Abstract
Objective
Telomeres protect against chromosomal end damage and shorten with each cell division; their length may be a marker of cardiovascular and overall biological aging. We examined the hypothesis that reduced telomere length is associated with increased coronary atherosclerosis in RA.
Methods
We performed a cross-sectional study in 145 patients with RA and 87 control subjects frequency-matched for age, race and sex. Coronary artery calcium score was determined by non-contrast cardiac computed tomography. Telomere length was measured from whole blood DNA, using real-time quantitative polymerase chain reaction and expressed as telomeric product to a single-copy gene product ratio (T/S ratio). Associations between telomere length, coronary artery calcium score, and disease activity (DAS28) were assessed with Spearman correlation, proportional odds logistic regression, and linear regression adjusting for age, race and sex.
Results
Telomere length was significantly inversely correlated with age in patients with RA (rho=−0.37, P<0.001) and control subjects (rho=−0.39, P=0.001). Among patients with RA, for every interquartile range (IQR) decrease in telomere length (T/S ratio), the odds of higher coronary artery calcium score increased by 38% (95% CI: 4, 60%), after adjusting for age, race and sex (P adjusted= 0.03). Telomere length was not associated with DAS28 (P adjusted= 0.17). Telomere length was not significantly different in patients with RA (median [IQR]: 1.02 units [0.9, 1.11 units]) compared to control subjects (1.05 units [0.95, 1.17 units]; P= 0.10).
Conclusion
Telomere length is inversely associated with coronary artery calcium score independent of age, race and sex in patients with RA.
Keywords: rheumatoid arthritis, telomere, atherosclerosis, cardiovascular, aging
Premature cardiovascular disease is the leading cause of early death in patients with rheumatoid arthritis (RA) (1). Traditional risk factors, such as age, do not fully account for the increased risk observed in RA (2). In many ways RA can be considered a disease of accelerated aging of both the immune and cardiovascular systems (3, 4). Thus, measures of biological aging, such as decreasing telomere length, may be altered in RA and better reflect cardiovascular age in these patients.
Telomeres are non-coding DNA sequences that cap the ends of chromosomes, protecting them from loss of DNA and from being recognized as double strand breaks (5). Telomeres shorten with every cell division, making them a form of biological clock (6). Indeed, telomere length is believed to be a better marker of biological age than chronological age (7-9), and thus may better estimate the risks of age-related diseases, such as cardiovascular disease. In fact, in many populations, short telomere length, independent of age, is associated with the presence and development of cardiovascular disease (10-13).
Shortened telomeres have been found in several cell types of patients with RA (14-16), but their association with cardiovascular disease has not been studied. We hypothesize that telomere length would be inversely associated with coronary atherosclerosis in patients with RA.
Methods
Study population
This was a cross-sectional study of 145 patients with RA and 87 control subjects. These subjects are part of a cohort in whom we have studied cardiovascular risk factors and coronary atherosclerosis (2, 17, 18). Recruitment and study procedures have been previously described (17). Subjects were 18 years of age or older and patients with RA met the American College of Rheumatology 1987 criteria for RA (19). Patients with RA and control subjects were matched for age, race and sex. Control subjects did not have a diagnosis of inflammatory disease. The study was approved by the Vanderbilt Institutional Review Board. All subjects gave written informed consent.
Study procedures
Clinical characteristics, laboratory measurements and coronary artery calcium scores were obtained as previously described (2, 17, 18). Briefly, the ten-year cardiovascular risk was determined by the Framingham risk score (20). Fasting lipid panels were performed in the hospital clinical laboratory. High sensitivity C-reactive protein (CRP) was measured by ELISA or the hospital clinical laboratory. Coronary artery calcium scores were determined by electron beam computed tomography and calculated in Agatston units (21).
DNA extracted from whole blood was used to measure telomere length of peripheral blood leukocytes. Telomere length was determined with a quantitative polymerase chain reaction based method by a reference laboratory (Telome Health, Inc.; Menlo Park, Ca, USA) (22, 23). Telomere product (T) was compared to a single-copy gene product (S) in each sample, and this ratio was compared to that of a genomic DNA standard, creating a T/S ratio which is proportional to the average telomere length (22, 23). Each sample was run in triplicate, standard reference DNA was run in quadruplicate, and 3 separate quality controls (mean T/S ratio range 0.78 to 2.26) were run in triplicate in each 384 well plate. The relative standard error for each sample was calculated. Samples with > 12.5% relative standard error were re-assayed. The inter-assay coefficient of variation for this assay is 6.45% (23).
Statistics
Based on 145 patients with RA and 87 control subjects and a T/S ratio standard deviation of 0.19, we had 90% power to detect a difference as small as a T/S ratio of 0.08 or higher, which is less than half a standard deviation in T/S ratio.
Descriptive statistics were calculated as median with interquartile range (median [interquartile range (IQR)]) for continuous variables, and frequency and proportions for categorical variables.
The independent association between telomere length and disease status (RA or control) was assessed with multivariable linear regression models with adjustment for age, race and sex. Additional analyses were performed only in the RA group. Spearman's rank correlation coefficients (rho) were calculated to assess the correlation between telomere length and continuous variables. Proportional odds logistic regression was used to assess association between telomere length and coronary artery calcium score as the outcome with adjustment for age, race and sex. Multivariable linear regression models were used to assess the association between telomere length and clinical and laboratory measures with adjustment for age, race and sex. Because age strongly influences telomere length and coronary artery calcification, age was included in models using restricted cubic splines to account for non-linear effects (24).
To assess whether the relationship between telomere length and coronary artery calcium score was modified by disease status, we used proportional odds models with independent variables including the cross-product term disease status (RA vs. control) and telomere length with adjustment for age, race and sex.
Triglyceride, CRP and urinary F2-isoprostane concentrations were natural logarithm-transformed to improve normality of residuals. Statistical analyses were performed using R version 2.15.1 (http://www.r-project.org) and IBM SPSS Statistics v22. Two-sided P values less than or equal to 0.05 were considered statistically significant.
Results
Clinical characteristics
Clinical characteristics of patients with RA (Table 1) have been previously reported (17). The patients with RA were a median [IQR] age of 53 years [45, 61 years], 88% Caucasian, 69% female, and the majority had moderate disease activity by DAS28 score (median [IQR]: 3.85 units [2.57, 4.75 units]).
Table 1.
Clinical characteristics of patients with RA
| RA (N=145) | |
|---|---|
| Age, years | 53 [45, 61] |
| Sex, # female | 100 (69) |
| Race, # Caucasian | 128 (88) |
| BMI, kg/m2 | 28.6 [24.2, 33.4] |
| Waist-hip ratio | 0.89 [0.81, 0.95] |
| Hypertension, # | 74 (51) |
| Systolic BP, mmHg | 132 [118.5, 145.5] |
| Diastolic BP, mmHg | 75 [68.5, 82] |
| Total cholesterol, mg/dl | 183 [155, 209] |
| HDL cholesterol, mg/dl | 43 [37, 54] |
| LDL cholesterol, mg/dl | 110 [89, 133] |
| Triglycerides, mg/dl | 110 [78, 158] |
| Smoking, pack years | 0 [0, 24] |
| Coronary calcium, Agatston units | 1 [0, 133] |
| DAS28, units | 3.85 [2.57, 4.75] |
| Disease duration, years | 3 [2, 13] |
| Rheumatoid factor, # positive | 98 (71) |
| CRP, mg/l | 4 [1.3, 10] |
Continuous variables are presented as median [interquartile range]. Categorical variables are presented as number (percent). BP= blood pressure, DAS28= disease activity score based on 28 joints and erythrocyte sedimentation rate, CRP=high sensitivity C-reactive protein.
Coronary artery calcium and telomere length in RA
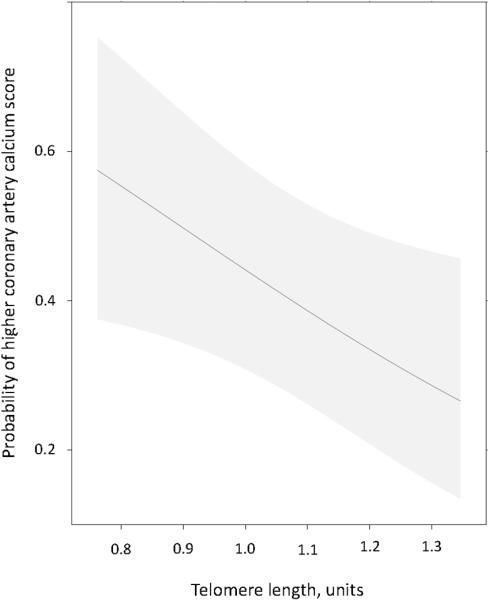
Telomere length was inversely correlated with coronary artery calcium score in univariate analysis (rho=−0.37, P<0.001) in patients with RA. After adjusting for age, race and sex, the odds of higher coronary artery calcium score increased by 38% (95% CI: 4, 60%, P adjusted 0.03) for every IQR decrease in telomere length (T/S ratio), (Figure 1).
Figure 1. Association between telomere length and coronary artery calcium score in patients with rheumatoid arthritis.
The probability of higher coronary artery calcium score increased significantly with decreasing telomere length, independent of age, race and sex (P=0.03). The figure was adjusted to a 53 year old, Caucasian, female. Shaded region represented the 95% confidence interval.
CV risk factors and telomere length in RA
Age was inversely correlated with telomere length (rho=−0.37, P<0.001), as expected, as was Framingham risk score (rho=−0.27, P=0.001) (Table 2, Supplemental Figure). The association between Framingham risk score and telomere length was no longer significant after adjustment for age, race, and sex (P adjusted=0.63). Telomere length was similar in males (median [IQR]: 1.01 units [0.88, 1.09]) and females (1.04 units [0.93, 1.12], P=0.35), including after adjustment for age and race (P=0.44). Lipid concentrations, including total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides, were not associated with telomere length in univariate or adjusted analyses (Table 2). Smoking history measured in pack-years was weakly inversely associated with telomere length (rho=−0.16, P=0.05, P adjusted=0.07). Diagnosis of hypertension, systolic blood pressure and diastolic blood pressure were not significantly associated with telomere length in univariate or adjusted analyses (All P>0.05) (Table 2).
Table 2.
Relationship between telomere length and cardiovascular risk factors in patients with rheumatoid arthritis
| Spearman rho | P* | Adjusted P** | |
|---|---|---|---|
| Framingham risk score | −0.27 | 0.001 | 0.63 |
| Total cholesterol | 0.03 | 0.71 | 0.59 |
| HDL cholesterol | 0.05 | 0.55 | 0.33 |
| LDL cholesterol | −0.02 | 0.85 | 0.99 |
| Triglycerides | 0.08 | 0.36 | 0.28 |
| Smoking, pack years | −0.16 | 0.05 | 0.07 |
| Hypertension | - | - | 0.28 |
| Systolic BP | −0.13 | 0.11 | 0.80 |
| Diastolic BP | −0.01 | 0.96 | 0.63 |
P value based on Spearman correlation.
Adjusted for age, race and sex using linear regression. BP= blood pressure.
Disease related variables and telomere length
There were no significant relationships between telomere length and disease-related factors or inflammation (Table 3). Disease activity measured by DAS28 score, disease duration, positive rheumatoid factor, CRP, cumulative corticosteroid dose, and cumulative methotrexate dose were not significantly associated with telomere length (P>0.05) (Table 3). Similarly, there was no significant association between current medication use (methotrexate, leflunomide, hydroxychloroquine, anti-tumor necrosis factor alpha agent, corticosteroids and non-steroidal anti-inflammatories) and telomere length (P>0.05) (Supplemental Table).
Table 3.
Relationship between telomere length and inflammation and RA disease related factors
| Spearman rho | P* | Adjusted P** | |
|---|---|---|---|
| DAS28 | −0.14 | 0.10 | 0.17 |
| Disease duration | −0.11 | 0.18 | 0.69 |
| RF positivity | - | - | 0.10 |
| CRP | −0.09 | 0.27 | 0.69 |
| CS, cumulative dose | −0.11 | 0.21 | 0.50 |
| MTX, cumulative dose | −0.08 | 0.34 | 0.32 |
P value based on Spearman correlation.
Adjusted for age, race and sex using linear regression. DAS= disease activity score, RF= rheumatoid factor (available for N= 139), CRP= high sensitivity C=reactive protein, CS= corticosteroids, MTX= methotrexate
Telomere length RA vs Controls
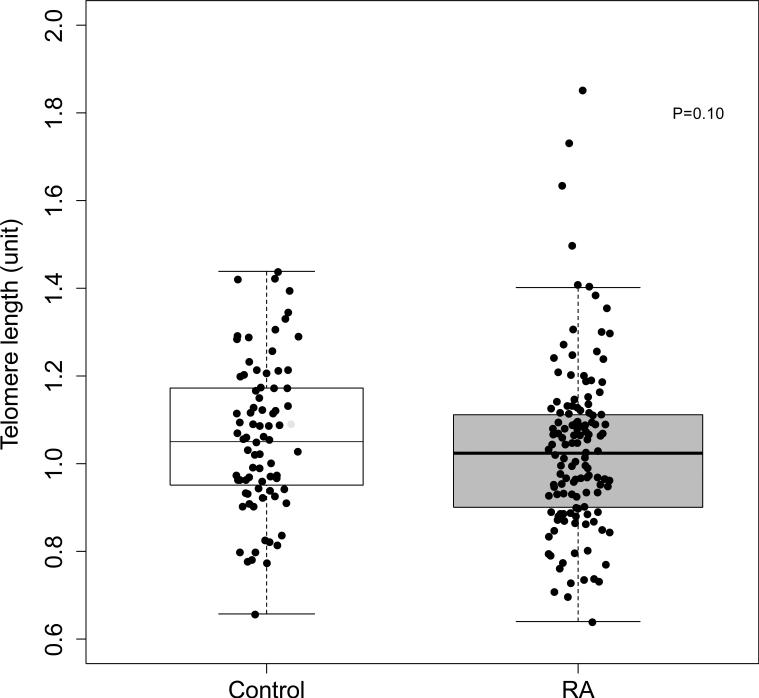
We compared telomere length among patients with RA to control subjects (N=87) matched for age, race and sex (median [IQR] age: 52 years [44, 58 years]; 84% Caucasian, and 64% female). Telomere length was not significantly different in patients with RA (median [IQR]: 1.02 units [0.9, 1.11 units] compared to control subjects (1.05 units [0.95, 1.17 units]; P=0.10) (Figure 2). Based on the previously published relationship between T/S ratio and terminal restriction fragment (TRF) length in kilobases (kb) (TRF in kb=2.16*(T/S ratio)+4.02) (23), the median telomere length was approximately 6.223 kb in RA and 6.288 kb in control subjects. In control subjects, as was the case in patients with RA, telomere length was inversely associated with age (rho=−0.39, P=0.001). In control subjects telomere length was not significantly inversely associated with coronary calcium score (rho=−0.18, P=0.10) or with adjustment for age, race and sex (P=0.25). Despite this difference between RA and control subjects, disease status did not significantly modify the association between telomere length and coronary calcium score by interaction analysis (P=0.69).
Figure 2. Telomere length in patients with rheumatoid arthritis and control subjects.
Telomere length was not significantly different in patients with RA (N=145) (1.02 units [0.9, 1.11 units]) compared to control subjects (N=87) (1.05 units [0.95, 1.17 units]), P=0.10.
Discussion
The major finding of this study is that telomere length is inversely associated with coronary artery calcium score independent of age, race, and sex in patients with RA. Telomere length is not significantly shorter in patients with RA compared to control subjects, matched for age, race and sex, and is not associated with RA disease related factors or cardiovascular risk factors.
Telomere length has been found to be shorter in patients with RA compared to control subjects in some cell populations such as naïve CD4 T-cells and CD34+ hematopoietic stem cells (HSC) (14-16). This is partially due to aberrant regulation of telomerase, an enzyme that elongates telomeres of some stem cells and other cells under highly proliferative conditions (25). For example, stimulated naïve CD4 T cells from patients with RA failed to upregulate telomerase which leads to shorter telomeres during clonal expansion (14). Such telomerase deficiency, however, is not present in all RA cell populations. Indeed, memory T cells have similar telomerase activity and similar telomere length comparing RA and control subjects (16).
An intriguing observation in studies that evaluated telomere length in RA in specific cell populations (14-16), or in peripheral leukocytes (26), was that there was no significant relationship between telomere length and disease activity, inflammation or disease duration. Our findings are consistent with those observations. Thus, it is likely that factors other than proliferative pressure from systemic inflammation drive telomere loss in some RA cell populations. Such factors may include impaired regulation of telomerase (14), deficiency of other DNA repair enzymes (27), and genetic factors (26, 28). Also, considering the reported association between atherosclerotic cardiovascular disease and shortened telomere length in other populations, this association in RA is of interest.
Others have also found that shorter leukocyte telomere length was significantly associated with more extensive coronary atherosclerosis measured by coronary artery calcification in non-RA populations, such as 325 subjects without diabetes, coronary heart disease, stroke or cancer (29), and a population based sample of 250 urban Palestinians (30). Shortened telomere length has also been associated with cardiovascular events in several patient populations, in both cross-sectional and prospective studies. For example, telomere length measured in peripheral blood leukocytes was significantly shorter in patients with premature myocardial infarction (N=203) compared to age-matched control subjects (N=180) (12). Moreover, in a prospective study that included 19,383 participants followed for up to 19 years, the hazard ratio for MI was 1.13 (95% CI, 1.04-1.23) independent of age and sex, for every 1000 bp decrease in telomere length at baseline (31).
Many suggest that telomere length is a better assessment of biological age than chronological age (7, 8), and this may explain its association with atherosclerotic cardiovascular disease which can be viewed as premature aging of the vasculature (9). RA is a disease associated with a prematurely aged immune system (4) as well as premature atherosclerosis (17). Indeed, coronary artery calcification has been viewed as a better indicator of vascular aging than chronological age (32); thus, investigation of telomere length as an indicator of cardiovascular age in RA is of interest. We found that shorter telomere length was associated with higher coronary calcium score, independent of age, race and sex. This finding may support the idea that, from a vascular perspective, telomere length is a better reflection of biological age than chronological age, or that shortened telomere length itself may be a driver of cardiovascular disease development. Conversely, shortened telomere length and coronary calcium may be common endpoints influenced by the same confounders; however, we did not find robust correlations between telomere length and cardiovascular risk factors. Regardless of pathway, shorter telomere length was associated with greater coronary atherosclerosis burden in patients with RA.
We found that telomere length in peripheral blood leukocytes was shorter, but not significantly so, in RA compared to matched control subjects. This contrasts with the finding of a prior study in which telomere length was shorter in 176 patients with RA compared to 1151 control subjects (26). The previous study found a 0.31 kb difference between RA and control subjects, and we found approximately a 0.07 kb difference. Based on an expected decreased in telomere length of approximately 30-40 base pairs/year (6), this would equate with a “biological age” difference between RA and control subjects of approximately 8-10 years in the previous study and approximately 2 years (though not significant) in our study. Differences in study design might explain these findings. In the prior study, although adjustments were made for age, the mean age of patients with RA was significantly higher than control subjects (63.8 compared to 48.2 years), and since age has the greatest influence on telomere length, this may have influenced those results. The previous study had a much larger control sample size; however, a post-hoc calculation indicated that our study had 90% power to detect the equivalent of a 0.17 kb difference in telomere length between patients with RA and control subjects. Thus, we had sufficient power to detect the larger difference of 0.31 kb found by the prior study. It is possible, however, that the true difference in peripheral blood leukocyte telomere length comparing RA to control subjects is smaller than our study was powered to detect.
This study has some limitations. Given the cross-sectional design, we could only show associations between telomere length and coronary calcium score and were unable to define the mechanisms underlying the observation. The telomere length was assessed in the whole blood, precluding examination of specific cell populations, and we didn't examine structural changes of the telomeres in RA. Also, we did not evaluate change in telomere length over time, which may have an influence on biological and cardiovascular aging. The patients with RA had relatively well-controlled disease, so we are limited in the ability to define the relationship between severe active disease and coronary calcium score. We did not stratify by HLA-DRB1 status, which may contribute to shortened telomere length (26, 28).
Conclusion
Telomere length is inversely associated with coronary artery calcium score independent of age, race and sex, in patients with RA, but is not significantly associated with other cardiovascular risk factors or disease activity.
Supplementary Material
Acknowledgments
Supported by grants: Arthritis Foundation Clinical to Research Transition Award, Rheumatology Research Foundation Innovative Research Award, NIH Grants: P60 AR056116, T32 AR059039, KL2TR000446, K23 AR068443, and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Authors’ Contributions: MJO contributed to study design, analysis and interpretation of data; JFS contributed to study design, and interpretation of data; AMO contributed to acquisition of patient-related data; AB participated in study design and performed statistical analysis; TG participated in study design and performed statistical analysis; AS participated in study design and performed statistical analysis; PR contributed to study design, and acquisition of coronary artery calcium data; CMS contributed to study design, data interpretation and oversaw all aspects of the study. All authors read and approved the final manuscript
Competing Interests: None
Contributor Information
Michelle J Ormseth, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Joseph F Solus, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Annette M Oeser, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Aihua Bian, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA.
Tebeb Gebretsadik, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA.
Ayumi Shintani, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA.
Paolo Raggi, Departments of Medicine and Radiology, University of Alberta, Edmonton, CA.
C Michael Stein, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA..
References
- 1.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.Chung CP, Oeser A, Avalos I, Gebretsadik T, Shintani A, Raggi P, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8:R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub RH, Scholmerich J, Cutolo M. The multiple facets of premature aging in rheumatoid arthritis. Arthritis Rheum. 2003;48:2713–21. doi: 10.1002/art.11290. [DOI] [PubMed] [Google Scholar]
- 4.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–58. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–60. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest. 2013;123:996–1002. doi: 10.1172/JCI66370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–37. [PMC free article] [PubMed] [Google Scholar]
- 9.Fyhrquist F, Saijonmaa O. Telomere length and cardiovascular aging. Ann Med. 2012;44(Suppl 1):S138–42. doi: 10.3109/07853890.2012.660497. [DOI] [PubMed] [Google Scholar]
- 10.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 11.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 12.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 13.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–5. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–8. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 18.Rho YH, Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, et al. Interaction between oxidative stress and high-density lipoprotein cholesterol is associated with severity of coronary artery calcification in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:1473–80. doi: 10.1002/acr.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell FE., Jr. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Springer; New York: 2001. [Google Scholar]
- 25.Xie Z, Jay KA, Smith DL, Zhang Y, Liu Z, Zheng J, et al. Early telomerase inactivation accelerates aging independently of telomere length. Cell. 2015;160:928–39. doi: 10.1016/j.cell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steer SE, Williams FM, Kato B, Gardner JP, Norman PJ, Hall MA, et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann Rheum Dis. 2007;66:476–80. doi: 10.1136/ard.2006.059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–49. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–6. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mainous AG, 3rd, Codd V, Diaz VA, Schoepf UJ, Everett CJ, Player MS, et al. Leukocyte telomere length and coronary artery calcification. Atherosclerosis. 2010;210:262–7. doi: 10.1016/j.atherosclerosis.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Kark JD, Nassar H, Shaham D, Sinnreich R, Goldberger N, Aboudi V, et al. Leukocyte telomere length and coronary artery calcification in Palestinians. Atherosclerosis. 2013;229:363–8. doi: 10.1016/j.atherosclerosis.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32:822–9. doi: 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- 32.Hecht HS. Coronary Artery Calcium Scanning: Past, Present, and Future. JACC Cardiovasc Imaging. 2015;8:579–96. doi: 10.1016/j.jcmg.2015.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.