compound use for the treatment of nicotine dependence, unrelated to the present research. JPK, AM and SS have no competing financial interests in relation to the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amitai N, Semenova S, Markou A. Clozapine attenuates disruptions in response inhibition and task efficiency induced by repeated phencyclidine administration in the intracranial self-stimulation procedure. Eur J Pharmacol. 2009;602:78–84. doi: 10.1016/j.ejphar.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry and clinical neurosciences. 2014;68:96–109. doi: 10.1111/pcn.12097. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Der-Avakian A, Markou A. Anhedonia, avolition, and anticipatory deficits: Assessments in animals with relevance to the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:744–758. doi: 10.1016/j.euroneuro.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto ICG, Viegas P, Ziff EB, Konkiewitz EC. Animal Models for Depression Associated with HIV-1 Infection. J Neuroimmune Pharm. 2014;9:195–208. doi: 10.1007/s11481-013-9518-9. [DOI] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B. Cerebrospinal fluid dopamine in HIV-1 infection. Aids. 1994;8:67–71. doi: 10.1097/00002030-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Beyene M, Carelli RM, Wightman RM. Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience. 2010;169:1682–1688. doi: 10.1016/j.neuroscience.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker R, Estler CJ, Maywald M, Weber D. Comparison of distribution of doxycycline in mice after oral and intravenous application measured by a high-performance liquid chromatographic method. Arzneimittel-Forschung. 1981;31:2116–2117. [PubMed] [Google Scholar]
- Carey AN, Liu X, Mintzopoulos D, Paris JJ, McLaughlin JP, Kaufman MJ. Conditional Tat Protein Brain Expression in the GT-tg Bigenic Mouse Induces Cerebral Fractional Anisotropy Abnormalities. Curr HIV Res. 2015;13:3–9. doi: 10.2174/1570162x13666150126125244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Liu XX, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, Kaufman MJ. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;43:49–54. doi: 10.1016/j.pnpbp.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. The Journal of pharmacology and experimental therapeutics. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat1-86, impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux microdialysis study. Neuroscience. 2009a;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In Vivo Microdialysis in Awake, Freely Moving Rats Demonstrates HIV-1 Tat-Induced Alterations in Dopamine Transmission. Synapse. 2009b;63:181–185. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Tuesta L, Kenny PJ. Role of alpha 5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology. 2013;229:503–513. doi: 10.1007/s00213-013-3235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse. 2000;38:432–437. doi: 10.1002/1098-2396(20001215)38:4<432::AID-SYN8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Wesselingh SL, Purcell DFJ. Astrocyte infection by HIV-1: Mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr Hiv Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2015;220:605–623. doi: 10.1007/s00429-013-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Markou A. Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. The Journal of pharmacology and experimental therapeutics. 2001;297:316–325. [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat(1-72) alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008;90:723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Charter GrpH. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500: neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, The HG. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Trujillo-Pisanty I, Cossette MP, Conover K, Shizgal P. Role of dopamine tone in the pursuit of brain stimulation reward. J Neurosci. 2012;32:11032–11041. doi: 10.1523/JNEUROSCI.1051-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. 2009;461:155–158. doi: 10.1016/j.neulet.2009.05.070. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, Markou A, Grant I, Semenova S. Methamphetamine Exposure Combined with HIV-1 Disease or gp120 Expression: Comparison of Learning and Executive Functions in Humans and Mice. Neuropsychopharmacology. 2015;40:1899–1909. doi: 10.1038/npp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Hubbard DT, Markou A, Semenova S. Expression of HIV gp120 protein increases sensitivity to the rewarding properties of methamphetamine in mice. Addict Biol. 2014;19:593–605. doi: 10.1111/adb.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S. Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neurotransmitter levels in mice. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.05.034. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan YW, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RM, Vidrine DJ, Danysh HE, Fletcher FE, McCurdy S, Arduino RC, Gritz ER. Factors Associated with Nonadherence to Antiretroviral Therapy in HIV-Positive Smokers. Aids Patient Care STDS. 2012;26:479–485. doi: 10.1089/apc.2012.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: A possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX, Li GH, Steiner J, Nath A. Role of Tat Protein in HIV Neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Loret EP, Darque A, Jouve E, Loret EA, Nicolino-Brunet C, Morange S, Castanier E, Casanova J, Caloustian C, Bornet C, Coussirou J, Boussetta J, Couallier V, Blin O, Dussol B, Ravaux I. Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial. Retrovirology. 2016;13:21. doi: 10.1186/s12977-016-0251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Ganno ML, Eans SO, Mizrachi E, Paris JJ. HIV-1 Tat Protein Exposure Potentiates Ethanol Reward and Reinstates Extinguished Ethanol-Conditioned Place Preference. Curr HIV Res. 2015;12:415–423. doi: 10.2174/1570162x1206150311160133. [DOI] [PubMed] [Google Scholar]
- Mediouni S, Marcondes MCG, Miller C, McLaughlin JP, Valente ST. The cross-talk of HIV-1 Tat and methamphetamine in HIV-associated neurocognitive disorders. Front Microbiol. 2015;6:24. doi: 10.3389/fmicb.2015.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98:587–597. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J. HIV-1 Tat Protein Decreases Dopamine Transporter Cell Surface Expression and Vesicular Monoamine Transporter-2 Function in Rat Striatal Synaptosomes. J Neuroimmune Pharm. 2012;7:629–639. doi: 10.1007/s11481-012-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Glasper ER, Harrell CS, Malviya SA, Otis JS, Neigh GN. Meloxicam Blocks Neuroinflammation, but Not Depressive-Like Behaviors, in HIV-1 Transgenic Female Rats. Plos One. 2014;9:9. doi: 10.1371/journal.pone.0108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panee J, Pang X, Munsaka S, Berry MJ, Chang L. Independent and Co-morbid HIV Infection and Meth Use Disorders on Oxidative Stress Markers in the Cerebrospinal Fluid and Depressive Symptoms. J Neuroimmune Pharm. 2015;10:111–121. doi: 10.1007/s11481-014-9581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of Conditional Central Expression of HIV-1 Tat Protein to Potentiate Cocaine-Mediated Psychostimulation and Reward Among Male Mice. Neuropsychopharmacology. 2014a;39:380–388. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Fenwick J, McLaughlin JP. Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Hormones Behav. 2014b;65:445–453. doi: 10.1016/j.yhbeh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology. 2014c;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek JR, Vigil O, Grant I, Heaton RK, Grp H. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Exp Neuropsychol. 2007;29:266–276. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A. The Intracranial Self-Stimulation Procedure Provides Quantitative Measures of Brain Reward Function #. T Mood and Anxiety Related Phenotypes in Mice. 2011;63:307–331. [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. Aids Patient Care STDS. 2008;22:233–243. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Dwoskin LP, Maragos WF. HIV-1 protein Tat inhibits vesicular monoamine transporter-2 activity in rat striatum. Synapse. 2012;66:755–757. doi: 10.1002/syn.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120-and tat(1-72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatr. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Ballard TM. Age-related impairments in operant DMTP performance in the PS2APP mouse, a transgenic mouse model of Alzheimer’s disease. Behav Brain Res. 2005;161:220–228. doi: 10.1016/j.bbr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat Protein-Induced Rapid and Reversible Decrease in H-3 Dopamine Uptake: Dissociation of H-3 Dopamine Uptake and H-3 2 beta-Carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) Binding in Rat Striatal Synaptosomes. The Journal of pharmacology and experimental therapeutics. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Midde NM, Gomez AM, Sun WL, Harrod SB. Intra-ventral tegmental area HIV-1 Tat(1-86) attenuates nicotine-mediated locomotor sensitization and alters mesocorticolimbic ERK and CREB signaling in rats. Front Microbiol. 2015;6:17. doi: 10.3389/fmicb.2015.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.