Abstract
Advances in DNA sequencing have created new opportunities to better understand the biology of cancers. Attention is currently focused on precision medicine: does a cancer carry a mutation that is targetable with already available drugs? But, when multiple, targetable mutations arise during the adenoma to carcinoma sequence remains unresolved. Borras and colleagues identified mutations and allelic imbalance in at-risk mucosa and early polyps in the human colon (this issue). Their analyses indicate that mutations in key genes can arise quite early during tumorigenesis and that polyps are often multi-clonal with at least two clones. These results are consistent with the “Big Bang” model of tumorigenesis, which postulates that intratumoral heterogeneity is a consequence of a mutational burst in the first few cell divisions following initiation that drives divergence from a single founder with unique but related clones co-evolving owing to neutral selection dynamics. Emerging questions center around the ancestry of the tumor and impact of early intratumoral heterogeneity on tumor establishment, growth, progression, and most importantly, response to therapeutic intervention. Additional sequencing studies in which samples, especially at-risk tissue and pre-malignant neoplasms, are analyzed from animal models and humans will further our understanding of tumorigenesis and lead to more effective strategies for prevention and treatment.
Keywords: Colorectal polyps and cancer, “Big Bang” model of tumorigenesis, intratumoral heterogeneity, tumor ancestry, whole exome sequencing
Commentary
Recent advances in DNA sequencing have created the opportunity to more fully assess changes in the genomes of normal tissue and early neoplasms. Borras and colleagues analyzed at-risk colonic mucosa and benign adenomas from patients with Familial Adenomatous Polyposis (FAP) for mutations and allelic imbalances (this issue). They found that the at-risk mucosa adjacent to tumors carried between 8 and 90 somatic mutations with 6% classified as damaging events, whereas they found adenomas carried between 9 and 186 somatic mutations with 9% classified as damaging events. Mutations were identified in known driver genes including APC, KRAS, and FBXW7 as well as new candidates including ALK, TCF7L2, and CNOT3. At least 23% of the somatic mutations in APC-driven adenomas arose prior to the overt initiation of tumorigenesis. Borras and colleagues inferred the number of clones in each adenoma from mutation frequencies and copy number data using computational tools; a majority (18/25) were multi-clonal with most consisting of at least one major clone and at least one minor clone. This early intratumoral heterogeneity mirrored that observed in Stage I colorectal cancers. Thus, sequencing analysis of both at-risk tissue and early neoplasms has identified new potential targets for prevention and provided insights into tumor biology.
Current treatments for colorectal cancer are combination therapies based on a 5-fluorouracil backbone. Unfortunately, only half of advanced Stage II and Stage III patients will benefit and there is currently no way to predict who will respond to combination treatment and who will not. Molecular features of the cancer can predict who is at greater risk of reoccurrence and consequently needs more aggressive treatment (1). In addition, molecular features are beginning to be used to predict who will benefit from treatment with newly developed targeted therapies. For example, patients with colorectal cancer benefit from treatment with cetuximab, which targets epidermal growth factor receptor, unless their cancer carries an activating mutation in KRAS (2). Similarly, patients benefit from treatment with pembrolizumab, which targets the programmed death pathway, unless their cancer is mismatch repair proficient (3). Recently, tumor texture as assessed by computed tomography (CT) has emerged as a non-invasive imaging biomarker, which has shown promise in predicting pathologic features, overall survival and response to therapy (4). Texture analysis provides an assessment of tumor heterogeneity by analyzing the distribution and relationship of pixel or voxel-gray levels in the image. Thus, features of the cancer including the molecular profile and complexity are beginning to be being used to help clinicians and their patients to choose among options for treatment but a deeper understanding of intratumoral heterogeneity is likely necessary to develop a cure.
Colon tumors are thought to be initiated through APC mutations and then progress from a benign to malignant state through the stepwise accumulation of additional driver mutations. Early sequencing studies in which whole tumors were analyzed found that specific mutations tightly correlated with distinct pathological states. For example, mutations in APC were often present in early adenomas, mutations in KRAS were present in some intermediate to late adenomas, and mutations in TP53 were present in cancers and metastatic lesions. This observation led Fearon and Vogelstein to propose the model that specific mutations provide a significant growth advantage and consequently drive clonal outgrowth (5). Some colorectal cancers might develop in this manner. These cancers would plausibly be more amenable to treatment because they would be relatively homogenous.
Sequencing studies also indicate that some colorectal cancers are highly heterogeneous. A recent study in which different regions of each cancer were analyzed found that one area could carry unique mutations compared to another area (6). This observation led Sottoriva and colleagues to propose the Big Bang model in which “private” mutations that can be detected within a subset of tumor cells likely arise within the first few cell divisions as an adenoma starts to form and the emerging clones co-exist as adenomas progress to cancers. Williams and colleague found evidence of neutral evolution in several other types (7). The study of Borras and colleagues is consistent with this new model of tumorigenesis. Firstly, they found mutations in several driver genes. Alterations in KRAS and PIK3CA were observed in 8% and 4% of the adenomas, respectively. Previously, such mutations were linked to later stages of tumorigenesis. Secondly, they found that 72% of the adenomas were multi-clonal by analyzing mutation frequency and copy number using the ABSOLUTE computational algorithm. The number of clones present in adenomas was estimated to be 1.72. This number was quite comparable to that of 2.06 estimated for Stage I cancers. Thus, intratumoral heterogeneity exists in the early adenoma and persists as distinct clones co-evolve.
An unresolved question is whether the distinct clones within a single tumor arise from one founder or multiple founders. Beginning in 1996, several studies demonstrated that colorectal polyps in humans could have a multi-ancestral architecture, meaning they were composed of cells derived from distinct founders. Novelli and colleagues analyzed polyps from a patient with FAP who was also a XY/XO mosaic (8). Some of the tumors were composed of cells from the XY lineage as well as cells from the XO lineage, which was interpreted as evidence of multiple founders. A criticism of this interpretation was that the XY/XO karyotype might be inherently unstable. Thirlwell and colleagues determined the clonal architecture of human polyps by sequencing APC in different regions of the same tumor (9). Some tumors carried distinct APC mutations in one region of a tumor versus another. Since colon polyps likely arise as a consequence of APC mutations, this finding further supported the notion that some tumors can be multi-ancestral “from birth”, derived from multiple founders, instead of becoming polyclonal as a consequence of divergence from a single founder. If some tumors can have a multi-ancestral origin at the time of neoplastic transformation, a mutational burst during the first few cell divisions is not necessary to explain unique clones with private mutations within a single tumor.
The laboratory mouse has been a powerful experimental tool to advance the understanding of the clonal origin of tumors (Figure 1). The study by Merritt and colleagues analyzed intestinal adenomas from aggregation chimeras that were generated by fusing an ApcMin/+ embryo with Rosa26 to an ApcMin/+ embryo without Rosa26 (10). The Min allele of Apc predisposes mice to the development of tumors throughout the intestinal tract. The Min mouse is an animal model of FAP. Rosa26 is a lineage marker that expresses the LacZ gene. Cells carrying this marker turn blue in the presence of the appropriate substrate, whereas those that do not are white. Some intestinal adenomas from aggregation chimeras were a mixture of blue and white neoplastic cells, indicating that they were derived from multiple founders. A follow up study revealed that the formation of multi-ancestral tumors was independent of tumor number, and not the result of random collision of white and blue tumors (11, 12).
Figure 1.
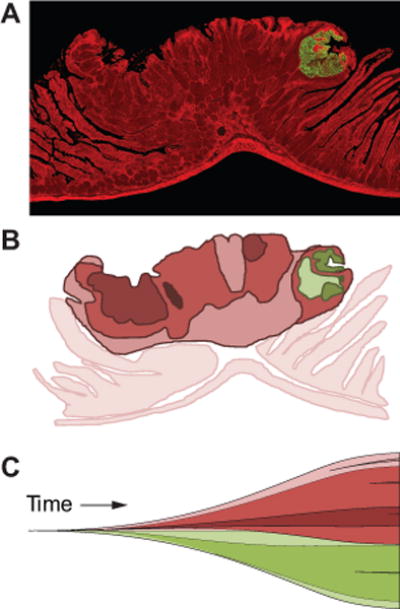
Mouse models in which lineage markers are mosaic allow the ancestry of tumors to be deduced. In one model, the intestinal tract is a patchwork of cells expressing red fluorescent protein and cells expressing green fluorescent protein (19). Tumors in this model are often a mixture of red and green neoplastic cells (A). Such tumors have a multi-ancestral origin with one red founder and one green founder. Conceptually, multi-ancestral tumors could be even more heterogeneous owing to clonal divergence driven by epigenetic and genetic changes (B; distinct subclones are different shades of red and green). Some of these changes could occur early during tumorigenesis as proposed in the “Big Bang” model of tumorigenesis (C). Thus, early intratumoral heterogeneity could reflect the involvement of multiple founders though recruitment as well as divergence driven by genetic alteration followed by co-evolution of existing and emerging clones. Presumably, complex cancers composed of different populations of neoplastic cells would be much more difficult to successfully treat.
Multi-ancestral tumors appear to form as a consequence of recruitment in which an initial founder facilitates the transformation of neighboring cells (13). Intestinal adenomas were a mixture of blue and white in aggregation chimeras that were generated by fusing an Apc+/+ embryo with Rosa26 lineage marker to an ApcMin/+ embryo without Rosa26. In fact, the percentage of multi-ancestral tumors was comparable to that observed in the Merritt study. Recruitment is not unique to intestinal tumors. Fomchenko and colleagues reported that recruited cells were truly transformed and could even overtake PDGF-induced gliomas in mice (14). Recruitment is not yet understood, but the consequent early intratumoral heterogeneity would likely have profound implications for prevention and treatment.
Somatic mutations in the at-risk mucosa might increase the likelihood that certain cells are recruited during tumorigenesis. Fischer and colleagues demonstrated that fields of APC-deficient cells favored the formation of adenomas in the intestine (15). The analysis of mutations that are in common between at-risk mucosa and early adenomas would provide further insight into the ancestry of human tumors. Borras and colleagues demonstrated that the at-risk mucosa carried numerous somatic mutations of which at least 23% were also present in adenomas. If all of the mutations in common between at-risk mucosa and adenomas have allele frequencies of 100% within the tumors, they either arose from a single founder or multiple founders with a shared ancestry. However, if some of the mutations in common are at a low allele frequency within the tumors, they likely arose from multiple founders. These founders could have been initiated independently of each other or one initiated founder could have recruited one or more neighbors. Recruited cells could contribute to resistance especially if the drug targets a specific driver mutation found only in the initial founder and its progeny and not in recruited clones.
Disruption of recruitment might be an effective strategy for prevention, especially if this process is mediated by paracrine oncogenic signaling. Borras and colleagues report that 80% of the adenomas from patients with FAP carried somatic alterations in genes involved in the WNT signaling pathway, known to be negatively regulated by APC. This high percentage is comparable to that observed in adenomas from patients with sporadic disease (16). In the context of chemoprevention, these observations focus attention on whether the WNT signaling pathway can be successfully inhibited. A recent study by Farin and colleagues indicates that WNT3 is transferred by direct contact between its source, the Paneth cell, and its target, the intestinal stem cell (17). Such short-range paracrine action provides at best a narrow window for intervention. In practical terms, chemopreventive programs are best focused on a defined at-risk patient population. It remains to be determined whether any of the early somatic genetic changes in colonic mucosa reported by Borras and colleagues are prognostic of neoplasia, give signals that can be detected by non-invasive methods, and can be leveraged into new chemopreventive strategies.
Advances in sequencing have created new opportunities at the bench and in the clinic. Attention is currently focused on precision medicine i.e., the use of sequence data to identify targetable mutations on a case but case basis. The study of Borras and colleagues emphasizes the need not to limit sequencing to advanced cancers but to more fully analyze at-risk normal appearing tissue and early neoplastic lesions.
They identified mutations in genes critical to intestinal tumorigenesis including APC, KRAS, and FBW7 as well as over 400 exonic mutations that were classified as damaging, recurrent potentially damaging, or potentially damaging events. The challenge is to determine which mutated genes in pre-malignancies are truly drivers and therefore targets (18). Additional studies leveraging the power of controlled laboratory animals and human samples are necessary to provide a deeper understanding of intratumoral heterogeneity, the unambiguous assessment of multi-ancestry, investigation of the mechanism of recruitment, and testing of responses to treatment for specific driver mutations, singly or in combination.
Acknowledgments
Financial support: This commentary was supported by Ride for Research (R.B. Halberg), Tomorrow’s Hope (R.B. Halberg), and National Cancer Institute grants T32 CA009135 (A.A. Leystra and C.K. Sievers) and P50 CA095013 (University of Wisconsin Carbone Cancer Center).
Footnotes
Potential conflicts of interest: none.
References
- 1.Clark-Langone KM, Wu JY, Sangli C, Chen A, Snable JL, Nguyen A, et al. Biomarker discovery for colon cancer using a 761 gene RT-PCR assay. BMC Genomics. 2007;8:279. doi: 10.1186/1471-2164-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology. 2013;266:177–84. doi: 10.1148/radiol.12120254. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 6.Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, et al. A Big Bang model of human colorectal tumor growth. Nat Genet. 2015;47:209–16. doi: 10.1038/ng.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MJ, Werner B, Barnes CP, Graham TA, Sottoriva A. Identification of neutral tumor evolution across cancer types. Nat Genet. 2016;48:238–44. doi: 10.1038/ng.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novelli MR, Williamson JA, Tomlinson IP, Elia G, Hodgson SV, Talbot IC, et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science. 1996;272:1187–90. doi: 10.1126/science.272.5265.1187. [DOI] [PubMed] [Google Scholar]
- 9.Thirlwell C, Will OC, Domingo E, Graham TA, McDonald SA, Oukrif D, et al. Clonality assessment and clonal ordering of individual neoplastic crypts shows polyclonality of colorectal adenomas. Gastroenterology. 2010;138:1441–54. doi: 10.1053/j.gastro.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Merritt AJ, Gould KA, Dove WF. Polyclonal structure of intestinal adenomas in ApcMin/+ mice with concomitant loss of Apc+ from all tumor lineages. Proc Natl Acad Sci U S A. 1997;94:13927–31. doi: 10.1073/pnas.94.25.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thliveris AT, Halberg RB, Clipson L, Dove WF, Sullivan R, Washington MK, et al. Polyclonality of familial murine adenomas: analyses of mouse chimeras with low tumor multiplicity suggest short-range interactions. Proc Natl Acad Sci U S A. 2005;102:6960–5. doi: 10.1073/pnas.0502662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton MA, Clipson L, Thliveris AT, Halberg RB. A statistical test of the hypothesis that polyclonal intestinal tumors arise by random collision of initiated clones. Biometrics. 2006;62:721–7. doi: 10.1111/j.1541-0420.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 13.Thliveris AT, Schwefel B, Clipson L, Plesh L, Zahm CD, Leystra AA, et al. Transformation of epithelial cells through recruitment leads to polyclonal intestinal tumors. Proc Natl Acad Sci U S A. 2013;110:11523–8. doi: 10.1073/pnas.1303064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fomchenko EI, Dougherty JD, Helmy KY, Katz AM, Pietras A, Brennan C, et al. Recruited cells can become transformed and overtake PDGF-induced murine gliomas in vivo during tumor progression. PLoS One. 2011;6:e20605. doi: 10.1371/journal.pone.0020605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer JM, Schepers AG, Clevers H, Shibata D, Liskay RM. Occult progression by Apc-deficient intestinal crypts as a target for chemoprevention. Carcinogenesis. 2014;35:237–46. doi: 10.1093/carcin/bgt296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–3. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahm CD, Szulczewski JM, Leystra AA, Paul Olson TJ, Clipson L, Albrecht DM, et al. Advanced Intestinal Cancers often Maintain a Multi-Ancestral Architecture. PLoS One. 2016;11:e0150170. doi: 10.1371/journal.pone.0150170. [DOI] [PMC free article] [PubMed] [Google Scholar]


