Abstract
Objectives
We examined whether a functional variant of the ADRA1A gene moderated cocaine-induced subjective effects in a group of cocaine-dependent individuals.
Methods
This study was a within-subject, double blind, placebo-controlled inpatient human laboratory evaluation of 65 non-treatment seeking, cocaine-dependent (DSM-IV) subjects, aged 18 to 55 years. Participants received both placebo (saline, IV) and cocaine (40mg, IV), and subjective responses were assessed 15 minutes prior to receiving an infusion and at 5-minute intervals for the subsequent 20 minutes. The rs1048101 variant of the α1A-adrenoceptor (ADRA1A) gene was genotyped and evaluated whether the Cys to Arg substitution at codon 347 in exon 2 (Cys347Arg) moderated the magnitude of the subjective effects produced by cocaine.
Results
Thirty (46%) subjects were found to have the major allele CC genotype, and 35 (44%) carried at least one minor T allele of rs1048101 (TT or TC genotype). Individuals with the CC genotype exhibited greater responses for ‘desire’ (p < 0.0001), ‘high’ (p < 0.0001), ‘any drug effect’ (p < 0.0001), ‘like cocaine” (p < 0.0001), and ‘likely to use cocaine if given access’ (p < 0.05) with experiment-wise significance.
Conclusions
This study indicates that ADRA1A genotype could be used to identify individuals for whom acute cocaine exposure may be more rewarding and by inference may result in greater difficulty in establishing and/or maintaining abstinence from cocaine.
Keywords: ADRA1A, genotype, polymorphism, cocaine, subjective effects
INTRODUCTION
The psychostimulant mechanism of action of cocaine is based upon its ability to inhibit reuptake of the dopamine, serotonin, and norepinephrine transporters, producing an increase in synaptic levels of these neurotransmitters.[1][2] Cocaine reward is mediated primarily through the mesocorticolimbic dopamine (DA) system, comprised of DA neurons originating in the ventral tegmental area (VTA) and projecting to target areas including nucleus accumbens (NAc), amygdala, and prefrontal cortex (PFC).[3] During acute intoxication, the subjective euphoria and reinforcing properties of cocaine are attributed to its ability to bind the dopamine transporter (DAT), inhibit reuptake of dopamine, and increase synaptic levels of DA.
Preclinical evidence suggests that stimulation of the noradrenergic system contributes to cocaine reward and reinforcement.[4] DAT-knockout (KO) mice continue to self-administer cocaine, suggesting that blockage of DAT alone is not sufficient to account for the reinforcing effects of cocaine and other neurotransmitter systems must contribute.[5] In a separate study, when compared to wild-type controls, norepinephrine transporter (NET) KO mice displayed a reduced response to acute cocaine administration, although behavioral sensitization to cocaine remained unchanged.[6] Similar findings were noted in alpha-1B adrenergic receptor KO mice, where cocaine-induced locomotor hyperactivity and behavioral sensitization were also found to be reduced.[7]
In addition to those findings which point to a role for the noradrenergic system in cocaine reward, significant evidence suggests that pharmacologic alteration of the NE system is associated with reductions in response to cocaine.[8] Preclinical evidence suggests a vital role for the β2 adrenergic system in stress-induced cocaine seeking [9], and intra-cerebral injection of the β1- and β2- adrenergic antagonists, betaxolol and ICI-118,551, respectively, was found to attenuate stress-induced reinstatement of cocaine-seeking behavior in rats.[10] Likewise, the alpha-2 agonists, clonidine, UK-14,304, and guanfacine, were found to decrease cue-induced reinstatement of cocaine seeking in rats, as was the combination of alpha-1 adrenergic antagonist, prazosin, and β-adrenergic antagonist, propranolol.[11] Under separate studies, prazosin demonstrated the ability to attenuate cocaine-induced reinstatement of cocaine-seeking behavior in rats [12,13] and decrease locomotor effects following acute administration of psychostimulants (cocaine and dextroamphetamine).[14] Additionally, the dopamine β-hydroxylase inhibitors, disulfiram and nepicastat, have demonstrated the ability to diminish cocaine-seeking behaviors in rats. While disulfiram appears to reduce these behaviors in response to drug (e.g., cocaine) [15], nepicastat has shown the ability to reduce cocaine-seeking in response to cocaine, cocaine-related cues, psychological stress (foot shock) and pharmacologic stress (yohimbine).[16]
These findings reflect the broader impact of cocaine’s NET antagonism – a functional coupling of the noradrenergic system to the dopaminergic system, mediated through the activation of α1-adrenoceptors, where cocaine-induced increases in synaptic levels of NE result in further increases in firing of DA neurons in the VTA and PFC.[17, 18] This connection has been further evidenced in studies where lesioning of noradrenergic neurons in locus coeruleus resulted in decreased DA release onto the NAc from the VTA, [19] and, conversely, activation of locus coeruleus NE neurons increased VTA DA neuronal firing.[20]
Activation of the noradrenergic system is likely influenced by the genetic subtype of the α1A-adrenoceptor gene. The gene ADRA1A, coding for α1A-adrenoceptor, has a functional polymorphism rs1048101 in exon 2 coding for the substitution of an arginine (ARG) for a cytosine (CYS) at codon 347 of the C-terminus [21] that may alter the activity of this receptor and impact disease expression and severity as well as responses to pharmacotherapy. Studies have examined the impact of rs1048101 on various clinical outcomes, including development of Alzheimer’s disease [22], risk of essential hypertension [23], response to irbesartan treatment of hypertension [24], hemodynamic responses to adrenaline [25], and severity of benign prostatic hyperplasia.[26] Additionally, in relation to cognition, this polymorphism may impact activation of α1A-adrenoceptors reported to influence critical functions for prevention of relapse in cocaine dependence including vigilance, impulsivity, and working memory.[27,28] It is for this reason that we selected ADRA1A rs1048101 for study.
In a recent study, our group found that disulfiram, which attenuates NE activity through its ability to inhibit dopamine-beta-hydroxylase and has been shown to block cocaine-induced reinstatement of cocaine seeking behavior in rats [15], significantly reduced the percentage of cocaine positive urines among T allele carriers (TT/TC) for rs1048101 of ADRA1A.[29] In CC homozygotes for ADRA1A, however, disulfiram showed no difference from placebo in regards to reduction of cocaine positive urines. These findings suggest preferential response to noradrenergic medications based upon α1A-adrenoceptor genetic subtype. However, the impact of receptor subtype on subjective responses to cocaine has yet to be elucidated.
In separate studies, our group recently reported that ankyrin repeat and kinase domain-containing 1 (ANKK1) and dopamine transporter (DAT1, SLC6A4) genotypes could be used to identify patient subpopulations who experience greater subjective effects following cocaine exposure.[30,31] As part of this larger study, and in order to further identify genetic subgroups in which there is enhanced response to cocaine administration in the laboratory, this present study examined whether the rs1048101 polymorphism enhances the subjective effects produced by cocaine in a group of cocaine-dependent individuals. We hypothesized that CC homozygotes for rs1048101 would report higher ratings of cocaine-induced subjective effects in the laboratory.
METHODS
Participants
Sixty-five non-treatment seeking, cocaine-dependent individuals were recruited from the greater Houston area from March 2010 through July 2012 and admitted to the Research Commons at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC). All participants signed informed consent documents, gave blood samples for genetic analysis, and were included in this portion of the study. At the time of screening, subjects underwent a full physical examination, psychiatric evaluation, and assessment of laboratory values. At intake, each participant was interviewed using the Mini International Neuropsychiatric Interview (MINI) and completed the Addiction Severity Index (ASI-Lite).[32,33] Subsequently, each participant met the following inclusion criteria: (a) male or female, (b) aged 18–55 years, (c) any race or ethnic origin, (d) history of cocaine use by either smoked or intravenous route, (e) diagnosis of cocaine dependence, as defined by the Diagnostic and Statistical Manual of Mental Disorders.[34] Exclusion criteria included current diagnosis of alcohol or other drug dependence (other than nicotine); inability to detect the effects of cocaine; serious medical conditions (i.e., asthma, heart disease, or AIDS, abnormal physical exam or laboratory findings, seizure disorder, history of head trauma); presence of any other Axis I psychiatric disorder; and pregnancy. Concomitant use of psychotropic medications or medications affecting blood pressure was not allowed. The study was approved by both the Institutional Review Boards of Baylor College of Medicine and the Research and Development Committee of the MEDVAMC.
Design and Procedures
Using a double-blind, placebo-controlled, within-subjects study design, participants were administered either saline or 40mg cocaine intravenously. The 40 mg dose of cocaine was selected on the basis of several prior publications from our lab and others showing that it engenders significant increases in cardiovascular responses and subjective effects, yet the dose is well within the range of doses that can be safely administered to cocaine-dependent patients in a clinical setting.[35,36,37] Each individual participated in both conditions, one dose given at 9 AM and the second at ~1 PM. Subjective effects were recorded 15 minutes prior to and at 5, 10, 15, and 20 minutes after administration. The effects were rated using a visual analog scale (VAS) with anchors ranging from 0, meaning “no effect,” to a maximum of 100, meaning “most ever.” Ratings were obtained for ‘high’ (“How high are you right now?”), ‘any drug effect’ (“Do you feel any drug effect ”), ‘stimulated’ (“How stimulated do you feel right now?”), ‘good effect’ (“Does the drug have any good effects right now?”), ‘like cocaine’ (“How much do you like the drug right now?”), ‘bad effect’ (“Does the drug have any bad effects right now?”), ‘anxious’ (“How anxious do you feel right now?”), ‘desire’ (“How much do you desire the drug right now?”), and ‘likely to use cocaine if had access’ (“If you had access to the drug right now how likely would you be to use it right now?”).
Genotyping
Genotyping of samples was conducted as previously described by our group.[30] DNA was isolated from blood using the Gentra Puregene Blood Kit (Qiagen, Germantown, MD) following the manufacturer’s recommendations. ADRA1A genotype was determined using 5’-fluorogenic exonuclease assays (TaqMan®, Applied Biosystems, Foster City, CA). The ADRA1A Cys347Arg genetic variant was genotyped using the TaqMan® primer-probe set (Applied Biosystems) ADRA1A rs1048101, Assay ID C_2696454_30. Polymerase chain reaction (PCR) amplifications were performed in duplicate using Platinum® quantitative PCR SuperMix-UDG ViiA7 (Invitrogen, Carlsbad, CA), and ViiA7 Software v1.1 was used for data analysis (Applied Biosystems). Sex was determined using an SRY rs11575897 (C_32310143_10, Applied Biosystems) TaqMan® assay. Population substructure was determined using TaqMan® SNP genotyping of ancestry informative markers (AIMs).[38]
Data Analysis
We compared baseline differences in demographics and drug use history using analysis of variance (ANOVA) for continuous variables, and Fisher’s exact test for categorical variables. The saline and 40mg subjective effects values were normalized to baseline (−15 min), and the difference between the 40mg and saline values evaluated across time. A repeated measures ANOVA was used to analyze the subjective effects scores over time for each participant. Population structure was included as a covariate in the statistical model. Data from each genotype group over time was analyzed to determine if the subjective effect scores were modulated by the ADRA1A genotype using R version 2.9.1 (R_Development_Core_Team, 2009). We compared ADRA1A genotype (0 = TT/TC genotype, 1 = CC genotype), time, and interactions between genotype and time. We analyzed all individuals with complete data, for the ADRA1A genotype (N=65). We calculated effect size as a partial eta-squared (denoted η2) statistic using condition or SNP variance over residual variance. The three general cut-offs for effect sizes are: large effect is ≥0.14, medium effect is ≥0.06, and a small effect is ≥0.01.[39] According to the Hardy-Weinberg equilibrium, the genotype frequencies observed were in the expected ratios (p = 0.789205). To determine population structure, our cohort was compared against CEPH-HGDP samples (1,035 subjects of 51 different regional populations including America, Europe, the Middle East, Central and East Asia, Oceania, and sub-Saharan Africa) as previously described.[38] Population structure was calculated by genotyping ten ancestry informative markers (AIMs). Previously, it was demonstrated that 94.6% of the maximum informational value is obtained using these ten AIMs.[40] The proportion of population substructure for each individual was determined using the STRUCTURE 2.3.3 software using four ancestral populations (K = 4), a burn-in period of 100,000 iterations, and 1 million Markov chain Monte Carlo replications after burn-in.[41,42] The proportion of population substructure represented in each individual was included in the analysis as a covariate to eliminate population stratification effects. Excluding the SRY assay and the ten ancestry informative markers, 27 variants have been examined for pharmacogenetic association using this dataset. Therefore, corrections for multiple testing were performed to evaluate experiment-wise significance (P <.05/27=0.0019) by applying the Bonferroni correction.
RESULTS
The demographic information is in Table 1. Although 66 subjects signed consent and gave blood samples for genetic analysis, one individual had an undefined genotype for ADRA1A and was subsequently removed from the data analysis. The genotypes of the participants included 30 CC homozygotes and 35 T-allele carriers (TC plus TT genotypes). The CC homozygote group was more likely to be older (p = 0.02), but there were no other differences between groups for demographics or drug use variables.
Table 1.
Demographic and clinical characteristics by ADRA1A rs1048101 genotype group
| Genotype group Characteristic |
CC | CT/TT |
|---|---|---|
| N | 30 | 35 |
| Male (%) | 76.7 | 82.9 |
| African American (%) | 86.7 | 62.9 |
| Caucasian (%) | 6.7 | 31.4 |
| Other (%) | 6.7 | 5.7 |
| Hispanic (%) | 3.3 | 14.3 |
| Education, years (SD) | 12.8 (1.8) | 12.5 (1.9) |
| Age (SD)* | 45.1 (5.9) | 41.0 (7.3) |
| Weight (SD) | 197.9 (42.9) | 181.9 (28.9) |
| Cocaine use, years (SD) | 19.0 (6.8) | 15.3 (8.1) |
| Cocaine use, daily, grams (SD) | 1.9 (1.2) | 2.5 (2.5) |
| Cocaine use, past 30 days (SD) | 18.3 (8.6) | 18.3 (7.5) |
| Nicotine use, years (SD) | 23.5 (6.3) | 19.4 (9.1) |
The only significant difference among the demographic variables is for Age, p = 0.0186. No significant baseline differences in any clinical characteristics were observed after adjusting for multiple testing (p > 0.05).
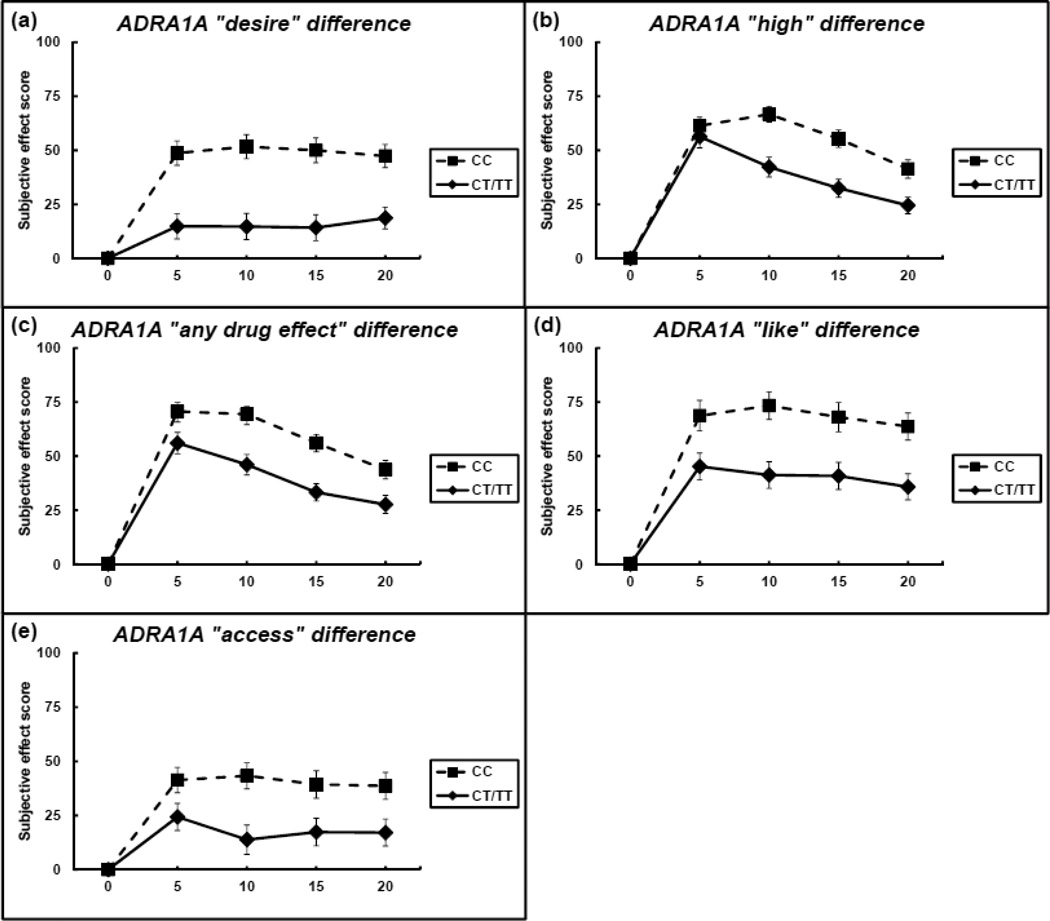
A dominant model was used for statistical analysis that grouped participants based upon presence or absence of the ADRA1A rs1048101 minor T allele (CC versus TC/TT). In comparison to saline, acute cocaine significantly increased heart rate and blood pressure, but there were no effects by genotype for any of these measures (data not shown). For the subjective effect ‘desire’, there was main effect of genotype (F = 37.06; df = 251; p = 4.26 × 10−9, η2=0.15) with an experiment-wise significance. The score for ‘desire’ in the CC genotype group increased to 48.67 ± 5.64 at 5 minutes into the trial and was sustained throughout the test period, while the value for ‘desire’ in the T-allele carrier group rose to 14.85 ± 5.85 at 5 minutes into the trial and later peaked at 18.63 ± 5.02 at 20 minutes after cocaine administration. The largest difference between the genotype groups for ‘desire’ occurred at 10 minutes after cocaine administration, where the CC genotype group had values of 51.67 ± 5.56 and the CT/TT genotypes group had values of 14.71 ± 6.04 (Figure 1A).
Figure 1. Subjective effect scores by ADRA1A rs1048101 genotype.
Subjective effect scores for ‘desire’ (panel A), ‘high’ (panel B), ‘any drug effect’ (panel C), ‘like’ (panel D), and ‘access’ (panel E) are shown for each time point starting with baseline at time zero. Scores for each time point in the CT/TT genotypes group (N = 35) are represented by the solid line, and time points for the CC genotype group (N = 30) are represented by the dashed line. Error bars indicate standard error of the mean.
For the subjective effect ‘high’, there was an experiment-wise significant main effect of genotype (F = 22.93; df = 251; p = 2.86 × 10−6, η2=0.09) and time (F = 25.29; df = 251; p = 9.41 × 10−7, η2=0.10); however, the interaction term was not significant (p = 0.79). The score for ‘high’ in the CC genotype group increased from baseline to 61.33 ± 3.97 at 5 minutes into trial, peaking at 66.67 ± 3.63 at 10 minutes into the test period. In the CT/TT genotypes group, the score for ‘high’ peaked at 56.29 ± 5.16 at 5 minutes into the trial and gradually decreased over the remainder of the test period. The largest difference between the genotype groups for ‘high’ occurred at 10 minutes into the trial where the CC genotype group had values of 66.67 ± 3.63 and the CT/TT genotypes group had values of 42.26 ± 4.55. (Figure 1B).
For the subjective effect ‘any drug effect’, there was an experiment-wise significant main effect of genotype (F = 22.57; df = 251; p = 3.42 × 10−6, η2=0.09) and time (F = 29.35; df = 251; p = 1.42 × 10−7, η2=0.12), and the interaction term was not significant (p = 0.92). The peak in ‘any drug effect’ for both groups occurred at 5 min following administration, where the CC genotype group had values of 70.67 ± 4.17 and the CT/TT genotypes group had values of 56.0 ± 4.96. The largest difference between the genotype groups for ‘any drug effect’ occurred at 10 minutes following administration where the CC genotype group had values of 69.33 ± 3.54 and the CT/TT genotypes group had values of 46.00 ± 4.74 (Figure 1C).
For the subjective effect ‘like cocaine’, there was an experiment-wise significant main effect of genotype (F = 21.04; df = 251; p = 7 × 10−6, η2=0.08), and the time and interaction terms were not significant. Similar to the subjective effects of cocaine ‘high’, ratings for ‘like cocaine’ continued to increase after the 5 minute time point and peaked at 10 minutes following administration in the CC homozygote group, while the T-allele carriers reported peak levels of ‘like cocaine’ at 5 minutes into the trial. The largest difference between genotype groups for ‘like cocaine’ occurred at 10 minutes following administration where the CC genotype group had values of 73.33 ± 6.36 and the CT/TT genotypes group had values of 41.23 ± 6.16 (Figure 1D).
For the subjective effect ‘access’ there was a significant main effect of genotype (F = 12.35; df = 251; p = 5.23 × 10−4, η2=0.05). The score for ‘access’ in the CC genotype group increased from baseline to 41.33 ± 5.87 at 5 minutes into trial, peaking at 43.33 ± 5.98 at 10 minutes into the test period. In the CT/TT genotypes group, the score for ‘high’ peaked at 24.34 ± 6.25 at 5 minutes into the trial and gradually decreased over the remainder of the test period. The largest difference between genotype groups occurred at 10 minutes following administration, where the CC genotype group had values of 43.33 ± 5.98 and the CT/TT genotypes group had values of 13.83 ± 6.79 (Figure 1E).
DISCUSSION
We found that homozygous individuals for the C allele of rs1048101 in the ADRA1A gene (ARG isoform) reported significantly elevated ratings of drug desire, high, and liking in response to acute cocaine administration in the laboratory, while those carrying at least one T allele of rs1048101 reported lower subjective effects. Additionally, in comparison to T-allele carriers, CC homozygotes also reported higher ratings of ‘any drug effect’ as well as higher likelihood of using cocaine if given ‘access’. We also found that in CC homozygotes, cocaine desire, high, and liking continue to peak for up to 10 minutes after cocaine administration, rather than peaking early (within 5 minutes) and gradually reducing. Thus, we confirmed our hypothesis that CC homozygotes would report higher subjective effects in response to cocaine.
According to the incentive salience theory of addiction, the activation of the mesocortical dopaminergic system is critical for drug ‘wanting’ (i.e., seeking for conditioned, drug-related rewards) and likely involves recruitment and modulation of ventral pallidum neurocircuitry as well as other structures (i.e., orbitofrontal and insular cortex, VTA, and NAc.[43,44,45] As stated above, both drug ‘liking’ and euphoria (i.e., the subjective, pleasurable effect of the substance) are thought to be mediated by activation of mesocorticolimbic DA, although not exclusively. The degree to which these collective drug effects occur is likely influenced by noradrenergic contributions. A study by Rothman et al. found that psychostimulants producing amphetamine-like subjective effects were more potent at NE release than DA release, and that psychostimulants produced these subjective effects at doses which activate the NE system, rather than the DA system.[46] Additionally, NE activity in the PFC was found to be critical for amphetamine-induced reward as well as DA release in the mesoaccumbens.[47] Further, in a separate study, NET-KO mice were found to self-administer cocaine at approximately four-fold the rate of wild type mice, suggesting that in the absence of NET, a decrease in the stimulant potency and reinforcement of the drug developed.[48]
The noradrenergic contribution to drug reward, and more specifically to drug wanting/liking, appears to be influenced by genetic factors. In a previous study, our group found that cocaine-dependent, CC homozygotes (those with the ARG configuration) did not experience a reduction in cocaine use when treated with disulfiram, while cocaine-dependent T-allele carriers demonstrated reduction in cocaine use in response to the medication.[29] Participants with the ARG configuration of ADRA1A likely experiences enhanced signaling in response to activation from NE, as there are no differences between the ARG and CYS isoforms in regards to physical conformation or binding affinity for NE.[21] These findings suggest ADRA1A in CC homozygotes does not have altered activation or signaling in the setting of pharmacologically reduced levels of synaptic NE. This persistent activity at ADRA1A is one possible explanation of the lack of response to disulfiram among CC homozygotes. Importantly, a similar phenomenon, referred to as ‘constitutive activity,’ has been described in α1-adrenoceptors, and is characterized by agonist-independent activity of the receptor, as well as increased binding affinity and intrinsic partial agonist activity.[49] In ADRA1A and ADRA1B receptors, specific mutations have been shown to result in increases in constitutive activity, agonist binding, and activation of intracellular second messengers.[50] Constitutively active wild-type ADRA1D receptors have also been described in rat fibroblasts [51] and vasculature (i.e., aorta, mesenteric arteries) [52], and conformational changes in the receptor have been linked with not only receptor activity, but plasma membrane expression.[53] Taken altogether, these findings suggest a potential mechanistic explanation of ADRA1A’s persistent activity, although constitutive activity in α1A- and α1B-adrenoceptors has not yet been determined in physiological systems.[49] Ultimately, in contrast, the CYS isoform of the receptor, seen in T-allele carriers, appears to be appropriately sensitive to synaptic levels of NE, and with pharmacologic reduction in NE levels and attenuation of activity at the receptor, there is an observed reduction in cocaine use.
Our present findings are consistent with this previous study. In CC homozygote participants, which likely experience enhanced signaling of ADRA1A, the subjective effects are reported as higher and more prolonged than in the T-allele carriers. Increased activation in the noradrenergic system would result in further downstream dopamine activity, since there is a functional coupling of these two neurotransmitter systems. As suggested by other studies, the degree of stimulation of D2 receptors translates directly into overall activity in the mesolimbic DA system and resultant feelings of euphoria and drug reward.[54] It follows that the higher and more prolonged cocaine euphoria, liking, and desire (i.e., wanting) observed among CC homozygote participants could confer higher addictive liability for this group, creating an even greater risk for development of cocaine dependence, or result in greater difficulty in establishing and/or maintaining abstinence from cocaine.
While these findings reveal the possible role of genetic factors in the positive subjective responses to acute cocaine, some important limitations should be noted. First, this study focuses on the effects of acute cocaine administration in cocaine-dependent participants and it might also be valuable to explore the impact of genetic factors on subjective effects produced by chronic or binge cocaine exposure in these individuals. Additionally, the administration of cocaine in the laboratory setting is quite different from use in a naturalistic setting. In our study, a smaller dose was given (40mg, IV) as compared to the typical amount of use reported by subjects (see Table 1). While this dose (40 mg) has been shown to increase heart rate, blood pressure and subjective effects produced by cocaine, future studies would provide more detailed information by analyzing effects produced by a range of doses. Lastly, the small size of our sample limits our ability to generalize to the broader population of cocaine-dependent patients. Nonetheless, the data are consistent with the suggestion that functional genetic polymorphisms determine response to substances of abuse.
In summary, this study demonstrates that CC homozygotes for the rs1048101 allele of the ADRA1A gene experience greater subjective effects upon cocaine administration in comparison to T-allele carriers. These findings may relate to differences in signaling and activation of the ADRA1A as determined by this genetic polymorphism. Combined with our previous results from the disulfiram pharmacogenetic trial, the ADRA1A polymorphism rs1048101 may help to identify a genetic subpopulation for which cocaine is more rewarding, but is also less likely to respond to noradrenergic medications for treatment of cocaine dependence. Ultimately, these findings enhance our understanding in the development of noradrenergic medications for the treatment of cocaine addiction and many of these efforts are ongoing in our laboratory and others.[55,56,57]
Acknowledgments
Source of Funding:
DS was supported in part by Career Development Award I01BX007080 from the United States (U.S) Department of Veterans Affairs Clinical Sciences Research and Development Service. DAN, TRK, TFN, and RDLG were supported by NIH/NIDA P50 DA018197, through MD Anderson’s Cancer Center Support Grant NIH/NIDA DA026120, and the Toomim Family Fund. This material is the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center.
Footnotes
Statement of Conflicts of Interest:
The authors have no conflicts of interest to declare.
AUTHORS CONTRIBUTIONS
DAN, TRK, TFN, and RDLG were responsible for overall study design. TFN and RDLG were responsible for implementation of the clinical protocols. DAN was responsible for design of the genetics protocols. DAN, TFN, and RDLG performed acquisition of data. SCH and EMN performed the statistical data analyses and aided in the interpretation of findings. DS drafted the manuscript. DAN, SCH, EMN, TRK, TFN, and RDLG aided in critical evaluation and revision of the manuscript.
References
- 1.Rothman R, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharm. 2013;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R., 2nd Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs. 2013;22:1549–1568. doi: 10.1517/13543784.2013.836488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- 4.Haile CN, Mahoney JJ, 3rd, Newton TF, De La Garza R., 2nd Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacol Ther. 2012;134:260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carboni E, Tanda GL, Frau R, DiChiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- 6.Mead AN, Rocha BA, Donovan DM, Katz JL. Intravenous cocaine induced-activity and behavioural sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur J Neurosci. 2002;16:514–520. doi: 10.1046/j.1460-9568.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- 7.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt KT, Weinshenker D. Adrenaline rush: The role of adrenergic receptors in stimulant-induced behaviors. Mol Pharmacol. 2014;85:640–650. doi: 10.1124/mol.113.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the nentral nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith RJ, Aston-Jones G. α(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XY, Kosten TA. Previous exposure to cocaine enhances self-administration in an alpha-1 adrenergic receptor dependent manner. Neuropsychopharmacology. 2007;32:638–645. doi: 10.1038/sj.npp.1301120. [DOI] [PubMed] [Google Scholar]
- 14.Drouin C, Blanc G, Villegier AS, Glowinski, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, et al. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine β-hydroxylase. Neuropsychopharmacol. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder JP, Epps SA, Grice TW, Weinshenker D. The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinshenker D, Schroeder JP. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 19.Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- 20.Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebral microdialysis. Brain Res. 1990;523:134–138. doi: 10.1016/0006-8993(90)91646-x. [DOI] [PubMed] [Google Scholar]
- 21.Lei B, Morris DP, Smith MP, Svetkey LP, Newman MF, Rotter JI, et al. Novel human alpha-1a-adrenocepter single nucleotide polymorphisms alter receptor pharmacology and biological function. Naunyn Schmiedebergs Arc Pharmacol. 2005;371:229–239. doi: 10.1007/s00210-005-1019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong CJ, Wang YC, Liu TY, Liu HC, Tsai SJ. A study of alpha-adrenoceptor gene polymorphisms and Alzheimer disease. J Neural Transm (Vienna) 2001;108:445–450. doi: 10.1007/s007020170065. [DOI] [PubMed] [Google Scholar]
- 23.Gu D, Ge D, Snieder H, He J, Chen S, Huang J, et al. Association of alpha1A adrenergic receptor gene variants on chromosome 8p21 with human stage 2 hypertension. J Hypertens. 2006;24:1049–1056. doi: 10.1097/01.hjh.0000226194.21311.2f. [DOI] [PubMed] [Google Scholar]
- 24.Jiang S, Mao G, Zhang S, Hong X, Tang G, Li Z, et al. Individual and joint association of alpha1A-adrenergic receptor Arg347Cys polymorphism and plasma irbesartan concentration with blood pressure therapeutic response in Chinese hypertensive subjects. Clin Pharmacol Ther. 2005;78:239–248. doi: 10.1016/j.clpt.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Snapir A, Koskenvuo J, Toikka J, Orho-Melander M, Hinkka S, Saraste M, et al. Effects of common polymorphisms in the alpha1A-, alpha2B-, beta1- and beta2-adrenoreceptors on haemodynamic responses to adrenaline. Clin Sci (Lond) 2003;104:509–520. doi: 10.1042/CS20020299. [DOI] [PubMed] [Google Scholar]
- 26.Klotsman M, Weinberg CR, Davis K, Binnie CG, Hartmann KE. A case-based evaluation of SRD5A1, SRD5A2, AR, and ADRA1A as candidate genes for severity of BPH. Pharmacogenomics J. 2004;4:251–259. doi: 10.1038/sj.tpj.6500248. [DOI] [PubMed] [Google Scholar]
- 27.Puumalu T, Riekkinen P, Sirvio J. Modulation of vigilance and behavioral activation by alpha-1 adrenoceptors in the rat. Pharmacol Biochem Behav. 1997;56:705–712. doi: 10.1016/s0091-3057(96)00408-x. [DOI] [PubMed] [Google Scholar]
- 28.Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 29.Shorter D, Nielsen DA, Huang W, Harding MJ, Hamon SC, Kosten TR. Pharmacogenetic randomized trial for cocaine abuse: Disulfiram and alpha-1A adrenoceptor gene variation. Eur Neuropsychopharmacol. 2013;23:1401–1407. doi: 10.1016/j.euroneuro.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spellicy CJ, Harding MJ, Hamon SC, Mahoney JJ, 3rd, Kosten TR, Newton TF, et al. A varian in ANKK1 modulates acute subjective effects of cocaine: a preliminary study. Genes Brain Behav. 2014;13:559–564. doi: 10.1111/gbb.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brewer AJ, Nielsen DA, Spellicy CJ, Hamon SC, Gingrich J, Thompson-Lake DG, et al. Genetic Variation of the Dopamine Transporter (DAT1) Influences the Acute Subjective Responses to Cocaine in Volunteers with Cocaine Use Disorders. Pharmacogenet Genomics. 2015;6:296–304. doi: 10.1097/FPC.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl):22–23. [PubMed] [Google Scholar]
- 33.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington DC: American Psychiatric Association; 2000. text revision (DSM-IV-TR) [Google Scholar]
- 35.De La Garza R, 2nd, Verrico CD, Newton TF, Mahoney JJ, 3rd, Thompson-Lake DG. Safety and preliminary efficacy of the acetylcholinesterase inhibitor Huperzine A as a treatment for cocaine use disorder. Int J Neuropsychopharmacol. 2015;19:pyv098. doi: 10.1093/ijnp/pyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De La Garza R, 2nd, Bubar MJ, Carbone CL, Moeller FG, Newton TF, Anastasio NC, et al. Evaluation of the dopamine β-hydroxylase (DβH) inhibitor nepicastat in participants who meet criteria for cocaine use disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:40–48. doi: 10.1016/j.pnpbp.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton TF, Haile CN, Mahoney JJ, 3rd, Shah R, Verrico CD, De La Garza R, 2nd, et al. Dopamine D3 receptor-preferring agonist enhances the subjective effects of cocaine in humans. Psychiatry Res. 2015;230:44–49. doi: 10.1016/j.psychres.2015.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, et al. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine-beta-hydroxylase. Biol Psych. 2013;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 40.Lao O, van Duijn K, Kersbergen P, de Knijff P, Kayser M. Proportioning whole-genome single-nucleotide-polymorphism diversity for the identification of geographic population structure and genetic ancestry. Am J Hum Genet. 2006;78:68–90. doi: 10.1086/501531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 44.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacol (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 45.Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23:1879–1885. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha BA. Stimulant and reinforcing effects of cocaine in monoamine transporter knockout mice. Eur J Pharmacol. 2003;479:107–115. doi: 10.1016/j.ejphar.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 49.Cotecchia S. Constitutive activity and inverse agonism at the α1 adrenoceptors. Biochem Pharmacol. 2007;73:1076–1083. doi: 10.1016/j.bcp.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Hwa J, Graham RM, Perez DM. Chimeras of alpha1-adrenergic receptor subtypes identify critical responses that modulate active state isomerization. J Biol Chem. 1996;271:7956–7964. doi: 10.1074/jbc.271.14.7956. [DOI] [PubMed] [Google Scholar]
- 51.McCune DF, Edelmann SE, Olges JR, Post GR, Waldrop BA, Waugh DJ, et al. Regulation of the cellular localization and signaling properties of the alpha(1B)- and alpha(1D)-adrenoceptors by agonists and inverse agonists. Mol Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- 52.Gisbert R, Ziani K, Miguel R, Noguera MA, Ivorra MD, Anxelmi E, et al. Pathological role of a constitutively active population of alpha(1D)-adrenoceptors in arteries of spontaneously hypertensive rats. Br J Pharmacol. 2002;135:206–216. doi: 10.1038/sj.bjp.0704447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Sainz JA, Romero-Avila MT, Medina Ldel C. α(1D)-Adrenergic receptors constitutive activity and reduced expression at the plasma membrane. Methods Enzymol. 2010;484:109–125. doi: 10.1016/B978-0-12-381298-8.00006-X. [DOI] [PubMed] [Google Scholar]
- 54.Seger D. Cocaine, metamfetamine, and MDMA abuse: the role and clinical importance of neuroadaptation. Clin Toxicol. 2010;48:695–708. doi: 10.3109/15563650.2010.516263. [DOI] [PubMed] [Google Scholar]
- 55.Haile CN, Hao Y, O’Malley P, Newton TF, Kosten TA. The α1-antagonist doxazosin alters the behavioral effects of cocaine in rats. Brain Sci. 2012;2:619–633. doi: 10.3390/brainsci2040619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haile CN, De La Garza R, 2nd, Mahoney JJ, 3rd, Nielsen DA, Kosten TR, Newton TF. The impact of disulfiram treatment on the reinforcing effects of cocaine: a randomized clinical trial. PLoS One. 2012;7:e47702. doi: 10.1371/journal.pone.0047702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newton TF, De La Garza R, II, Brown G, Kosten TR, Mahoney JJ, III, Haile CN. Noradrenergic α1 Receptor Antagonist Treatment Attenuates Positive Subjective Effects of Cocaine in Humans: A Randomized Trial. PLoS ONE. 2012;7:e30854. doi: 10.1371/journal.pone.0030854. [DOI] [PMC free article] [PubMed] [Google Scholar]