Abstract
Background
Tendon injuries are one of the most common musculoskeletal conditions in active patients. Platelet rich plasma (PRP) has shown some promise in the treatment of tendon disorders, but little is known as to the mechanisms by which PRP can improve tendon regeneration. PRP contains numerous different growth factors and cytokines which activate various cellular signaling cascades, but it has been difficult to determine precisely which signaling pathways and cellular responses are activated following PRP treatment. Additionally, macrophages play an important role in modulating tendon regeneration, but the influence of PRP on determining whether macrophages assume a proinflammatory or antiinflammatory phenotype remains unknown.
Hypothesis/Purpose
We used genome wide expression profiling, bioinformatics and protein analysis to determine the cellular pathways activated in fibroblasts treated with PRP. We also evaluated the effect of PRP on macrophage polarization.
Study Design
Controlled laboratory study.
Methods
Tendon fibroblasts or macrophages from rats were cultured and treated with either platelet poor plasma (PPP) or PRP. RNA or protein was isolated from cells and analyzed using microarrays, qPCR, immunoblot or bioinformatics techniques.
Results
Pathway analysis determined that the most highly induced signaling pathways in PRP treated tendon fibroblasts was the TNFα and NFκB pathways. PRP also downregulated the expression of ECM genes and induced the expression of autophagy related genes and reactive oxygen species (ROS) genes and protein markers in tendon fibroblasts. PRP failed to have a major impact on markers of macrophage polarization.
Conclusions
PRP induces an inflammatory response in tendon fibroblasts which leads to the formation of ROS and activation of oxidative stress pathways. PRP does not appear to significantly modulate macrophage polarization.
Clinical Relevance
PRP might act by inducing a transient inflammatory event which could then trigger a tissue regeneration response.
Keywords: platelet rich plasma, tendon, tendinopathy, oxidative stress, inflammation, autophagy
Introduction
Acute and chronic tendon injuries are relatively common problems in the general population secondary to sports participation and other physical activity, and may be the source of significant morbidity8,24. Platelet rich plasma (PRP) is a commonly used biological treatment in sports medicine, specifically with with interstitial tendon tears and chronic tendinopathies8,25. Initially described for use in oral and maxillofacial surgery, PRP is a refined product of autologous blood with a platelet concentration greater than that of whole blood, typically isolated via differential centrifugation1. Platelets are important in the injury response, as they release growth factors which initiate and modulate wound healing in both soft and hard tissues. The justification for the use of PRP clinically stems from an attempt to recapitulate or augment this natural biologic process1. Despite numerous clinical outcome studies on the effects of PRP in sports medicine, there remains a paucity of information on its mechanism of action19,25.
Two cell types, fibroblasts and macrophages, appear to predominate and coordinate the healing process in injured and diseased tendons10,37,39. Fibroblasts function as the principal cell involved in tendon maintenance and repair, while macrophages help to break down damaged tendon tissue, and can secrete cytokines and other signaling molecules that modulate the activity of fibroblasts26,28,38,39. Macrophages exhibit two phenotypes, a proinflammatory (M1) and antiinflammatory (M2) phenotype28,38. In response of tissue to injury, the M1 population of macrophages predominates initially, mediating phagocytosis and apoptosis while M2 macrophages appear later and become the more prevalent population that coordinates the repair process and promotes fibroblast proliferation7,28,39. There have been several in vitro studies on the effect of PRP on tendon cells, measuring the expression of specific genes or proteins related to tendon function40,41, and to our knowledge no studies of the effect of PRP on macrophage polarization. As PRP is a dense milieu of numerous growth factors and other signaling molecules, and only a limited number of signaling pathways have been studied in tendon cells treated with PRP, we sought to determine transcriptome-wide changes in gene expression using microarrays and informative bioinformatics analyses to evaluate which cellular signaling pathways were activated by PRP in an unbiased fashion. Furthermore, because macrophages appear to play an important role in tendon inflammation and repair, we determined the effect of PRP on macrophage polarization. We hypothesized that PRP would activate signaling pathways involved in ECM synthesis and remodeling, and would polarize macrophages to an antiinflammatory M2 phenotype.
Methods
Animals
This study was approved by the University of Michigan IACUC, and followed PHS guidelines for the ethical treatment of animals. Male retired breeder inbred Lewis rats were obtained Charles River (Wilmington, MA) and housed under specific pathogen free conditions. The inbred Lewis strain was selected to avoid adverse immune reactions from blood pooling29. Rats were deeply anesthetized with sodium pentobarbital to obtain blood and tendon tissue, and then humanely euthanized via anesthetic overdose and induction of bilateral pneumothorax.
Plasma Preparation
Blood was obtained via cardiac puncture and collected into sodium citrate vacutainer tubes (BD, Franklin Lakes, NJ). Platelet poor plasma (PPP) and platelet rich plasma (PRP) were prepared from whole blood under sterile conditions. As no widely used, commercially available clinical system is available to prepare PRP from rats, a manual preparation approach modified from previous studies1,11 was used. Briefly, blood was centrifuged at 500 × g for 5 minutes at 4°C, followed by a 5 minute rest period, and then another cycle again at 700 × g for 17 minutes at 4°C. The supernatant above the packed cells contained two visibly different layers, the uppermost and nearly transparent layer containing PPP and a lower, partially flocculent layer containing PRP. A total of three separate batches of PRP and PPP were prepared. A HemaVet 950 system (Drew Scientific, Oxford, CT) was used to quantify platelet densities. The mean concentration of platelets from PRP was 1.4×106±0.5×105 platelets/μL and PPP was 3.0×104±0.5×103 platelets/μL, which resulted in PRP having a approximately 4-fold elevation in platelet concentration compared to whole blood. PRP and PPP were frozen at −80°C until use.
Tendon Fibroblast Culture
Fibroblasts were isolated and from tail tendons as previously described31. Tail tendons develop from the same population of somitic progenitor cells as limb tendons33, and were used as a source of tendon fibroblasts in order to get a large number of low passage cells. Tendon fascicles were finely minced and placed in DMEM containing 0.2% type II collagenase (Life Technologies, Grand Island, NY) and vigorously agitated for 3 hours at 37°C. An equal volume of growth medium (GM) composed of DMEM containing 10% fetal bovine serum and 1% antibiotic/antimycotic (Life Technologies) was added to the digested tissue, which was then filtered through a 100μm strainer. Cells were centrifuged at 1000 × g for 10 minutes, resuspended in GM and plated on 100mm type I collagen coated dishes (BD). All cells were cultured in humidified incubators maintained at 37°C and 5% CO2. Fibroblasts were grown to 70% confluence, collected from dishes using TrypLE (Life Technologies) and resuspended in 3D type I collagen gels. The collagen for these gels was prepared and extracted as described18. Briefly, rat tail tendons were excised and placed in 0.2% acetic acid at 4°C. After 5 days, the collagen solution was centrifuged at 24,000 × g for 30 min, the supernatant was collected, lyophilized and dissolved again in 0.2% acetic acid to a final concentration of 2.7 mg/ml. To prepare the collagen gel, the collagen solution was combined with 10× MEM (Life Technologies) and 0.34 N NaOH in an 8:1:1 ratio at 4°C. Tendon fibroblasts were resuspended in this mixture and 300 μl containing 2×105 cells was added to each well of a 24-well plate (BD). The plate was then placed in the humidified incubator at 37°C for 45 minutes for gelling to occur.
After being embedded in 3D collagen gels, fibroblasts were cultured in GM for 3 days. The media was then changed to contain DMEM plus 1% antibiotic/antimycotic and 10% PPP or PRP clot releaseate. The clot releasate was prepared by treating PPP or PRP with 30mM CaCl2 to activate the coagulation cascade. Activated PPP and PRP were vigorously agitated for 1 hour at 4°C and then spun down at 12000 × g for 10 minutes at 4°C. The supernatant containing the clot releasate was collected, added to DMEM plus 1% antibiotic/antimycotic and passed through a 0.22μm filter to remove any small fibrin clumps. The resulting PPP or PRP containing media was added to wells containing tendon fibroblasts and changed every 2 days.
Macrophage Culture
Rat resident peritoneal macrophages were purchased from Cell Biologics (Chicago, IL) and cultured in macrophage medium containing basal medium supplemented with GM-CSF, 10% FBS and 1% antibiotic/antimycotic (Cell Biologics). Cells were thawed and cultured on plasma treated dishes (BD) for 3 days, after which the 10% FBS in the media was substituted for either 10% PPP or PRP for 2 days prior to RNA isolation.
RNA Isolation and Gene Expression
Tendon fibroblasts or macrophages were treated with PPP or PRP for 24 hours, and RNA was isolated as previously described16,36 using a miRNeasy micro kit (Qiagen, Valencia, CA). All RNA had an A260/A280 ratio > 1.8 (Nanodrop, ThermoFisher, Waltham, MA) and RIN values > 8.0 measured (Bioanalyzer, Agilent, Santa Clara, CA). After reverse transcription of RNA with iScript supermix (Bio-Rad, Hercules, CA), quantitative PCR (qPCR) was conducted in a CFX96 real time thermal cycler using iTaq SYBR green supermix reagents (BioRad). The 2−ΔCt technique was used to normalize the expression of mRNA transcripts to the stable housekeeping gene β-actin. A listing of RNA transcripts and primer sequences is provided in Supplementary Table 1.
Microarray Analysis
Microarray measurements of PPP or PRP treated tendon fibroblasts was performed by the University of Michigan DNA Sequencing Core following manufacturer recommendations. Equal amounts of RNA isolated from three individual wells was pooled into a single sample for microarray analysis, and two pooled samples of PPP and two pooled samples of PRP were analyzed. RNA was pooled as gene expression from a pooled RNA sample is similar to the average from the individual samples comprising the pooled sample5,23. RNA was prepared for microarray analysis using a GeneChip Pico WT kit (Affymetrix, Santa Clara, CA) and hybridized to Rat Gene ST 2.1 strips (Affymetrix). Raw microarray data was loaded into ArrayStar version 12.1 (DNASTAR, Madison, WI) to calculate fold-changes in gene expression data. The microarray dataset is available through the NIH GEO database (ascension number GSE70918). The Upstream Regulator module of Ingenuity Pathway Analysis software (Qiagen) was used to determine the transcriptional regulators that could explain the observed change in gene expression measurements obtained from microarrays. This module examines the number of known targets of each transcription regulator that are present in the microarray dataset, along with the direction of change to predict likely relevant transcriptional regulators. If the observed direction of the fold-change in expression is consistent with a particular activation state of the transcriptional regulator, then a prediction is made about whether the pathway is activated or inhibited. A full listing of the IPA Upstream Regulator analysis results is listed in Supplemental Table 2.
Protein Isolation and Measurements
Fibroblasts in 3D collagen gels were treated with media containing PPP or PRP for 5 days. Collagen gels were homogenized in ice cold RIPA buffer (Sigma Aldrich, St. Louis, MO) supplemented with 1:100 protease and phosphatase inhibitor cocktail (Life Technologies) and 1% NP-40 (Sigma Aldrich). After vigorous homogenization, samples were vortexed for 10 minutes at 4°C and then spun down at 12000 × g for 10 minutes at 4°C. The supernatant was collected and protein concentration was measured using a BCA assay (Life Technologies). Proteins were diluted in Laemmli sample buffer (Bio-Rad) and 10μg of total protein was loaded into AnyKD gels (Bio-Rad). Proteins were separated with electrophoresis, and the gels were either stained with Coomassie Brilliant Blue (Bio-Rad) or transferred to membranes for immunoblotting (Bio-Rad). For NFκB immunoblots, nitrocellulose membranes were blocked in 2% goat serum and incubated with rabbit anti-phospho-NFκB antibodies (S536, 1:1000 dilution) and goat-anti-rabbit HRPO-conjugated secondary antibodies (1:2000) dilution. For detection of carbonylated proteins, an OxiSelect Protein Carbonyl Immunoblot kit (Cell Biolabs, San Diego, CA) was used following manufacturers directions. Briefly, PVDF membranes were blocked in 5% powdered milk, treated with dinitrophenylhydrazine which reacts with carbonylated amino acid residues in proteins to produce dinitrophenol residues. Membranes were then incubated with anti-dinitrophenol residue antibodies (1:1000 dilution) and HRPO-conjugated secondary antibodies (1:1000 dilution). Membranes were treated with Clarity enhanced chemiluminescence solution (ECL, Bio-Rad) to activate HRPO. Gels and membranes were visualized in a ChemiDoc XRS system (Bio-Rad), and densitometry analysis was performed using ImageLab 5.2 software (Bio-Rad).
Protein Array
A rat cytokine antibody array (C2, RayBiotech, Norcross, GA) was used to measure the abundance of 34 proteins in PPP and PRP samples. A total of 100μL of PPP or PRP was used per assay, which followed the instructions of the manufacturer. Membranes were developed with ECL solution and quantified as described above.
Statistics
Data is presented as mean±SD. Differences between PPP and PRP groups were assessed using t-tests (α=0.05) in GraphPad Prism 6.0 (La Jolla, CA).
Results
Protein Abundance in PPP versus PRP
The relative difference in cytokines, growth factors and other proteins important in tissue inflammation, macrophage activity in platelet poor plasma (PPP) and platelet rich plasma (PRP) was determined. Of the 34 proteins analyzed, 26 were significantly higher in PRP compared to PPP, including CCL2, CCL20, CXCL5, IL1α, IL1β, IL6, IL10, PDGF-AA and TNFα (Table 1).
Table 1.
Relative abundance of 34 different proteins from platelet poor plasma (PPP) and platelet rich plasma (PRP).
| Protein | PPP | PRP |
|---|---|---|
| Activin A | 1.00±0.51 | 2.07±0.21* |
| Advanced glycosylation end product receptor (RAGE) | 1.00±0.56 | 2.09±0.22* |
| Agrin | 1.00±0.32 | 1.95±0.04* |
| Brain-derived neurotrophic factor (BDNF) | 1.00±0.54 | 2.01±0.12* |
| Cluster of differentiation 86 (CD86) | 1.00±0.39 | 1.79±0.07* |
| Chemokine (C-C motif) ligand 2 (CCL2) | 1.00±0.48 | 2.87±0.08* |
| Chemokine (C-C motif) ligand 20 (CCL20) | 1.00±0.30 | 2.04±0.11* |
| Chemokine (C-X-C motif) ligand 1 (CXCL1) | 1.00±0.51 | 1.49±0.19 |
| Chemokine (C-X-C motif) ligand 2 (CXCL2) | 1.00±0.35 | 1.31±0.22 |
| Chemokine (C-X-C motif) ligand 3 (CXCL3) | 1.00±0.45 | 1.31±0.25 |
| Chemokine (C-X-C motif) ligand 5 (CXCL5) | 1.00±0.48 | 6.32±0.05* |
| Chemokine (C-X-C motif) ligand 7 (CXCL7) | 1.00±0.29 | 1.36±0.11 |
| Ciliary neurotrophic factor (CNTF) | 1.00±0.53 | 2.00±0.26* |
| Colony-stimulating factor 2 (CSF2) | 1.00±0.52 | 2.29±0.06* |
| Fas Ligand | 1.00±0.79 | 2.83±0.36* |
| Fractalkine | 1.00±0.57 | 3.04±0.17* |
| Intercellular adhesion molecule 1 (ICAM1) | 1.00±0.49 | 2.74±0.01* |
| Interferon gamma (IFNγ) | 1.00±0.45 | 2.22±0.01* |
| Interleukin-1 receptor-like 2 (IL1RL2) | 1.00±0.56 | 2.12±0.08* |
| Interleukin 1 alpha (IL1α) | 1.00±0.32 | 1.90±0.05* |
| Interleukin 1 beta (IL1β) | 1.00±0.43 | 2.17±0.07* |
| Interleukin 2 (IL2) | 1.00±0.42 | 1.69±0.11 |
| Interleukin 4 (IL4) | 1.00±0.46 | 1.94±0.19* |
| Interleukin 6 (IL6) | 1.00±0.40 | 2.01±0.17* |
| Interleukin 10 (IL10) | 1.00±0.40 | 2.02±0.21* |
| Interleukin 13 (IL13) | 1.00±0.56 | 1.45±0.32 |
| L-Selectin | 1.00±0.46 | 2.83±0.05* |
| Leptin | 1.00±0.46 | 2.43±0.03* |
| Matrix metalloproteinase-8 (MMP8) | 1.00±0.27 | 4.17±0.01* |
| Platelet derived growth factor AA (PDGFAA) | 1.00±0.32 | 1.73±0.01* |
| Prolactin receptor | 1.00±0.50 | 1.53±0.05 |
| Tissue inhibitor of metalloproteinases 1 (TIMP1) | 1.00±0.40 | 3.01±0.26* |
| Tumor necrosis factor alpha (TNFα) | 1.00±0.52 | 2.98±0.24* |
| Vascular endothelial growth factor A (VEGF) | 1.00±0.52 | 1.57±0.01 |
Values are relative intensities normalized to the PPP group and are presented as mean±SD. N=3 replicates from each group.
significantly different from PPP group (P<0.05).
Microarray and Bioinformatics Analysis of PRP Treatment
We analyzed the effect of PRP treatment on global changes in gene expression patterns in tendon fibroblasts. Using microarrays, we determined that PRP treatment resulted in an upregulation greater then 1.5-fold of 315 genes, and a downregulation greater than 1.5-fold of 460 genes (Figure 1A). To gain more information about the biological significance of the microarray results and identify the signaling pathways predicted to be activated or inhibited by PRP, we analyzed fold-changes in global gene expression with the Upstream Regulator module of Ingenuity Pathway Analysis (IPA) software. This analysis identified the TNFα pathway (P=6.6×10−6) and the NFκB pathway (P=9.5×10−19) as the two pathways activated in fibroblasts treated with PRP (Figure 1B).
Figure 1.

Microarray and bioinformatics analysis of tendon fibroblasts treated with PRP or PPP. (A) Microarray analysis identified 3296 genes of 36685 that were significantly different (P<0.05) between PRP and PPP treated cells. Of the 3296 genes that were significantly different, 315 genes were greater than 1.5 fold upregulated and 460 genes were greater than 1.5 fold downregulated in PRP treated cells compared to PPP. (B) Ingenuity Pathway Analysis identified two pathways that were predicted to be highly activated in cells treated with PRP compared to PPP, the TNFα pathway (P=6.6×10−6) and the NFκB pathway (P=9.5×10−19). The merged pathways are presented.
PRP Effects on Tendon Fibroblast Gene Expression
After performing microarrays, we sought to validate the fold-change values of specific genes of relevance to tendon biology and inflammation with qPCR. For genes related to tendon growth, PRP upregulated BMP7, but did not change the expression of TGFβ and downregulated CTGF and IGF1 (Figure 2). The inflammation- and immune-modulating cytokines CCL2, CCL7, IL1α, IL6, IL10 and TNFα were upregulated in response to PRP, while no difference in IL1β or VEGF was observed, and IL15 was downregulated (Figure 2).
Figure 2.

Gene expression of growth factor and cytokine transcripts from PPP or PRP treated tendon fibroblasts. Target gene expression was normalized to β-actin. Values are mean±SD. N=6 replicates from each group. *, significantly different from PPP group (P<0.05).
The expression of genes involved with ECM synthesis and remodeling was also quantified. PRP had no effect on the expression of the hyaluronic acid synthase enzymes, HAS1 and HAS2 (Figure 3). Elastin expression was downregulated along with a slight elevation in the cross-linking enzyme LOX (Figure 3). The major fibrillar collagens, type 1 and type 3 collagen, along with genes associated with collagen fibril assembly, CILP, fibromodulin, and collagen type 12 and 14, were downregulated in PRP treated fibroblasts, while the basement membrane type 8 collagen and the proteoglycan lubricin were upregulated (Figure 3). PRP induced the expression of the major collagenase MMP13, along with the stromelysins MMP3 and MMP10 and the gelatinase MMP9, with no difference observed in the expression of the collagenase MMP8 and the gelatinase MMP2, nor the TIMP genes, TIMP1 or TIMP2 (Figure 3).
Figure 3.
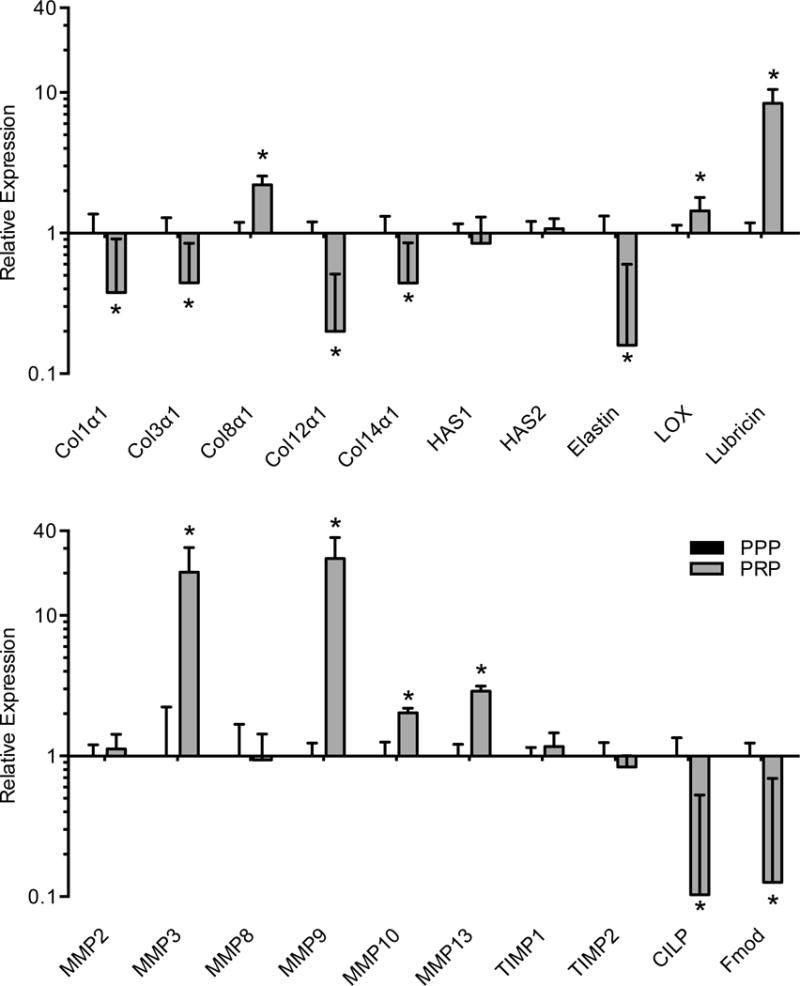
Gene expression of ECM structural and remodeling transcripts from PPP or PRP treated tendon fibroblasts. Target gene expression was normalized to β-actin. Values are mean±SD. N=6 replicates from each group. *, significantly different from PPP group (P<0.05).
Subsequently, the expression of genes involved in various cell functions including fibroblast proliferation, differentiation, autophagy and inflammation was assessed. The cell proliferation marker Ki67 was slightly elevated in response to PRP treatment, but a downregulation in the expression of genes involved in tendon fibroblast specification and differentiation including EGR1, EGR2, scleraxis and tenomodulin was observed (Figure 4). For genes involved with autophagy, there was an induction in the expression of Atg10, Bnip1 and GABARAPL2, with no difference in beclin1 or Trim13 levels (Figure 4). Transcription factors involved with inflammation, Fosb, Fosl1 and c-Jun were induced by PRP, but no difference in the expression of the deacetylase Sirt1 or the nitric oxide producing gene iNOS were observed (Figure 4). Genes involved with prostaglandin production were upregulated by PRP treatment, including PLD1, PTGES, Cox1 and Cox2, while no difference in the leukotriene synthesis enzyme 5-Lox was observed (Figure 4). PRP also induced the expression of markers of elevated reactive oxygen species production, including SOD1, SOD2, NFE2L2 and peroxiredoxin 1 (Figure 4).
Figure 4.
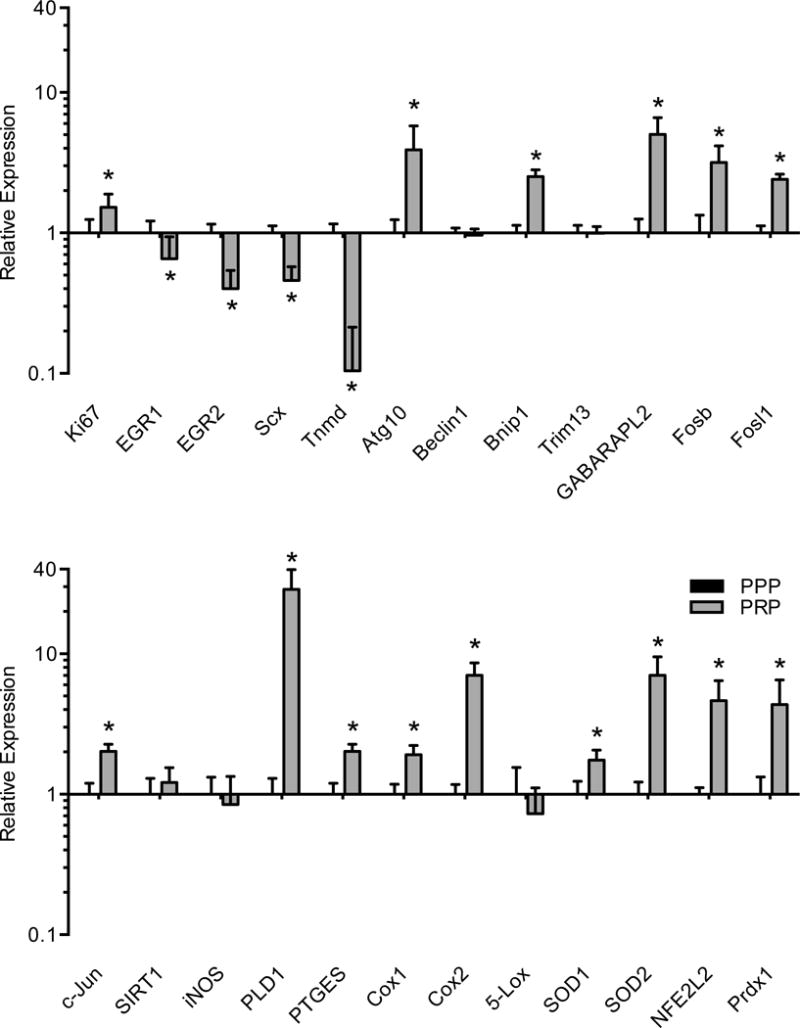
Gene expression of cell proliferation, differentiation, autophagy and inflammatory transcripts from PPP or PRP treated tendon fibroblasts. Target gene expression was normalized to β-actin. Values are mean±SD. N=6 replicates from each group. *, significantly different from PPP group (P<0.05).
PRP Effects on NFκB Activation and Protein Carbonylation
To verify that an elevation in reactive oxygen species was indeed present, and that the predicted elevation of NFκB did occur, we measured the levels of carbonylated proteins and the abundance of phospho-NFκB using immunoblots, and observed an induction in both the amount of carbonylated proteins and in activated NFκB protein levels (Figure 5).
Figure 5.

Immunoblots for carbonylation and NFκB activation. (A) Immunoblots for total carbonylated proteins and phospho NFκB activation from tendon fibroblasts treated with PRP or PPP. A Coomassie stained gel of the same samples is shown for validation of equal protein loading. Band densitometry analysis for (B) total carbonylated proteins and (C) phospho NFκB blots. Values are mean±SD. N=4 replicates from each group. *, significantly different from PPP group (P<0.05).
PRP and Macrophage Polarization
Finally, the expression of transcripts involved in the polarization of macrophages to a proinflammatory or antiinflammatory phenotype was assessed. PRP resulted in an induction in the expression of the M1 proinflammatory markers iNOS, IL1β and VEGF, with no changes in the expression of CCR7, CD11b, CD68, IL15 or TNFα (Figure 6A). However, there was also a modest induction in several M2 antiinflammatory markers including arginase 1, IL10, CD163 and CD14, with no change in FGF2, CD206, CD168, TGFβ or IGF1 expression (Figure 6B).
Figure 6.

Gene expression of markers of (A) proinflammatory and (B) antiinflammatory polarization from PPP or PRP treated macrophages. Target gene expression was normalized to β-actin. Values are mean±SD. N=6 replicates from each group. *, significantly different from PPP group (P<0.05).
Discussion
PRP is commonly used in the treatment of acute and chronic tendon injuries and diseases8,25, and to our knowledge, this is the first study that investigated global transcriptomic changes in tendon fibroblasts following PRP administration. We hypothesized that PRP would activate signaling pathways involved in ECM synthesis and remodeling. Surprisingly, the only two pathways predicted to be activated were the proinflammatory TNFα and NFκB pathways. Genes related to cellular proliferation and tendon collagen remodeling were also increased, suggesting PRP may activate fibroblast activity and collagen remodeling, but not collagen synthesis. We also hypothesized that PRP would polarize macrophages to an antiinflammatory M2 phenotype, but unexpectedly failed to observe any clear effect of PRP treatment on macrophage polarization. While more studies are necessary, these results provide important insight into the regulation of tendon cell activity by PRP treatment and suggest that PRP might act by inducing an intermittent bout of inflammation which may then trigger a tissue regeneration response.
PRP contains numerous growth factors and cytokines that can activate various signaling pathways in cells. Some of the signaling components that are downstream of individual receptors can act to inhibit or further enhance the activation of other pathways regulated by different receptors. For example, PRP contains both IL1β and IL10. While IL1β is a well known activator of proinflammatory intracellular signaling cascades, IL10 signaling pathways are able to inhibit the pathways activated by IL1β and reduce the expression of proinflammatory genes22. With the use of expression data from the entire transcriptome, the IPA-based bioinformatics approach employed in this study allowed us to evaluate the complex relationship between various individual signaling cascades to identify which pathways were most highly enriched following PRP treatment. Interestingly, the highly related TNFα and NFκB pathways were the only two pathways that were predicted to be functionally activated in response to PRP. Based on the role that the TNFα and NFκB pathways play in regulating ECM remodeling, oxidative stress and inflammation, we then chose to further evaluate and characterize these responses in greater detail.
Both acute tendon tendon tears and chronic degenerative tendinopathies involve a damaged or disordered ECM that must be remodeled and repaired by tendon fibroblasts8,17. Hyaluronic acid (HA) is a glycosaminoglycan that serves as a template for new ECM synthesis3,36, and PRP treatment had no effect on the expression of the major HA synthesis enzymes, HAS1 and HAS2. PRP downregulated the expression of the major collagens in tendon, collagen 1 and 3 as well as elastin, which has an important role in restoring ECM organization after being stretched. In addition to these genes, several transcripts that help to assemble mature collagen fibrils, including the FACIT collagens 12 and 14, as well as CILP and fibromodulin were downregulated after PRP treatment. These results are in general agreement with collagen expression reported in previous work9,40 and indicate that PRP treatment reduces the expression of major ECM components in tendon fibroblasts. PRP also induced the expression of several major MMPs, including MMP3, MMP9, MMP10 and MMP13 which together degrade the major fibrillar collagens, minor collagens and other ECM structural proteins8. While we are still in the early stages of understanding the networks of transcription factors and signaling pathways that regulate tendon fibroblast specification and proliferation, EGR1, EGR2 and scleraxis are transcription factors known to play crucial roles in tendon development, growth and remodeling16,20,36, and in the current study PRP downregulated the expression of all three of these genes. Tenomodulin, which is a marker of differentiated fibroblasts20, was also downregulated by PRP. Autophagy is a catabolic cellular process that is important in tissue remodeling14,34, and multiple genes that are important in the initiation and maturation of autophagosomes were upregulated by PRP, including Atg10, Bnip1 and GABARAPL2. The combined changes in ECM, MMP, tenogenesis and autophagy gene expression suggest that PRP treatment likely actually results in atrophied ECM and reduced fibroblast activity, which is largely consistent with a state of elevated acute tissue inflammation8.
The TNFα/NFκB signaling cascade is a well known mediator of inflammation. Binding of TNFα to its receptor TNFR1 triggers the activation of the IKK complex which is responsible for activating the p65 transcription factor subunit of NFκB through a combination of degradation of the inhibitor IκB complex, and phosphorylation of NFκB2,35. Once activated, NFκB translocates to the nucleus and induces the expression of numerous genes, many of which are associated with inflammation. Oxidative stress is also able to activate NFκB, often having an additive effect to TNFα signaling27. In the current study, treatment of tendon fibroblasts with PRP resulted in an elevation of genetic markers of oxidative stress including SOD1, SOD2, NFE2L2 and peroxiredoxin 1 as well as a chronic phosphorylation and activation of NFκB. Consistent with this, we observed a marked increase in carbonylated proteins which are sensitive markers of elevated oxidative stress30. While TNFα is elevated in PRP and is able to induce oxidative stress through induction of proinflammatory pathways21, since platelets can produce and release hydrogen peroxide13, it is possible that PRP also contains endogenous peroxides which can produce reactive oxygen species and further enhance oxidative stress in tendon fibroblasts. No change in iNOS expression was observed, and combined with elevations in SOD1, SOD2, NFE2L2 and Prdx1 this suggests the elevated oxidative stress was likely due to peroxide-mediated processes instead of nitric oxide. Among the more potent proinflammatory genes that are induced in response to NFκB activation are the prostaglandin synthesis enzymes PTGES, Cox1 and Cox227. PRP treatment potently induced the expression of these three enzymes, but had no effect on 5-Lox expression, suggesting a role for prostaglandins but not leukotrienes in PRP-mediated inflammation. PRP did not change the expression of SIRT1, which is an important and potent inhibitor of NFκB activity21. Several other proinflammatory transcription factors were also upregulated by PRP treatment, including Fosb, Fosl1 and c-Jun. These results together suggest that PRP treatment induces a robust and heady induction of inflammatory and oxidative stress pathways in tendon fibroblasts.
Macrophages appear to play an important role in the repair and regeneration of both acute tendon injuries and chronic degenerative tendinopathies7,10,39. Dragoo and colleagues12 also observed that PRP injection into otherwise healthy tendons resulted in an acute inflammatory response and infiltration of macrophages into the injected tissue. This is consistent with findings in the current manuscript, as PRP contains elevated levels of several chemokine ligand proteins that are involved in the recruitment of macrophages to tissue. CCL2 and CCL7 expression were also highly induced in fibroblasts treated with PRP. Many of the individual components of PRP are also able to polarize cultured macrophages into a specific phenotype in isolation. For example, IFNγ and TNFα can prime macrophages to a M1 phenotype, while IL4 and IL10 are able to polarize macrophages to a M2 phenotype28. There are no widely accepted and specific and definitive binary markers of the M1 or M2 phenotype, and a panel of various markers and the fold change in these markers are typically used to assess phenotypic changes in macrophage polarization28,32,38. Within the M2 phenotype there are sub-phenotypes, including M2a macrophages which are generally regarded as antiinflammatory macrophages that function to resolve the M1 response, and M2c macrophages which function to promote tissue repair and regeneration28. The specific markers for these sub-phenotypes are less well defined than those that define M1 versus M2 macrophages, however Arg1 and FGF2 are generally considered M2a markers while CD14, CD163, CD168, CD206, IGF-1, IL10 and TGF β can mark M2c macrophages28,42. We observed that PRP treatment modestly increased the expression of the M1 markers iNOS and IL1β along with a robust increase in VEGF. However there were also modest increases in the M2a marker Arg1 and M2c markers CD14, IL10 and CD163. With the exception of VEGF, no macrophage phenotype marker that was evaluated showed tremendous changes in expression. In the absence of robust changes in more specific markers, we do not feel that VEGF alone can sufficiently mark the M1 phenotype, and conclude that PRP did not have a marked impact on macrophage polarization. Interestingly, despite a substantial change in VEGF observed in macrophages, PRP treatment did not change VEGF expression in tendon fibroblasts. Neovascularization is often observed in acute and chronic tendon disorders37,39, and it is possible that macrophages are the cells responsible for signaling for blood vessel ingrowth into damaged areas of tendons.
This work has several limitations. These studies were conducted in cultured rat cells, and while we attempted to simulate a native environment as much as possible in vitro, it is possible that cells would respond to PRP differently in vivo. We also used a single dose of PRP added to culture media and did not determine if there were dose dependent effects of PRP on cell behavior. For most measures we evaluated changes in gene expression and not protein, and it is possible that changes in gene expression do not result in changes in protein levels. There are no commercially available PRP kits that have been validated for rats. Further, growth factor and cytokine levels in PRP can vary widely depending on the specific kit that is used4. Despite these limitations, this study provided important information related to the mechanism of action of PRP in tendon fibroblasts and macrophages. Future studies that use human cells or in vivo animal studies, prepare PRP from commercial kits, use multiple doses and time points, and that analyze more changes at the protein level will provide further insight into the biology of PRP and hopefully further refine its clinical use.
Conclusion
PRP has been used as a therapy for the treatment of tendon injuries and chronic diseases, but meta-analyses of numerous clinical trials do not indicate a clear benefit for the use of PRP in treating tendon disorders25. A major reason for this is the scarcity of trials that have enrolled large cohorts of patients, as well as substantial variation in PRP preparation and delivery, patient demographics, and the chronicity and site of injury25. Another limitation to the widespread acceptance of PRP for use in clinical practice is an inadequate understanding of its biological mechanism of action. This study provided cellular and biochemical data on the mechanism of action for PRP and reported that it appears to work by inducing a massive inflammatory reaction in tendon fibroblast cells. Inflammation is generally thought of in a negative fashion, but it also plays an important role in triggering a regeneration and repair response15. While exacerbation of inflammation in an acute tendon injury may not be beneficial, inducing an acute bout of inflammation in chronic tendinopathies may end up initiating a subsequent regenerative response. PRP is not unique in this manner – prolotherapy and needle fenestration are used in the treatment of chronic tendinopathies and have been proposed to work by a similar mechanism of action6. Considering the time and expense required in preparing PRP, future large clinical trials that evaluate the ability of PRP to treat chronic tendon disorders in comparison to prolotherapy or fenestration are warranted.
Supplementary Material
What is known about the subject
PRP has previously been shown to impact the activity of tendon fibroblasts, but a mechanism of action was not clearly defined.
What this study adds to existing knowledge
We provide additional information about the mechanism of action for PRP, and suggest that it appears to work by inducing a massive inflammatory response. This mechanism of action is similar to other treatments of chronic tendinopathies like needle fenestration and prolotherapy.
Acknowledgments
This work was supported by NIH grants R01-AR063649, F31-AR065931 and F32-AR067086.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, University of Michigan Medical School, Ann Arbor
References
- 1.Amable PR, Carias RBV, Teixeira MVT, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss H, Handschick K, Jurrmann N, et al. Cyclin-dependent kinase 6 phosphorylates NF-κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. Viola JP, ed. PLoS ONE. 2012;7(12):e51847. doi: 10.1371/journal.pone.0051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. AJP – Cell Physiology. 2012;303(5):C577–C588. doi: 10.1152/ajpcell.00057.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo TN, Pouliot MA, Kim H-J, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266–271. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 5.Chaillou T, Jackson JR, England JH, et al. Identification of a conserved set of upregulated genes in mouse skeletal muscle hypertrophy and regrowth. J Appl Physiol. 2015;118(1):86–97. doi: 10.1152/japplphysiol.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiavaras MM, Jacobson JA. Ultrasound-guided tendon fenestration. Semin Musculoskelet Radiol. 2013;17(1):85–90. doi: 10.1055/s-0033-1333942. [DOI] [PubMed] [Google Scholar]
- 7.Dakin SG, Werling D, Hibbert A, et al. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS ONE. 2012;7(2):e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol. 2013;115(6):884–891. doi: 10.1152/japplphysiol.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 10.Dean BJF, Snelling SJB, Dakin SG, Murphy RJ, Javaid MK, Carr AJ. Differences in glutamate receptors and inflammatory cell numbers are associated with the resolution of pain in human rotator cuff tendinopathy. Arthritis Res Ther. 2015;17(1):176. doi: 10.1186/s13075-015-0691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delos D, Leineweber MJ, Chaudhury S, Alzoobaee S, Gao Y, Rodeo SA. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am J Sports Med. 2014;42(9):2067–2074. doi: 10.1177/0363546514540272. [DOI] [PubMed] [Google Scholar]
- 12.Dragoo JL, Braun HJ, Durham JL, et al. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40(6):1274–1281. doi: 10.1177/0363546512442334. [DOI] [PubMed] [Google Scholar]
- 13.Finazzi-Agrò A, Menichelli A, Persiani M, Biancini G, Del Principe D. Hydrogen peroxide release from human blood platelets. Biochim Biophys Acta. 1982;718(1):21–25. doi: 10.1016/0304-4165(82)90004-6. [DOI] [PubMed] [Google Scholar]
- 14.Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, Claflin DR, Bedi A, Mendias CL. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30(12):1963–1970. doi: 10.1002/jor.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumucio JP, Flood MD, Phan AC, Brooks SV, Mendias CL. Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J Appl Physiol. 2013;115(4):539–545. doi: 10.1152/japplphysiol.00374.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumucio JP, Phan AC, Ruehlmann DG, Noah AC, Mendias CL. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol. 2014;117(11):1287–1291. doi: 10.1152/japplphysiol.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumucio JP, Sugg KB, Mendias CL. TGF-β Superfamily Signaling in Muscle and Tendon Adaptation to Resistance Exercise. Exerc Sport Sci Rev. 2015;43(2):93–99. doi: 10.1249/JES.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149(6):1309–1323. doi: 10.1083/jcb.149.6.1309.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739–748. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 20.Huang AH, Lu HH, Schweitzer R. Molecular regulation of tendon cell fate during development. In: Andarawis-Puri N, Flatow EL, Soslowsky LJ, editors. J Orthop Res. 6. Vol. 33. 2015. pp. 800–812. [DOI] [PubMed] [Google Scholar]
- 21.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Kelly A, Lynch A, Vereker E, et al. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem. 2001;276(49):45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- 23.Kendziorski C, Irizarry RA, Chen KS, Haag JD, Gould MN. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci USA. 2005;102(12):4252–4257. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan K, Cook J. The painful nonruptured tendon: clinical aspects. Clin Sports Med. 2003;22(4):711–725. doi: 10.1016/s0278-5919(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 25.Khan M, Bedi A. Cochrane in CORR (®): Platelet-rich Therapies for Musculoskeletal Soft Tissue Injuries (Review) Clin Orthop Relat Res. 2015;473(7):2207–2213. doi: 10.1007/s11999-015-4207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 27.Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol. 2013;64(4):409–421. [PubMed] [Google Scholar]
- 28.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22(8):1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marco ML. The Fischer-Lewis model of chronic allograft rejection–a summary. Nephrol Dial Transplant. 2006;21(11):3082–3086. doi: 10.1093/ndt/gfl451. [DOI] [PubMed] [Google Scholar]
- 30.McDonagh B, Sakellariou GK, Smith NT, Brownridge P, Jackson MJ. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J Proteome Res. 2014;13(11):5008–5021. doi: 10.1021/pr5006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendias CL, Gumucio JP, Lynch EB. Mechanical loading and TGF-β change the expression of multiple miRNAs in tendon fibroblasts. J Appl Physiol. 2012;113(1):56–62. doi: 10.1152/japplphysiol.00301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134(14):2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 34.Neel BA, Lin Y, Pessin JE. Skeletal muscle autophagy: a new metabolic regulator. Trends Endocrinol Metab. 2013;24(12):635–643. doi: 10.1016/j.tem.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelzer C, Thome M. IKKα takes control of canonical NF-κB activation. Nat Immunol. 2011;12(9):815–816. doi: 10.1038/ni.2082. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz AJ, Sarver DC, Sugg KB, Dzierzawski JT, Gumucio JP, Mendias CL. p38 MAPK Signaling in Postnatal Tendon Growth and Remodeling. In: Philp A, editor. PLoS ONE. 3. Vol. 10. 2015. p. e0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- 38.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32(7):944–951. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Qiu Y, Triffitt J, Carr A, Xia Z, Sabokbar A. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: an in vitro and in vivo study. J Orthop Res. 2012;30(6):982–990. doi: 10.1002/jor.22016. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Wang JH-C. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 42.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189(7):3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


