Abstract
Purpose
Pancreatic adenocarcinoma is a highly aggressive cancer, currently treated with limited success and dismal outcomes. New diagnostic and treatment strategies offer the potential to reduce cancer mortality. Developing highly-specific non-invasive imaging probes for pancreatic cancer is essential to improving diagnostic accuracy and monitoring therapeutic intervention.
Experimental Design
A bispecific heterodimer was synthesized by conjugating an anti-tissue factor (TF) Fab with an anti-CD105 Fab, via the bioorthogonal “click” reaction between tetrazine (Tz) and trans-cyclooctene (TCO). The heterodimer was labeled with 64Cu for positron emission tomography (PET) imaging of nude mice bearing BXPC-3 xenograft and orthotopic pancreatic tumors.
Results
PET imaging of BXPC-3 (TF/CD105+/+) xenograft tumors with 64Cu-labeled heterodimer displayed significantly enhanced tumor uptake (28.8 ± 3.2 %ID/g; n = 4, SD) at 30 h post-injection (p.i.), as compared to each of their monospecific Fab tracers (12.5 ± 1.4 and 7.1 ± 2.6 %ID/g; n = 3, SD). In addition, the activity-concentration ratio allowed for effective tumor visualization (tumor/muscle ratio 75.2 ± 9.4 at 30 h p.i.; n = 4, SD). Furthermore, 64Cu-NOTA-heterodimer enabled sensitive detection of orthotopic pancreatic tumor lesions with an uptake of 17.1 ± 4.9 %ID/g at 30 h p.i. and tumor/muscle ratio of 72.3 ± 46.7.
Conclusions
This study demonstrates that dual targeting of TF and CD105 provided synergistic improvements in binding affinity and tumor localization of the heterodimer. Dual-targeted imaging agents of pancreatic and other cancers may assists in diagnosing pancreatic malignancies as well as reliable monitoring of therapeutic response.
Keywords: Dual-targeting, pancreatic cancer, positron emission tomography (PET), tissue factor, CD105 (endoglin)
Introduction
Pancreatic adenocarcinoma is one of the most lethal cancers worldwide, with an overall 5-year survival rate of approximately 7% (1). Despite aggressive treatment protocols, including surgical intervention, chemotherapy and radiotherapy, 5-year survival rates have remained unchanged over the last decade. These dismal outcomes have been linked, in part, to ineffective diagnostic tools detecting pancreatic malignancies and monitoring therapeutic response (2). Currently, there are no clinically reliable serum biomarkers available for the recognition of early symptoms of pancreatic cancer in patients (3). Initial diagnoses and disease staging are commonly accomplished using computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS) (4, 5). As over 80% of patients are diagnosed with unresectable disease, there is an urgent need for sensitive and specific imaging agents for pancreatic cancer (6).
Molecular imaging allows for the noninvasive assessment of physiological and pathological processes at the cellular or molecular level in living organisms (7). PET imaging using 18F-FDG can effectively detect primary pancreatic tumors and hepatic metastases commonly missed by CT and MRI (8). While 18F-FDG PET is a sensitive diagnostic modality for detecting pancreatic malignancies, this radioactive sugar molecule is limited by poor specificity and high off-target uptake in inflammatory diseases (9, 10). To improve upon the limitations of 18F-FDG PET, immunoPET employs radioactive monoclonal antibodies (mAbs) or antibody fragments for selective targeting of malignancies. Antibody-based imaging agents offer high specificity for epitopes known to be differentially expressed on cancer cells (11).
Furthermore, bispecific antibody fragments are promising alternatives to conventional antibody therapeutics, as they allow for simultaneous recognition of two antigens. The bispecific properties of heterodimers promote enhanced tumor uptake through improved specificity (12, 13). Although bispecific antibodies have been proposed for combination therapy in clinical trials, their potential utilization as molecular imaging agents remains largely unexplored (14, 15).
Concurrent targeting of tumor vasculature and cancer cells can provide additional benefits for molecular imaging. A majority of pancreatic adenocarcinomas are associated with arterial thrombosis, migratory thrombophlebitis, tumor angiogenesis, and rapid metastasis (16, 17). Tissue factor (TF), which serves as the primary initiator of the extrinsic pathway of blood coagulation, is overexpressed in most solid tumors, including pancreatic cancer (18). In general, it is thought that overexpression of TF contributes to higher incidence rates of thrombotic complications in cancer patients (19). Similarly, CD105 (also called endoglin) is a proliferation-associated cell-surface protein highly expressed on activated endothelial cells. Overexpression of CD105 is known to be associated with decreased patient survival for most cancers (20). Given the heterogeneous nature and complex stromal microenvironment of pancreatic cancer, targeting of individual tumor-specific antigens may result in suboptimal imaging agent accumulation (21).
In the current manuscript, we describe the development and evaluation of a novel bispecific heterodimer through conjugation of two mAb Fab fragments, respectively targeting TF and CD105. Antibody fragments were generated through enzymatic digestion of ALT-836, an anti-TF chimeric mAb, and TRC105, a mAb recognizing both human and murine CD105. The conjugation of two mAb fragments was accomplished through the inverse-electron-demand Diels–Alder reaction between electron-deficient tetrazine (Tz) and strained trans-cyclooctene (TCO), taking advantage of the emerging TCO/Tz “click chemistry” platform. In addition, we have shown that the biological activity of the heterodimer was maintained after the conjugation reaction. To date, there are no investigations into the simultaneous targeting of TF and CD105 for molecular imaging. We hypothesized that this TF/CD105 heterodimer would synergistically harness the targeting capabilities of ALT-836-Fab and TRC105-Fab. To test this hypothesis, we examined the advantages of dual TF/CD105 targeting in terms of tumor-binding affinity and specificity in a human pancreatic cancer mouse model.
Materials and Methods
Chemicals
TRC105 and ALT-836 were respectively provided by TRACON Pharmaceuticals Inc. (San Diego, CA, USA) and Altor Bioscience Corporation (Miramar, FL, USA). 2-S-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) was obtained from Marocyclics, Inc. (Dallas, TX, USA). TCO-PEG4-NHS ester and tetrazine-PEG5-NHS ester were purchased from Click Chemistry Tools (Scottsdale, AZ, USA). Pierce immobilized papain, protein A column, and all other reaction buffers and chemicals were purchased from Thermo Fisher Scientific (Carlsbad, CA, USA).
Fab generation and characterization
ALT-836 (2-mg/mL) and TRC105 (2-mg/mL) were individually digested in reaction buffer (20-mM sodium phosphate monobasic, 10-mM disodium ethylenediaminetetraacetic acid (EDTA), and 80-mM cysteine, HCl) for 4 h at 37 °C, with immobilized papain/total volume at a ratio of 1:10. The reaction mixture was centrifuged at 13,200-rpm for 5-min to remove immobilized papain. The reaction was filtered through a Millipore 0.22-μm syringe filter (EMD Millipore, Darmstadt, Germany) before the supernatant was purified by size exclusion chromatography using a HiPrep 16/60 Sephacryl S-100 HR (GE Healthcare, Madison, WI, USA). The collected fraction (~50-kDa) was concentrated using a 10-kDa molecular weight cut-off (MWCO) spin-filter (Amicon Ultra-4, Millipore) and purified with a protein A column (NAb Protein A Spin Kit; Thermo Fisher Scientific, Carlsbad, CA, USA). The purity of ALT-836-Fab and TRC105-Fab was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie brilliant blue R-250 staining (Thermo Fisher Scientific, Carlsbad, CA, USA).
Heterodimer synthesis and purification
Ten-nmol ALT-836-Fab was mixed with 20-nmol TCO-PEG4-NHS ester in phosphate buffered saline (PBS) at pH 8.5 and incubated for 2 h at 25 °C. Concurrently, 10-nmol of TRC105-Fab was incubated with 20-nmol of tetrazine-PEG5-NHS ester using the same conditions described for TCO-PEG4-NHS ester. Reaction mixtures were individually passed through PD-10 columns for collection of activated ALT-836-Fab and TRC105-Fab, respectively. Activated ALT-836-Fab and TRC105-Fab were mixed at an equimolar ratio in PBS buffer and incubated at 25 °C for 2 h. The heterodimers were separated by gel filtration on a HiPrep 16/60 Sephacryl S-100 HR and purity was evaluated by 10%-SDS-PAGE gel.
NOTA conjugation and 64Cu labeling
Chelation of Fab conjugates was accomplished with a reaction ratio of p-SCN-Bn-NOTA: Fab of 10:1 at pH 9.0. NOTA-heterodimer was purified using a PD-10 column (GE Healthcare Life Sciences, Piscataway, NJ, USA) with PBS as the mobile phase. High-specific activity (> 5 Ci/μmol) 64Cu was produced in a CTI RDS 112 cyclotron via 64Ni(p,n)64Cu reaction. For radiolabeling, 50-100-μg of NOTA-Fab was reacted with 74-148-MBq (2–4 mCi) of 64CuCl2 in 300-μL of sodium acetate buffer (0.1 M, pH 4.5) at 37 °C for 30 min under constant agitation (400 rpm). 64Cu-NOTA-Fab was purified from free activity using a PD-10 column with PBS as the mobile phase.
Cell lines and animal model
The human pancreatic adenocarcinoma cell line (BXPC-3) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured according to the supplier's protocol. All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. For implantation, 5 × 106 tumor cells were mixed with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at a ratio of 1:1 before subcutaneously injected into the forelimb axillary of 4-to-5-wk-old female athymic nude mice (Envigo, Cambridgeshire, PE28 4HS United Kingdom). Over the subsequent 3–4 weeks post-inoculation, tumors were monitored and allowed to grow to 5–8 mm in diameter.
For orthotopic tumor models, the splenic portion of the pancreas was carefully exposed and 2×106 tumor cells in Matrigel: PBS (ratio 1:1, total volume of 30 μl) were injected into the tail of the pancreas. The incision was closed in 2 layers (peritoneum and abdominal wall) with interrupted sutures. After implantation, nude mice were inspected daily for bleeding or wound complications. After 2 weeks, mice were examined for tumor formation twice a week by palpation. After 5–12 weeks, tumors were confirmed by PET imaging and histology to be pancreatic adenocarcinoma.
Flow cytometry
In vitro TF and CD105 binding affinity/specificity of the heterodimer was evaluated by flow cytometry in BXPC-3 cells. Briefly, cells were harvested and suspended in PBS supplemented with 2% BSA at a concentration of 5 × 106 cells/mL. Cells were incubated with 100 nmol fluorescein isothiocyanate (FITC)-labeled heterodimer and Fab conjugates for 30 min at 25 °C. After washing, samples were analyzed with a FACS Calibur 4-color cytometer (BD Biosciences, Franklin Lakes, NJ, USA). FlowJo software was employed for analysis of flow cytometry data (Tree Star Inc., Ashland, OR, USA).
PET imaging and biodistribution studies
PET and PET/CT scans were performed using an Inveon microPET/microCT rodent scanner (Siemens Healthcare, Erlangen, Germany). Image reconstruction and region-of-interest (ROI) analysis of each PET image was carried out as previously described (22). BXPC-3 tumor-bearing mice were intravenously injected with 5-10-MBq (~150 – 300-μCi) of either 64Cu-NOTA-heterodimer, 64Cu-NOTA-ALT-836-Fab, or 64Cu-NOTA-TRC105-Fab. Sequential static PET scans were acquired at 3, 15, 24, and 30 h post-injection (p.i.) of tracers. Twenty million coincidence events per mouse were acquired for each static PET emission scan. CT-based attenuation correction was performed, and the registered CT image set fused with the PET image set for anatomic orientation. After the last PET scan at 30 h p.i., mice were euthanized for biodistribution analysis. Tissues were collected and wet-weighed before the activity was assayed using an automated γ-counter (2470 WIZARD 2; Perkin Elmer, Waltham, MA, USA). The activity concentration was decay corrected to the time of injection, and the results were expressed as a percentage of injected radioactivity dose per gram of tissue (% ID/g) (mean ± SD; ≥ 3 mice per group).
Histology
Slices of tissue at 5-μm thickness were fixed with cold acetone for 10 min and air-dried for 30 min. After rinsing with PBS and blocking with 10% donkey serum for 30 min at 25 °C, the slides were incubated with 20-nM FITC-labeled Fab conjugates for TF or CD105 staining. Then, rat anti-mouse CD31 antibody (Thermo Fisher Scientific, Carlsbad, CA, USA) and Cy3-labeled donkey anti-rat IgG (Thermo Fisher Scientific, Carlsbad, CA, USA) were used for CD31 staining (red). 4',6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Carlsbad, CA, USA) was used to stain cell nuclei and confocal fluorescence images were acquired with an Eclipse Ti microscope (Nikon, Melville, NY, USA).
Statistical analyses
Quantitative data were expressed as mean ± standard deviation (SD). Means were compared using the unpaired Student t test. P values of less than 0.05 were considered statistically significant.
Results
Synthesis and characterization of heterodimer
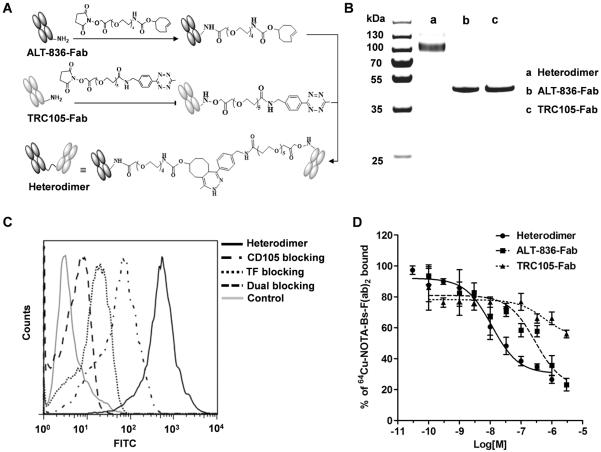
Monovalent Fab antibody fragments were created by papain digestion of intact ALT-836 or TRC105 mAb and separated by size exclusion chromatography. Purification of fragments was accomplished with protein A affinity columns. Derived Fab fragments were reacted with Diels-Alder orthogonal reactive pair tetrazine (Tz)/ transcyclooctene (TCO) for the generation of bispecific heterodimer (Fig. 1A). The reaction was monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and a band at ~100-kDa was observed, corresponding to the Fab dimer molecular weight. Bispecific heterodimer antibody fragments were purified by size exclusion chromatography from unreacted Fabs, and the reaction efficiency was calculated to be 38% (Supplementary Fig. 1). Purity and identity of the heterodimer (M[H]+:103.50-kDa) were confirmed by SDS-PAGE and MALDI-TOF mass spectra (Fig. 1B and Supplementary Fig. 2).
Figure 1.
Synthesis and characterization of the heterodimer. A, Schematic representation of the synthesis of the heterodimer. B, SDS-PAGE gel confirming the identity and purity of heterodimer. C, Flow cytometry analysis in BXPC-3 after 1 h incubation of FITC-Fab conjugates (50 nM) confirmed the dual-targeting of TF and CD105 and specificity of the heterodimer. D, Competitive binding assay comparing heterodimer (circles), ALT-836-Fab (squares), and TRC105-Fab (triangles) binding affinities. IC50 values were markedly lower for the heterodimer (11.35 ± 1.04 nM) compared to ALT-836-Fab (288.9 ± 18 nM) and TRC105-Fab (583.9 ± 36 nM).
In vitro bispecificity of the heterodimer
To evaluate the binding affinity and bispecificity of the heterodimer, FITC-labeled Fab conjugates were used to separately visualize the binding to human pancreatic cancer cells (BXPC-3), which express high levels of both TF and CD105. Compared to TRC105-Fab and ALT-Fab, the heterodimer revealed significantly stronger binding to BXPC-3 cells (Fig. 1C and Supplementary Fig. 3), which was effectively blocked with a saturating dose of ALT-836 or TRC105 antibody or both. Together, these results demonstrate that dual-targeting of TF and CD105 resulted in enhanced binding affinity and specificity of the heterodimer.
Finally, a competitive binding assay was performed to determine and compare the binding affinities of the heterodimer, ALT-836-Fab, and TRC105-Fab to BXPC-3 cells (Fig. 1D). The results of the binding isotherm showed a concentration dependent displacement of bound 64Cu-NOTA-heterodimer with IC50 values of 11.35 ± 1.04, 288.9 ± 18, and 583.9 ± 36-nM for heterodimer, ALT-836-Fab, and TRC105-Fab, respectively. Only a partial displacement of the bound radioligand at high concentrations (μM) of the competing antibody fragments demonstrated the ambivalent nature of heterodimer binding.
Heterodimer shows enhanced tumor-specific targeting in vivo
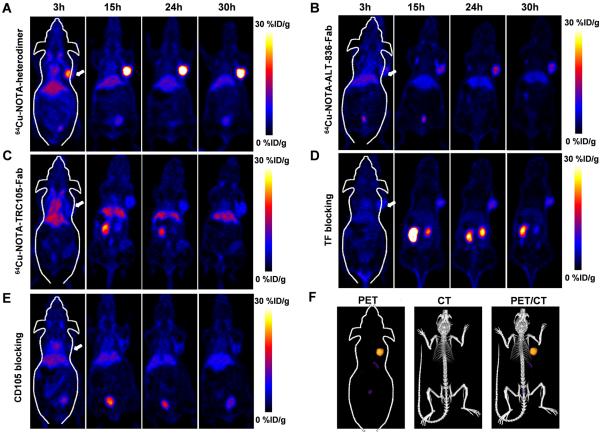
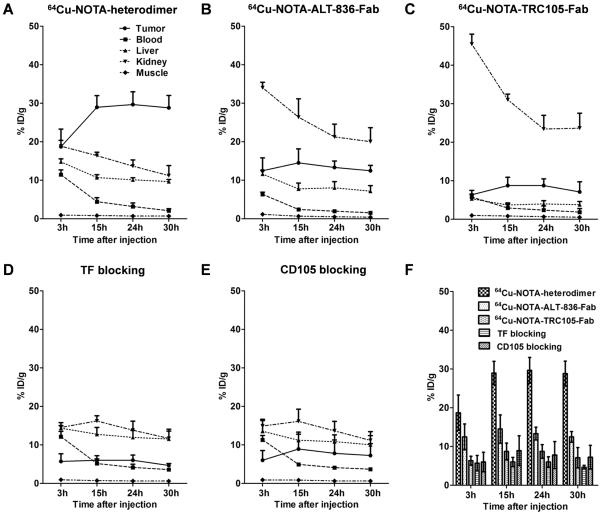
To evaluate the dual-targeting properties and high specificity of the heterodimer in vivo, each antibody fragment was conjugated to the chelator NOTA and radiolabeled with 64Cu. The average number of NOTA per antibody fragment was 3.6 ± 0.4 (Supplementary Fig. 4), and radiolabeling yield was 91.5% after 15 min incubation with 64Cu, as determined by radio-TLC. 200-300-μCi of 64Cu-NOTA-heterodimer, 64Cu-NOTA-ALT-836-Fab, or 64Cu-NOTA-TRC105-Fab were administered to BXPC-3 (TF/CD105 +/+) tumor-bearing athymic nude mice and time points of 3, 15, 24 and 30 h post-injection (p.i.) were chosen for serial static PET scans imaging, based upon previous PET imaging results using mono- and divalent antibody fragments (23, 24). Coronal PET images of BXPC-3 tumor-bearing mice showed rapid tumor accumulation of all three tracers with sharp delineation of tumor xenografts. The 64Cu-NOTA-heterodimer displayed significantly higher tumor accumulation, in comparison to the two monovalent fragments (Fig. 2A and Movie S1). Also, a representative PET/CT image of a BXPC-3 tumor-bearing mouse at 30 h p.i with 64Cu-NOTA-heterodimer is shown in Figure 2F. Region-of-interest (ROI) analyses of the images were performed for quantification of tracer uptake and expressed as %ID/g in BXPC-3 tumors as well as non-target tissues, including blood pool, liver, kidney, and muscle. As clearly indicated in PET images (n = 4; Fig. 2A and 3A), 64Cu-NOTA-heterodimer displayed high tumor accumulation at early time points (18.7 ± 4.6 %ID/g at 3 h p.i.) that peaked at 29.7 ± 3.3 %ID/g, 24 h p.i. of tracer. Maximum tumor uptake values for 64Cu-NOTA-ALT-836-Fab and 64Cu-NOTA-TRC105-Fab were significantly lower (P < 0.01) at 13.3 ± 1.7 %ID/g and 8.7 ± 1.8 %ID/g, respectively (n = 3; Fig. 2B,C and Fig. 3B,C). In contrast, the peak tumor uptake of free copper was 2.0 ± 1.8 %ID/g, which was significantly lower than that of this Fab conjugate (n = 3; Supplementary Fig. 5). Consistent with its higher molecular weight, 64Cu-NOTA-heterodimer displayed a longer blood half-life, evidenced by the initially elevated heart activity concentrations of 11.5 ± 1.2 %ID/g and 2.1 ± 0.5 %ID/g at 3 and 30 h p.i., respectively (n = 4; Fig. 3A). Kidney uptake of 64Cu-NOTA-ALT-836-Fab and 64Cu-NOTA-TRC105-Fab was higher than that of 64Cu-NOTA-heterodimer, demonstrating renal clearance as the major excretion pathway for Fab fragments (Fig. 3B and C). Liver uptake was comparable between 64Cu-NOTA-heterodimer and 64Cu-NOTA-ALT-836-Fab; however, two-fold lower values were registered for 64Cu-NOTA-TRC105-Fab, indicating less dominant hepatic clearance of this Fab conjugate.
Figure 2.
In vivo PET imaging of dual TF and CD105 expression with heterodimer in BXPC-3 bearing mice. A, Serial coronal PET images of 64Cu-NOTA-heterodimer at 3, 15, 24 and 30 h p.i. of each tracer. B, Serial coronal PET images of 64Cu-NOTA-ALT-836-Fab. C, Serial coronal PET images of 64Cu-NOTA-TRC105-Fab. D, TF blocking resulted in a significant decrease in 64Cu-NOTA-heterodimer tumor uptake. E, CD105 blocking also resulted in decreased tumor uptake of 64Cu-NOTA-heterodimer. F, A representative PET/CT image of BXPC-3 tumor-bearing mice at 30 h p.i. of 64Cu-NOTA-heterodimer (n ≥ 3).
Figure 3.
Quantitative ROI analysis of in vivo imaging data. A, Time-activity curves of the BXPC-3 tumor, blood, liver, kidney and muscle following intravenous administration of 64Cu-NOTA-heterodimer B, 64Cu-NOTA-ALT-836-Fab or C, 64Cu-NOTA-TRC105-Fab. D, Time-activity curves of BXPC-3 tumor and tissues after intravenous administration of 64Cu-NOTA-heterodimer after TF or E, 64Cu-NOTA-heterodimer after CD105 blocking. F, Comparison of tumor uptake in above all groups by quantitative analysis of the PET data (n ≥ 3).
To demonstrate that 64Cu-NOTA-heterodimer retained its in vivo specificity towards both TF and CD105, a large (40-mg/kg) dose of either ALT-836 or TRC105 or both ALT-836 and CD105 intact antibody was administered in mice 12 h prior to injection of the bispecific tracer. Peak tumor uptake values of 64Cu-NOTA-heterodimer significantly diminished to 6.0 ± 1.3 %ID/g, 8.9 ± 3.8 %ID/g and 2.8 ± 1.0 %ID/g after TF, CD105, and TF plus CD105 blockade (n = 3; Fig. 2D,E, Fig. 3D,E and Supplementary Fig. 6), respectively. Tracer uptake in all major organs was similar between 64Cu-NOTA-heterodimer and 64Cu-NOTA-heterodimer with TF or CD105 blockade, except the BXPC-3 tumor (significantly higher in the former), further confirming the TF and CD105 dual-specificity of 64Cu-NOTA-heterodimer (Fig. 3F). PET data demonstrated that dual-targeting using the heterodimer tracer offers a significant advantage in terms of absolute tumor uptake, target specificity, and off-target uptake compared to monospecific Fab fragments.
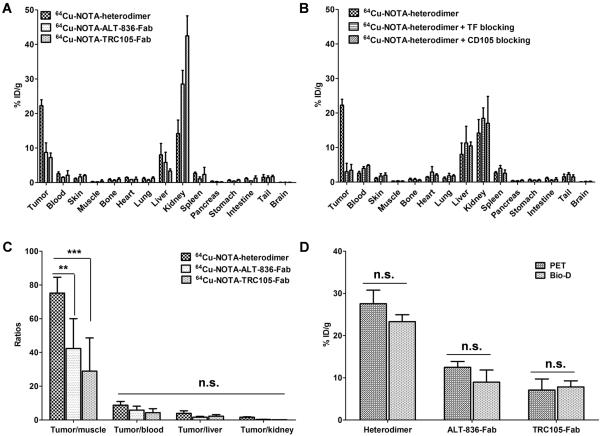
After terminal scans at 30 h p.i., ex vivo biodistribution and histology studies were performed to validate in vivo PET data and confirm the biodistribution profile of tracers (Fig. 4A and B). 64Cu-NOTA-heterodimer showed prominent tumor and low background uptake, with an excellent tumor/muscle ratio of 75.2 ± 9.4. This was superior to those of 64Cu-NOTA-ALT-836-Fab (42.4 ± 17.6) and 64Cu-NOTA-TRC105-Fab (29.0 ± 19.7), achieved at 30 h p.i. (Fig. 4C). Fig. 4D shows no statistically significant difference between PET-derived and biodistribution data, suggesting that ROI analyses of the PET images accurately reflected tracer distribution in vivo as well as the dual-specificity of 64Cu-NOTA-heterodimer.
Figure 4.
Ex vivo biodistribution validates the results of PET imaging. A, Biodistribution of 64Cu-NOTA-heterodimer, 64Cu-NOTA-ALT-836-Fab, and 64Cu-NOTA-TRC105-Fab in BXPC-3 bearing mice, 30 h p.i. (n ≥ 3). B, Biodistribution of 64Cu-NOTA-heterodimer after TF or CD105 blocking (n ≥ 3). C, Tumor-to-normal tissue comparison of 64Cu-NOTA-heterodimer and 64Cu-labeled Fab fragments. D, Comparison of tumor uptake of 64Cu-NOTA-heterodimer and Fab conjugate tracers between microPET and biodistribution data, 30 h p.i. Differences were not statistically significant (p > 0.05).
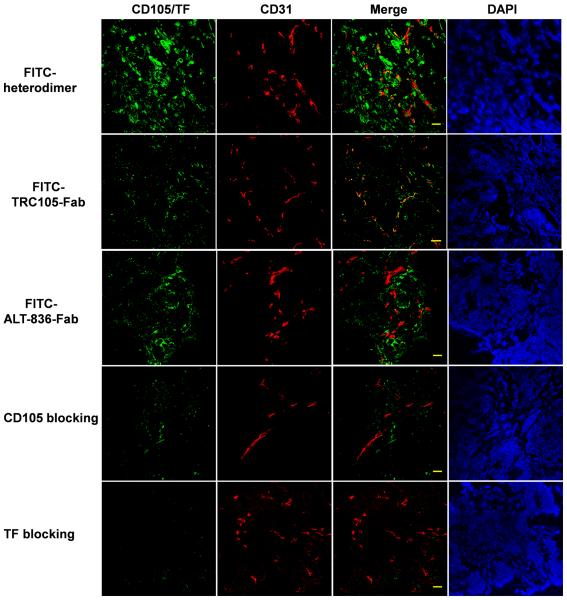
Immunofluorescence staining of resected BXPC-3 tumor sections was carried out to correlate PET-tracer uptake with in situ TF/CD105 expression (Fig. 5). FITC-labeled Fab conjugates were visualized using fluorescence microscopy, which showed localization consistent with TF and CD105 in situ expression profiles. FITC-TRC105-Fab uptake was localized within tumor vasculature, confirmed by co-localization with CD31, and on the cells, whereas FITC-ALT-836-Fab was found on the membrane of TF-expressing BXPC-3 cells. In agreement with its TF/CD105 bivalent property, FITC-heterodimer staining showed a stronger fluorescent signal that was distributed in both BXPC-3 cells and tumor-as4)sociated vasculature in the tissue. Blocking with ALT-836 or TRC105 resulted in low-intensity signals of TF/CD105 staining, confirming the TF/CD105 bi-specificity of 64Cu-NOTA-heterodimer.
Figure 5.
Immunofluorescence staining of BXPC-3 tumors sections. FITC-heterodimer, FITC-ALT-836-Fab, FITC-TRC105-Fab and FITC-heterodimer with TF or CD105 blocking were used for TF/CD105 staining (green). Rat anti-mouse CD31 antibody and Cy3-labeled donkey anti-rat IgG were used for CD31 staining (red). DAPI was used to stain cell nuclei (bar: 30 μm).
Heterodimer shows enhanced detection of orthotopic pancreatic tumors
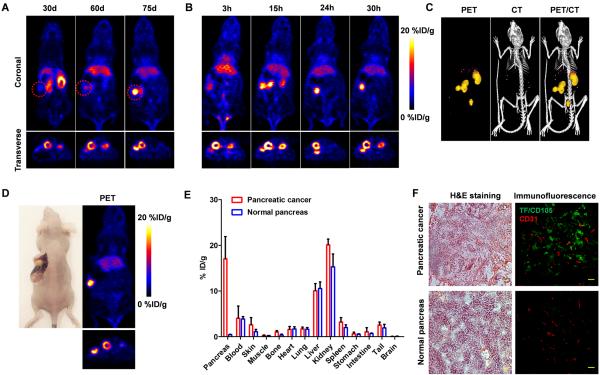
To investigate the potential of 64Cu-NOTA-heterodimer for sensitive detection of pancreatic adenocarcinomas, an orthotopic tumor model was established by stereotactically seeding BXPC-3 cells in the pancreas. The 64Cu-NOTA-heterodimer was examined for its ability to detect in vivo pancreatic malignancies. Growth kinetics of BXPC-3 orthotopic tumors was monitored using the heterodimer imaging agent and the tumors were shown to steadily increase in size from day 30 to 75 (Fig. 6A). Sequential coronal and transverse images of slices containing BXPC-3 orthotopic tumors showed a sharp definition of tumor contours, whereas negligible radioactivity was observed surrounding the kidney position (Fig. 6B and Movie S2). ImmunoPET/CT imaging was performed on mice with confirmed tumors, approximately 2-3 mm in diameter (Fig. 6C). Posterior quantitative analysis of the images in orthotopic mice revealed a persistently high tumor uptake of 64Cu-NOTA-heterodimer from 9.5 ± 1.8 %ID/g at 3 h p.i. to the peak value of 17.8 ± 1.5 %ID/g at 24 h p.i.. This is in contrast with no 64Cu-NOTA-heterodimer accumulation at the pancreas of normal mice (Supplementary Fig. 7, 8 and Movie S3). Altogether, these data suggest that 64Cu-NOTA-heterodimer offers sufficient sensitivity for the detection of TF/CD105-positive orthotopic pancreatic tumors.
Figure 6.
Heterodimer-based PET imaging in orthotopic BXPC-3 tumors. A, Serial coronal PET images of 64Cu-NOTA-heterodimer in mice at 30, 60 and 75 d of seeding BXPC-3 cells into the tail of the pancreas. B, Coronal and transverse PET images of mice bearing orthotopic BXPC-3 tumor at 3, 15, 24 and 30 h following injection of 64Cu-NOTA-heterodimer. C, Co-registered PET/CT images of mice bearing orthotopic BXPC-3 tumor at 30 h p.i. of 64Cu-NOTA-heterodimer. D, Ex vivo PET imaging of excised pancreas from the mouse bearing orthotopic BXPC-3 tumor at 30 h p.i. E, ex vivo biodistribution of 64Cu-NOTA-heterodimer in orthotopic BXPC-3 tumor-bearing mice and normal mice at 30 h p.i. (n = 3); F, Hematoxylin and eosin (H&E) and immunofluorescence staining of normal pancreatic tissue and pancreatic tumors (bar: 30 μm).
After the last imaging time point (30 h p.i.), ex vivo imaging in mice with orthotopic pancreatic tumors and biodistribution studies were performed. Primary tumors in the pancreas were clearly visible and displayed high activity further validating in vivo PET data (Fig. 6D). Ex vivo 64Cu-NOTA-heterodimer biodistribution studies at 30 h p.i. showed a tissue distribution of tracer similar to that of the subcutaneous xenograft models (Fig. 6E). H&E staining of pancreas sections confirmed the presence of a localized tumor mass, consistent with the observation of a sharply defined tumor in PET/CT images (Fig. 6F). Additionally, TF/CD105 immunofluorescence revealed a strong fluorescent signal that distributed in both tumor cell and tumor-associated vasculature (co-localized with CD31). Overall, small animal PET/CT using 64Cu-NOTA-heterodimer enabled highly specific and sensitive detection of aggressive BXPC-3 pancreatic tumors.
Discussion
Most patients with pancreatic cancer are diagnosed with metastatic disease, and only a small fraction of patients are suitable candidates for surgical resection (6). Although a recent randomized phase III trial showed that gemcitabine, in combination with nab-paclitaxel, significantly improved overall survival of patients with metastatic pancreatic cancer, advanced pancreatic cancer often becomes highly resistant to chemotherapy (25). To enhance patient survival, there is an urgent need for improved diagnostic tools for simplified detection of pancreatic malignancies. Molecular imaging not only has the potential to improve the diagnostic imaging and staging of malignancies but can also provide valuable intraoperative guidance to enhance patient survival during surgical intervention (7). The specificity of molecular imaging probes is imperative to accurately diagnose and stage pancreatic cancer.
Imaging disease-related biomarkers enables more accurate patient classification and better prognostication of treatment success. A link between pancreatic cancer and venous thromboembolisms has been well established and is thought to be associated with tumor growth and angiogenesis (26). Overexpression of TF in pancreatic cancer plays a critical role in the pathophysiology of cancer-related thrombosis and angiogenesis (27). Due to this correlation, combined targeting of TF and angiogenic pathways is an appealing strategy to potentially circumvent treatment resistance and improve patient survival. In pancreatic cancer, overexpression of angiogenic factors, such as vascular endothelial growth factor (VEGF), CD105, and integrin correlates with disease progression (20, 28). However, treatments targeting known oncogenes or growth factors such as K-Ras, VEGF, and EGF/EGFR in pancreatic cancer have resulted in suboptimal therapeutic response (29). Upregulation of CD105 is common in most solid tumors, so the concurrent targeting of TF and CD105 may offer new possibilities for effective treatment of pancreatic malignancies.
In this study, we sought to investigate the benefits of simultaneous targeting of TF and CD105 for potential imaging and therapy of pancreatic cancer. By fusing two Fab fragments from mAbs against TF and CD105 respectively, we created a heterobifunctional construct possessing excellent in vivo tumor-homing capabilities. Our results from noninvasive PET imaging with 64Cu-NOTA-heterodimer demonstrated a significantly higher tumor uptake of the heterodimer compared to that of either the Fab fragment or whole antibody (24, 30). This indicated that dual-TF/CD105 targeting provides a synergistic tumor-targeting advantage in BXPC-3 tumors (Fig. 3 and 4). More importantly, this targeting advantage did not occur at the expense of increased non-specific tracer accumulation in normal organs, as indicated by a high tumor/muscle ratio of 75.2 ± 9.4 at 30 h after 64Cu-NOTA-heterodimer administration. BXPC-3 orthotopic tumor nodules were easily identifiable, owing to high tracer uptake (17.1 ± 4.9 %ID/g at 30 h p.i) and tumor/muscle ratio (72.3 ± 46.7). These findings may have significant implications for cancer therapy, in which combined TF and antiangiogenic inhibition therapies may effectively address current therapeutic limitations, including TF inhibitor resistance and the heterogeneous expression of TF in pancreatic cancer.
An emerging trend in the clinical implementation of multifunctional pharmaceutics is theranostics, a combined diagnostic and therapeutic approach designed to eliminate multi-step procedures, reduce delays in treatment, and facilitate patient care overall (31). Recently, a bispecific antibody known as Blincyto (blinatumomab, AMGEN) was approved by the Food and Drug Administration (FDA) for treating B-cell acute lymphoblastic leukemia. This has much interest in the application of bispecific antibodies for cancer diagnostics and treatment (14). The simplified molecular engineering platform we presented in this study is not limited to antibodies, yet is widely applicable to other disease targeting ligands, including peptides, small molecular weight proteins, aptamers, and many nanoplatforms.
In conclusion, our novel heterodimer demonstrated that dual-targeting enhanced tumor accumulation, augmented targeting specificity, and improved diagnostic sensitivity. This enabled the detection of 2-3-mm pancreatic malignant lesions in orthotopic tumor-bearing mice. In the future, this paradigm may be expanded for the construction of novel heterodimers using previously failed clinical antibody candidates. In turn, those failed antibodies may be revived for improving therapeutic outcomes.
Supplementary Material
Statement of Clinical Translation.
The high mortality rate of pancreatic cancer can be attributed to insufficient diagnostic tools for early disease detection. Several novel imaging agents have been pre-clinically evaluated for detecting pancreatic cancer, yet many of these agents are targeted to single receptors with limited specificity. In this study, we investigate the advantages of using a novel heterodimeric imaging construct, co-targeted to the tumor vasculature (CD105) and the surface of malignant cells (tissue factor), for molecular imaging. The heterodimer was shown to be a more effective PET imaging agent, in comparison to single-targeted CD105 or tissue factor antibody tracers, in both xenograft and orthotopic mouse models. Currently, the clinical benefits of heterodimeric imaging agents for the recognition of early disease symptoms and monitoring of therapeutics response in patients have been unexplored.
Acknowledgements
This work is supported, in part, by the University of Wisconsin–Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520, 5T32GM08349, and T32CA009206), the Department of Defense (W81XWH-11-1-0644 and W81XWH-11-1-0648), the National Science Foundation (DGE-1256259), and the American Cancer Society (125246-RSG-13-099-01-CCE).
Footnotes
Authorship Contributions HL, CGE, and, HWC planned the research, performed experiments, and analyzed data; SS, RH, BL, CPT, HC, RJN, and WC were involved in planning and supporting components of the research; SAG and RJN provided radioisotopes; CGE and WC planned and supervised the research; and HL, CGE, and WC wrote the paper. All the authors approved the final version.
Conflicts of Interest Bai Liu and Hing C. Wong are employees of Altor Bioscience Corporation. Charles P. Theuer is an employee of TRACON Pharmaceuticals, Inc. The other authors declare that they have no conflicts of interest. No other potential conflicts of interest relevant to this article are reported.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Zamboni G, Hirabayashi K, Castelli P, Lennon AM. Precancerous lesions of the pancreas. Best Pract Res Clin Gastroenterol. 2013;27:299–322. doi: 10.1016/j.bpg.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Goggins M. Markers of pancreatic cancer: working toward early detection. Clin Cancer Res. 2011;17:635–7. doi: 10.1158/1078-0432.CCR-10-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holzapfel K, Reiser-Erkan C, Fingerle AA, Erkan M, Eiber MJ, Rummeny EJ, et al. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom Imaging. 2011;36:179–84. doi: 10.1007/s00261-010-9633-5. [DOI] [PubMed] [Google Scholar]
- 5.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain T, Nguyen QT. Molecular imaging for cancer diagnosis and surgery. Adv Drug Deliv Rev. 2014;66:90–100. doi: 10.1016/j.addr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248–56. doi: 10.1053/j.semnuclmed.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NQ, Bartholomeusz DF. 18F-FDG-PET/CT in the assessment of pancreatic cancer: is the contrast or a better-designed trial needed? J Gastroenterol Hepatol. 2011;26:613–5. doi: 10.1111/j.1440-1746.2011.06625.x. [DOI] [PubMed] [Google Scholar]
- 10.Kramer-Marek G, Gore J, Korc M. Molecular imaging in pancreatic cancer--a roadmap for therapeutic decisions. Cancer Lett. 2013;341:132–8. doi: 10.1016/j.canlet.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowles SM, Wu AM. Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol. 2012;30:3884–92. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–97. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo H, Hong H, Yang SP, Cai W. Design and applications of bispecific heterodimers: molecular imaging and beyond. Mol Pharm. 2014;11:1750–61. doi: 10.1021/mp500115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheridan C. Amgen's bispecific antibody puffs across finish line. Nat Biotechnol. 2015;33:219–21. doi: 10.1038/nbt0315-219. [DOI] [PubMed] [Google Scholar]
- 15.Garber K. Bispecific antibodies rise again. Nat Rev Drug Discov. 2014;13:799–801. doi: 10.1038/nrd4478. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 17.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–63. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 18.Nitori N, Ino Y, Nakanishi Y, Yamada T, Honda K, Yanagihara K, et al. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2005;11:2531–9. doi: 10.1158/1078-0432.CCR-04-0866. [DOI] [PubMed] [Google Scholar]
- 19.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 20.Hong H, Chen F, Zhang Y, Cai W. New radiotracers for imaging of vascular targets in angiogenesis-related diseases. Adv Drug Deliv Rev. 2014;76:2–20. doi: 10.1016/j.addr.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 22.Luo H, Hong H, Slater MR, Graves SA, Shi S, Yang Y, et al. PET of c-Met in Cancer with (6)(4)Cu-Labeled Hepatocyte Growth Factor. J Nucl Med. 2015;56:758–63. doi: 10.2967/jnumed.115.154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Orbay H, Yang Y, Graves SA, Nayak TR, Hong H, et al. PET Imaging of Abdominal Aortic Aneurysm with 64Cu-Labeled Anti-CD105 Antibody Fab Fragment. J Nucl Med. 2015;56:927–32. doi: 10.2967/jnumed.114.153098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong H, Zhang Y, Nayak TR, Engle JW, Wong HC, Liu B, et al. Immuno-PET of tissue factor in pancreatic cancer. J Nucl Med. 2012;53:1748–54. doi: 10.2967/jnumed.112.105460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thota R, Pauff JM, Berlin JD. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncology (Williston Park) 2014;28:70–4. [PubMed] [Google Scholar]
- 26.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 27.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. 2013;4:253–63. doi: 10.3978/j.issn.2078-6891.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni X, Yang J, Li M. Imaging-guided curative surgical resection of pancreatic cancer in a xenograft mouse model. Cancer Lett. 2012;324:179–85. doi: 10.1016/j.canlet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Hong H, Orbay H, Valdovinos HF, Nayak TR, Theuer CP, et al. PET imaging of CD105/endoglin expression with a (6)(1)/(6)(4)Cu-labeled Fab antibody fragment. Eur J Nucl Med Mol Imaging. 2013;40:759–67. doi: 10.1007/s00259-012-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palekar-Shanbhag P, Jog SV, Chogale MM, Gaikwad SS. Theranostics for cancer therapy. Curr Drug Deliv. 2013;10:357–62. doi: 10.2174/1567201811310030013. [DOI] [PubMed] [Google Scholar]
- 32.Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1:539–47. doi: 10.4161/mabs.1.6.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.