Abstract
BACKGROUND
Hypoalbuminemia is common in patients with chronic heart failure, and is a marker of disease severity associated with an adverse prognosis. Whether hypoalbuminemia contributes to (or is associated with) worse outcomes in AHF is unclear. We sought to determine the implications of low serum albumin in patients receiving decongestive therapies for acute heart failure (AHF).
METHODS
Baseline serum albumin levels were measured in 456 AHF subjects randomized in the DOSE-AHF and ROSE-AHF trials. We assessed the relationship between admission albumin levels (both as a continuous variable and stratified by median albumin [≥3.5 g/dL]) and worsening renal function [WRF], worsening heart failure [WHF], and clinical decongestion by 72 hours; 7-day cardiorenal biomarkers; and post-discharge outcomes.
RESULTS
The mean baseline albumin level was 3.5±0.5 g/dL. Albumin was not associated with WRF, WHF, or clinical decongestion by 72 hours. Furthermore, there was no association between continuous albumin levels and symptom change by visual analog scale or weight change by 72 hours. Albumin was not associated with 60-day mortality, rehospitalization or unscheduled emergency room visits.
CONCLUSIONS
Baseline serum albumin levels were not associated with short-term clinical outcomes for AHF patients undergoing decongestive therapies. These data suggest serum albumin may not be a helpful tool to guide decongestion strategies.
Keywords: heart failure, proteins, diuretics
INTRODUCTION
Human serum albumin is a 65-kilodalton protein that comprises over 50% of the total plasma protein concentration1. Albumin binds to exogenous particles and may have both anti-oxidant and antiinflammatory properties, and it is responsible for nearly 70–80% of the plasma oncotic pressure.1
Hypoalbuminemia is common in patients with chronic heart failure 2, 3 with a prevalence of approximately 25%, and may be even more common in the elderly or frail.4 In acute heart failure (AHF), hypoalbuminemia may facilitate increased peripheral edema and pulmonary congestion at lower left atrial pressures.5 Hypoalbuminemia (< 3–3.5 g/dL) has been associated with incident worsening renal function (WRF) during decongestive therapy for AHF.6, 7 In particular, low albumin levels have been postulated to cause an inability to regulate volume status and lead to intravascular volume losses and reduced renal perfusion.6 Furthermore, hypoalbuminemia in AHF is associated with a higher incidence of adverse outcomes, and its prognostic impact may be more pronounced in patients with reduced left ventricular ejection fraction.8 However, whether the phenotypic presentation and clinical course during decongestion in AHF vary according to albumin levels need further clarification.
We hypothesize that lower baseline albumin levels will be associated with incident WRF, worsening heart failure (WHF), and less response to decongestive therapies. The Diuretic Strategies in Patients with Acute Decompensated Heart Failure (DOSE-AHF) and the Low-dose Dopamine or Low-dose Nesiritide in Acute Heart Failure with Renal Dysfunction (ROSE-AHF) trials provide a well-characterized AHF cohort with adjudicated outcome data to study these relationships.
METHODS
Study Population
We included two studies conducted within the NHLBI-sponsored Heart Failure Clinical Trials Network. The protocols were approved by the Institutional Review Board at each site and written informed consent was obtained from all patients prior to randomization. All trials were conducted in the United States and Canada.
DOSE-AHF and ROSE-AHF were prospective double-blinded trials that tested the effectiveness and renal consequences of different decongestive strategies in AHF patients with clinical evidence of congestion. The diagnosis of AHF was based on the presence of at least one sign (rales, peripheral edema, ascites, or radiographic evidence of pulmonary congestion) and one symptom (dyspnea, orthopnea or edema), regardless of ejection fraction. DOSE-AHF tested high vs. low dose loop diuretic and bolus vs. continuous infusion intravenous loop diuretic dosing in hospitalized patients with AHF, using a 2x2 factorial design.9 Of the 308 patients randomly assigned, 151 were assigned to low dose loop diuretic, 157 to high dose loop diuretic, 156 to bolus dosing, and 152 to continuous infusion dosing. The ROSE-AHF trial tested the effectiveness of additional low-dose dopamine (2 μg/kg/min) or low-dose nesiritide (0.005 μg/kg/min) in hospitalized patients with AHF and renal dysfunction (glomerular filtration rate 15–60mL/min/1.73 m2 as estimated by the Modification of Diet and Renal Disease equation).10 Of the 360 patients randomly assigned, 122 were assigned to low-dose dopamine and 119 to low-dose nesiritide which were both compared to placebo.
In both trials, patients with advanced chronic kidney disease were excluded. In DOSE-AHF this was defined as a serum creatinine >3.0 mg/dL and in ROSE-AHF as an estimated GFR of <15 mL/min/1.73m2. Patients with a terminal illness other than heart failure with an expected survival of < 1 year were also excluded from both trials. There were no exclusion criteria for liver disease in either trial.
Cohort selection criteria
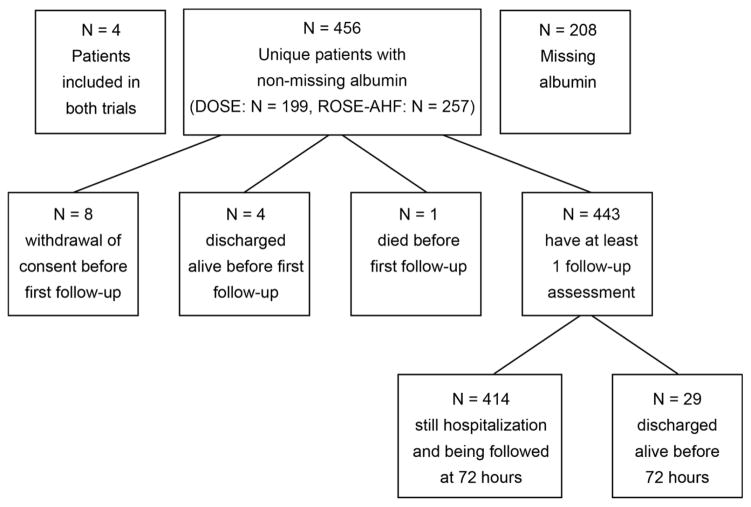
All randomly assigned patients with albumin levels checked locally at the enrolling sites (N=456) were included in this analysis. If patients were enrolled in both trials, only the observations from DOSE-AHF were included as this was the first trial enrollment.
Outcome assessment
All outcomes were assessed from randomization. WRF was defined as an increase in serum creatinine of >0.3 mg/dl from baseline until 72 hours. WHF was defined as the need for rescue therapy (additional open label loop diuretic, addition of a thiazide, vasoactive therapy, ultrafiltration, or mechanical circulatory or respiratory support from baseline until 72 hours). Freedom from congestion was defined as JVP <8 cm H2O, no orthopnea, and, at most, trace peripheral edema after 72 hours of treatment. The effectiveness of decongestive therapies was determined by improvement in symptoms (as measured by dyspnea and global well-being analogue scales); net fluid loss and weight change; and diuretic efficiency (net fluid loss produced per 40 mg of furosemide equivalents) until 72 hours.11
Cardiorenal biomarkers included serum creatinine, cystatin C, and amino terminus pro-B-type natriuretic peptide (NT-proBNP). These biomarkers were measured at baseline and, for DOSE, 7 days after enrollment. They were analyzed at a biomarker core laboratory at the University of Vermont, Burlington, VT.
Post-hospitalization clinical outcomes were previously adjudicated as part of the clinical trials.9, 10 They included death, rehospitalization, and emergency department visits at 60 days.
Statistical analysis
Continuous variables were expressed as medians with the 25th and 75th percentile. Categorical variables were expressed as frequencies with percentages. Baseline characteristics of albumin levels ≥ or < 3.5 g/dL (median value) were compared by the Wilcoxon rank sum test or Pearson’s chi square test where appropriate. Logistic and general linear regression were used to determine the association between albumin levels and clinical decongestion endpoints, symptom change and fluid status by 72 hours, and for change in cardio-renal biomarkers by 7 days. All models were adjusted for the clinical trial with additional adjustment for baseline values when modeling a change for a continuous variable. Interactions with left ventricle ejection fraction (LVEF) > or ≤45% and albumin level were checked. Cox-proportional hazards models were used to determine the association between baseline albumin and death, rehospitalization, or unscheduled emergency department visits by 60-days. Two-sided P-values <.05 were considered statistically significant and double-sided P-values <.1 were used to identify potential interactions. Statistical analyses were completed using SAS software, version 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Baseline Characteristics
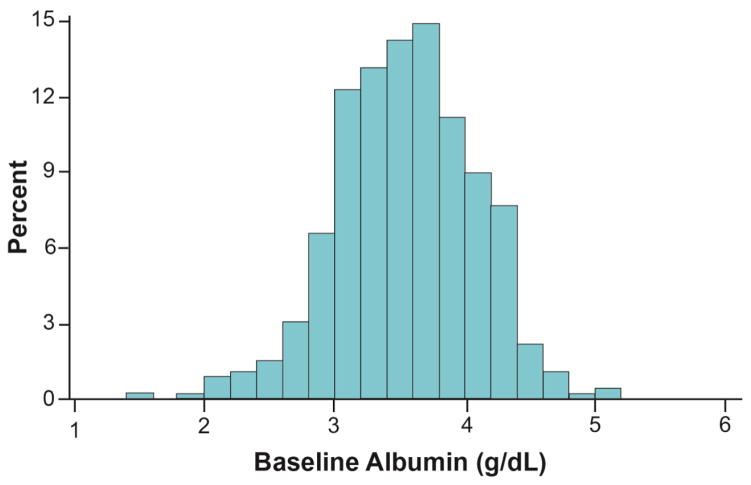
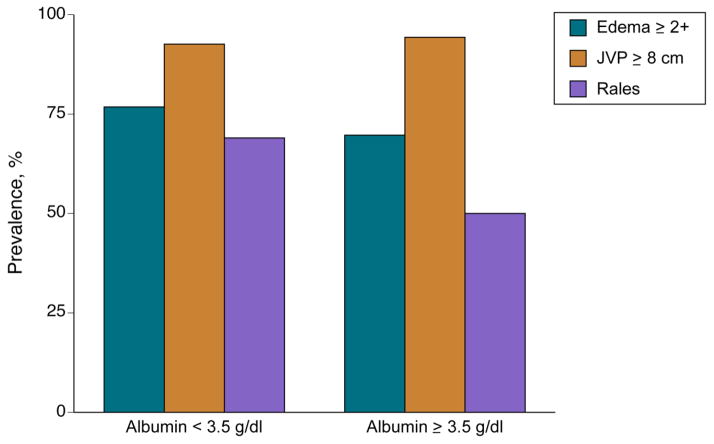
Serum albumin levels were available for 456 subjects (Figure 1). Mean baseline albumin level Figure 2) was 3.5±0.5 g/dL. Baseline characteristics stratified by albumin level are described in Table 1. Lower albumin was associated with a higher prevalence of rales on auscultation (P<0.001), a trend towards increased significant peripheral edema (P=0.09), but not JVP ≥ 8 cm H2O (P=0.45, Figure 3).
Figure 1.
Consort Diagram
Figure 2.
Baseline Albumin Levels
Table 1.
Baseline Characteristics
| Characteristic | Albumin < 3.5 g/dl (N=204) | Albumin ≥ 3.5 g/dl (N=252) | p-value* |
|---|---|---|---|
| Demographics | |||
| Age, years | 68 (57.5, 78) | 68 (59, 77) | 0.80 |
| Male sex | 151/204 (74.0) | 192/252 (76.2) | 0.59 |
| White race | 124/204 (60.8) | 197/252 (78.2) | <0.001 |
| Clinical History | |||
| Ejection fraction, % | 33 (20, 55) | 29.5 (20, 50) | 0.23 |
| Ischemic etiology | 102/204 (50.0) | 150/252 (59.5) | 0.042 |
| Diabetes | 115/204 (56.4) | 138/252 (54.8) | 0.73 |
| ICD | 63/204 (30.9) | 128/252 (50.8) | <0.001 |
| Chronic Liver Disease | 8/204 (3.9) | 8/252 (3.2) | 0.67 |
| Malignancy | 11/204 (5.4) | 19/252 (7.5) | 0.36 |
| Tricuspid regurgitation | 0.84 | ||
| None/trivial | 71/202 (35.1) | 85/251 (33.9) | |
| Mild | 49/202 (24.3) | 58/251 (23.1) | |
| Moderate | 50/202 (24.8) | 60/251 (23.9) | |
| Severe | 32/202 (15.8) | 48/251 (19.1) | |
| NYHA classification at baseline | 0.051 | ||
| II | 8/192 (4.2) | 8/242 (3.3) | |
| III | 123/192 (64.1) | 181/242 (74.8) | |
| IV | 61/192 (31.8) | 53/242 (21.9) | |
| Medications | |||
| ACE inhibitor or ARB | 117/204 (57.4) | 140/252 (55.6) | 0.70 |
| Beta-blocker | 170/204 (83.3) | 205/252 (81.3) | 0.58 |
| Aldosterone antagonist | 52/204 (25.5) | 80/252 (31.7) | 0.14 |
| Oral diuretic dose pre-hospitalization, furosemide equivalents in mg/day | 80 (80, 160) | 80 (80, 160) | 0.088 |
| HF Clinical Assessment | |||
| Body mass index, kg/m2 | 31.4 (26.7, 36.8) | 30.6 (26.2, 37.3) | 0.64 |
| Self-assessment | |||
| Global VAS at baseline | 49 (34, 63) | 50 (30, 69) | 0.48 |
| Dyspnea VAS at baseline | 50 (33, 75) | 57 (38, 76) | 0.063 |
| Local Labs | |||
| Sodium, mg/L | 138 (136, 141) | 138 (136, 141) | 0.47 |
| Hemoglobin, g/dL | 11.2 (9.9, 12.6) | 11.8 (10.6, 13.2) | 0.002 |
| Blood urea nitrogen, mg/dl | 31.5 (24, 47.5) | 34 (24, 50) | 0.20 |
| Core Labs | |||
| Creatinine, mg/dl | 1.49 (1.17, 1.94) | 1.58 (1.22, 1.90) | 0.60 |
| NT-proBNP, pg/ml | 5268 (3071, 10703) | 4240 (1923, 9618) | 0.030 |
| Cystatin C, mg/L | 1.59 (1.27, 2.08) | 1.58 (1.25, 2.04) | 0.70 |
Variables are expressed as median (25th and 75th percentile) or n/N (%)
P-values by Wilcoxon Test, Fisher’s Exact, or Chi-square
Abbreviations: ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; VAS, visual analogue scale; and NT-proBNP, amino terminal pro-B-type natriuretic peptide.
Figure 3.
The Relationship of Albumin Levels to Physical Examination Findings
P-values by Pearson’s chi square test For edema ≥2+ P=0.09; for JVP ≥ 8 cm water P=0.45; and for rales P<0.001.
Clinical decongestion endpoints
At 72 hours the incidence of WHF or WRF in the combined studies was 29.6% (N=131, Table 2). Individually, the incidence of WHF was 13.1% (N=58) and the incidence of WRF was 19.8% (N=88). This incidence of WRF was similar in DOSE-AHF and ROSE-AHF (19.5% and 20.0%, respectively). However, DOSE-AHF had a higher incidence of WHF than ROSE-AHF (21.2% and 6.8%, respectively). Only a minority of patients were free of congestion at the end of 72 hours: 13.9% (N=56). Baseline albumin levels were not associated with the incidence of WHF, WRF, or freedom from congestion (P>.05 for all, Table 2) and there were no interactions between albumin and LVEF for each (p>0.1 for all). The results were similar when albumin was analyzed as a continuous variable. In a sensitivity analysis, the assumption of linear risk-relationship of continuous albumin levels with respect to each outcome was tested. We were unable to reject the null hypothesis of a linear relationship with albumin modeled for WRF or WHF, P=0.68; for WRF, P=0.70; for WHF, P=0.55; or for freedom from congestion, P=0.39. Thus, there were no signs of a threshold effect for albumin.
Table 2.
Clinical decongestion endpoints
| 72 hour endpoints | Albumin < 3.5 g/dl | Albumin ≥ 3.5 g/dl | OR (95% CI) for albumin ≥ 3.5 g/dl | p-value (binary albumin) | OR (95% CI) for 1 g/dl increase in albumin | p-value (continuous albumin) |
|---|---|---|---|---|---|---|
| Incident WHF or WRF | 60/198 (30.3%) | 71/245 (29.0%) | 0.99 (0.65, 1.49) | 0.95 | 0.96 (0.65, 1.42) | 0.84 |
| WHF | 26/198 (13.1%) | 32/246 (13.0%) | 1.12 (0.63, 1.97) | 0.71 | 0.98 (0.58, 1.66) | 0.94 |
| WRF | 43/200 (21.5%) | 45/245 (18.4%) | 0.82 (0.51, 1.31) | 0.40 | 0.85 (0.55, 1.33) | 0.48 |
| Free of congestion | 23/172 (13.4%) | 33/230 (14.3%) | 1.10 (0.62, 1.96) | 0.74 | 1.35 (0.77, 2.37) | 0.30 |
OR for albumin≥3.5 g/dl
Abbreviations: WHF, worsening heart failure; and WRF, worsening renal function
Symptom change, fluid loss, and change in cardiorenal biomarkers
Albumin levels were not associated with changes in dyspnea or global well-being by visual analogue scales by 72 hours (Table 3). Baseline albumin levels were associated with net fluid loss (Δ −578 mL/ albumin g/dL, P=0.048) but not weight change (P=0.43) or lower diuretic efficiency (P=0.053). All three outcomes were not associated with baseline albumin ≥ 3.5 g/dl (P>.05 for all). Further, there were no associations between baseline albumin levels and change in measured cardiorenal biomarkers at 7 days: creatinine (P=0.75), cystatin C (P=0.76), nor NT-proBNP (P=0.60). Comparable findings were seen for albumin ≥ 3.5 g/dl (P>.05 for all). There were no interactions with LVEF and albumin for any of the continuous 72-hour or 7-day outcomes (p>0.1 for all).
Table 3.
The association of admission albumin with symptom change and fluid loss by 72 hours and change in cardio-renal biomarkers by 7 days
| Endpoints | N | Estimate (95% CI) for albumin ≥ 3.5 g/dl | p-value (binary albumin) | Estimate (95% CI) per 1 g/dl albumin | p-value (continuous albumin) |
|---|---|---|---|---|---|
| Change in dyspnea VAS at 72h* | 382 | 0.06 (−4.07, 4.18) | 0.98 | 0.05 (−3.92, 4.03) | 0.98 |
| Change in global VAS at 72h* | 377 | −0.77 (−5.15, 3.61) | 0.73 | 1.04 (−3.17, 5.25) | 0.63 |
| Net fluid loss at 72 hrs | 391 | −437 (−1036, 162) | 0.15 | −578 (−1151, −6) | 0.048 |
| Weight change at 72 hours (lbs)* | 388 | 0.74 (−0.97, 2.46) | 0.39 | 0.67 (−1.00, 2.35) | 0.43 |
| Diuretic efficiency (randomization-72h) | 390 | −59 (−135, 17) | 0.13 | −72 (−144, 1) | 0.053 |
| Change in creatinine at discharge or 7 days*† | 185 | 0.01 (−0.11, 0.12) | 0.87 | 0.02 (−0.09, 0.13) | 0.75 |
| Change in cystatin C at discharge or 7 days*† | 186 | −0.01 (−0.14, 0.12) | 0.87 | 0.02 (−0.10, 0.14) | 0.76 |
| Change in log(NTproBNP) at discharge or 7 days*† | 185 | −0.01 (−0.20, 0.18) | 0.94 | −0.05 (−0.22, 0.13) | 0.60 |
Additionally adjusts for baseline value
Additionally adjusts for day of discharge/day 7 biomarker measure
All models adjusted for trial
Diuretic efficiency = net fluid output produced per 40 mg of furosemide equivalents
Post-discharge hospitalization events
The composite outcome--death, rehospitalization, unscheduled ER visit--occurred in 195 patients (43.4%) over 60 days of follow-up. Individually, there were 40 deaths (8.8%), 144 rehospitalizations (32.3%), and 51 unscheduled ER visits (11.4%, Table 4). Baseline albumin ≥3.5 g/dL was not associated with the composite outcome (HR 0.97, 95% CI 0.73–1.29, P=0.84), nor rehospitalizations (HR 1.05, 95% CI 0.76–1.47, P=0.76). There was a non-significant trend for a reduced risk of death by 60-days for albumin ≥3.5 g/dL (HR 0.64, 95% CI 0.37–1.12, P=0.12. Otherwise, all other findings were comparable when albumin was analyzed as a continuous variable.
Table 4.
Association of albumin and post-hospitalization events
| Endpoint | N | Events | HR (95% CI) for albumin ≥ 3.5 g/dl | p-value (binary albumin) | HR (95% CI) for 1 g/dl increase in albumin | p-value (continuous albumin) |
|---|---|---|---|---|---|---|
| Any death, rehospitalization, unscheduled or ER visit | 449 | 195 | 0.97 (0.73, 1.29) | 0.84 | 0.98 (0.75, 1.27) | 0.88 |
| Patient died within the first 60 days post-randomization | 456 | 40 | 0.61 (0.33, 1.15) | 0.13 | 0.64 (0.37, 1.12) | 0.12 |
| Any rehospitalization during follow-up | 446 | 144 | 1.05 (0.76, 1.47) | 0.76 | 0.95 (0.70, 1.29) | 0.73 |
| Any Death or Rehospitalization during study follow-up | 452 | 165 | 0.96 (0.70, 1.30) | 0.77 | 0.88 (0.67, 1.17) | 0.40 |
After multivariable adjustment (age, SBP, lnBUN), the HR (95% CI) for albumin ≥ 3.5 g/dl is 0.55 (0.29, 1.05), p=0.071. The HR (95% CI) for 1 g/dl increase in albumin is 0.57 (0.33, 0.97), p=0.038.
DISCUSSION
This analysis has several important findings which inform our understanding of what the clinical implications of serum albumin levels are in patients hospitalized for AHF. First, serum albumin levels were largely within the normal range in this AHF trial population. Despite this, lower albumin levels within this range were associated with physical exam findings of peripheral congestion but not central venous congestion (JVP ≥ 8 cm H2O), suggesting that the former is more affected by oncotic pressure and the latter by hydrostatic pressure. Second, besides net fluid loss at 72 hours, admission albumin level was not associated with short-term clinical endpoints, symptom change, nor change in cardiorenal biomarkers with decongestive therapies. Third, baseline serum albumin level was not associated with mortality, rehospitalization or ER visits. Taken in aggregate, these data suggest that although patients with lower serum albumin may have more peripheral edema upon presentation, serum albumin may not influence short-term clinical and cardiorenal changes during decongestive therapies for AHF.
In AHF, venous congestion is a result of elevated cardiac filling pressures in addition to salt and water retention by the kidney. This causes increased hydrostatic pressure in capillary beds throughout the body, which counterbalances the osmotic gradient between the intravascular space and the interstitium resulting in a low protein edema.12 Our finding that lower albumin levels were associated with increased peripheral and pulmonary edema on exam, but not central congestion supports the assertion that lower plasma oncotic pressure further augments the hydrostatic-mediated extravasation of fluid into the interstitium and that central venous pressure is not the sole determinant of pulmonary or peripheral edema.13, 14
These findings conflict with prior reports suggesting that lower albumin levels were associated with incident WRF during treatment for AHF. A single-center, prospective cohort of 80 patients with AHF had a 26% incidence of WRF, (increase in serum creatinine ≥0.3 or 25%).6 Their analysis suggested that serum albumin <3.5 g/dL was independently associated with WRF. Another retrospective study of 177 patients hospitalized with AHF receiving continuous loop diuretic infusions demonstrated a 27% incidence of WRF, suggested that a serum albumin ≤3.0 g/dL was a strong independent predictor.7 In contrast, our cohort has a much larger sample size (N=456) with more outcomes (N=88) comprised of randomized patients from multiple centers receiving protocolized decongestive therapy, therefore reducing potential bias related to regional treatment and practice patterns. Along similar lines, this discrepancy may also be explained by these studies having broader, non-trial populations. Further strengthening this assertion was the lack of association between albumin and serum creatinine or cystatin C change.
Based on these prior studies6, 7 and the notion that plasma oncotic pressure may lead to dysregulation of intravascular volume with subsequent decrements in renal blood flow and more peripheral and pulmonary edema, we hypothesized that lower albumin would be associated with both WRF and WHF. However, this was not the case in the present analysis. Low albumin may be a minor contributor to WRF as renal impairment in heart failure results from a complex interplay between both hemodynamic and non-hemodynamic factors.15
In addition to the preservation of renal function, decongestion and preventing clinical worsening is paramount during AHF treatment. WHF during the course of AHF treatment identifies patients with either worsening symptoms or poor response to initial therapy in whom treating clinicians may intensify treatment.16 As such, this outcome encompasses both heart failure pathophysiology and the clinician’s interpretation of the patient’s clinical status and response to treatment. Given the observation that albumin was not associated with subjective change in symptoms, it is not surprising that we found no association between baseline albumin levels and WHF.
Pharmacologically, albumin may interact with decongestive therapies. Hypoalbuminemia has been postulated to contribute to loop diuretic resistance as albumin-loop diuretic binding facilitates drug delivery to the kidney.17 From this, intravenous albumin administration may increase diuretic efficacy, but there are mixed reports regarding its clinical benefit. Any increase in diuretic efficacy may be primarily in patients with chronic renal dysfunction and nephrotic syndrome or cirrhosis and the benefit may only be within the first 24 hours.18–20 In contrast, our results suggest that patients with lower albumin levels had higher net fluid loss and a trend towards increased diuretic efficiency. Potential explanations for this include: 1) serum albumin levels in heart failure patients are relatively higher in comparison to patients with cirrhosis or nephrotic syndrome whereby albumin-facilitated loop diuretic delivery to the kidney plays a minor role in the development of diuretic resistance in heart failure. 2) AHF patients with lower albumin have more peripheral edema, hence clinicians may continue decongestive therapies longer. Similar results have previously been reported. A retrospective analysis of 162 patients with AHF showed that hypoalbuminemia (albumin ≤ 3 g/dL) had no association with diuretic effectiveness in patients receiving continuous infusions of loop diuretics.21 Taken in aggregate, albumin levels in general AHF populations, without significant renal or hepatic abnormalities, may not significantly impact decongestive treatment.
Hypoalbuminemia in HF may result from inflammatory stress,22, 23 hepatic congestion and right heart failure,24 and malnutrition resulting in impaired protein synthesis.25 Although low baseline albumin levels were associated with higher NT-proBNP and a trend towards worse NYHA status, we found no association between baseline albumin levels and prognosis in this cohort.4, 8 This finding may be representative of selection bias given the inclusion criteria of DOSE-AHF and ROSE-AHF and, therefore, not generalizable to more advanced heart failure. As such, prior cohorts demonstrating the prognostic role of lower albumin should not be discredited.
This analysis must be interpreted within the context of several limitations inherent to its design. This is a post-hoc analysis of a composed cohort from two randomized controlled, double-blinded trials (DOSE-AHF and ROSE-AHF), which were not adequately powered to detect clinical endpoints according to baseline albumin levels. Yet, all short-term and post-discharge clinical endpoints were adjudicated within the confines of a clinical trial, supporting the validity of these findings. In contrast to prior analyses with highly heterogenous AHF cohorts, the present study represents carefully selected AHF populations with prospectively collected outcomes and, therefore, minimizes unintential biases and other factors that may have confounded the albumin-risk relationship. Along the same lines, these results may not be generalizable to a more severe AHF phenotype, those with hypotension requiring intravenous vasoactive or inotropic therapies or those with significant hepatic or renal dysfunction.
CONCLUSION
Patients with lower serum albumin levels on admission have evidence of peripheral congestion upon presentation. However, there was no association with albumin levels and in-hospital endpoints, long-term endpoints, symptomatic change, or change in cardiorenal biomarkers. In populations without severe hypoalbuminemia, the intention to achieve adequate diuresis may overcome the impact of lower albumin during acute therapy for decompensation. It is not known whether therapy directed specifically to improve nutrition would improve post-discharge outcomes.
Highlights.
Binding of albumin to diuretics are key to delivery to the nephron, and low albumin levels diminish intravascular oncotic pressures necessary to maintain intravascular volume for effective diuresis.
Based on prospectively collected data from two acute heart failure clinical trials (DOSE-AHF and ROSE-AHF), this may not be the case in acute heart failure populations largely free of nephrotic syndrome or cirrhosis.
Our data from two well characterized cohorts of patients with acute heart failure suggested serum albumin may not be a helpful tool to guide decongestion strategies or determine effectiveness of therapy.
Acknowledgments
Sources of funding: The DOSE-AHF and CARRESS-HF studies were funded by the National Heart, Lung, and Blood Institute, U.S. National Institutes of Health. All the authors have access to all the data in its entirety and approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–9. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 2.Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. European journal of heart failure. 2009;11:170–7. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–9. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Arques S, Roux E, Sbragia P, Gelisse R, Pieri B, Ambrosi P. Usefulness of serum albumin concentration for in-hospital risk stratification in frail, elderly patients with acute heart failure. Insights from a prospective, monocenter study. Int J Cardiol. 2008;125:265–7. doi: 10.1016/j.ijcard.2007.07.094. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circulation research. 1959;7:649–57. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- 6.Valdespino-Trejo A, Orea-Tejeda A, Castillo-Martinez L, Keirns-Davis C, Montanez-Orozco A, Ortiz-Suarez G, Delgado-Perez DA, Marquez-Zepeda B. Low albumin levels and high impedance ratio as risk factors for worsening kidney function during hospitalization of decompensated heart failure patients. Experimental and clinical cardiology. 2013;18:113–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke MM, Dorsch MP, Kim S, Aaronson KD, Koelling TM, Bleske BE. Baseline albumin is associated with worsening renal function in patients with acute decompensated heart failure receiving continuous infusion loop diuretics. Pharmacotherapy. 2013;33:583–8. doi: 10.1002/phar.1241. [DOI] [PubMed] [Google Scholar]
- 8.Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL., Jr Serum albumin and mortality in acutely decompensated heart failure. Am Heart J. 2010;160:1149–55. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM Network NHFCR. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM Network NHFCR. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. Jama. 2013;310:2533–43. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WH. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circulation Heart failure. 2014;7:261–70. doi: 10.1161/CIRCHEARTFAILURE.113.000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witte CL, Witte MH, Dumont AE, Cole WR, Smith JR. Protein content in lymph and edema fluid in congestive heart failure. Circulation. 1969;40:623–30. doi: 10.1161/01.cir.40.5.623. [DOI] [PubMed] [Google Scholar]
- 13.Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, Young JB, Califf RM, Nohria A. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circulation Heart failure. 2008;1:170–7. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breidthardt T, Irfan A, Klima T, Drexler B, Balmelli C, Arenja N, Socrates T, Ringger R, Heinisch C, Ziller R, Schifferli J, Meune C, Mueller C. Pathophysiology of lower extremity edema in acute heart failure revisited. The American journal of medicine. 2012;125:1124.e1–1124.e8. doi: 10.1016/j.amjmed.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Damman K, Testani JM. The kidney in heart failure: an update. European heart journal. 2015 doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torre-Amione G, Milo-Cotter O, Kaluski E, Perchenet L, Kobrin I, Frey A, Rund MM, Weatherley BD, Cotter G. Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. Journal of cardiac failure. 2009;15:639–44. doi: 10.1016/j.cardfail.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, Nagase S, Morino Y. Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int. 1987;32:198–203. doi: 10.1038/ki.1987.192. [DOI] [PubMed] [Google Scholar]
- 18.Kitsios GD, Mascari P, Ettunsi R, Gray AW. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: a meta-analysis. J Crit Care. 2014;29:253–9. doi: 10.1016/j.jcrc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Elwell RJ, Spencer AP, Eisele G. Combined furosemide and human albumin treatment for diuretic-resistant edema. Ann Pharmacother. 2003;37:695–700. doi: 10.1345/aph.1C320. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani N, Gorski JC, Horlander JC, Sr, Craven R, Hoen H, Maya J, Brater DC. Effects of albumin/furosemide mixtures on responses to furosemide in hypoalbuminemic patients. J Am Soc Nephrol. 2001;12:1010–6. doi: 10.1681/ASN.V1251010. [DOI] [PubMed] [Google Scholar]
- 21.Bleske BE, Clark MM, Wu AH, Dorsch MP. The effect of continuous infusion loop diuretics in patients with acute decompensated heart failure with hypoalbuminemia. J Cardiovasc Pharmacol Ther. 2013;18:334–7. doi: 10.1177/1074248412474347. [DOI] [PubMed] [Google Scholar]
- 22.Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–34. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 23.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–9. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 24.Carr JG, Stevenson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1989;63:709–13. doi: 10.1016/0002-9149(89)90256-7. [DOI] [PubMed] [Google Scholar]
- 25.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]