Abstract
Objectives
To assess the relationship and directionality between mobility and cognitive performance.
Method
Cross-sectional analysis of a racially/ethnically diverse sample of 327 community-dwelling adults (mean age=68.9±9.9yrs.; range:40–100) categorized as having no mobility dysfunction; upper extremity (UE) impairment; lower extremity (LE) impairment; or mobility limitation (both UE and LE impairments), and compared by global cognition with multiple hierarchical linear regression adjusted for socio-demographic, health, and mood factors. Bootstrapping mediation analysis investigated the directionality of the mobility-cognition association.
Results
LE (Est.=−2.95±0.77, p=0.001) but not UE impairment (Est.= −1.43±1.05, p=0.175) was associated with poorer global cognitive performance/impairment. The presence of mobility limitation had the strongest effect on cognition (Est.= −3.78±1.09 (p<0.001) adjusting for socio-demographic factors, body composition, comorbidities, and mood. Mediation analysis indicated that the relationship between cognition and mobility likely operates in both directions.
Discussion
The association between cognitive function and mobility follows a dose-response pattern in which the likelihood of poor global cognition increases with progression of mobility dysfunction with evidence that LE impairments may be better indicators of impaired cognitive status than UE impairments. Using brief, valid tools to screen older patients for early signs of mobility dysfunction especially when the lower extremity is affected is feasible, may provide the first detectable stage of future cognitive impairment, and provide actionable steps for interventions to improve performance, reduce burden, and prevent development of physical disability and loss of independence.
Keywords: muscle strength, mobility, physical dysfunction stage, cognition, cross-sectional studies
Introduction
Cognitive impairment and the subsequent development of dementia likely result from the culmination of several pathological processes that begin decades before symptoms become clinically apparent1. Mobility loss is often a more readily clinically recognizable medical condition that frequently occurs in an age-related fashion2. The loss of one’s cognitive abilities and mobility poses a serious threat to the well-being and the ability of older adults to remain independent, and are among the greatest fears of individuals as they age.
Adding to the complexity of age-related functional loss is that in many cases cognition and mobility appear to decline in a complex, interactive, and likely bi-directional relationship. The ability to perform activities of daily living (ADL) particularly the more complex activities such as shopping, preparing meals, using appliances, and balancing a checkbook, rely a great deal on maintaining cognitive and mobility function. The recently proposed Central Benefit Model of exercise in falls prevention provides a bio-psychological approach to understanding the intricate association between cognitive and physical dysfunction3. According to this model, reduced executive functioning may lead to gait and balance problems as a direct effect of impaired attention, central processing, and execution of postural response or via loss of motivation leading to reduced physical activity participation and in turn to muscle waste and weakness. A feedback loop is proposed with impaired mobility and loss of motivation potentially leading to further cognitive decline3.
Models examining mobility decline suggest that age-related changes in muscle cells and loss of strength lead to decreased mobility performance measured by slower gait and poor balance, and finally difficulties performing normal daily activities (i.e. mobility-related disability).4 In this model, cognitive impairment may play the role of risk factor, moderator, or outcome of mobility decline, thus it is important to better understand the relationship between cognitive function and mobility dysfunction at its different stages. While cognitive impairment has been linked to measures of mobility dysfunction spanning the disablement process, from poor muscle strength5, to slower gait6, poor balance7, and mobility8, many previous studies have focused on later stages of disability9–10, or failed to address the importance of the relationship between stage of mobility dysfunction and cognitive abilities. In addition, although there seems to be more evidence for cognitive loss preceding or concurring physical loss11, recent reports of a reverse association10,12–13 suggest that the directionality of the cognition-mobility association remains unclear as do the mechanisms linking the two processes which are likely complex and involve behavioral, stress-related, and inflammatory pathways14–16
Understanding the interconnectedness between mobility and cognitive impairment and the sequence in which loss occurs are important as stage of mobility dysfunction may provide valuable information for the risk, diagnosis, and prognosis of significant neurocognitive disorders. In the same vein, the presence of early cognitive impairment may indicate the need for interventions to prevent development of future mobility impairments. To aid these efforts, the current cross-sectional study was designed to assess whether and how stage of mobility dysfunction relates to cognitive performance and to investigate the directionality of the cognition-mobility association in a relatively large and ethnically diverse sample of community-dwelling older adults.
Methods
Study participants were community-dwelling adults aged 40 years and older recruited to participate in research projects studying (1) the use of screening tests to detect cognitive and functional impairment in a multicultural community, and (2) corresponding biomarkers of cognitive decline at New York University (NYU) Langone Medical Center between February 2012 and March 2014. Participants were recruited via word-of-mouth, solicitation at educational seminars on dementia and other age-related conditions, collaborations with local senior centers, housing projects, churches, and other organizations advocating for the betterment of seniors, particularly minorities and other disadvantaged groups, and advertisements to individuals registered into the Research Match database maintained by NYU Clinical Science and Translational Institute. Exclusion criteria included: primary language other than English or Spanish, established diagnosis of a primary Axis I psychiatric (e.g., schizophrenia, bipolar disease), or degenerative neurologic (i.e., Parkinson’s disease, amyotrophic lateral sclerosis) disorder that could impact cognitive and/or physical performance or cooperation with study procedures. A total of 350 participants were evaluated in one of two settings: a clinic visit in our research lab, or at the location they were initially recruited (e.g. senior center, church). All procedures were identical and data collected included socio-demographic characteristics, cognitive, physical, emotional, and functional aspects of health using standardized, portable assessment tools. Of these, 332 (95%) had valid data on mobility with all but five participants also contributing data on cognitive function. A total of 327 participants were therefore included in the data analysis. The protocol was approved by the NYU Institutional Review Board and all study participants provided signed informed consents. While one of the research projects from which participants were recruited in the current study used an inclusion cutoff age of 40 years (to allow for a representative population sample to map the aging brain), most participants were aged 50 years and older (312 out of 327 or 95%). With the exception of one study in which a quarter were recruited to have MCI, participants were not targeted based on their cognitive status.
Cognitive function
Global cognitive function was measured using the Montreal Cognitive Assessment (MoCA)17- a tool validated for use in older adults. The MoCA assesses performance on 10 individual items for a total of 30 points with higher scores indicating better performance. To account for educational differences, an extra point is allowed for those with ≤12 years of education. These items measure different cognitive aspects including visuospatial skills, executive function, attention, language, memory, and orientation with the total score used as a measure of global cognitive performance. The total MoCA score was used in data analysis as a measure of global cognitive performance along with an impairment measure using the widely accepted cutoff of <2617.
Stage of mobility dysfunction
To test our hypotheses regarding the association between mobility dysfunctional stage and cognitive performance, we created a variable that combines muscle strength and LE performance to measure dysfunction at different stages of disablement. Handgrip strength was measured with a handheld dynamometer (Baseline® Digital Smedley Spring Dynamometer, Patterson Medical, Warrenville, IL) in each hand and expressed in pounds (lbs.), with the mean of the two being used in data analysis. Handgrip strength (GS) correlates with other measures of muscle strength including lower extremity strength18 and is often used in aging studies as an indicator of overall muscle strength19. Muscle impairment was defined as being in the bottom 20th gender-specific percentile of sample distribution. The mini Physical Performance Test (mPPT)20 includes the following tasks: pick-up-penny, 50-ft usual-pace walking test, 5 complete chair-raises, and the progressive Romberg balance-test, each ranging from 0 to 4, with 4 indicating highest level of performance. A score of <12 on this scale (range: 0–16) was used as an indicator of impaired LE performance20. Since the progression of disablement4 implies that higher level dysfunction includes the presence of a lower level of dysfunction, we classified being impaired in both GS and LE performance as an indicator of later stage mobility dysfunction. In contrast, impairment in either GS or LE performance would capture earlier stages of dysfunction. In this context, we considered the group with low GS but normal LE performance as having upper extremity (UE) impairment, while those in the bottom 20th on the performance test as having LE impairment. The mini PPT includes items that rely heavily on LE strength such as walking test, chair stands, picking up a penny from the floor, and balance) and therefore could be used as a measure of LE strength. While UE strength is associated with LE strength, it only accounts for about 40% of variation in LE strength21 supporting the idea that declines in UE and LE strength may represent similar but separate processes. Therefore, our staging variable includes the following four levels: 0=no mobility dysfunction; 1= UE impairment (i.e. low GS but normal LE performance); 2= LE impairment (low LE performance but normal GS); and 3=mobility limitation (i.e. low GS and low LE performance).
Covariates
Potentially important covariates were included in the analyses: increasing age (in years), gender (Female vs. Male) and racial/ethnicity status (1=Non-Hispanic White; 2=Non-Hispanic Black; 3=Hispanic). Education was not included as a covariate due to its collinearity with racial/ethnicity status in this sample. Body Mass Index (BMI=weight/height2); proportion visceral fat and muscle mass (expressed in lbs.) derived from bioelectrical impedance analysis with the BC-558 Ironman Segmental Body Composition Monitor (Tanita Corporation, Arlinton Heights, IL); abdominal and hip girth were measured and their ratio was used as a covariate; and total number of co-morbid medical conditions (including cardiovascular risk factors and conditions spanning multiple body systems) ascertained by self-report as part of medical history. Finally, given the co-existence of depressive symptomatology with poor physical22 and cognitive function23, we also included depression as measured by the Hospital Anxiety and Depression Scale (HADS)24 as a covariate.
Data analysis
Relationships between covariates, predictor, and outcome were assessed with chi square test, variance analysis or correlation analysis depending on level of measurement to help identify potential covariates to be included in further analyses (Tables 1–2). MoCA scores were linearly regressed on the 4-level predictor variables controlling for the effect of significant covariates and potential mediators (Table 3). We used a multiple hierarchical approach in which the effect of stage of mobility dysfunction on the outcome was initially assessed in an unadjusted model (Model 1). To this model, socio-demographic and health factors were added to assess the impact of expected confounding factors (Model 2). In the final model, mood was added to Model 2 to determine its potential mediating role (Model 3). In addition, likelihood of cognitive impairment was estimated with logit regression modeling using the same hierarchical approach. Given the hypothesized progressiveness and dynamicity of the disablement process and the role that cognitive impairment might play, we then separated the two components of mobility impairment (muscle strength and LE performance) to investigate their mediating role as a means of assessing the directionality of the cognition-mobility association. A potential mediating role for muscle strength for example would likely reflect a cognitive impairment-as initiator role (cognitive impairment leads to muscle strength loss and in turn to lower extremity performance). Similarly, mediation by LE performance would suggest a cognitive impairment-as consequence of mobility impairment role. In the latter context, age-related muscle loss is expected to lead to poor LE performance and disability (not measured in the current study) which in turn may lead to cognitive loss. These mediation analyses were conducted using bootstrapping techniques (SAS code available from http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html), which involve re-sampling the data multiple times (we used 1000 resamples) to obtain an empirical estimation of the indirect effects across the resamples with confidence intervals around it to assess its statistical significance25 (Figure 1). Advantages of this technique include obtainment of a quantitative indirect effect and lax requirements regarding the sampling distribution of indirect effects. Finally, sensitivity analyses were employed to assess the impact of (a) including middle-aged individuals and (b) those with missing data on the outcome and the predictor.
Table 1.
Distribution of variables of interest by stage of physical impairment
| Characteristics | None Mean (±SD) |
UE impairment Mean (±SD) |
LE impairment Mean (±SD) |
Mobility limitation Mean (±SD) |
P value | |
|---|---|---|---|---|---|---|
| N | 187 | 31 | 81 | 33 | 332 | |
| Age | 65.0 (±9.18) | 72.6 (±8.8) | 73.2 (±7.5) | 79.2 (±9.0) | <0.001 | |
| Female, % | 65.2 | 64.5 | 76.5 | 78.8 | 0.160 | |
| Race, % | White, non-Hispanic | 45.3 | 55.2 | 26.6 | 30.3 | 0.003 |
| Black, non-Hispanic | 17.7 | 3.5 | 34.2 | 27.3 | ||
| Hispanic | 37.1 | 41.4 | 39.2 | 42.4 | ||
| BMI | 27.8 (±5.4) | 26.8 (±4.9) | 29.0 (±5.9) | 28.3 (±3.9) | 0.252 | |
| Medical conditions | 5.0 (±2.9) | 5.3 (±2.7) | 6.0 (±3.0) | 5.9 (±3.3) | 0.109 | |
| Depression | 4.8 (±3.6) | 5.5 (±3.6) | 6.2 (±4.1) | 5.6 (±4.8) | 0.082 | |
| Visceral fat | 11.3 (±3.9) | 11.9 (±3.7) | 12.9 (±4.2) | 12.6 (±2.7) | 0.022 | |
| Girth ratio | 0.87 (±0.1) | 0.91 (±0.1) | 0.90 (±0.1) | 0.89 (±0.1) | 0.030 | |
| Muscle mass | 103.4 (±23.1) | 93.6 (±18.6) | 97.4 (±20.1) | 87.2 (±13.1) | <0.001 | |
| MoCA | 24.1 (±4.5) | 22.0 (±6.2) | 20.5 (±5.3) | 19.6 (±7.0) | <0.001 | |
Abbreviations: UE=upper extremity; LE=lower extremity; SD=standard deviation; BMI=body mass index (weight/height2); MoCA=Montreal Cognitive Assessment (range: 0–30).
Notes: Likelihood of older age, muscle wasting, and poor cognitive performance increased with increasing mobility impairment. Girth ratio=abdominal girth/hip girth
Table 2.
Correlation coefficients among covariates, components of the predictor (Mini PPT and Handgrip strength), and MoCA score
| Age | BMI | TMC | Depression | VF | GR | MM | mPPT | GS | MoCA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | −0.076 (0.176) |
0.271 (<.001) |
0.059 (0.288) |
0.296 (<.001) |
0.061 (0.274) |
−0.307 (<.001) |
−0.463 (<.001) |
−0.427 (<.001) |
−0.233 (<0.001) |
| BMI | - | 1.000 | 0.136 (0.016) |
0.080 (0.167) |
0.678 (<.001) |
0.268 (<.001) |
0.293 (<.001) |
−0.163 (0.004) |
−0.029 (0.603) |
−0.018 (0.888) |
| TMC | - | - | 1.000 | 0.153 (0.006) |
0.209 (<0.001) |
−0.011 (0.843) |
0.044 (0.435) |
−0.171 (0.002) |
−0.103 (0.058) |
0.088 (0.109) |
| Depression | - | - | - | 1.000 | 0.100 (0.084) |
0.143 (0.011) |
−0.042 (0.465) |
−0.153 (0.006) |
−0.104 (0.063) |
−0.140 (0.012) |
| VF | - | - | - | - | 1.000 | 0.503 (<.001) |
0.510 (<.001) |
−0.188 (<0.001) |
0.129 (0.021) |
−0.122 (0.030) |
| GR | - | - | - | - | - | 1.000 | 0.439 (<.001) |
−0.128 (0.022) |
0.154 (0.005) |
−0.182 (0.001) |
| MM | - | - | - | - | - | - | 1.000 | 0.188 (<.001) |
0.670 (<.001) |
0.072 (0.204) |
| mPPT | - | - | - | - | - | - | - | 1.000 | 0.403 (<.001) |
0.371 (<.001) |
| GS | - | - | - | - | - | - | - | - | 1.000 | 0.175 (.001) |
| MoCA | - | - | - | - | - | - | - | - | - | 1.000 |
Abbreviations: BMI=body mass index; TMC=total number of medical conditions; VF=visceral fat; GR=girth ratio; MM=muscle mass; mPPT=mini PPT (range: 0–16); GS=grip strength; MoCA=Montreal Cognitive Assessment (range: 0–30).
Notes: Factors found to be significantly (<0.05) associated with physical and cognitive performance were included in linear regression models.
Table 3.
Association between physical impairment staging and cognitive performance with and without adjustment for covariates
| N | Intercept | No physical dysfunction |
UE impairment |
LE impairment |
Mobility limitation |
|
|---|---|---|---|---|---|---|
| Cognitive performance | ||||||
| Model 1 | 327 | 24.06±0.38 (p<0.001) |
0 | −2.06±1.01 (p=0.043) |
−3.59±0.69 (p<0.001) |
−4.41±1.00 (p<0.001) |
| Model 2 | 295 | 35.12±3.80 (p<0.001) |
0 | −1.56±1.07 (p=0.147) |
−2.99±0.75 (p<0.001) |
−3.69±1.09 (p<0.001) |
| Model 3 | 279 | 35.47±3.77 (p<0.001) |
0 | −1.43±1.05 (p=0.175) |
−2.95±0.77 (p<0.001) |
−3.78±1.09 (p<0.001) |
| Cognitive impairment | ||||||
| Model 1 | 327 | 2.37±0.26 (p<0.001) |
0 | −0.76±0.56 (p=0.171) |
−1.48±0.36 (p<0.001) |
−1.63±0.46 (p<0.001) |
| Model 2 | 295 | 7.87±2.57 (p=0.002) |
0 | −0.41±0.66 (p=0.529) |
−1.11±0.43 (p=0.010) |
−1.15±0.57 (p=0.047) |
| Model 3 | 279 | 8.44±2.71 (p=0.002) |
0 | −0.44±0.67 (p=0.515) |
−1.27±0.46 (p=0.006) |
−1.27±0.61 (p=0.038) |
Notes:
N=number of subjects; values represent estimated effect on outcome ±standard error (p value); GS=handgrip strength; LE=lower extremity;
Model 1 adjusted for age, gender, and race/ethnicity; Model 2 adjusted for age, gender, race/ethnicity, height, abdominal/hip girth ratio, and total number of comorbidities; Model 3 adjusted for age, gender, race/ethnicity, height, abdominal/hip girth ratio, total number of comorbidities, and depressive symptoms. Physical limitation was measured by presence of impairment in both grip strength and lower extremity.
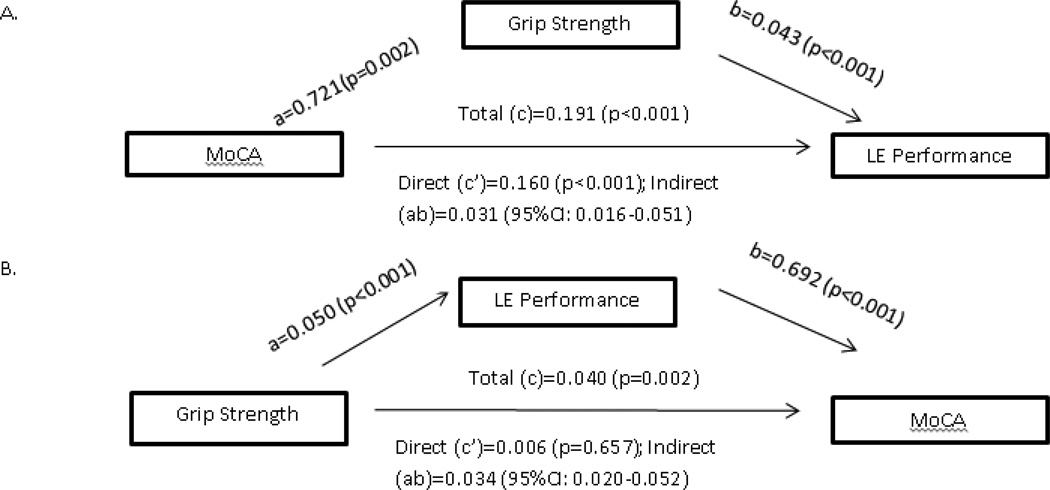
Figure 1.
Mediation analysis assessing A. grip strength as the mediator of the cognitive-physical performance association and B. physical performance as mediator of the grip strength-cognitive performance association; a paths reflect the association between predictor and mediator; b paths the direct effects of mediator on the outcome with adjustment for predictor; c paths the total effects of predictor on outcome without adjustment for mediator; c’ the direct effect of predictor on outcome with adjustment for mediator, and ab paths reflect the bias corrected indirect effect of predictor on outcome.
Results
The sample characteristics across the four groups are presented in Table 1. More than half of subjects had no mobility impairment, while about 10% had UE impairment, 24% had LE impairment, and 10% had mobility limitations (both UE and LE impairments). Participants with indication of mobility limitation were significantly older and less likely to be Caucasian than the other groups. They also had significantly more visceral fat, lower muscle mass, and performed poorest on MoCA. Modeling strategies regarding potential covariates to be included in further analyses were based on these associations as well as those revealed by inspecting correlation coefficients among the different measured factors suggesting specific effects (e.g. age, race/ethnicity, body composition, disease burden) that should be accounted for when assessing the mobility-cognition association (Table 2). BMI was not considered a covariate of interest in this analysis based on a lack of association with either the outcome or the predictor. Girth ratio was the only body composition measure included in Model 2 in light of its larger impact on MoCA and relatively large collinearity with visceral fat. To minimize the risk of type II error, we included disease burden as a potential correlate based on an a priori assumption of an impact on cognitive function in spite of a weak effect on the outcome..
General linear models revealed a significant trend toward a stepwise-like association with a smaller impact of early vs. later stage of physical dysfunction on cognitive performance (Table 3). In the first model, unadjusted MoCA scores declined by a factor of -2 in the UE impairment group but by more than 4-fold in the mobility limitation group, with the LE impairment group faring in the middle. Adjustment for socio-demographic, body composition and health factors only slightly reduced these effects. Inclusion of depressive symptoms had no impact. Similar results were obtained when the likelihood of cognitive impairment was assessed using the same modeling strategy (Model 3).
Figure 1 presents the results of our bootstrapping analyses investigating muscle strength as mediator of the cognition-LE performance association and separately LE performance as mediator of the GS-cognition association. We found a significant association between predictor, mediator, and outcome in both associations assessed. When the former association was assessed, the effect of the predictor (i.e. MoCA) on the outcome (i.e. LE performance) was reduced by 16% ((c path - c’ path)/c path) while in the case of the latter association, a 85% reduction in the effect of the predictor (i.e. GS) on the outcome (i.e. MoCA) was noted when the 2 mediators were included (muscle strength and LE performance, respectively). The non-significant c’ term for the effect of GS on MoCA suggests that its effect is due entirely to an impact on mobility performance while the effect of MoCA on mobility performance in only partially due to lower MS in those with poorer cognitive performance. Adjustment for confounders and potential mediators had no impact on the indirect effect of GS on MoCA and only slightly reduced the indirect effect of MoCA on LE performance from 0.031 to 0.027 (13% reduction), which remained significant (bias corrected 95% CI: 0.014–0.045).
Discussion
Our results suggest that the association between cognitive function and mobility follows a dose-response format with increasing likelihood of poor cognition as an individual progresses through different stages of mobility dysfunction. While our data support associations suggested by the Central Benefit Model, we also found evidence that LE impairment may better capture the effects of mobility impairment on cognition than UE impairment as measured by low GS and that these effects likely operate in both directions. This is encouraging as evidence of a connection between poor cognitive performance and early stage mobility dysfunction suggests that strategies to prevent future physical limitations and disability in individuals with low cognitive function may be more efficient when focused on addressing events that constitute the first steps in the disablement process. This in turn may reduce the risk of further cognitive decline and potentially the development of dementia possibly through an effect on other health-related factors.
Associations between cognition and different measures of mobility have been long recognized. Individuals with evidence of impaired cognitive function tend to perform poorly on physical tests26. Even in older adults without evidence of cognitive impairment, faster performance on mobility tests is associated with better cognitive abilities27. This strong link between cognitive function and mobility has been interpreted as suggestive of an underlying aging process that accounts for declines observed across various systems including cognition and physical function28. Results pooled from longitudinal studies, however, suggest that although changes in physical and cognitive functioning are associated, the evidence is not consistent enough to provide support for the common cause hypothesis but rather suggests a true dynamic relationship between these changes in which the temporal ordering may operate in both directions29. The authors of the latter meta-analysis however, acknowledge the general lack of studies that directly assessed the order of decline and indicated the need for a detailed examination of the direction in which these changes occur.
Within the confines of a cross-sectional analysis, our mediation findings provide support for the idea that the longitudinal association between cognitive and physical aspects of functioning is likely to operate in both directions. Using a bootstrapping as mediation approach, we found strong evidence that poor cognitive performance may lead to poor physical performance directly but also indirectly through its impact on muscle strength. Similarly, we found that poor muscle strength is associated with poor cognition, mostly through the effect on LE (mobility) performance. We interpreted these indirect effects as evidence of temporal ordering favoring the idea of dynamicity of the longitudinal association between cognitive and physical function in old age. These relationships should be examined in longitudinal studies that are better equipped to separate between- from within-person changes and employ other complex analytical methods to correct for bias and allow fitting of alternative models of change29.
The public health relevance of our findings suggests that the association between mobility and cognitive impairment follows a step-wise pattern. Although the strongest association may be observed between dementia and mobility disability (i.e. inability to perform mobility-related ADLs), it has been previously suggested that early measures of mobility dysfunction such as poor muscle strength can be used to identify individuals with poor cognitive performance who may be at risk for development of future cognitive impairment and dementia. While a consistent association between cognition and mobility has already been reported5, 30–32, what previous work failed to establish is a clear link between stage of mobility dysfunction and cognitive abilities and the pattern in which it unfolds. Although poor muscle strength can be used as an indication that the person may have reached the first stage of mobility dysfunction, the possibility that the person may in fact be much further down the process cannot be ruled out unless measures of dysfunction at more advanced stages are also taken into account. To address this issue, we constructed a variable that combines muscle strength and physical performance to better identify the stage of dysfunction in which participants fit best. Given the progressive nature of disablement in which downstream events rely on presence of upstream events and also the multifactorial nature of poor physical performance21, this categorization scheme allows separation of physical impairment and limitation stages and supports a further subcategorization of the impairment state.
Most importantly, the dose-response association observed between stage of mobility dysfunction and cognitive performance suggests that progression through the disablement process (i.e. going from impairment to limitation to disability) corresponds to worsening cognitive functioning. Taken together, these associations suggest that progression through the stages of physical disablement goes along with progression to dementia, which given the bidirectional nature of the association as evidenced by our mediation analyses and other previous work29 indicates the potential for targeting individuals with early evidence of dysfunction in either aspect of functionality for interventions to mitigate or prevent decline and progression in the other.
While our modeling approach does not allow us to clearly distinguish confounders from mediators, the interpretation depending on a priori assumptions, the lack of an impact of depression (our assumed mediator) suggest that other factors may better help clarify the mechanistic pathways linking physical dysfunction to cognitive impairment including physical inactivity14–15, stress and inflammatory processes16. Clarification of these likely complex mechanisms would inform interventions designed to maintain functionality and prevent both physical and cognitive decline.
Limitations of our study need to be considered when interpreting these results. First, the cross-sectional design of our study does not allow us to establish but rather to suggest a temporal ordering of decline in two aspects of functionality. We were able to demonstrate that late stage mobility dysfunction is associated with the poorest cognitive performance. However, whether physical impairment was the result of cognitive decline or actually triggered the cognitive decline cannot be parceled out in our study, although our mediation results were indicative of both scenarios being possible. Second, inclusion of middle-aged adults may raise questions of whether our physical performance measure, validated for use in older populations would lead to ceiling effects in this subgroup. However, of the 98 participants younger than 65 years only 1 scored a perfect 16 on the mini PPT, suggesting limited ceiling effects. These findings are in line with other reports of good performance of similar measures in non-disabled middle-aged adults33. Moreover, when we excluded participants younger than 65 years, the results remained unchanged (e.g. Est.UE=−0.55±1.23, p=0.655; Est.LE=−2.46±0.90, p=0.007; Est.ML=−3.57±1.27, p=0.005 for the fully-adjusted model predicting cognitive performance). Third, excluding participants with missing data on either the outcome or/and predictor of interest could have an impact on our findings, especially in light of significant differences in mean MoCA performance (18.5±4.0 vs. 22.6±5.4 for missing and non-missing respectively, p=0.013). To address this we replaced their missing data with the sample mean (i.e. MoCA) or sample median (i.e. stage of mobility dysfunction) and reassessed the association between mobility dysfunction stage and MoCA performance. The results remained unchanged (unadjusted model: Est.UE=−1.69±0.99, p=0.089; Est.LE=−3.19±0.67, p<0.001; Est.ML=−3.89±0.96, p<0.001). Further adjustment had little impact on the results. Forth, we assumed that presence of poor LE performance in the absence of GS impairment may be considered an indicator of LE impairment. This state may alternatively reflect orthopedic problems or chronic pain syndromes that do not affect either muscle strength or cognition or other factors such as low motivation or confidence in ability to perform functional tests rather than ‘true’ physical limitations as these would require presence of impairments such as poor muscle function34–35. This possibility highlights the importance of finding ways to better separate the motivational component of physical performance from the actual physical inability to perform the physical tasks. Advantages include the relatively large sample size of multicultural, community-dwelling older adults, the use of validated tools to measure mobility and cognitive function, and our staging of mobility dysfunction strategy which reduces the likelihood of miscategorization.
Our findings support the idea of a complex association between cognition function and mobility in later life in which mobility decline may reflect pathological changes in the brain but also may lead to further cognitive decline and in turn resulting in even more physical decline. Finding ways to mitigate this vicious cycle of mobility decline is imperative as it may help alleviate the burden of disability and dementia, the leading causes of distress in older adults and those caring for them. Using brief, valid tools to screen older patients for early signs of mobility dysfunction is feasible and may provide the first detectable stage of future cognitive impairment. Physical performance measurements particularly those measuring physical performance should be encouraged during routine annual wellness examinations as this may help identify those in early stages of cognitive impairment for whom interventions to improve symptoms and prevent development of physical disability and loss of independence may be more successful.
Acknowledgments
Both authors had full access to all the data in the study, gave final approval of the current version to be published, and take responsibility for the conduct of the research. Both authors contributed to study concept, design, and interpretation of data. Dr. Tolea drafted the manuscript and conducted data analysis. Dr. Galvin – the principal investigator takes responsibility for the integrity of the data and the accuracy of the data analysis, has the right to publish any and all data, separate and apart from the guidance of any sponsor and provided critical revision of the manuscript for important intellectual content, obtained funding, and provided study supervision.
Study funding: This work was supported by the National Institutes of Health (5R01AG040211-02), the Michael J. Fox Foundation for Parkinson’s Research, and the Morris and Alma Schapiro Foundation.
Funding:
This work was supported by grants from the NIH (R01 AG040211 and P30 AG008051), Morris and Alma Schapiro Fund, Michael J Fox Foundation and the New York State Department of Health (DOH-2011-1004010353).
Footnotes
None of the authors have personal, financial or potential conflicts of interest.
References
- 1.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1979;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 2.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, Ligaments and Tendons Journal. 2013;3(4):346–350. [PMC free article] [PubMed] [Google Scholar]
- 3.Liu-Ambrose T, Nagamatsu LS, Liang Hsu, et al. Emerging concept: 'central benefit model' of exercise in falls prevention. Br J Sports Med. 2013;47:115–117. doi: 10.1136/bjsports-2011-090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagi SZ. Some conceptual issues in disability and rehabilitation. In: Sussman MB, editor. Sociology and Rehabilitation. Washington, DC: American Sociological Association; 1965. [Google Scholar]
- 5.Alfaro-Acha A, Al Snih S, Raji MA, et al. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61(8):859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 7.Montero-Odasso M, Verghese J, Beauchet O, et al. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mograbi DC, de Assis Faria C, Charchat Fichman H, et al. Relationship between activities of daily living and cognitive abilities in a sample of older adults with heterogeneous educational level. Annals of Indian Academy of Neurology. 2014;17(1):71–76. doi: 10.4103/0972-2327.128558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford K, Sowers M, Seeman TE, et al. Cognitive functioning is related to physical functioning in a longitudinal study of women at midlife. Gerontology. 2010;56(3):250–258. doi: 10.1159/000247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauth EB, Schwartz S, Tschanz JT, et al. Baseline disability in activities of daily living predicts dementia risk even after controlling for baseline global cognitive ability and depressive symptoms. Int J Geriatr Psychiatry. 2013;28(6):597–606. doi: 10.1002/gps.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: Results from the Women's Health Initiaive Memory Study. J of Gerontol A Biol Sci Med Sc. 2010;65A(3):300–306. doi: 10.1093/gerona/glp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkins CH, Roe CM, Morris JC, et al. Mild physical impairment predicts future diagnosis of dementia of the Alzheimer’s type. J Am Geriatric Soc. 2013;61(7):1055–1059. doi: 10.1111/jgs.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrman S, Ebmeier KP. Can exercise prevent cognitive decline? Practitioner. 2014;258(1767):17–21. 12–13. [PubMed] [Google Scholar]
- 15.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2012;67(6):671–676. doi: 10.1093/gerona/glr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Bohannon RW, Magasi SR, Bubela DJ, et al. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46(4):555–558. doi: 10.1002/mus.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23(2):132–137. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins CH, Roe CM, Morris JC. A brief clinical tool to assess physical function: the mini-physical performance test. Arch Gerontol Geriatr. 2010;50(1):96–100. doi: 10.1016/j.archger.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manini TM, Clark BC. Dynapenia and aging: an updata. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stegenga BT, Nazareth I, Torres-Gonzalez F, et al. Depression, anxiety and physical function: exploring the strength of causality. J Epidemiol Community Health. 2012;66(7):e25. doi: 10.1136/jech.2010.128371. [DOI] [PubMed] [Google Scholar]
- 23.Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70(3):374–382. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 26.McGough EL, Kelly VE, Logsdon RG, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed "up & go" test. Phys Ther. 2011;91(8):1198–1207. doi: 10.2522/ptj.20100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berryman N, Bherer L, Nadeau S, et al. Executive functions, physical fitness and mobility in well-functioning older adults. Exp Gerontol. 2013;48(12):1402–1409. doi: 10.1016/j.exger.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Christensen H, Mackinnon AJ, Korten A, et al. The "common cause hypothesis" of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16(4):588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- 29.Clouston SA, Brewster P, Kuh D, et al. The Dynamic Relationship Between Physical Function and Cognition in Longitudinal Aging Cohorts. Epidemiol Rev. 2013 doi: 10.1093/epirev/mxs004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle PA, Buchman AS, Wilson RS, et al. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11):1339–1344. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raji MA, Kuo F, Snih SA, et al. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53(9):1462–1468. doi: 10.1111/j.1532-5415.2005.53457.x. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 33.Hearty TM, Schenkman ML, Kohrt WM, et al. Continuous Scale Physical Functional Performance Test: Appropriateness for Middle-Aged Adults with and without Parkinson’s Disease. JNPT. 2007;31:64–70. doi: 10.1097/NPT.0b013e3180676afa. [DOI] [PubMed] [Google Scholar]
- 34.Ajzen I. The Theory of Planned Behavior. Organizational Behavior and human decision processes. 1991;50:179–211. [Google Scholar]
- 35.Mullen SP, McAuley E, Satariano WA, et al. Physical activity and functional limitations in older adults: the influence of self-efficacy and functional performance. J Gerontol B Psychol Sci Soc Sci. 2012;67(3):354–361. doi: 10.1093/geronb/gbs036. [DOI] [PMC free article] [PubMed] [Google Scholar]