Abstract
Most human Δ9-tetrahydrocannabinol (THC) use is via inhalation, and yet few animal studies of inhalation exposure are available. Popularization of non-combusted methods for the inhalation of psychoactive drugs (Volcano®, e-cigarettes) further stimulates a need for rodent models of this route of administration. This study was designed to develop and validate a rodent chamber suitable for controlled exposure to vaporized THC in a propylene glycol vehicle, using an e-cigarette delivery system adapted to standard size, sealed rat housing chambers. The in vivo efficacy of inhaled THC was validated using radiotelemetry to assess body temperature and locomotor responses, a tail-flick assay for nociception and plasma analysis to verify exposure levels. Hypothermic responses to inhaled THC in male rats depended on the duration of exposure and the concentration of THC in the vehicle. The temperature nadir was reached after ~40 min of exposure, was of comparable magnitude (~3 °Celsius) to that produced by 20 mg/kg THC, i.p. and resolved within 3 hours (compared with a 6 hour time course following i.p. THC). Female rats were more sensitive to hypothermic effects of 30 min of lower-dose THC inhalation. Male rat tail-flick latency was increased by THC vapor inhalation; this effect was blocked by SR141716 pretreatment. The plasma THC concentration after 30 min of inhalation was similar to that produced by 10 mg/kg THC i.p.. This approach is flexible, robust and effective for use in laboratory rats and will be of increasing utility as users continue to adopt “vaping” for the administration of cannabis.
Keywords: marijuana, cannabis, vape, tetrad, in vivo, substance abuse
Graphical abstract
1. Introduction
Marijuana is used by human populations to self-administer Δ9-tetrahydrocannabinol (THC) via inhalation in the majority of cases and smoking the combusted plant material continues to be the most common method (Etter, 2015; Schauer et al, 2016). Despite the occasional effort to expose laboratory animals to marijuana smoke (Lichtman et al, 2001; Paule et al, 1992) the vast majority of studies have been conducted using parenteral injections (Maldonado, 2002; Pertwee, 2005; Scallet, 1991). Recent broad availability of non-combusting devices (e-cigarettes, Volcano® vaporizers, etc) capable of vaporizing or aerosolizing drugs has led to increasing adaptation for using marijuana materials (Etter, 2015; Giroud et al, 2015; Lee et al, 2016; Morean et al, 2015), including with populations using marijuana for medical relief (Borodovsky et al, 2016). Adverse health effects of these novel devices may span the range from the differential drug effects caused by the route of administration to effects of the vehicles in which drugs are vaporized. Thus, an inhalation method for a broad range of scientific investigation is needed and new, well controlled laboratory animal models are of significant interest to enhance translational inference.
Although a limited number of prior studies have investigated the exposure of laboratory animals to marijuana or THC via varied inhalation methods, there are no prior methods reported for THC exposure via e-cigarette vapor. Efforts to deliver cannabinoids using combusted marijuana, Volcano® type vaporizers or similar with rats (Manwell et al, 2014a; Manwell et al, 2014b) or mice (Lichtman et al, 2000; Marshell et al, 2014; Wilson et al, 2002) have been attempted but may have limitations. For example two recent studies using Volcano® vaporizers reported all-or-nothing behavioral effects and thus do not demonstrate an ability to differentially control dose within an effective range (Manwell et al, 2014a; Manwell et al, 2014b). Some studies have used physical restraint (Lichtman et al, 2001; Wiebelhaus et al, 2012) which may induce stress effects. Other investigations failed to find differential cannabinoid effects versus placebo on some of the tetrad measures, while confirming effects on other endpoints (Lichtman et al, 2000; Lichtman et al, 2001; Marshell et al, 2014). An additional limitation is that prior work has used non-standardized one-off delivery models that would be difficult to reproduce with fidelity (Boni et al, 1991; Lichtman et al, 2001; Varvel et al, 2006; Wiebelhaus et al, 2012) or use a cumbersome method to store the vapor (Manwell et al, 2014a; Manwell et al, 2014b). Thus, the present work was undertaken to determine if physiologically relevant amounts of THC, the primary psychoactive constituent of cannabis, could be delivered to rats using an e-cigarette type vaporizer.
Prior studies of the inhalation of exogenous cannabinoids are sparse, particularly in rats. Any given study will have limits in both design and outcome which limits the ability to compare results to a much larger set of studies featuring injected THC. Comparison of dose-effect relationships between routes of administration is an obvious goal. One set of studies used a “metered dose inhaler” for aerosolized THC in mice and found that the dose which produces hypothermia or hypolocomotion is above that necessary for antinociception (Lichtman et al, 2000; Wilson et al, 2002). However exposure to marijuana smoke decreased mouse temperature to the same extent as placebo marijuana smoke (and there was no effect of SR141716 pretreatment) suggesting not all inhalation models prove effective for study of all cannabinoid-related outcomes (Lichtman et al, 2001). Studies using a Volcano device found that conditioned place preference was only produced under one exposure condition in rats (Manwell et al, 2014a) and inhaled THC had a small stimulant effect on locomotor behavior (Manwell et al, 2014b). In total, these prior attempts have shown uneven efficacy, used marijuana smoke in some cases (which may be aversive), failed to demonstrate dose-response relationships and/or required restraint (which may invoke stress responses). Study of the effects of inhaled THC would therefore benefit from an e-cigarette inhalation model, particularly one that adapts current products designed for human use.
This study was conducted to provide in vivo validation of a method for delivering physiologically relevant amounts of THC to rats using commercial e-cigarette technology. The validation measures (body temperature, activity and nociception) were selected from the traditional tetrad used to assess cannabinoid activity in rodents (Wiley et al, 2014). Marshell and colleagues (2014) compared inhaled and intraperitoneal THC in mice and also found that dose effect relationships on individual tetrad components did not coincide, in the most extreme case inhaled THC produced no catalepsy at doses that altered temperature, activity and nociception (Marshell et al, 2014); for this reason catalepsy was not assessed in the present study.
2. Methods
2.1 Subjects
Male Sprague Dawley (N=16; Harlan, CA, USA) and Wistar rats (N=34 male, N=5 female; Charles River, NC, USA) were housed in humidity and temperature-controlled (23±1 °C) vivaria on 12:12 hour light:dark cycles. Animals entered the laboratory at ~10 weeks of age. Animals had ad libitum access to food and water in their home cages. All procedures were conducted in the animals’ dark (active) cycle under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and in a manner consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al, 2011).
2.2 Radiotelemetry
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and sterile radiotelemetry transmitters (Data Sciences International, St. Paul, MN; TA-F40) were implanted in the abdominal cavity through an incision along the abdominal midline posterior to the xyphoid space. Absorbable sutures were used to close the abdominal muscle incision and the skin incision was closed with the tissue adhesive. For the first three days of the recovery period, an antibiotic (cephazolin; 0.4 g/ml; 2.0 ml/kg, s.c.) and an analgesic (flunixin; 2.5 mg/ml; 2.0 ml/kg, s.c.) were administered daily. A minimum of 7 days was allowed for surgical recovery prior to starting any experiments.
Activity and temperature responses were evaluated in clean standard plastic home cages (thin layer of bedding) in a dark testing room, separate from the vivarium, during the (vivarium) dark cycle. Radiotelemetry transmissions were collected via telemetry receiver plates placed under the cages as previously described (Aarde et al, 2013b; Miller et al, 2013; Wright et al, 2012).
2.3 Intravenous catheterization
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and prepared with chronic intravenous catheters as described previously (Aarde et al, 2013a; Aarde et al, 2015b; Miller et al, 2012). Briefly, the catheters consisted of an 14-cm length of polyurethane based tubing (Micro-Renathane®, Braintree Scientific, Inc, Braintree MA, USA) fitted to a guide cannula (Plastics One, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3 cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal's back to the right jugular vein. Catheter tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 3M St Paul, MN). A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, an antibiotic (cephazolin) and an analgesic (flunixin) were administered daily. During testing and training, intravenous catheters were flushed with ~0.2–0.3 ml heparinized (166.7 USP/ml) saline before sessions and ~0.2–0.3 ml heparinized saline containing cefazolan (100 mg/mL) after sessions.
2.4 Apparatus
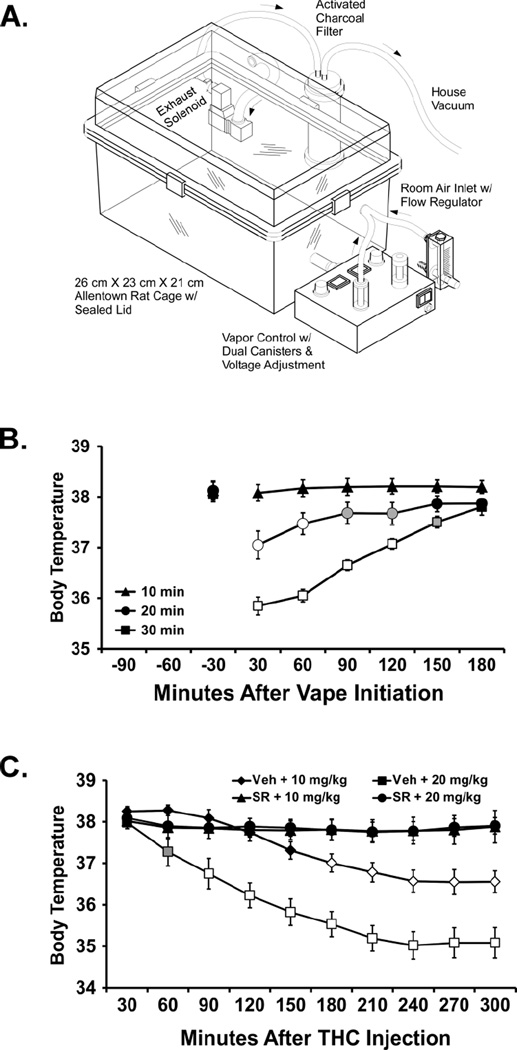
Sealed exposure chambers were modified from the 259mm × 234mm × 209mm Allentown, Inc (Allentown, NJ) rat cage to regulate airflow and the delivery of vaporized drug to rats using e-cigarette devices (see Figure 1A; N.b., the described studies were conducted in a version that used a single-cartridge delivery system and the activated charcoal filter was a large floor model -- Carbtrol Corporation G-0.5, Bridgeport CT). The latter included the Protank 3 Atomizer by Kanger Tech (Shenzhen Kanger Technology Co.,LTD; Fuyong Town, Shenzhen, China) and the 510 DCT Tank Atomizer by Ego E-Cigs (Joyetech USA, Irvine, CA, USA). A custom interface has been developed by La Jolla Alcohol Research, Inc (La Jolla, CA, USA) to permit triggering of the device by MedPC IV software (Med Associates, St. Albans, VT USA) but direct attachment of these commercial e-cigarettes to the chamber was used for some experiments. The chamber air was vacuum controlled by a chamber exhaust valve (i.e., a “pull” system) to flow room ambient air through an intake valve at 1 L per minute. This also functioned to ensure that vapor entered the chamber on each device triggering event. The vapor stream was integrated with the ambient air stream once triggered.
Figure 1.
A) Schematic of the apparatus used, adapted from a patent application filed by La Jolla Alcohol Research, Inc. Minor details varied in the present study which was conducted during development of this device. B) Mean (N=8; ±SEM) body temperature of male Sprague-Dawley rats following THC vapor inhalation for 10, 20 or 30 min. A significant difference from both the baseline and the other exposure conditions is indicated by the open symbols and from the 10 min condition by the shaded symbols. C) Mean (N=7) body temperature following intraperitoneal injection of vehicle (Veh) or SR141716 (SR) and then THC (10, 20 mg/kg). A significant difference from all other conditions is indicated by open symbols and from the Veh + 10 mg/kg condition by the shaded symbol.
2.5 Drugs
The inhalation exposure was to Δ9-tetrahydrocannabinol (THC; 25, 50, 100, 200 mg/mL) or crude marijuana extract (400 mg/mL; analyzed by supplier at 29% THC, thus ~116 mg/mL) in propylene glycol (PG) vehicle using e-cigarette type cartridges. Four 10-s vapor puffs were delivered with 2-s intervals every 5 minutes, which resulted in use of approximately 0.125 ml in a 40 min exposure session. The vacuum was turned off for the 4:12 min interval between vapor deliveries. Dosing conditions (concentrations, time of exposure, pairing for exposure, etc) for the experiments were determined based on initial pilot experiments.
For intraperitoneal administration, THC (0.3, 3.0, 10, 20 mg/kg) or SR141716 (4, 10 mg/kg), was suspended in a vehicle of 95% ethanol, Cremophor EL and saline in a 1:1:8 ratio. THC doses were selected based on prior pharmacokinetic and thermoregulatory findings (Irimia et al, 2015; Taffe et al, 2015). Treatment conditions (including vehicle and solo-drug challenges) were quasi-randomized within the Experiments (inhalation conditions by rat pairs), with active THC doses administered no more frequently than a 7 day interval for any individual inclusive of all studies for that individual. The THC and crude extract was provided by the U.S. National Institute on Drug Abuse’s Drug Supply Program and the SR141716 was purchased from Cayman Chemical.
2.6 Evaluation of plasma Δ9-THC content
Plasma THC content was quantified using fast liquid chromatography/mass spectrometry (LC/MS) adapted from (Irimia et al, 2015) and (Lacroix and Saussereau, 2012). 50 µL of plasma was mixed with 100 µL of deuterated internal standard (200 ng/mL THC-d3) and plasma cannabinoids were extracted into 400 µL ethyl acetate/acetonitrile (1:1) and then dried. The samples were reconstituted in 100 µL acetonitrile and underwent derivatization using 20 µL dabsyl chloride and 20 µL 0.1M NaOH in a hot water bath at 70 °C for 5 min. Separation was performed on an Agilent Eclipse XDB-C18 (3.5 µM, 2.1mm × 100mm; 35 °C) using gradient elution with water and acetonitrile, both in 0.2% formic acid (0.25mL/min; 80–100% acetonitrile). THC was quantified using an Agilent 1100 single quadrupole MSD using electrospray ionization and selected ion monitoring [THC m/z=602.3; THC-d3 m/z=605.3]. Calibration curves were conducted for each assay at a concentration range of 12.5–400 ng/mL and the observed correlation coefficients ranged from 0.996–0.999. Individual samples were run in a randomized order with respect to subject, time-point and dosing condition.
2.7 Experiments
2.7.1 Experiment 1
(Duration of exposure) was conducted in a group (N=8) of male Sprague-Dawley (Harlan) rats which were experimentally naïve at the start of these studies. Animals were placed individually in telemetry recording cages to establish baselines for at least 30 min prior to radiotelemetry studies. For all vapor drug exposure, they were transferred in pairs to the inhalation chamber for the designated treatment conditions and thereafter returned to the individual recording cages. Recording continued for up to 4 hours after the start of vapor inhalation. The telemetry data taken in the 30 minutes prior to moving the rat to the inhalation chamber were used as the pre-treatment baseline for statistical analysis purposes. A subsequent experiment in this group determined the effect of pre-treatment with 4 mg/kg, i.p., SR141716 (Rimonabant) or vehicle (in a balanced order) 15 min prior to intraperitoneal administration of THC (10, 20 mg/kg). This was included to provide a comparison of the magnitude of effects produced by vapor inhalation with a more commonly used route of administration in prior rat studies.
2.7.2 Experiment 2
(Female Rat Efficacy) was conducted in a group of female (N=5) and male (N=8) adult Wistar (Charles River) rats implanted with intravenous catheters and radiotelemetry devices and previously used in experiments determining the effects of THC (0.1, 0.3, 1.0 mg/kg, i.v.) on temperature and activity combined with blood sampling for pharmacokinetic distribution studies. A subsequent experiment in this group determined the effect of pre-treatment with 4 mg/kg, i.p., SR141716 (Rimonabant) or vehicle (in a balanced order) 15 min prior to administration of THC (0.1, 0.3, 1.0 mg/kg, i.v.). For the present experiment the rats were exposed for 30 min to THC vapor (Females: 25, 50 mg/mL; Males: 50, 100 mg/mL) or the PG vehicle alone in a balanced order. The variation in THC concentration (and reduction relative to Experiment 1) with a fixed inhalation duration was incorporated to further determine if dose-related differences could be produced by this method.
2.7.3 Experiment 3
(Crude marijuana extract) was conducted in a second group (N=8) of male Sprague-Dawley (Harlan) rats that was originally obtained and implanted for telemetry in a single cohort with the Experiment 1 animals. This group was subjected to i.p. challenges with doses of 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone reported in (Aarde et al, 2015a) prior to the presently reported study. The rats were exposed to crude marijuana extract, THC (50 mg/mL) and the PG vehicle alone in a balanced order.
2.7.4 Experiment 4
(Pharmacokinetics) was conducted in a group of male adult Wistar (Charles River) rats (N=6) prepared with intravenous catheters for blood sampling following vapor inhalation and a second group of male Wistar rats (N=6) previously used in intravenous self-administration experiments (involving methylone as the training drug; (Nguyen et al, 2016)) was used for PK determinations after IP THC dosing. Male Wistar blood was obtained from some subjects by acute venipuncture under gas inhalation anesthesia due to non-patent catheters.
2.7.5 Experiment 5
(Nociception) was conducted in a group of male adult Wistar (Charles River) rats (N=14) previously used in intravenous self-administration experiments (involving mephedrone training). Tail flick latency was assessed after vapor inhalation of THC (200 mg/mL in PG; 4 ten second puffs every 5 min for 10–30 min) or i.p. injection of THC (0.3, 1.0, 3.0 mg/kg) using a 52°C water bath and a 15 s maximum immersion interval per assessment (Gold et al, 1994). The experiment to determine the effect of pre-treatment with 4 mg/kg,i.p., SR 141716 was conducted in a group of male adult Sprague-Dawley (Harlan) rats (N=8). The i.p. experiment was conducted to provide a reference for the magnitude of effects produced by vapor inhalation.
2.8 Data Analysis
The body temperature and activity rate (counts per minute) were collected via the radiotelemetry system on a 5-minute schedule and analyzed in either 5-minute bins or 30 min averages (in the graphs the timepoint refers to the ending time, i.e. 60 = average of 35–60 min samples). Any missing temperature values were interpolated from preceding and succeeding values. Plasma THC levels are represented as ng/mL. The data were analyzed with two way repeated measures Analysis of Variance (ANOVA) with factors for the Drug Treatment Condition and the Time post-initiation of vapor, or postinjection (i.p.). Any significant effects were followed with post-hoc analysis using Tukey correction for all comparisons. All analysis used Prism 6 for Windows (v. 6.02; GraphPad Software, Inc, San Diego CA).
3. Results
3.1 Experiment 1 (Duration of exposure)
The first experiment (Figure 1B) demonstrated that vapor exposure to THC (200 mg/mL) reduced body temperature in drug-naïve, adult male Sprague-Dawley rats (mean weight 433 g; SEM 3.5) in a duration-dependent manner -- 10 minutes of vapor did not alter body temperature but a significant reduction was observed after 20 or 30 minutes of inhalation exposure. The ANOVA of the 30 min averages confirmed significant effects of Time post-vapor initiation [F (6, 42) = 43.11; P < 0.0001], Duration of exposure [F (2, 14) = 37.87; P < 0.0001] and the interaction of factors [F (12, 84) = 19.87; P < 0.0001]. The post-hoc test further confirmed that temperature differed between all three conditions from 30–120 min after the start of inhalation and between the 10 and 30 min conditions at 150 min. Temperature differed from the respective pre-inhalation baseline after 20 min (30, 60 min post-initiation) or 30 min (30–150 min post-initiation) of THC inhalation. Locomotor activity was not affected in an exposure-duration dependent manner in this study.
The next experiment in this group (N=7; one animal died unexpectedly for unknown reasons before completing) was a four condition repeated-measures study in which 10 or 20 mg/kg THC, i.p., was preceded 15 min beforehand with injection of SR141716 (4 mg/kg, i.p.) or the vehicle. No baseline was recorded in this experiment due to the anticipated duration of effect thus all measurements are post-injection, relative to time of THC administration, and analysis was conducted on 30 min averages up to 300 min post-injection. The ANOVA confirmed there was a significant effect of Time [F (9, 54) = 40.61; P < 0.0001], of Drug Treatment [F (3, 18) = 22.13; P < 0.0001] and the interaction [F (27, 162) = 10.73; P < 0.0001] on body temperature (Figure 1C). The post-hoc test confirmed that temperature differed between all dose conditions except the two SR pre-treatment conditions from 180–300 min post-injection. In addition, body temperature after Veh+20 mg/kg THC was lower than all other conditions from 90–150 min post-injection and lower than the Veh+10 mg/kg THC condition 60 min post-injection. There was a significant effect of Time [F (9, 54) = 42.82; P < 0.0001] but not of Drug Treatment or the interaction on activity (not shown). The post-hoc test of the marginal means confirmed that activity was lower from 60–300 min compared with the 30 min time point and from 120–300 min compared with the 60 min time point.
3.2 Experiment 2 (Female Rat Efficacy)
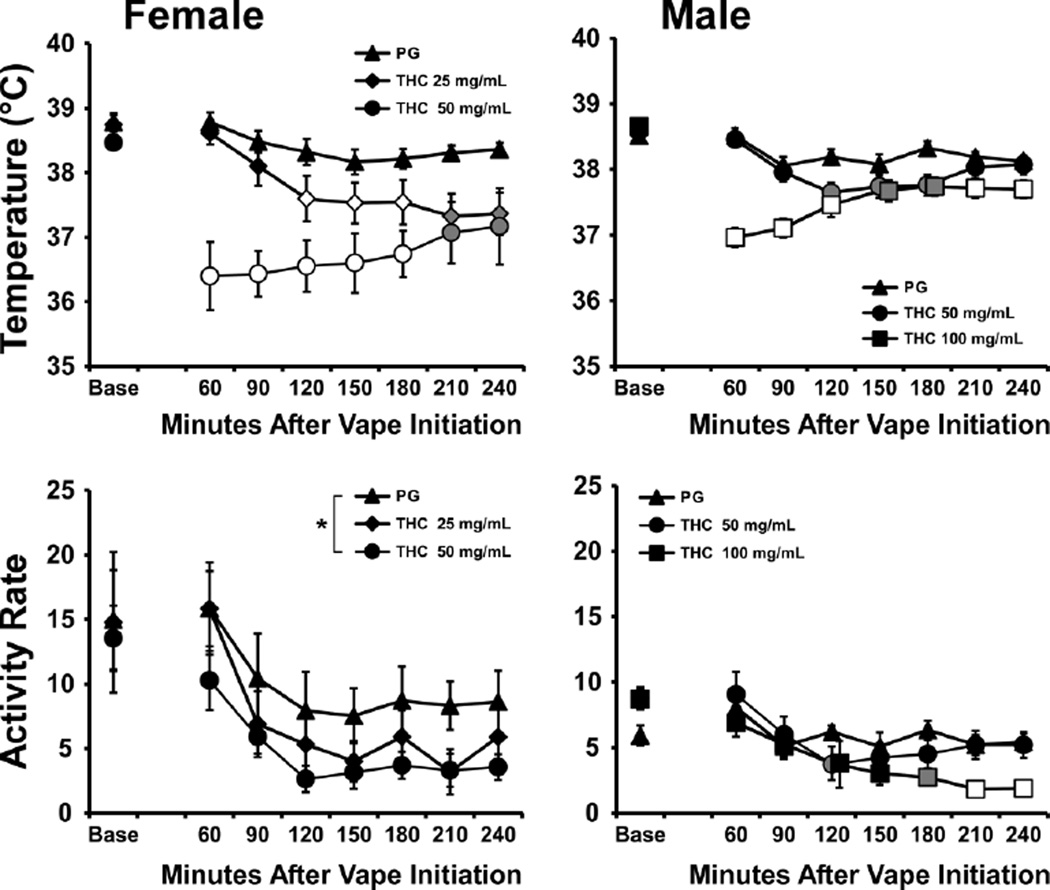
The body temperature of the female Wistar rats (mean weight 272 g; 6.3 SEM) was reduced by inhalation of the THC for 30 min (4 10 sec puffs every 5 min), but not by inhalation of the PG vehicle (Figure 2). The ANOVA (including the average of three baseline samples, −15 to −5, and then in 30 min bins post-initiation of vapor) confirmed main effects of Time post-initiation [F (7, 28) = 9.45; P < 0.0001], Drug Inhalation Condition [F (2, 8) = 14.54; P = 0.005] and the interaction of factors [F (14, 56) = 5.10; P < 0.0001]. The post-hoc test confirmed temperature was lower after inhalation of THC (25 mg/mL) 120–240 min after initiation compared with PG and compared with the baseline. Temperature was also significantly reduced following inhalation of THC (50 mg/mL) 60–240 min post-initiation compared with PG or with the baseline for that condition. The body temperature also differed between the two THC inhalation conditions 60–180 min post-initiation. There were no changes in body temperature relative to the baseline confirmed for the PG inhalation condition. The female rats’ activity rate was also significantly affected by Time post-initiation [F (7, 96) = 7.35; P < 0.0001] and by Drug Inhalation Condition [F (2, 96) = 6.42; P < 0.005], but not by the interaction of factors. The post-hoc test of the marginal means confirmed that less activity was observed in the THC (50 mg/mL) condition relative to the PG condition.
Figure 2.
Mean body temperature (upper panels) and activity rates (lower panels) of female (N=5; ±SEM) and male (N=8; ±SEM) Wistar rats after inhalation of PG or THC (50 mg/mL in PG) vapor inhalation for 30 min. A significant difference from baseline and both of the other conditions is indicated by the open symbols and from the baseline and the PG condition by the shaded symbols. Marginal mean differences are indicated by *. Base = baseline.
The body temperature of male Wistar rats (mean weight 462 g; 13.6 SEM) was significantly altered by Time post-initiation [F (7, 49) = 18.77; P < 0.0001], by Drug Inhalation Condition [F (2, 14) = 8.98; P < 0.005] and by the interaction of factors [F (14, 98) = 12.24; P < 0.0001]. The post-hoc test confirmed temperature was lower after inhalation of THC (50 mg/mL) 120–180 min after initiation compared with PG and 90–240 min compared with the baseline. Temperature was also significantly reduced following inhalation of THC (100 mg/mL) 60–240 min post-initiation compared with PG or with the baseline for that condition. The body temperature also differed between the two THC inhalation conditions 60–90 and 210–240 min post-initiation. Temperature in the PG condition was lower than baseline 90 and 150 min post-initiation. The activity rate of the male rats was significantly affected by Time post-initiation [F (7, 49) = 13.13; P < 0.0001] and the interaction of factors [F (14, 98) = 3.01; P < 0.001]. The post-hoc test confirmed significant differences in activity rate compared with PG inhalation in the THC (50 mg/mL) inhalation condition (Baseline and 120 min post-initiation) or THC (100 mg/mL) inhalation condition (Baseline and 180–240 min post-initiation). Activity also differed between THC concentrations in the 210–240 min timepoints. Within inhalation condition, activity was significantly different from the baseline after THC (50 mg/mL) inhalation (120–240 min post-initiation) or THC (100 mg/mL) inhalation (90–240 min post-initiation) but not after PG.
3.3 Experiment 3 (Crude marijuana extract)
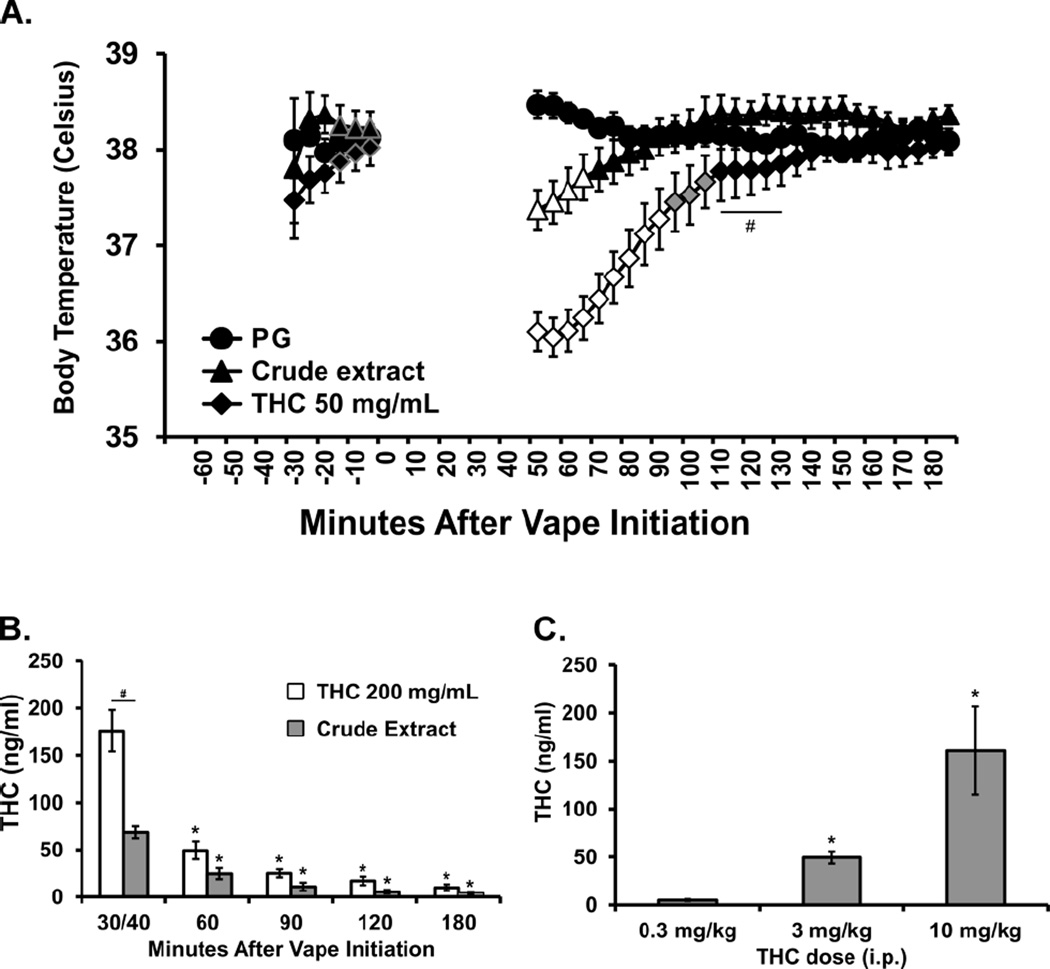
The body temperature of the second group of Sprague-Dawley male rats (mean weight 523 g; SEM 13.6) was also reduced by inhalation of the Crude marijuana extract (400 mg/mL; analyzed at 29% THC, thus ~116 mg/mL) and by THC (50 mg/mL) for 40 min (4 10 sec puffs every 5 min), but not by inhalation of the PG vehicle (Figure 3A). The ANOVA (including three baseline samples, −15 to −5, and 50–180 min post-initiation of vapor) confirmed main effects of Time post-initiation [F (29, 203) = 41.03; P < 0.0001], Drug Inhalation Condition [F (2, 14) = 11.91; P < 0.005] and the interaction of factors [F (58, 406) = 27.51; P < 0.0001]. The Tukey post-hoc test first confirmed that body temperature was lower than all three baseline measurements in the Crude (50–65 min after vapor initiation) and THC 50 mg/mL (50–90 min after vapor initiation) conditions but not after PG exposure. The post-hoc test further confirmed that body temperature was lower in the THC 50 mg/mL condition compared with PG (50–105 min after vapor initiation) and Crude (50–130 min post-initiation) treatments and that temperature differed among all three conditions from 50–65 min after the initiation of vapor. Activity was not significantly affected by drug inhalation condition (not shown).
Figure 3.
A) Mean (N=8; ±SEM) body temperature of male Sprague-Dawley rats after inhalation of PG, Crude marijuana extract or THC vapor inhalation for 40 min. Temperature is represented in the 5 min samples as collected. A significant difference from both the baseline and the other exposure conditions is indicated by the open symbols, from the PG Vehicle condition by the shaded symbols and from the Crude condition by #. The three baseline samples for the THC and crude extract conditions are outlined in grey for clarity. Also shown are B) mean (N=5; ±SEM) plasma THC concentrations after vapor inhalation of THC for 30 min or of crude extract for 40 min; and C) mean (N=6) plasma concentrations of THC after injection of THC (0.3, 3, 10 mg/kg, i.p.). A significant difference from the first time point or the 0.3 mg/kg, i.p. dose is indicated by * and between THC and Crude materials by #.
3.4 Experiment 4 (Pharmacokinetics)
Six adult male Sprague-Dawley rats were used to determine effects of 30 min of exposure to two source Materials [THC alone in PG (200 mg/mL; 4 ten-second puffs per 5 min for 30 min) and Crude marijuana extract (400 mg/mL in PG; 4 ten-second puffs per 5 min for 40 min)], with N=5 completing each exposure condition / source Material (Figure 3B). The ANOVA confirmed that there were significant effects of source Material [F (1, 8) = 19.21; P < 0.005] and Time [F (4, 32) = 82.72; P < 0.0001] as well as the interaction of Material with Time [F (4, 32) = 16.12; P < 0.0001] on the plasma THC levels. The post-hoc test confirmed that plasma THC levels were significantly lower than the first time point from 60–180 min after vapor initiation for both source materials. Plasma THC was also significantly lower following crude extract vapor in the first time point compared with the THC exposure.
An additional study analyzed plasma THC levels in a group of adult male Wistar rats (N = 6) collected 30 min after injection of THC (0.3, 3.0, 10.0 mg/kg, i.p.) (Figure 3C). Effects were dose dependent with the 10 mg/kg dose producing plasma THC levels similar to that produced by 30 min of inhalation exposure to THC in PG. The one-way ANOVA confirmed a main effect of Dose [F (1.022, 5.110) = 9.43; P < 0.05] and the post-hoc test (Tukey) confirmed plasma levels were higher after both 3 and 10 mg/kg compared with the 0.3 mg/kg dose.
3.5 Experiment 5 (Nociception)
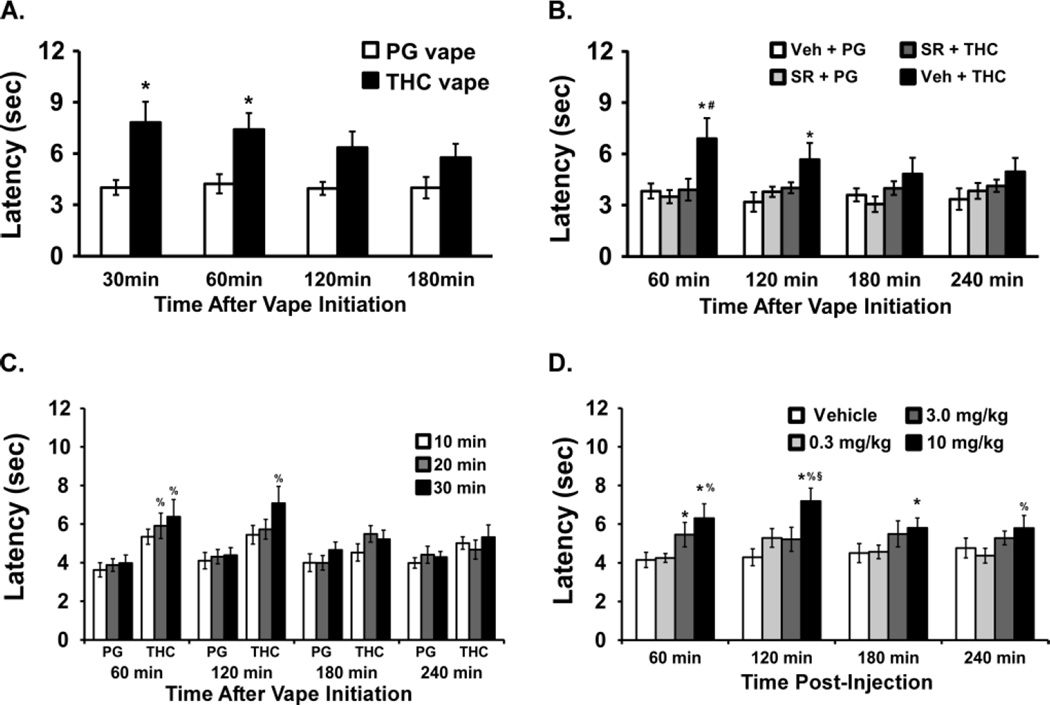
The effects of THC vapor vs PG on nociception (Figure 4A) were determined in a group of male Wistar rats (N=14; mean weight 574 g; SEM 11.9). The ANOVA confirmed there was a main effect of Drug Inhalation Condition [F (1, 26) = 9.92; P < 0.005] but not of Time or the interaction on tail-flick latency. The post hoc test confirmed that tail flick latency was longer 30 and 60 min after the initiation of THC vs PG exposure for 20 min. Thereafter a subset of the pilot study group (N=8) was assessed for tail-flick latency after 20 min of vapor exposure to the PG vehicle or THC, with vapor initiated 15 min after pre-treatment with 4 mg/kg SR141716, i.p., or the injection vehicle (Figure 4B). The ANOVA confirmed a main effect of Drug Inhalation Condition [F (3, 28) = 5.03; P < 0.01] but not of Time or the interaction. The post-hoc test confirmed that tail flick latency was longer after 20 min of THC (Veh pretreatment) compared with all three other pre-treatment/inhalation conditions at 60 min and elevated relative to Veh + PG at 120 min.
Figure 4.
Mean tail-flick latency measured following A) 20 min of PG or THC exposure (N=14); B) 20 min of exposure with pre-treatment with SR141716 (SR; 4 mg/kg, i.p.) or Vehicle (N=8); C) 10, 20 or 30 min of PG or THC exposure (N=14); D) Injection of THC (N=10; 0.3–10 mg/kg, i.p.) Significant differences compared with respective vehicle condition are indicated by *, differences from SR+THC vapor by #, from the lowest dose (10 min or 0.3 mg/kg) by % and from the 3.0 mg/kg dose by §.
The entire group (N=14) next completed a study in which the tail-flick latency was assessed 60, 120, 180 and 240 min after 10, 20 or 30 min of vapor THC exposure (Figure 4C). The ANOVA confirmed main effects of Inhalation Duration [F (5, 78) = 4.11; P = 0.0023], of Time after vapor initiation [F (3, 234) = 3.85; P < 0.05] and of the interaction [F (15, 234) = 2.03; P = 0.05] on tail-flick latency. The post-hoc test further confirmed that tail-flick latency was slower after 30 min of THC inhalation compared with any of the three PG durations from 60–120 after vapor initiation and slower after 20 min of THC compared with the 10 or 20 min PG exposures at the 60 min time point.
Finally, a subset of the group (N=10) was evaluated for tail-flick latency after intraperitoneal administration (Figure 4D) of the vehicle or THC (0.3, 3.0, 10.0 mg/kg). The ANOVA confirmed a main effect of Drug Dose Condition [F (3, 27) = 6.79; P < 0.005] but not of Time or the interaction. The post-hoc of the marginal means confirmed that latency was slower after the 10.0 mg/kg dose in comparison with the vehicle and 0.3 conditions. The post-hoc test also confirmed that latency was longer after 10 mg/kg THC compared with vehicle (60–180 min post-injection), 0.3 mg/kg THC (60–120, 240 min post-injection) and 3.0 mg/kg THC (120 min post-injection). Tail-flick also was longer 60 min after 3.0 mg/kg THC compared with vehicle injection.
4. Discussion
The majority of human intoxication with Δ9-tetrahydrocannabinol (THC) continues to be via marijuana smoke inhalation, and the ever widening availability of e-cigarette products on the consumer market has supported the adaptation of these devices for use with cannabis extracts (Etter, 2015; Giroud et al, 2015; Morean et al, 2015). Relatively few studies using inhalation exposure to THC have been conducted in animal models and none have used e-cigarette approaches. It is therefore of significant interest to further explore effects of inhaled THC in rodents and to develop suitable pre-clinical models of e-cigarette use. The present study demonstrates that intrapulmonary delivery of THC, using e-cigarette “vape” technology and propylene glycol as the vehicle, produces some of the major cannabinoid-typical effects in the rat. The vapor inhalation of THC significantly reduced the body temperature of male and female rats, just as it does when administered by injection in mice (Abel, 1973; Pertwee and Tavendale, 1977), rats (Taylor and Fennessy, 1977, 1982) or monkeys (Matsuzaki et al, 1987; McMahon et al, 2005; Taffe, 2012). The magnitude of hypothermia depended on the amount of time during which rats were exposed to vapor (Figure 1B) and the concentration in the vehicle (Figure 2), thereby demonstrating a dose-dependent relationship. Inhaled THC also decreased locomotor activity in the male and female rats in Experiment 2. Hypothermic effects were also produced by the inhalation of a crude marijuana extract (Figure 3A) which confirms efficacy (on this measure) using materials more similar to street preparations being used by humans. Furthermore, vapor inhalation of THC decreased nociception as assessed with the tail-flick assay, similar to effects of injected THC in rats in prior (Lichtman and Martin, 1991; Mason et al, 1999; Novelli et al, 1983) and the present (Figure 4D) studies. The anti-nociceptive effects lasted up to two hours after vapor initiation, depended on exposure duration and were blocked by pre-inhalation administration of the CB1 antagonist SR141716. In sum, effects of THC inhalation generalized across males and females (Experiment 2), across two rat strains (Experiment 1 vs Experiment 2) and across minor differences in age, weight and prior experimental history (Experiment 1 vs Experiment 3). The method is effective and could be readily duplicated using widely available commercial e-cigarette products with minor modification of commercially available rat cages.
The vapor/i.p. relationships for the in vivo measures coincide with the similar plasma THC levels found 30 min after initiation of vapor THC (200 mg/mL) inhalation and 30 min after 10 mg/kg THC, i.p. (Figure 3). For a translational comparison, humans reach >100 ng/ml plasma THC concentrations after intentionally smoking marijuana or using a vaporizing device with ethanolized (pure) THC (Abrams et al, 2007; Zuurman et al, 2008), a peak of 62 ng/mL plasma THC after ad lib consumption of vaporized cannabis (Hartman et al, 2015) and 162 ng/mL plasma THC using a paced marijuana smoking procedure in which consumption was fixed (Huestis et al, 1992). Peak plasma levels in the rats in this study approximated those values via inhalation (and also i.p. injection) enhancing translational validity. The relatively rapid onset and offset of the body temperature response relative to intraperitoneal THC administration in this (Figure 1C) and a prior study (Taffe et al, 2015) shows that this method may provide a route of administration that matches human use more closely. It is unclear at present why the plasma levels of THC were lower after crude extract vapor inhalation and additional investigation with a wider range of exposure conditions and concentrations in the PG would be required to fully resolve this question. For the purposes of this validation it is most important that the lower plasma levels coincide with the smaller decrease in body temperature relative to the THC inhalation conditions.
This study found a partial dissociation between the thermoregulatory or antinociceptive effects and the locomotor inhibitory effects of THC in male rats since there were no effects of THC on locomotor behavior confirmed for the Experiment 1 and 3 inhalation studies. Nevertheless, female and male rats exhibited suppressed locomotor activity after inhalation in Experiment 2 and we’ve shown previously that i.p. THC can significantly reduce activity of male rats using a similar radiotelemetry approach (Taffe et al, 2015). Although most tetrad testing for cannabinoid action has used relatively high doses that produced significant effects on all measures (temperature, activity, nociception and catalepsy), such studies obscured the independent dose-relationship for each outcome by failing to describe threshold dosing across all measures. In a recent mouse study, inhaled THC produced no catalepsy at doses that altered temperature, activity and nociception (Marshell et al, 2014). Still, it may be the case that the locomotor assay used for this study is not the most sensitive and may have underrepresented locomotor suppressive effects of THC.
The major caveat to this study is that the experiments do not cover every possible avenue of investigation outlined by a decades-long literature of the effects on injected THC in mice or rats. It may be that other in vivo endpoints may be of greater interest to some audiences. Nevertheless, the main goal was to provide broad validation of the approach using the most common rodent indices of cannabinoid activity, and the data support the conclusion that pharmacologically active doses of THC were inhaled. As one specific example for additional exploration, there was an apparent sex-difference in this study (Figure 2) with females appearing more sensitive to a 30 min, 50 mg/mL THC inhalation condition. This is most likely due to significant differences in the bodyweight (females averaged 59% of the male weight), and therefore the administered per-kilogram THC dose. Other studies involving i.v. THC administration in these groups confirm a similar magnitude of thermoregulatory response between the sexes on a mg/kg basis (not shown) and Figure 1C shows that a 50% lower mg/kg dose results in lesser effect on hypothermia within a single group. This interpretation assumes a relatively similar amount of THC was taken up via inhalation in male and female rats. It would be of interest to further determine the presence or absence of sex-differences in the effects of inhaled THC. Similarly, it would be of interest to further explore the role of non-THC constituents in the effects of inhaling crude marijuana extracts.
In conclusion, these studies validate a new model of intrapulmonary THC delivery by showing hypothermic, hypolocomotive and antinociceptive effects. This method has several advantages. Inhalation (increasingly via e-cigarettes) administration is in widespread human use and this work establishes an inhalation model suitable for rats. The time course of effects is much shorter than with i.p. administration and there is no need for the surgical implantation of catheters required for intravenous administration. The flexibility of the vehicle permits the evaluation of crude extracts, mixtures of cannabinoids and potentially combinations of THC with, other drugs e.g., nicotine. This study therefore represents a significant advance in the preclinical study of the effects of THC on behavior and physiology.
Highlights.
Few animal studies of Δ9-tetrahydrocannabinol (THC) inhalation are available.
This study validated an e-cigarette system for delivering vaporized THC to rats.
Hypothermia, anti-nociception and hypolocomotor effects were produced.
This approach is flexible, robust and effective for use in laboratory rats.
Acknowledgments
Funding: This work was funded by support from the United States Public Health Service National Institutes of Health (R01 DA024105, R01 DA035482 and R44 DA041967) which had no direct input on the design, conduct, analysis or publication of the findings. Development of the apparatus was supported by La Jolla Alcohol Research, Inc and MC is inventor on a patent for this device. SAV consults for La Jolla Alcohol Research, Inc. This is manuscript #29252 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The remaining authors declare no competing financial interests.
Literature Cited
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, et al. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013a;18(5):786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 2015a;232(16):3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013b;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2015b;232(11):1867–1877. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel EL. Chronopharmacology of delta9-tetrahydrocannabinol hypothermia in mice. Experientia. 1973;29(12):1528–1529. doi: 10.1007/BF01943897. [DOI] [PubMed] [Google Scholar]
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007;82(5):572–578. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- Boni JP, Barr WH, Martin BR. Cocaine inhalation in the rat: pharmacokinetics and cardiovascular response. J Pharmacol Exp Ther. 1991;257(1):307–315. [PubMed] [Google Scholar]
- Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int J Drug Policy. 2016 doi: 10.1016/j.drugpo.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF. Electronic cigarettes and cannabis: an exploratory study. Eur Addict Res. 2015;21(3):124–130. doi: 10.1159/000369791. [DOI] [PubMed] [Google Scholar]
- Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B. E-Cigarettes: A Review of New Trends in Cannabis Use. Int J Environ Res Public Health. 2015;12(8):9988–10008. doi: 10.3390/ijerph120809988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253(1–2):45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, et al. Controlled Cannabis Vaporizer Administration: Blood and Plasma Cannabinoids with and without Alcohol. Clin Chem. 2015;61(6):850–869. doi: 10.1373/clinchem.2015.238287. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16(5):276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Irimia C, Polis IY, Stouffer D, Parsons LH. Persistent effects of chronic Delta9-THC exposure on motor impulsivity in rats. Psychopharmacology (Berl) 2015;232(16):3033–3043. doi: 10.1007/s00213-015-3942-x. [DOI] [PubMed] [Google Scholar]
- Lacroix C, Saussereau E. Fast liquid chromatography/tandem mass spectrometry determination of cannabinoids in micro volume blood samples after dabsyl derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;905:85–95. doi: 10.1016/j.jchromb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Lee DC, Crosier BS, Borodovsky JT, Sargent JD, Budney AJ. Online survey characterizing vaporizer use among cannabis users. Drug Alcohol Depend. 2016;159:227–233. doi: 10.1016/j.drugalcdep.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther. 1991;258(2):517–523. [PubMed] [Google Scholar]
- Lichtman AH, Peart J, Poklis JL, Bridgen DT, Razdan RK, Wilson DM, et al. Pharmacological evaluation of aerosolized cannabinoids in mice. Eur J Pharmacol. 2000;399(2–3):141–149. doi: 10.1016/s0014-2999(00)00321-6. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR. The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend. 2001;63(2):107–116. doi: 10.1016/s0376-8716(00)00205-2. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacology & therapeutics. 2002;95(2):153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vaporized Delta-tetrahydrocannabinol (Delta-THC) delivery system part I: Development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods. 2014a doi: 10.1016/j.vascn.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE. A vapourized Delta-tetrahydrocannabinol (Delta-THC) delivery system part II: Comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods. 2014b doi: 10.1016/j.vascn.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, et al. In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Delta-THC in mice: Inhalation versus intraperitoneal injection. Pharmacol Biochem Behav. 2014;124C:40–47. doi: 10.1016/j.pbb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DJ, Jr, Lowe J, Welch SP. Cannabinoid modulation of dynorphin A: correlation to cannabinoid-induced antinociception. Eur J Pharmacol. 1999;378(3):237–248. doi: 10.1016/s0014-2999(99)00479-3. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Casella GA, Ratner M. delta 9-Tetrahydrocannabinol: EEG changes, bradycardia and hypothermia in the rhesus monkey. Brain research bulletin. 1987;19(2):223–229. doi: 10.1016/0361-9230(87)90087-6. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav Pharmacol. 2005;16(5–6):363–372. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721–728. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, et al. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2012;121(1–2):90–96. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Krishnan-Sarin S. High School Students' Use of Electronic Cigarettes to Vaporize Cannabis. Pediatrics. 2015 doi: 10.1542/peds.2015-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals: Eigth Edition. 8th. Washington, D.C: National Academies Press; 2011. p. xxv.p. 220. [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA. Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict Biol. 2016 doi: 10.1111/adb.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli GP, Peduto VA, Bertol E, Mari F, Pieraccioli E. Analgesic interaction between nitrous oxide and delta-9-tetrahydrocannabinol in the rat. Br J Anaesth. 1983;55(10):997–1000. doi: 10.1093/bja/55.10.997. [DOI] [PubMed] [Google Scholar]
- Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, et al. Chronic marijuana smoke exposure in the rhesus monkey. II: Effects on progressive ratio and conditioned position responding. J Pharmacol Exp Ther. 1992;260(1):210–222. [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handbook of experimental pharmacology. 2005;(168):1– 51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Tavendale R. Effects of delta9-tetrahydrocannabinol on the rates of oxygen consumption of mice. Br J Pharmacol. 1977;60(4):559–568. doi: 10.1111/j.1476-5381.1977.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallet AC. Neurotoxicology of cannabis and THC: a review of chronic exposure studies in animals. Pharmacol Biochem Behav. 1991;40(3):671–676. doi: 10.1016/0091-3057(91)90380-k. [DOI] [PubMed] [Google Scholar]
- Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am J Prev Med. 2016;50(1):1–8. doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Delta9-Tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012;201:125–133. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, Vandewater SA. Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Delta(9) -tetrahydrocannabinol in Sprague-Dawley rats. Br J Pharmacol. 2015;172(7):1783–1791. doi: 10.1111/bph.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR. Biphasic nature of the effects of delta9-tetrahydrocannabinol on body temperature and brain amines of the rat. Eur J Pharmacol. 1977;46(2):93–99. doi: 10.1016/0014-2999(77)90244-8. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR. Time-course of the effects of chronic delta 9-tetrahydrocannabinol on behaviour, body temperature, brain amines and withdrawal-like behaviour in the rat. J Pharm Pharmacol. 1982;34(4):240–245. doi: 10.1111/j.2042-7158.1982.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, et al. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 2006;186(2):226–234. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic "marijuana" produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126(3):316–323. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014;97(1):55–63. doi: 10.1016/j.lfs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Peart J, Martin BR, Bridgen DT, Byron PR, Lichtman AH. Physiochemical and pharmacological characterization of a Delta(9)-THC aerosol generated by a metered dose inhaler. Drug Alcohol Depend. 2002;67(3):259– 267. doi: 10.1016/s0376-8716(02)00078-9. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PloS one. 2012;7(8):e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurman L, Roy C, Schoemaker RC, Hazekamp A, den Hartigh J, Bender JC, et al. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol. 2008;22(7):707–716. doi: 10.1177/0269881108089581. [DOI] [PubMed] [Google Scholar]