Abstract
Autoinflammatory diseases are hyperinflammatory, immune-dysregulatory conditions that typically present in early childhood with fever and rashes and disease-specific patterns of organ inflammation. This review provides a historic background of autoinflammatory disease research, an overview of the currently genetically defined autoinflammatory diseases, and insights into treatment strategies derived from understanding of the disease pathogenesis. The integrative assessment of autoinflammatory conditions led to the identification of innate pro-inflammatory cytokine “amplification loops” as the cause of the systemic and organ-specific disease manifestations, which initially centered around increased IL-1 production and signaling. More recently additional innate proinflammatory cytokine amplification loops resulting in increased Type I IFN, IL-17, IL-18 or IL-36 signaling or production have led to the successful use of targeted therapies in some of these conditions. Clinical findings such as fever patterns, type of skin lesions, genetic mutation testing, and the prevalent cytokine abnormalities can be used to group autoinflammatory diseases.
Introduction
History
The concept of “autoinflammatory diseases” was introduced by Dr. Daniel Kastner after the discoveries of the genetic causes of the most prevalent periodic fever syndrome worldwide, familial Mediterranean fever (FMF), in 1997, and of the TNF receptor associated periodic syndrome (TRAPS) in 1999 (Consortium, 1997a, Consortium, 1997b, McDermott et al., 1999). This concept aimed to distinguish autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) that were thought to be caused by adaptive immune dysregulation, from the two hereditary fever syndromes, FMF and TRAPS that lack features of adaptive immune dysregulation such as high-titer autoantibodies and auto-reactive lymphocytes in affected tissues.
In 2000/2001, the discovery that gain-of-function (GOF) mutations in the first recognized human intracellular sensor of microbial danger signals, NLRP3, causes the disease spectrum of cryopyrin associated autoinflammatory syndromes (CAPS), led to a paradigm shift in thinking about autoinflammatory disease (Hoffman et al., 2001). Upon activation, NLRP3 sensors nucleate an IL-1 activating platform, the inflammasome, a discovery that provided the molecular link between innate immune sensing and the production of the pro-inflammatory cytokine IL-1 (Broderick et al., 2015, Martinon et al., 2002) and led to the use of IL-1 inhibiting therapies that changed many patients’ lives. The periodic fever syndromes, the cryopyrinopathies (CAPS) and the deficiency of interleukin-1 receptor antagonist syndrome (DIRA) are “prototypic” autoinflammatory diseases that illustrate a key role of IL-1 in autoinflammation. Early proof of concept studies with the IL-1 blocking agent anakinra (Kineret®) in patients with cryopyrinopathies/CAPS showed impressive clinical responses, and led to the FDA approval of three IL-1 blocking agents for the treatment of CAPS (rilonacept (Arcalyst, canakinumab (Ilaris®), and anakinra (Kineret®) (Jesus and Goldbach-Mansky, 2014). More recently mutations in pathways that affect Type I IFN production, and IL-18 and IL-36 signaling have led to the recognition of other proinflammatory cytokine amplification loops in causing autoinflammatory disease phenotypes.
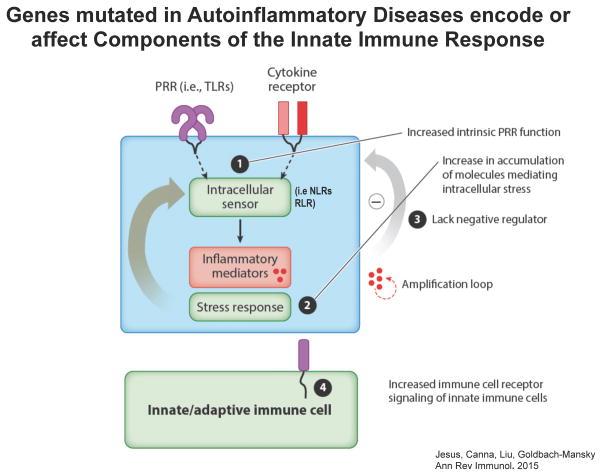
The genetic “mutations” leading to autoinflammatory phenotypes affect components of the innate immune responses as depicted in Figure 1 (de Jesus et al., 2015)
Figure 1. Principles of immune dysregulation in Autoinflammatory Diseases.
The innate immune system relies on danger recognition by germ line-encoded pattern recognition receptors (PRRs) such as membrane bound receptors (i.e. TLRs), and intracellular sensors (NLRs and RLRs). Genes that are mutated and lead to autoinflammatory phenotypes contribute to the innate immune dysregulation in specific ways as outlined below. (see accompanying text)
Gain-of function (GOF) mutations in genes encoding pattern recognition receptors (PRRs) referred to as “sensors” that recognize microbial or intracellular danger signals, or mutations in their adaptors lead to increased production of inflammatory mediators. GOF-mutations in the intracellular sensors (component 1, Figure 1): NLRP3, MEFV and NLRC4 that are linked to increased IL-1 production or in the viral RIG-Like receptors/sensors (RLR), IFIH1/MDA5 and DDX58/RIG-I, or the adaptor molecule, TMEM173/STING that are linked to increased Type I IFN production cause autoinflammatory diseases.
Loss-of-function (LOF) mutations in molecules that control cellular pathways result in cell maladaptation and stress (component 2, Figure 1) that cause autoinflammatory phenotypes. LOF mutations in enzymes or molecules that affect protein homeostasis (i.e. protein misfolding, endoplasmic reticulum transport, protein degradation and clearance); mitochondrial function and oxidative stress production; the cell migration; intracellular trafficking; autophagy; cell differentiation; and nucleotide metabolism/degradation cause autoinflammatory diseases.
LOF mutations resulting in loss of negative regulators of an immune response can also lead to autoinflammatory phenotypes (component 3, Figure 1).
Increased signaling through receptors that control innate immune cell function lead to hyper-responsiveness to immune signals (component 4, Figure 1). As the signaling abnormalities affect innate and adaptive immune cells, patients with these latter mutations often present with overlapping clinical features of autoinflammation, mild immunodeficiencies, and/or autoimmunity (McGonagle et al., 2009).
Clinical classification of autoinflammatory diseases
Although the genetic analyses provide the ultimate diagnosis of the monogenic diseases described below, it can take weeks to months to obtain the genetic results. However, a number of clinical characteristics distinguish the different autoinflammatory diseases and can be used to group them based on the fever pattern, skin rashes and the inflammatory organ manifestations and can provide early clues to the underlying cytokine dysregulation. Table 1 is an expansion of a previously proposed grouping now including additional novel diseases (Almeida de Jesus and Goldbach-Mansky, 2013).
Table 1.
Clinical Features of Mendelian Autoinflammatory Diseases
| Clinical and laboratory findings | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cytokine Mediator | Gene affected/Inheritance |
Systemic inflammation |
Mucocutaneous | Musculoskeletal | Gastrointestinal | Liver, spleen and lymph nodes |
Other/Specific features | |
| IL-1 mediated autoinflammatory diseases | ||||||||
|
| ||||||||
| IL-1β |
1. The classic hereditary “periodic fever syndromes”
|
|||||||
| FMF | MEFV/AR or AD | Fever, increased inflammatory markers | Erysipellas-like erythema (FMF and TRAPS); maculopapular or purpuric exanthema (HIDS); recurrent oral ulcers may occur (HIDS) | Large joints episodic arthritis (FMF); arthralgia, non-erosive acute polyarthritis (HIDS and TRAPS); migratory myalgia (TRAPS) | Abdominal pain, diarrhea, constipation (FMF, HIDS, TRAPS); acute peritonitis (FMF and TRAPS); vomiting (HIDS) | Hepatosplenomegaly and painful cervical adenopathy (HIDS) | Pleuritis, pericarditis, epididymitis (FMF and TRAPS); periorbital edema, conjunctivitis (TRAPS); recurrent or severe infections (HIDS) | |
| HIDS | MVK/AR | |||||||
| TRAPS | TNFRSF1A/AD | |||||||
|
| ||||||||
|
2. The neutrophilic urticaria of the cryopyrin associated periodic syndromes (CAPS)
| ||||||||
| FCAS | NLRP3/AD | Fever, increased inflammatory markers | Neutrophilic urticaria (NOMID, MWS, FCAS); recurrent oral ulcers (MWS) | Myalgia, arthralgia, oligoarticular arthritis (FCAS and MWS); epiphyseal and patella enlargement, periostitis, chronic arthropathy (NOMID) | Abdominal pain (NOMID and MWS) | Occasional lymphadenopathy (NOMID and MWS); hepatomegaly during disease exacerbations (NOMID) | Headache, conjunctivitis (FCAS, MWS, NOMID); sensorineural hearing loss, episcleritis, optic disk edema (MWS and NOMID); delayed mental development, chronic aseptic meningitis, progressive amurosis (NOMID) | |
| MWS | ||||||||
| NOMID/CINCA | ||||||||
|
| ||||||||
|
3a. Pustular skin dermatoses, IL-1-mediated
| ||||||||
| DIRA | IL1RN/AR | Occasional fever, increased inflammatory markers | Pustular dermatitis | Recurrent multifocal aseptic osteomyelitis | Uncommon | Uncommon | Deformity of clavicles and ribs, absence of odontoid process, venous thrombosis, CNS vasculitis (DIRA); dyserythropoietic anemia (Majeed) | |
| Majeed | LPIN2/AR | |||||||
|
| ||||||||
|
3b. Pustular skin dermatoses, partially IL-1 mediated
| ||||||||
| PAPA | PSTPIP1/AD | Occasional fever, increased inflammatory markers | Pyoderma gangrenosum, severe acne | Deforming aseptic pyogenic arthritis | Uncommon | Uncommon | Aseptic pyogenic arthritis responsive to IL-1 inhibition; skin disease partially responsive to TNF-α inhibition | |
| HA20 | TNFAIP3/AD | Fever, increased inflammatory markers | Folliculitis, pathergy, frequent episodes of oral and genital ulcers | Non-deforming polyarthritis | Colitis | Not reported | Anterior uveitis, retinal vasculitis, CNS vasculitis, | |
|
| ||||||||
|
3c. Pustular skin dermatoses, non- IL-1 mediated
|
||||||||
| IL-36 | DITRA | IL36RN/AR | Fever, increased inflammatory markers | Generalized pustular psoriasis; oral mucosa pustulas may occur | Uncommon | Uncommon | Uncommon | Fever of elevated temperature, secondary skin infections |
| IL-17/IL23 | CAMPS | CARD14/AD | Fever with skin secondary infections | Plaque or pustular psoriasis | Arthritis | Uncommon | Uncommon | Rare systemic manifestations |
| Lack of IL-10 | EO-IBD | IL10RA/AR | Fever, increased inflammatory markers | Folliculitis; oral aphtous lesions | Arthritis | Severe colitis with bloody diarrhea, abscesses, perianal fistula | Occasional lymphadenopathy | Recurrent infections |
| IL10RB/AR | ||||||||
| IL10/AR | ||||||||
|
| ||||||||
|
IL-18/IL-1 mediated Diseases
| ||||||||
|
4. Diseases with the propensity to develop macrophage activation syndrome
|
||||||||
| IL-18/IL-1/(late in disease IFNγ) | NLRC4-MAS | NLRC4/AD | Fever, increased inflammatory markers | Rare dermographism, urticarial rash | Arthralgia | Early-onset non-specific enterocolitis with variable severity | Hepatosplenomegaly and occasional lymphadenopathy | Coagulopathy, pancytopenia, hyperferritinemia, hypertriglyceridemia |
|
| ||||||||
| Type-I IFN mediated autoinflammatory diseases | ||||||||
|
5a. Vasculopathy and panniculitis
|
||||||||
| Type I interferon | CANDLE/PRAAS | PSMB8, PSMA3, PSMB4, PSMB9/AR or digenic, POMP/AD | Fever, increased inflammatory markers | Nodular exanthema, panniculitis, lipodystrophy | Myositis, arthralgia, arthritis, joint contractures | Increased intra-abdominal fat | Hepatosplenomegaly | Basal ganglion calcifications, dyslipidemia, pancreatic abnormalities, microcytic anemia, cytopenias, eyelids edema and erythema |
|
|
||||||||
|
5b. Vasculitis/vasculopathy and/or livedo reticularis
|
||||||||
| SAVI | TMEM173/AD | Fever, increased inflammatory markers | Erythemato-purpuric lesions, ishemic ulcerative skin disease, necrosis of extremities, loss of tissue. Recurrent oral ulcers may occur | Arthralgia, myositis | Uncommon | Occasional lymphadenopathy | Interstitial lung disease, lung fibrosis, emphysema, paratracheal adenopathy; basal ganglia calcifications (rare); typical involvement of cheeks, ear lobes and tip of nose; flares triggered by cold; nasal septum, perforation; anemia; lymphopenia; hypergammaglobulinemia | |
| TREX1-associated AGS (AGS1) | TREX1/AR or AD | Occasional fever | Chilblain lesions, livedo reticularis | Uncommon | Uncommon | Hepatosplenomegaly (rare) | Basal ganglia calfications, variable degrees of white matter abnormalities and cerebral atrophy. | |
| TREX1-associated Familial Chilblain Lupus (FCL), or TREX1-associated SLE | TREX1/AD | Occasional fever, increased inflammatory markers | Chilblain lesions, livedo reticularis, malar rash, photosensitivity, oral and nasal ulcers, livedo reticularis | Arthralgia, acute and chronic (rare) arthritis | Uncommon | Uncommon (FCL), or hepatosplenomegaly and lymphadenopathy | Cold-induced, bluish-red lesions on the hands, feet and ears that may ulcerate and result in tissue loss (FCL). About 0.5% of SLE patients have TREX1 mutations and fulfill ACR classification criteria for SLE; their clinical manifestations do not differ from non-monogenic SLE. | |
| TREX1-mediated retinal vasculopathy with cerebral leukodystrophy (RVCL) | TREX1/AD (caused by specific C-terminal frameshift mutations) | Not reported | Not reported | Not reported | Not reported | Not reported | Adult-onset; retinal vasculopathy leading to blindness in middle age; cerebral white matter and mass lesions. | |
| AGS2, AGS3, AGS4 | RNASEH2B, RNASEH2C, RNASEH2A/AR | Occasional fever | Chilblain lesions, livedo reticularis | Uncommon | Uncommon | Hepatosplenomegaly (rare, in AGS3 and AGS4) | AGS3 and AGS4 have an earlier disease onset and higher mortality than AGS2 | |
| SAMHD1-associated AGS (AGS5) | SAMHD1/AR | Occasional fever | Chilblain lesions, livedo reticularis, peripheral calcinosis (rare), partial lipodystrophy | Arthritis, joint contractures | Uncommon | Uncommon | Cerebral vasculopathy, early-onset stroke, basal ganglion calcifications; glaucoma, cataract, cortical blindness | |
| ADAR-associated AGS (AGS6) | ADAR/AR or AD | Occasional fever | Chilblain lesions, livedo reticularis | Uncommon | Uncommon | Hepatosplenomegaly (rare) | Later onset, lower morbidity and mortality, variable CNS manifestations | |
| IFIH1-assocated AGS (AGS7) | IFIH1/AD | Occasional fever | Chilblain lesions, livedo reticularis | Arthritis (rare) | Uncommon | Hepatosplenomegaly (rare) | Variable severity of CNS disease; thrombocytopenia; asymptomatic carriers reported | |
| IFIH1 (MDA5)- associated Singleton- Merten disease | IFIH1/AD | Not reported | Delayed primary tooth exfoliation and permanent tooth eruption, truncated tooth root formation, early-onset periodontal disease, severe root and alveolar bone resorption associated with dysregulated mineralization, and tooth loss; psoriasiform skin lesions (only in IFIH-SMS) Not reported in DDX58-SMS. | Arthritis, joint contractures, calcific tendinitis, erosive changes in the terminal tufts of the distal phalanges; hypotonia; osteoporosis | Not reported | Not reported | Aortic and valvular calcification; glaucoma | |
| DDX58 (RIG-I)-associated Singleton-Merten disease | DDX58/AD | |||||||
| SPENCDI | ACP5/AR | Uncommon | Uncommon | Radiolucent and irregular spondylar and metaphyseal lesions representing islands of chondroid tissue within bone | Colitis | Uncommon | Sinopulmonary infections, opportunistic viral infections, ITP, thyroiditis, spasticity, developmental delay, late-onset cerebral calcifications | |
| IFN +? | DADA2 | CECR1/AR | Fever, increased inflammatory markers | Livedo reticularis, purpuric lesions and ischemic an necrotic skin disease | Myalgia, arthralgia | Abdominal pain, diarrhea, ascites (rare) | Hepatosplenomegaly and occasional lymphadenopathy | Ischemic or hemorrhagic stroke, testicular pain, portal hypertension, lymphopenia, low IgM, recurrent infections, amaurosis |
| Other | ||||||||
|
| ||||||||
|
6. Granulomatous skin diseases
|
||||||||
| IL-1+ other | PGA/Blau | NOD2 (CARD15)/AD | Rare fever, increased inflammatory markers | Ichthyosis-like exanthema | Polyarthritis, hypertrophic tenosynovitis | Uncommon | Hepatospleneomegaly and lymphadenopathy may be observed | Chronic uveitis, cataract, glaucoma, amaurosis; interstitial lung disease (rare); transient neuropathy; parotitis; pericarditis; arterial hypertension |
| IL-1+ other | PLAID | PLCG2/AD | Variable, fever due to infections | Cold-induced urticaria and/or granulomatous skin rash | Uncommon | Uncommon | Uncommon | Positive autoantibodies and autoimmune manifestations, recurrent and/or severe infections, allergic disease |
| APLAID | Erythematous plaques and vesicopustular lesions, cellulitis | Arthralgia | Abdominal pain and bloody diarrhea | Uncommon | Interstitial lung disease; corneal erosions, ulcerations, intraocular hypertension, cataracts; mild immunodeficiency | |||
|
|
||||||||
|
7. Miscellaneous
|
||||||||
| ? | Cherubism | SH3BP2/AD | No fever reported | Dental impact includes disruption of primary or secondary dentition, absent teeth, rudimentary development of teeth, abnormally shaped teeth, delayed or ectopically erupting teeth. | Multilocular radioluscent expansive lesions of mandible and maxila. Involvement of zygomatic arches, condyles and ribs are rarely observed. | Not reported | Enlarged submandibular and cervical lymph nodes | Fibro-osseous tissue extension into the orbital walls with displacement of the globe and retraction of eyelids and invasion of retrobulbar spaces of the orbits causing displacement of optic nerves and proptosis may occur in more severe forms. Upper airway obstruction due to tongue displacement or obliteration of the nasal airway may rarely occur. |
| ? | Monogenic sJIA | LACC1/AR | Characteristic quotidian fever and increased inflammatory markers | Erythematous maculopapular rash | Chronic polyarthritis | Not reported | Hepatosplenomegaly and lymphadenopathy was observed in the minority of the patients. | Patients reported fulfilled classification criteria for systemic JIA |
| ? | SIFD | TRNT1/AR | Recurrent fever and increased inflammatory markers | Icthyotic skin lesions in one patient | Not reported | Colitis | Splenomegaly | Congenital sideroblastic anemia, developmental delay, cardiomyopathy, B-cell immunodeficiency, sensorineural hearing loss, seizures, ataxia, cerebral atrophy, retinitis pigmentosa, aminoaciduria |
Abbreviations used: FMF- familial Mediterranean fever; HIDS- hyperimmunoglobulinemia D with periodic fever syndrome; TRAPS- TNF receptor-associated periodic syndrome; FCAS- familial cold autoinflammatory syndrome; MWS- Muckle-Wells syndrome; NOMID- neonatal-onset mutlisystem inflammatory disease; CINCA- chronic infantile neurological cutaneous and articular syndrome; DIRA- deficiency of interleukin-1 receptor antagonist; PAPA- pyogenic arthritis, pyoderma gangrenosum and acne syndrome; HA20- haploinsufficiency of A20; DITRA- deficiency of IL-36 receptor antagonist; CAMPS- CARD14-mediated psoriasis; EO-IBD- early-onset inflammatory bowel disease; CANDLE- chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; PRAAS- proteasome-associated autoinflammatory syndrome; SAVI- STING-associated vasculopathy with onset in infancy; AGS- Aicardi-Goutières syndrome; SLE- systemic lupus erythematosus; SPENCDI- spondyloenchondrodysplasia with immune dysregulation; DADA2- deficiency of adenosine deaminase 2; MAS- macrophage activation syndrome; PGA- pediatric granulomatous arthritis; PLAID- PLCG2-associated antibody deficiency and immune dysregulation; APLAID- PLCG2-associated autoinflammation, antibody deficiency and immune dysregulation; sJIA- systemic juvenile idiopathic arthritis; SIFD- Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and developmental delay; AR- autosomal recessive; AD- autosomal dominant; CNS- central nervous system; ACR- American College of Rheumatology; ITP- immune thrombocytopenic purpura; AIHA- autoimmune hemolytic anemia.
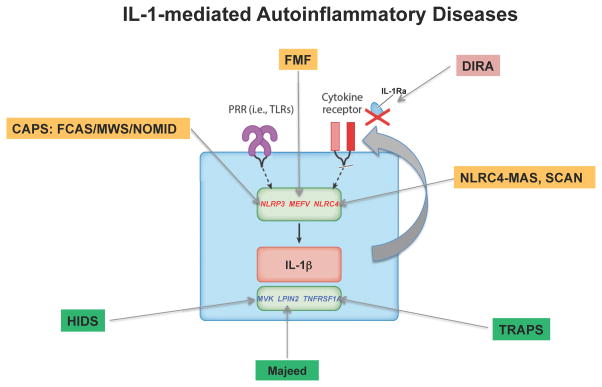
IL-1 mediated diseases (Figure 2A)
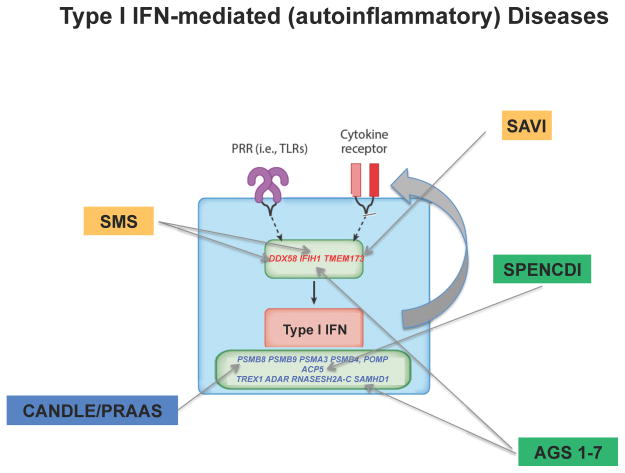
Figure 2.
Figure 2A and B. IL-1 mediated (2A) and IFN Type I (2B) mediated autoinflammatory diseases and their genetic causes. A and B. IL-1 or Type I IFN mediated diseases caused by GOF mutations in sensors are highlighted in yellow and LOF mutations in cellular/metabolic pathways that result in stress are highlighted in green. The diseases caused by LOF mutation in a negative regulator are highlighted in red.
Abbreviations used: IL-1 mediated diseases: CAPS- cryopyrin associated periodic syndrome (FCAS- familial cold autoinflammatory syndrome, MWS- Muckle-Wells syndrome, NOMID- neonatal-onset multisystem inflammatory disease); FMF- familial Mediterranean fever; DIRA- deficiency of interleukin-1 receptor antagonist; NLRC4-MAS (macrophage activation syndrome) (also IL-18 mediated) or SCAN- syndrome of enterocolitis and autoinflammation associated with mutation in NLRC4; HIDS- hyperimmunoglobulinemia D and periodic fever syndrome; TRAPS-,TNF receptor associated periodic syndrome
Type I IFN mediated: SMS- Singleton-Merten syndrome; SAVI- STING-associated vasculopathy with onset in infancy; CANDLE- chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature/PRAAS- proteasome-associated autoinflammatory syndrome; AGS- Aicardi-Goutières syndrome 1–7; SPENCDI- spondyloenchondrodysplasia with immune dysregulation
1. The classic “periodic fever syndromes” and 2. The cryopyrin associated periodic syndromes
Among the periodic fever syndromes, Familial Mediterranean fever (FMF) and Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) present with recurrent fever attacks of short duration of 3–4 days in FMF, and 3–7 days in HIDS, that recur every 4 to 6 weeks (Simon and van der Meer, 2007, Drenth et al., 1994). The fever attacks in TNF receptor associated periodic syndrome (TRAPS) are longer, lasting a week up to several weeks. FMF is the most prevalent monogenic autoinflammatory disease with more than 100,000 affected persons worldwide. It is caused by autosomal recessive, mostly missense mutations in the MEFV gene which encodes an IL-1 forming inflammasome (Consortium, 1997a, Consortium, 1997b) however autosomal dominant forms of FMF are also recognized. The prevalence of FMF is high in eastern Mediterranean populations including Sephardic Jews, Armenian, Turkish and Arabian descendants (Onen, 2006, Chae et al., 2009), hence the name “Mediterranean Fever”. HIDS is caused by recessive loss-of-function (LOF) mutations in MVK gene (Houten et al., 1999) and TRAPS by LOF mutations in the TNFRSF1A gene, which encodes the p55 TNF receptor (McDermott et al., 1999).
The 3 cryopyrinopathies or cryopyrin-associated periodic syndromes (CAPS): Familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and Neonatal-onset multisystem inflammatory disease (NOMID) also called chronic infantile, neurological, cutaneous and articular (CINCA) syndrome (Aksentijevich et al., 2002) comprise a clinical disease continuum and were the first disease group to be treated with IL-1 blocking therapies. CAPS is caused by autosomal dominant gain-of-function (GOF) mutations in NLRP3/CIAS1 (Hoffman et al., 2001). While the milder diseases, FCAS and MWS are mostly familial, most severe cases of NOMID are sporadic. Of patients with “clinical NOMID”, over 70% of patients who are “mutation-negative” by Sanger sequencing have somatic mosaicism (Tanaka et al., 2011, Saito et al., 2005).
Most patients develop disease in childhood but adult onset is seen in FMF and TRAPS. Disease flares present with fever, and elevation of acute phase reactants that correlate with disease activity. In FMF the disease flares are associated with sterile peritonitis presenting as acute abdominal pain and with large joint arthritis. More variable symptoms include myalgia and other forms of serositis including pleuritis and pericarditis. The most common cutaneous manifestation in FMF is erysipelas-like erythema, which is only present in 7 to 40% of patients in various cohorts; the rash is frequently misdiagnosed as cellulitis (Fonnesu et al., 2009). In contrast HIDS patients present with tender, cervical lymphadenopathy (Bader-Meunier et al., 2011), abdominal pain and diarrhea, vomiting, polyarthralgia and non-erosive large joint arthritis. Skin lesions including papular, urticarial, nodular or purpuric rashes (Bader-Meunier et al., 2011) while serositis, myalgia, and oral and genital ulcers are rarely observed. HIDS flares can be triggered by immunizations, trauma, surgery or stress and are characterized by high-grade fever with chills (Drenth et al., 1994). In most instances, HIDS has a benign evolution (van der Hilst et al., 2008). TRAPS fever attacks present with abdominal pain that similar to FMF can mimic an acute abdomen, and migratory pain that is associated with an overlying tender erythematous macular, edematous or urticarial skin rash that is caused by an underlying monocytic fasciitis (Hull et al., 2002b). Half of TRAPS patients have ocular manifestations (periorbital edema, recurrent conjunctivitis or anterior uveitis). Arthritis and pleuritis are seen in 30–50% of the patients. Neurological manifestations are rare.
The classic presentation in the CAPS spectrum includes fever and neutrophilic urticaria, conjunctivitis, arthralgia, and a marked increase of acute phase reactants during disease activity. The inflammatory attacks in FCAS are cold induced and subside within several hours, while in MWS and NOMID, low-grade inflammation and fever are continuous in between disease exacerbations (Hoffman et al., 2004). During disease flares, patients may complain of headaches, MWS patients develop sensorineural hearing loss in the 2–3rd decade and NOMID patients in the 1st decade of their lives. Severe neurological involvement is a diagnostic feature of NOMID and includes chronic aseptic neutrophilic meningitis with increased intracranial pressures (Neven et al., 2008, Goldbach-Mansky et al., 2006). Untreated, organ damage develops starting in childhood (Sibley et al., 2012) including sensorineural hearing loss (Hawkins et al., 2004), hydrocephalus, brain atrophy, and optic nerve atrophy with progressive vision loss (Goldbach-Mansky et al., 2006). Many NOMID patients present with a deforming arthropathy.
Abnormal laboratory findings of the IL-1 mediated autoinflammatory diseases include marked leukocytosis with neutrophilia and thrombocytosis, and increased acute phase reactants, (erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)). Chronic anemia develops in patients with persistent inflammation, and secondary amyloidosis occurs in up to 13% of Turkish patients with FMF (Tunca et al., 2005), in up to 64% of TRAPS patients (McDermott et al., 1999) with mutations in cysteine residues (Hull et al., 2002a), and in 25 to 33% of untreated MWS patients living in Europe (Aganna et al., 2002) who are not appropriately treated. It is rarely observed in HIDS.
IL-1 inhibition is standard treatment for patients with CAPS (Jesus and Goldbach-Mansky, 2014, Levy et al., 2015) and needs to be initiated early to prevent the development and progression of organ damage (Sibley et al., 2012). Colchicine remains the treatment of choice for FMF, but is ineffective in HIDS and TRAPS. However, the empiric use of IL-1 blocking agents in the treatment of the 3 classic periodic fever syndromes (FMF, HIDS, TRAPS) (Jacobelli et al., 2007), and a subset of conditions presenting with pustular dermatosis and aseptic osteomyelitis (DIRA and Majeed) see below, confirmed an important role of IL-1 in these diseases. Patients typically experience dramatic improvement of inflammatory symptoms, fever, skin rash, and acute phase reactants (Goldbach-Mansky et al., 2006), however some differences in CNS penetration of the drugs exist (Sibley et al., 2014).
3. Syndromes presenting with pustular skin rashes and episodic or continuous fever episodes
These conditions comprise a pathogenically heterogeneous group.
3a. IL-1 mediated
Deficiency of interleukin 1 receptor antagonist (DIRA) is caused by autosomal recessive LOF mutations in the IL1RN gene, which encodes the IL-1 receptor antagonist (IL-1Ra) (Aksentijevich et al., 2009, Reddy et al., 2009) and Majeed syndrome by autosomal recessive LOF mutations in LPIN2 gene, which encodes a phosphatase that catalyzes the conversion of phosphatidic acid to diacylglycerol in the endoplasmic reticulum membrane (Ferguson et al., 2005). DIRA patients present with pustular dermatitis in isolated crops or generalized with multifocal periosteitis and osteomyelitis resulting in vertebral block formation, and marked elevations of acute phase reactants. Other features include onychomadesis, widened ribs and clavicles (Aksentijevich et al., 2009, Reddy et al., 2009). A potential complication includes atlantoaxial subluxation due to odontoid destruction. Majeed syndrome presents with neonatal-onset recurrent osteitis and multifocal osteomyelitis, and congenital dyserythropoietic anemia. Skin involvement is more variable (El-Shanti and Ferguson, 2007). Both conditions rapidly and completely resolve on IL-1 inhibition (Aksentijevich et al., 2009, Reddy et al., 2009),(Herlin et al., 2012).
3b. Partially IL-1 mediated
Pyogenic sterile arthritis, pyoderma gangrenosum and acne (PAPA) syndrome is caused by autosomal dominant mutations in PSTPIP1 gene (Wise et al., 2002) and presents with painful cutaneous ulcers (pyoderma gangrenosum) and a sterile pyogenic arthritis. Cystic acne and pathergic pustules or skin abscesses can develop at trauma or needle injection site (Wise et al., 2002). Fever is rarely present. HA20 is caused by autosomal dominant LOF mutations in TNFAIP3, which causes haploinsufficiency in the NF-κB regulatory protein A20 leading to prolonged signaling after TNF stimulation. Patients present with oral and genital ulcers between infancy and adolescence. Other disease manifestations include folliculitis, pathergy, polyarthritis, anterior uveitis, fever, colitis, circulating autoantibodies, such as ANA, lupus anticoagulant, anti-dsDNA and anti-RNP are seen.
Laboratory findings include leukocytosis and increased ESR and CRP during flares (Wise et al., 2002). Treatment of PAPA and HA20 is challenging and optimal therapies have not been developed (Demidowich et al., 2012). Anti-TNF antibodies (infliximab and adalimumab) are often necessary to treat skin manifestations of PAPA syndrome (Demidowich et al., 2012). The joint manifestations respond to IL-1 inhibition. Treatment of HA20 includes colchicine, anti-TNF agents and anti-IL-1 therapy in a patient refractory to anti-TNF (Zhou et al., 2015).
3c. Not IL-1 mediated
Deficiency of interleukin-36 receptor antagonist (DITRA) is caused by homozygous LOF mutations in IL36RN, encoding the IL-36 receptor antagonist (Marrakchi et al., 2011). Most patients develop a generalized erythematous and pustular skin rash, associated with fever up to 40–42°C during childhood that are often triggered by viral or bacterial infections (Marrakchi et al., 2011). Increased CRP and white blood cell count are seen (Marrakchi et al., 2011) and secondary skin infections and sepsis are complications (Marrakchi et al., 2011). The mutation suggests a causative role of IL-36 in the skin disease but drugs targeting IL-36 signaling do not exist. Acitretin, oral steroids, methotrexate, cyclosporin, and TNF inhibitors have been reported with partial responses (Marrakchi et al., 2011).
CARD14 mediated psoriasis (CAMPS) is caused by autosomal dominant GOF mutations in CARD14 gene causing a monogenic form of plaque and pustular psoriasis. It is also the cause of familial pityriasis rubra pilaris, once thought to be an early-onset “psoriasis mimic” with resistance to many psoriasis treatments (Jordan et al., 2012b, Jordan et al., 2012a, Fuchs-Telem et al., 2012). Patients present with typical plaque psoriasis with variable severities and with generalized pustular psoriasis. Fever and other systemic manifestations are generally not present but can occur with superinfections (Jordan et al., 2012a, Jordan et al., 2012b). Treatment of CAMPS includes drugs used for the treatment of moderate-to-severe psoriasis, such as, methotrexate, cyclosporine and anti-TNF agents, and biologics targeting IL17 and IL23.
Early onset inflammatory bowel disease (EO-IBD) is caused by autosomal recessive LOF mutations in IL-10R and IL-10 encoding genes (Glocker et al., 2009). Patients present before 3 months of age with severe enterocolitis (bloody diarrhea, colonic abscesses, perianal fistula and oral ulcers) associated with recurrent fever and failure to thrive (Kotlarz et al., 2012). Musculoskeletal manifestations include acute recurrent arthritis of large joints(Kotlarz et al., 2012) and recurrent folliculitis (Kotlarz et al., 2012). Recurrent infections may indicate a defect in the immune responses in patients with EO-IBD (Glocker et al., 2009, Kotlarz et al., 2012). EO-IBD is refractory to standard immunosuppressants, and hematopoietic stem cell transplantation (HSCT) has resulted in complete clinical remission in most transplanted patients (Kotlarz et al., 2012, Engelhardt et al., 2012).
IL-18/IL-1 mediated Diseases
4. Diseases with the propensity to develop macrophage activation syndrome (NRLC4-MAS)
NLRC4-MAS is caused by activating heterozygous GOF mutations in the innate immune sensor NLRC4, which assembles a caspase-1 activating inflammasome (Canna et al., 2014),(Romberg et al., 2014). Patients have variable, early-onset enterocolitis followed by recurrent febrile episodes. Macrophage activation syndrome (MAS) attacks can be triggered by infections or physical stress. MAS presentations include pancytopenia, hepatitis, splenomegaly, and hyperferritinemia. In contrast to the CAPS patients with NLRP3 mutations, extraordinary elevation of serum IL-18 levels (10- to 100-fold higher than in CAPS) are detected even during clinical quiescence, a finding also seen in sJIA/Still’s disease patients at risk for MAS (Ichida et al., 2014, Shimizu et al., 2015). The role of IL-18 in the development of MAS and as a therapeutic target is being evaluated. The fever flares are responsive to corticosteroids and IL-1 inhibition.
Type-I IFN mediated autoinflammatory diseases (Figure 2B)
5a. Vasculopathy and Panniculitis/lipoatrophy
Proteasome associated autoinflammatory syndromes (PRAAS) or Chronic Atypical Neutrophilic Dermatitis with Lipodystrophy and Elevated temperatures (CANDLE) also called Nakajo-Nishimura syndrome is caused by recessive or digenic mutations in proteasome subunits, PSMB8 (Agarwal et al., 2010),(Arima et al., 2011),(Kitamura et al., 2011),(Liu et al., 2012) PSMB9, PSMA3, PSMB4 and the proteasome assembly molecule, POMP (Brehm et al., 2015). Patients present with early-onset recurrent fever, violaceous cutaneous rashes, periorbital edema and erythema, lipodystrophy, arthritis or arthralgia, myositis and with increased acute phase reactants (Torrelo et al., 2010). Marked finger swelling, in early infancy with conjunctivitis or episcleritis, lymphocytic aseptic meningitis, basal ganglia calcifications, lymphadenopathy, hepatomegaly, prominent abdomen and low weight and height are additional features (Liu et al., 2012). Laboratory abnormalities include thrombocytosis, neutropenia, lymphopenia, thrombocytopenia and hypertriglyceridemia with flares (Liu et al., 2012). Long-term organ damage from untreated patients who survive into adulthood includes the development of muscle atrophy, cardiac arrhythmias and dilated cardiomyopathy (Arima et al., 2011, Kitamura et al., 2011). Partial responses to high doses of steroids (Liu et al., 2012), NSAIDs, colchicine, dapsone, methotrexate, tacrolimus and azathioprine and biologics including anti-TNF, anti–IL-1 and anti-IL-6 agents are seen (Liu et al., 2012) but lipodystrophy progresses despite therapies(Liu et al., 2012). Laboratory data suggesting increased IFN signaling led to the development of a compassionate use protocol with the JAK1/JAK2 inhibitor baricitinib (clinicaltrials.gov/NCT01683409).
5b. Vasculitis and/or livedo retiularis
A number of syndromes presenting with vasculopathies, vasculitis and severe livido reticularis are caused by dysregulation of viral sensing pathways and are linked to Type-I IFN production marked by the presence of a strong IFN response gene signature (IRS) in the blood. STING-associated vasculopathy with onset in infancy (SAVI) is an autoinflammatory disease caused by de-novo GOF mutations in adaptor protein in the cytosolic DNA-sensing pathway, TMEM173, which encodes the stimulator of interferon (IFN) genes (STING) (Liu et al., 2014) and pointed to the important role of IFN in these diseases. Patients with SAVI present with early-onset vasculitis that affects small dermal vessels in cold sensitive acral areas including fingers, toes, ears, kneecaps that lead to vasoocclusion and gangrene. Most patients develop progressive interstitial lung disease (ILD) with variable severity (Liu et al., 2014),(Jeremiah et al., 2014). Elevated autoantibody levels are variably seen in patients; however, titers do not correlate with the presence or severity of the disease. SAVI patients present with a strong IRS in whole-blood suggesting a critical role of chronic IFN stimulation in the disease pathogenesis (Liu et al., 2014),(Jeremiah et al., 2014). Aicardi-Goutières Syndromes (AGS) 1–7 are a rare group of diseases caused by autosomal recessive LOF mutations in genes encoding enzymes in the nucleotide metabolism, the exonuclease TREX1, the ribonucleases RNASEH2A, RNASEH2B, and RNASEH2C; a nuclease, SAMHD1, and the dsRNA-specific adenosine deaminase ADAR1. More recently, autosomal dominant GOF mutations in the viral sensors, IFIH1, encoding MDA5, and DDX58 encoding RIG-I, have been found to cause AGS (Crow and Manel, 2015). Patients who develop disease in early infancy present with subacute encephalomyelitis mimicking viral infections that rapidly lead to demyelination, spastic paraplegia and neurological decline which dominate the clinical picture. Most patients follow a non-progressive, chronic course after the initial acute phase. On MRI patients have basal ganglion calcifications and severe white matter disease, up to 40% continue to have vascular rashes, including “chilblain lesions” on hands and feet. Many patients have low-titer autoantibodies (Crow and Manel, 2015). When the disease manifests later in life or presents as autosomal dominant form, the devastating CNS manifestations do not develop and the clinical phenotype is more variable with prominent vascular manifestations (see Table 1). Later-onset conditions include a syndrome referred to in the literature as Singleton-Merten Syndrome (SMS) characterized by abnormalities of blood vessels, teeth, and bone that is caused by autosomal dominant mutations in IFIH1 or DDX58. Calcifications of the aorta, and the aortic and mitral valves, glaucoma and acro-osteolysis are typical features. Delayed primary tooth exfoliation and permanent tooth eruption, truncated tooth root formation, early-onset periodontal disease, severe root and alveolar bone resorption associated with dysregulated mineralization leading to tooth loss are frequently observed in IFIH1-associated SMS (Rutsch et al., 2015, Jang et al., 2015). AGS-causing mutations affect enzymes that regulate nucleotide metabolism and cause accumulation of immunogenic nucleic acids. It is hypothesized that these self nucleic acids trigger chronic Type I IFN production through the viral DNA and RNA sensors. Strategies that are based on the understanding of the disease mechanism are currently being explored as treatment options and include reduction in transcription of retroviral sequences inhibition of chronic Type I IFN signaling (Crow and Manel, 2015). SPENCDI, Spondyloenchondrodysplasia with immune dysregulation is a syndrome of bone dysplasia; central nervous system involvement (cerebral calcifications); and immune dysregulation that is caused by LOF mutations in tartrate-resistant phosphatase (TRAP; encoded by ACP5) (Lausch et al., 2011, Briggs et al., 2011). Patients present with a prominent IRS features of immunodeficiency, including upper respiratory and pulmonary infections and interstitial fibrosis, fulminant hemorrhagic chickenpox; and autoimmunity including idiopathic thrombocytopenic purpura (ITP), thyroid disease (Briggs et al., 2011). DADA2, Deficiency of adenosine deaminase 2 is caused by autosomal recessive mutations in CECR1, encoding the enzyme adenosine deaminase 2 (ADA2), cause an early-onset vasculopathy resembling polyarteritis nodosa (Zhou et al., 2014, Navon Elkan et al., 2014). Patients can present with early-onset stroke, livedo reticularis, recurrent fever, hepatosplenomegaly, arterial hypertension, ophthalmologic manifestations and myalgia. CECR1 is essential for vascular integrity and neutrophil development consistent with observations that DADA2 patients have a defect in small vessel endothelial integrity and impaired of M2-like macrophage differentiation and polarization towards inflammatory M1-like macrophages and monocytes (Navon Elkan et al., 2014, Zhou et al., 2014). Therapeutic interventions include anti-TNF agents, fresh-frozen plasma and hematopoietic stem cell transplantation (HSCT) (Van Eyck et al., 2015, Zhou et al., 2014, Navon Elkan et al., 2014).
6. Syndromes presenting with granulomatous skin lesions and minimal or low-grade fever attacks also represent a heterogeneous group of diseases
Blau syndrome/early-onset sarcoidosis (pediatric granulomatous arthritis, PGA) is caused by autosomal dominant GOF mutations in NOD2/CARD15 (Alonso et al., 2003). PGA can be inherited in an autosomal dominant pattern (Blau syndrome) or occur sporadically (referred to as early-onset Sarcoidosis) (Rose et al., 2009, Rose et al., 2011). PGA patients present with granulomatous inflammation of eyes, joints and skin leading to the classical triad of chronic uveitis, arthritis and dermatitis. Uveitis is often the most recalcitrant disease manifestation, presenting as panuveitis (Arostegui et al., 2007). Cataract, glaucoma and irreversible blindness are frequent complications of untreated disease. Most patients with PGA develop an ichthyosis-like exanthema (Rose et al., 2009, Rose et al., 2011). Laboratory exams demonstrate persistent leukocytosis, thrombocytosis and increased ESR and CRP. Synovial, skin and liver biopsies may show non-caseating granulomata(Arostegui et al., 2007). Optimal therapy is not well defined. Systemic corticosteroids are used for severe disease biologics targeting TNF and IL-1 are beneficial especially in patients with refractory uveitis (Rose et al., 2011). PLAID and APLAID are caused by autosomal dominant mutations in different positions in PLCG2. PLAID is clinically characterized by a cold-induced urticarial and/or a granulomatous skin rashes atopic manifestations, positive autoantibodies, and recurrent opportunistic infections while APLAID presents with recurrent erythematous plaques and vesicopustular skin lesions, granulomas on skin biopsy, arthralgia, uveitis, and recurrent sinopulmonary infections. Treatment of PLAID includes cold avoidance, anti-histamines and IVIG. APLAID patients partially respond to anakinra and high-dose corticosteroids (Ombrello et al., 2012),(Zhou et al., 2012).
7. Miscellaneous
Cherubism is caused by autosomal dominant mutations in SH3BP2 and approximately 50% of the cases occur de novo. SH3BP2 is a cytoplasmic adaptor protein that interacts with TNFAIP3/A20 and with protein tyrosine kinases such as ABL1 and SYK that regulate transcriptional activity in immune cells. Patients develop symmetrical multilocular and radioluscent lesions in the mandible and the maxilla that expand and first appear in childhood in the presence of submandibular and cervical lymphadenopathy. Patients can present with significant dental problems. The majority of cherubism cases regress spontaneously after puberty (Papadaki et al., 2012, Meng et al., 2005). Monogenic Still’s disease is caused by homozygous LOF mutations in LACC1, which encodes the enzyme laccase (multicopper oxidoreductase) domain-containing 1. Patients present clinically with features of systemic juvenile idiopathic arthritis (JIA) including fever, erythematous maculopapular rashes, chronic polyarthritis, leukocytosis, thrombocytosis, and elevated markers of inflammation (Wakil et al., 2015). All patients reported were unresponsive to treatment with NSAIDs, systemic corticosteroids, methotrexate, biologics targeting TNF, IL-6 or rituximab (Wakil et al., 2015). Congenital Sideroblastic anemia, B-cell Immunodeficiency, periodic Fevers, and Developmental delay (SIFD) is a mitochondrial disease caused by autosomal recessive LOF mutations in TRNT1 (Chakraborty et al., 2014). Patients present in infancy with transfusion-dependent sideroblastic anemia, recurrent noninfectious fever episodes, B-cell lymphopenia with hypogammaglobulinemia causing recurrent sinopulmonary bacterial infections, and with progressive developmental delay (Wiseman et al., 2013). Occult multiorgan failure and/or cardiomyopathy are seen; early allogenic bone marrow transplant was curative in one patient (Wiseman et al., 2013). TRNT1 encodes an enzyme that adds two cytosine- and one adenosine-(CCA) residues to the 3′ end mitochondrial and cytosolic tRNA molecules, which is necessary for tRNA aminoacylation (Sasarman et al., 2015, Chakraborty et al., 2014). The disease-causing mutations lead to a reduction in CCA enzyme activity, defective mitochondrial translation, and the inability to detect tRNAs with backbone damage (Sasarman et al., 2015). This defect is thought to result in a ‘loss of quality-control-mechanisms’ that recognize and prevent damaged tRNA from CCA maturation and from entering the ribosome machinery of protein synthesis, thus suggesting a role of CCA addition in intracellular stress responses (Sasarman et al., 2015).
Conclusion
In the past 15 years the pathogenesis of a number of immune dysregulatory disorders that present with fever, systemic and organ-specific inflammation were clinically and genetically defined which provided insights into their pathogenesis and gave rise to novel targeted agents in the treatment of these conditions and the important role of IL-1 in causing the disease spectrum. But unresponsiveness to IL-1 blocking agents in a growing group of autoinflammatory diseases/phenotypes led to the search for additional dysregulated cytokine pathways and resulted in the discovery of dysregulation in Type I IFN production, IL-18 over-secretion, lack of IL-10 signaling and unopposed IL-36 signaling. In a number of diseases oral ulcers and other oral cavity and tooth abnormalities are present and may provide clues to these disorders (Figure 3). It is thus necessary that caring clinicians who see young infants and children be familiar with the clinical aspects of these diseases in order to recognize them, to initiate appropriate referrals to tertiary care centers so that early therapy can be started to prevent physical sequela, mortality from untreated diseases and guarantee a better quality of life for these patients.
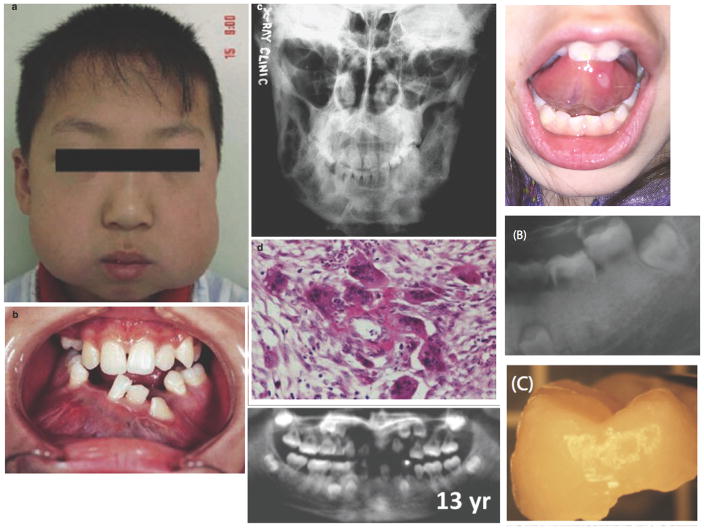
Figure 3. Features of oral disease manifestations.
(A) “Chubby cheek” appearance due to mandibular enlargement in a boy with cherubism (From Meng et al. (2005). Reprinted with permission from Elsevier). (B) Expansion of the mandible in cherubism with resultant dental abnormalities (From Meng et al. (2005). Reprinted with permission from Elsevier). (C) This plain film reveals multiple large radiolucent lesions with surrounding sclerosis in the mandible of a female with cherubism (From Jain and Sharma (2006). Reprinted with permission from Springer Science + Business Media). (D) Histologic findings in cherubism of multinucleated giant cells in collagen stromal tissue (From Meng et al. (2005). Reprinted with permission from Elsevier). (E) Periodontitis and root resorption in a patient with Singleton-Merten syndrome (SMS) (From Rutsch et al (2015). Reprinted with permission from Elsevier). (F) Oral ulcer in a patient with an undifferentiated autoinflammatory disease. (G) Root resorption and delayed tooth eruption in a young adult with SMS (From Lu C et al (2014). Reprinted with permission from Taylor & Francis Group). (H) Exfoliated tooth with root resorption in a patient with SMS (From Lu C et al (2014). Reprinted with permission from Taylor & Francis Group).
List of Abbreviations of disorders
- FMF
Familial Mediterranean Fever
- TRAPS
TNF receptor associated periodic syndrome
- MKD
Mevalonate kinase deficiency
- HIDS
Hyperimmunoglobulinemia D and periodic fever syndrome
- CAPS
Cryopyrin associated periodic syndromes
- FCAS
Familial cold autoinflammatory syndrome
- MWS
Muckle-Wells syndrome
- NOMID
Neonatal-onset multisystem inflammatory disease
- CINCA
Chronic infantile neurologic, cutaneous and arthritis syndrome
- DIRA
Deficiency of interleukin 1 receptor antagonist Majeed syndrome
- PAPA
Pyogenic arthritis, pyoderma gangrenosum and acne (syndrome)
- DITRA
Deficiency of interleukin 36 receptor antagonist
- CAMPS
CARD14- mediated psoriasis
- PRAAS
Proteasome associated autoinflammatory syndrome
- CANDLE
Chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperatures
- DADA2
Deficiency of ADA2
- SAVI
STING- associated vasculopathy with onset in infancy
- SPENCDI
Spondyloenchondrodysplasia with immune dysregulation
- PGA
Pediatric granulomatous arthritis
- PLAID
PLCG2-associated antibody deficiency and immune dysregulation
- APLAID
PLCG2-associated autoinflammation, antibody deficiency and immune dysregulation
- HA20
Haploinsufficiency of A20
- SIFD
Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and developmental delay
- AGS
Aicardi-Goutières syndrome
References
- AGANNA E, MARTINON F, HAWKINS PN, ROSS JB, SWAN DC, BOOTH DR, LACHMANN HJ, BYBEE A, GAUDET R, WOO P, FEIGHERY C, COTTER FE, THOME M, HITMAN GA, TSCHOPP J, MCDERMOTT MF. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445–52. doi: 10.1002/art.10509. [DOI] [PubMed] [Google Scholar]
- AGARWAL AK, XING C, DEMARTINO GN, MIZRACHI D, HERNANDEZ MD, SOUSA AB, MARTINEZ DE VILLARREAL L, DOS SANTOS HG, GARG A. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87:866–72. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKSENTIJEVICH I, MASTERS SL, FERGUSON PJ, DANCEY P, FRENKEL J, VAN ROYEN-KERKHOFF A, LAXER R, TEDGARD U, COWEN EW, PHAM TH, BOOTY M, ESTES JD, SANDLER NG, PLASS N, STONE DL, TURNER ML, HILL S, BUTMAN JA, SCHNEIDER R, BABYN P, EL-SHANTI HI, POPE E, BARRON K, BING X, LAURENCE A, LEE CC, CHAPELLE D, CLARKE GI, OHSON K, NICHOLSON M, GADINA M, YANG B, KORMAN BD, GREGERSEN PK, VAN HAGEN PM, HAK AE, HUIZING M, RAHMAN P, DOUEK DC, REMMERS EF, KASTNER DL, GOLDBACH-MANSKY R. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKSENTIJEVICH I, NOWAK M, MALLAH M, CHAE JJ, WATFORD WT, HOFMANN SR, STEIN L, RUSSO R, GOLDSMITH D, DENT P, ROSENBERG HF, AUSTIN F, REMMERS EF, BALOW JE, JR, ROSENZWEIG S, KOMAROW H, SHOHAMN G, WOOD G, JONES J, MANGRA N, CARRERO H, ADAMS BS, MOORE TL, SCHIKLER K, HOFFMAN H, LOVELL DJ, LIPNICK R, BARRON K, O’SHEA JJ, KASTNER DL, GOLDBACH-MANSKY R. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE JESUS A, GOLDBACH-MANSKY R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147:155–74. doi: 10.1016/j.clim.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALONSO D, ELGART GW, SCHACHNER LA. Blau syndrome: a new kindred. J Am Acad Dermatol. 2003;49:299–302. doi: 10.1067/s0190-9622(02)61772-4. [DOI] [PubMed] [Google Scholar]
- ARIMA K, KINOSHITA A, MISHIMA H, KANAZAWA N, KANEKO T, MIZUSHIMA T, ICHINOSE K, NAKAMURA H, TSUJINO A, KAWAKAMI A, MATSUNAKA M, KASAGI S, KAWANO S, KUMAGAI S, OHMURA K, MIMORI T, HIRANO M, UENO S, TANAKA K, TANAKA M, TOYOSHIMA I, SUGINO H, YAMAKAWA A, TANAKA K, NIIKAWA N, FURUKAWA F, MURATA S, EGUCHI K, IDA H, YOSHIURA K. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A. 2011;108:14914–9. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AROSTEGUI JI, ARNAL C, MERINO R, MODESTO C, ANTONIA CARBALLO M, MORENO P, GARCIA-CONSUEGRA J, NARANJO A, RAMOS E, DE PAZ P, RIUS J, PLAZA S, YAGUE J. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum. 2007;56:3805–13. doi: 10.1002/art.22966. [DOI] [PubMed] [Google Scholar]
- BADER-MEUNIER B, FLORKIN B, SIBILIA J, ACQUAVIVA C, HACHULLA E, GRATEAU G, RICHER O, FARBER CM, FISCHBACH M, HENTGEN V, JEGO P, LAROCHE C, NEVEN B, LEQUERRE T, MATHIAN A, PELLIER I, TOUITOU I, RABIER D, PRIEUR AM, CUISSET L, QUARTIER P. Mevalonate kinase deficiency: a survey of 50 patients. Pediatrics. 2011;128:e152–9. doi: 10.1542/peds.2010-3639. [DOI] [PubMed] [Google Scholar]
- BREHM A, LIU Y, SHEIKH A, MARRERO B, OMOYINMI E, ZHOU Q, MONTEALEGRE G, BIANCOTTO A, REINHARDT A, ALMEIDA DE JESUS A, PELLETIER M, TSAI WL, REMMERS EF, KARDAVA L, HILL S, KIM H, LACHMANN HJ, MEGARBANE A, CHAE JJ, BRADY J, CASTILLO RD, BROWN D, CASANO AV, GAO L, CHAPELLE D, HUANG Y, STONE D, CHEN Y, SOTZNY F, LEE CC, KASTNER DL, TORRELO A, ZLOTOGORSKI A, MOIR S, GADINA M, MCCOY P, WESLEY R, ROTHER K, HILDEBRAND PW, BROGAN P, KRUGER E, AKSENTIJEVICH I, GOLDBACH-MANSKY R. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest. 2015;125:4196–211. doi: 10.1172/JCI81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGGS TA, RICE GI, DALY S, URQUHART J, GORNALL H, BADER-MEUNIER B, BASKAR K, BASKAR S, BAUDOUIN V, BERESFORD MW, BLACK GC, DEARMAN RJ, DE ZEGHER F, FOSTER ES, FRANCES C, HAYMAN AR, HILTON E, JOB-DESLANDRE C, KULKARNI ML, LE MERRER M, LINGLART A, LOVELL SC, MAURER K, MUSSET L, NAVARRO V, PICARD C, PUEL A, RIEUX-LAUCAT F, ROIFMAN CM, SCHOLL-BURGI S, SMITH N, SZYNKIEWICZ M, WIEDEMAN A, WOUTERS C, ZEEF LA, CASANOVA JL, ELKON KB, JANCKILA A, LEBON P, CROW YJ. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43:127–31. doi: 10.1038/ng.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODERICK L, DE NARDO D, FRANKLIN BS, HOFFMAN HM, LATZ E. The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol. 2015;10:395–424. doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- CANNA SW, DE JESUS AA, GOUNI S, BROOKS SR, MARRERO B, LIU Y, DIMATTIA MA, ZAAL KJ, SANCHEZ GA, KIM H, CHAPELLE D, PLASS N, HUANG Y, VILLARINO AV, BIANCOTTO A, FLEISHER TA, DUNCAN JA, O’SHEA JJ, BENSELER S, GROM A, DENG Z, LAXER RM, GOLDBACH-MANSKY R. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–6. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAE JJ, AKSENTIJEVICH I, KASTNER DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol. 2009;146:467–78. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKRABORTY PK, SCHMITZ-ABE K, KENNEDY EK, MAMADY H, NAAS T, DURIE D, CAMPAGNA DR, LAU A, SENDAMARAI AK, WISEMAN DH, MAY A, JOLLES S, CONNOR P, POWELL C, HEENEY MM, GIARDINA PJ, KLAASSEN RJ, KANNENGIESSER C, THURET I, THOMPSON AA, MARQUES L, HUGHES S, BONNEY DK, BOTTOMLEY SS, WYNN RF, LAXER RM, MINNITI CP, MOPPETT J, BORDON V, GERAGHTY M, JOYCE PB, MARKIANOS K, RUDNER AD, HOLCIK M, FLEMING MD. Mutations in TRNT1 cause congenital sideroblastic anemia with immunodeficiency, fevers, and developmental delay (SIFD) Blood. 2014;124:2867–71. doi: 10.1182/blood-2014-08-591370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSORTIUM FF. A candidate gene for familial Mediterranean fever. Nat Genet. 1997a;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- CONSORTIUM TIF. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997b;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- CROW YJ, MANEL N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429–40. doi: 10.1038/nri3850. [DOI] [PubMed] [Google Scholar]
- DE JESUS AA, CANNA SW, LIU Y, GOLDBACH-MANSKY R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–74. doi: 10.1146/annurev-immunol-032414-112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMIDOWICH AP, FREEMAN AF, KUHNS DB, AKSENTIJEVICH I, GALLIN JI, TURNER ML, KASTNER DL, HOLLAND SM. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) Arthritis Rheum. 2012;64:2022–7. doi: 10.1002/art.34332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRENTH JP, HAAGSMA CJ, VAN DER MEER JW. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine (Baltimore) 1994;73:133–44. [PubMed] [Google Scholar]
- EL-SHANTI HI, FERGUSON PJ. Chronic recurrent multifocal osteomyelitis: a concise review and genetic update. Clin Orthop Relat Res. 2007;462:11–9. doi: 10.1097/BLO.0b013e3180986d73. [DOI] [PubMed] [Google Scholar]
- ENGELHARDT KR, SHAH N, FAIZURA-YEOP I, KOCACIK UYGUN DF, FREDE N, MUISE AM, SHTEYER E, FILIZ S, CHEE R, ELAWAD M, HARTMANN B, ARKWRIGHT PD, DVORAK C, KLEIN C, PUCK JM, GRIMBACHER B, GLOCKER EO. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2012;131(3):825–30. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- FERGUSON PJ, CHEN S, TAYEH MK, OCHOA L, LEAL SM, PELET A, MUNNICH A, LYONNET S, MAJEED HA, EL-SHANTI H. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005;42:551–7. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONNESU C, CERQUAGLIA C, GIOVINALE M, CURIGLIANO V, VERRECCHIA E, DE SOCIO G, LA REGINA M, GASBARRINI G, MANNA R. Familial Mediterranean Fever: a review for clinical management. Joint Bone Spine. 2009;76:227–33. doi: 10.1016/j.jbspin.2008.08.004. [DOI] [PubMed] [Google Scholar]
- FUCHS-TELEM D, SARIG O, VAN STEENSEL MA, ISAKOV O, ISRAELI S, NOUSBECK J, RICHARD K, WINNEPENNINCKX V, VERNOOIJ M, SHOMRON N, UITTO J, FLECKMAN P, RICHARD G, SPRECHER E. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012;91:163–70. doi: 10.1016/j.ajhg.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCKER EO, KOTLARZ D, BOZTUG K, GERTZ EM, SCHAFFER AA, NOYAN F, PERRO M, DIESTELHORST J, ALLROTH A, MURUGAN D, HATSCHER N, PFEIFER D, SYKORA KW, SAUER M, KREIPE H, LACHER M, NUSTEDE R, WOELLNER C, BAUMANN U, SALZER U, KOLETZKO S, SHAH N, SEGAL AW, SAUERBREY A, BUDERUS S, SNAPPER SB, GRIMBACHER B, KLEIN C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBACH-MANSKY R, DAILEY NJ, CANNA SW, GELABERT A, JONES J, RUBIN BI, KIM HJ, BREWER C, ZALEWSKI C, WIGGS E, HILL S, TURNER ML, KARP BI, AKSENTIJEVICH I, PUCINO F, PENZAK SR, HAVERKAMP MH, STEIN L, ADAMS BS, MOORE TL, FUHLBRIGGE RC, SHAHAM B, JARVIS JN, O’NEIL K, VEHE RK, BEITZ LO, GARDNER G, HANNAN WP, WARREN RW, HORN W, COLE JL, PAUL SM, HAWKINS PN, PHAM TH, SNYDER C, WESLEY RA, HOFFMANN SC, HOLLAND SM, BUTMAN JA, KASTNER DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKINS PN, LACHMANN HJ, AGANNA E, MCDERMOTT MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–12. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- HERLIN T, FIIRGAARD B, BJERRE M, KERNDRUP G, HASLE H, BING X, FERGUSON PJ. Efficacy of anti-IL-1 treatment in Majeed syndrome. Ann Rheum Dis. 2012;72(3):410–3. doi: 10.1136/annrheumdis-2012-201818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN HM, MUELLER JL, BROIDE DH, WANDERER AA, KOLODNER RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN HM, ROSENGREN S, BOYLE DL, CHO JY, NAYAR J, MUELLER JL, ANDERSON JP, WANDERER AA, FIRESTEIN GS. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–85. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUTEN SM, KUIS W, DURAN M, DE KONING TJ, VAN ROYEN-KERKHOF A, ROMEIJN GJ, FRENKEL J, DORLAND L, DE BARSE MM, HUIJBERS WA, RIJKERS GT, WATERHAM HR, WANDERS RJ, POLL-THE BT. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet. 1999;22:175–7. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- HULL KM, DREWE E, AKSENTIJEVICH I, SINGH HK, WONG K, MCDERMOTT EM, DEAN J, POWELL RJ, KASTNER DL. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002a;81:349–68. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- HULL KM, WONG K, WOOD GM, CHU WS, KASTNER DL. Monocytic fasciitis: a newly recognized clinical feature of tumor necrosis factor receptor dysfunction. Arthritis Rheum. 2002b;46:2189–94. doi: 10.1002/art.10448. [DOI] [PubMed] [Google Scholar]
- ICHIDA H, KAWAGUCHI Y, SUGIURA T, TAKAGI K, KATSUMATA Y, GONO T, OTA Y, KATAOKA S, KAWASUMI H, YAMANAKA H. Clinical manifestations of Adult-onset Still’s disease presenting with erosive arthritis: Association with low levels of ferritin and Interleukin-18. Arthritis Care Res (Hoboken) 2014;66:642–6. doi: 10.1002/acr.22194. [DOI] [PubMed] [Google Scholar]
- JACOBELLI S, ANDRE M, ALEXANDRA JF, DODE C, PAPO T. Failure of anti-TNF therapy in TNF Receptor 1-Associated Periodic Syndrome (TRAPS) Rheumatology (Oxford) 2007;46:1211–2. doi: 10.1093/rheumatology/kel298. [DOI] [PubMed] [Google Scholar]
- JANG MA, KIM EK, NOW H, NGUYEN NT, KIM WJ, YOO JY, LEE J, JEONG YM, KIM CH, KIM OH, SOHN S, NAM SH, HONG Y, LEE YS, CHANG SA, JANG SY, KIM JW, LEE MS, LIM SY, SUNG KS, PARK KT, KIM BJ, LEE JH, KIM DK, KEE C, KI CS. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet. 2015;96:266–74. doi: 10.1016/j.ajhg.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEREMIAH N, NEVEN B, GENTILI M, CALLEBAUT I, MASCHALIDI S, STOLZENBERG MC, GOUDIN N, FREMOND ML, NITSCHKE P, MOLINA TJ, BLANCHE S, PICARD C, RICE GI, CROW YJ, MANEL N, FISCHER A, BADER-MEUNIER B, RIEUX-LAUCAT F. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124:5516–20. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de JESUS AA, GOLDBACH-MANSKY R. IL-1 blockade in autoinflammatory syndromes. Annu Rev Med. 2014;65:223–44. doi: 10.1146/annurev-med-061512-150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN CT, CAO L, ROBERSON ED, DUAN S, HELMS CA, NAIR RP, DUFFIN KC, STUART PE, GOLDGAR D, HAYASHI G, OLFSON EH, FENG BJ, PULLINGER CR, KANE JP, WISE CA, GOLDBACH-MANSKY R, LOWES MA, PEDDLE L, CHANDRAN V, LIAO W, RAHMAN P, KRUEGER GG, GLADMAN D, ELDER JT, MENTER A, BOWCOCK AM. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet. 2012a;90:796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN CT, CAO L, ROBERSON ED, PIERSON KC, YANG CF, JOYCE CE, RYAN C, DUAN S, HELMS CA, LIU Y, CHEN Y, MCBRIDE AA, HWU WL, WU JY, CHEN YT, MENTER A, GOLDBACH-MANSKY R, LOWES MA, BOWCOCK AM. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012b;90:784–95. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITAMURA A, MAEKAWA Y, UEHARA H, IZUMI K, KAWACHI I, NISHIZAWA M, TOYOSHIMA Y, TAKAHASHI H, STANDLEY DM, TANAKA K, HAMAZAKI J, MURATA S, OBARA K, TOYOSHIMA I, YASUTOMO K. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121:4150–60. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTLARZ D, BEIER R, MURUGAN D, DIESTELHORST J, JENSEN O, BOZTUG K, PFEIFER D, KREIPE H, PFISTER ED, BAUMANN U, PUCHALKA J, BOHNE J, EGRITAS O, DALGIC B, KOLHO KL, SAUERBREY A, BUDERUS S, GUNGOR T, ENNINGER A, KODA YK, GUARISO G, WEISS B, CORBACIOGLU S, SOCHA P, USLU N, METIN A, WAHBEH GT, HUSAIN K, RAMADAN D, AL-HERZ W, GRIMBACHER B, SAUER M, SYKORA KW, KOLETZKO S, KLEIN C. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347–55. doi: 10.1053/j.gastro.2012.04.045. [DOI] [PubMed] [Google Scholar]
- LAUSCH E, JANECKE A, BROS M, TROJANDT S, ALANAY Y, DE LAET C, HUBNER CA, MEINECKE P, NISHIMURA G, MATSUO M, HIRANO Y, TENOUTASSE S, KISS A, ROSA RF, UNGER SL, RENELLA R, BONAFE L, SPRANGER J, UNGER S, ZABEL B, SUPERTI-FURGA A. Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet. 2011;43:132–7. doi: 10.1038/ng.749. [DOI] [PubMed] [Google Scholar]
- LEVY R, GERARD L, KUEMMERLE-DESCHNER J, LACHMANN HJ, KONE-PAUT I, CANTARINI L, WOO P, NASELLI A, BADER-MEUNIER B, INSALACO A, AL-MAYOUF SM, OZEN S, HOFER M, FRENKEL J, MODESTO C, NIKISHINA I, SCHWARZ T, MARTINO S, MEINI A, QUARTIER P, MARTINI A, RUPERTO N, NEVEN B, GATTORNO M, FOR P EUROFEVER. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. 2015;74:2043–9. doi: 10.1136/annrheumdis-2013-204991. [DOI] [PubMed] [Google Scholar]
- LIU Y, JESUS AA, MARRERO B, YANG D, RAMSEY SE, MONTEALEGRE SANCHEZ GA, TENBROCK K, WITTKOWSKI H, JONES OY, KUEHN HS, LEE CC, DIMATTIA MA, COWEN EW, GONZALEZ B, PALMER I, DIGIOVANNA JJ, BIANCOTTO A, KIM H, TSAI WL, TRIER AM, HUANG Y, STONE DL, HILL S, KIM HJ, ST HILAIRE C, GURPRASAD S, PLASS N, CHAPELLE D, HORKAYNE-SZAKALY I, FOELL D, BARYSENKA A, CANDOTTI F, HOLLAND SM, HUGHES JD, MEHMET H, ISSEKUTZ AC, RAFFELD M, MCELWEE J, FONTANA JR, MINNITI CP, MOIR S, KASTNER DL, GADINA M, STEVEN AC, WINGFIELD PT, BROOKS SR, ROSENZWEIG SD, FLEISHER TA, DENG Z, BOEHM M, PALLER AS, GOLDBACH-MANSKY R. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–18. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y, RAMOT Y, TORRELO A, PALLER AS, SI N, BABAY S, KIM PW, SHEIKH A, LEE CC, CHEN Y, VERA A, ZHANG X, GOLDBACH-MANSKY R, ZLOTOGORSKI A. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64:895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARRAKCHI S, GUIGUE P, RENSHAW BR, PUEL A, PEI XY, FRAITAG S, ZRIBI J, BAL E, CLUZEAU C, CHRABIEH M, TOWNE JE, DOUANGPANYA J, PONS C, MANSOUR S, SERRE V, MAKNI H, MAHFOUDH N, FAKHFAKH F, BODEMER C, FEINGOLD J, HADJ-RABIA S, FAVRE M, GENIN E, SAHBATOU M, MUNNICH A, CASANOVA JL, SIMS JE, TURKI H, BACHELEZ H, SMAHI A. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–8. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- MARTINON F, BURNS K, TSCHOPP J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- MCDERMOTT MF, AKSENTIJEVICH I, GALON J, MCDERMOTT EM, OGUNKOLADE BW, CENTOLA M, MANSFIELD E, GADINA M, KARENKO L, PETTERSSON T, MCCARTHY J, FRUCHT DM, ARINGER M, TOROSYAN Y, TEPPO AM, WILSON M, KARAARSLAN HM, WAN Y, TODD I, WOOD G, SCHLIMGEN R, KUMARAJEEWA TR, COOPER SM, VELLA JP, AMOS CI, MULLEY J, QUANE KA, MOLLOY MG, RANKI A, POWELL RJ, HITMAN GA, O’SHEA JJ, KASTNER DL. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–44. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- MCGONAGLE D, AZIZ A, DICKIE LJ, MCDERMOTT MF. An integrated classification of pediatric inflammatory diseases, based on the concepts of autoinflammation and the immunological disease continuum. Pediatr Res. 2009;65:38R–45R. doi: 10.1203/PDR.0b013e31819dbd0a. [DOI] [PubMed] [Google Scholar]
- MENG XM, YU SF, YU GY. Clinicopathologic study of 24 cases of cherubism. Int J Oral Maxillofac Surg. 2005;34:350–6. doi: 10.1016/j.ijom.2004.09.006. [DOI] [PubMed] [Google Scholar]
- NAVON ELKAN P, PIERCE SB, SEGEL R, WALSH T, BARASH J, PADEH S, ZLOTOGORSKI A, BERKUN Y, PRESS JJ, MUKAMEL M, VOTH I, HASHKES PJ, HAREL L, HOFFER V, LING E, YALCINKAYA F, KASAPCOPUR O, LEE MK, KLEVIT RE, RENBAUM P, WEINBERG-SHUKRON A, SENER EF, SCHORMAIR B, ZELIGSON S, MAREK-YAGEL D, STROM TM, SHOHAT M, SINGER A, RUBINOW A, PRAS E, WINKELMANN J, TEKIN M, ANIKSTER Y, KING MC, LEVY-LAHAD E. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370:921–31. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- NEVEN B, PRIEUR AM, QUARTIER DIT MAIRE P. Cryopyrinopathies: update on pathogenesis and treatment. Nat Clin Pract Rheumatol. 2008;4:481–9. doi: 10.1038/ncprheum0874. [DOI] [PubMed] [Google Scholar]
- OMBRELLO MJ, REMMERS EF, SUN G, FREEMAN AF, DATTA S, TORABI-PARIZI P, SUBRAMANIAN N, BUNNEY TD, BAXENDALE RW, MARTINS MS, ROMBERG N, KOMAROW H, AKSENTIJEVICH I, KIM HS, HO J, CRUSE G, JUNG MY, GILFILLAN AM, METCALFE DD, NELSON C, O’BRIEN M, WISCH L, STONE K, DOUEK DC, GANDHI C, WANDERER AA, LEE H, NELSON SF, SHIANNA KV, CIRULLI ET, GOLDSTEIN DB, LONG EO, MOIR S, MEFFRE E, HOLLAND SM, KASTNER DL, KATAN M, HOFFMAN HM, MILNER JD. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366:330–8. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONEN F. Familial Mediterranean fever. Rheumatol Int. 2006;26:489–96. doi: 10.1007/s00296-005-0074-3. [DOI] [PubMed] [Google Scholar]
- PAPADAKI ME, LIETMAN SA, LEVINE MA, OLSEN BR, KABAN LB, REICHENBERGER EJ. Cherubism: best clinical practice. Orphanet J Rare Dis. 2012;7(Suppl 1):S6. doi: 10.1186/1750-1172-7-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY S, JIA S, GEOFFREY R, LORIER R, SUCHI M, BROECKEL U, HESSNER MJ, VERBSKY J. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–44. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMBERG N, AL MOUSSAWI K, NELSON-WILLIAMS C, STIEGLER AL, LORING E, CHOI M, OVERTON J, MEFFRE E, KHOKHA MK, HUTTNER AJ, WEST B, PODOLTSEV NA, BOGGON TJ, KAZMIERCZAK BI, LIFTON RP. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46:1135–9. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE CD, AROSTEGUI JI, MARTIN TM, ESPADA G, SCALZI L, YAGUE J, ROSENBAUM JT, MODESTO C, CRISTINA ARNAL M, MERINO R, GARCIA-CONSUEGRA J, CARBALLO SILVA MA, WOUTERS CH. NOD2-associated pediatric granulomatous arthritis, an expanding phenotype: study of an international registry and a national cohort in Spain. Arthritis Rheum. 2009;60:1797–803. doi: 10.1002/art.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE CD, MARTIN TM, WOUTERS CH. Blau syndrome revisited. Curr Opin Rheumatol. 2011;23:411–8. doi: 10.1097/BOR.0b013e328349c430. [DOI] [PubMed] [Google Scholar]
- RUTSCH F, MACDOUGALL M, LU C, BUERS I, MAMAEVA O, NITSCHKE Y, RICE GI, ERLANDSEN H, KEHL HG, THIELE H, NURNBERG P, HOHNE W, CROW YJ, FEIGENBAUM A, HENNEKAM RC. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet. 2015;96:275–82. doi: 10.1016/j.ajhg.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO M, FUJISAWA A, NISHIKOMORI R, KAMBE N, NAKATA-HIZUME M, YOSHIMOTO M, OHMORI K, OKAFUJI I, YOSHIOKA T, KUSUNOKI T, MIYACHI Y, HEIKE T, NAKAHATA T. Somatic mosaicism of CIAS1 in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2005;52:3579–85. doi: 10.1002/art.21404. [DOI] [PubMed] [Google Scholar]
- SASARMAN F, THIFFAULT I, WERAARPACHAI W, SALOMON S, MAFTEI C, GAUTHIER J, ELLAZAM B, WEBB N, ANTONICKA H, JANER A, BRUNEL-GUITTON C, ELPELEG O, MITCHELL G, SHOUBRIDGE EA. The 3′ addition of CCA to mitochondrial tRNASer(AGY) is specifically impaired in patients with mutations in the tRNA nucleotidyl transferase TRNT1. Hum Mol Genet. 2015;24:2841–7. doi: 10.1093/hmg/ddv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU M, NAKAGISHI Y, INOUE N, MIZUTA M, KO G, SAIKAWA Y, KUBOTA T, YAMASAKI Y, TAKEI S, YACHIE A. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin Immunol. 2015;160:277–81. doi: 10.1016/j.clim.2015.06.005. [DOI] [PubMed] [Google Scholar]
- SIBLEY CH, CHIOATO A, FELIX S, COLIN L, CHAKRABORTY A, PLASS N, RODRIGUEZ-SMITH J, BREWER C, KING K, ZALEWSKI C, KIM HJ, BISHOP R, ABRAMS K, STONE D, CHAPELLE D, KOST B, SNYDER C, BUTMAN JA, WESLEY R, GOLDBACH-MANSKY R. A 24-month open-label study of canakinumab in neonatal-onset multisystem inflammatory disease. Ann Rheum Dis. 2014;74(9):1714–9. doi: 10.1136/annrheumdis-2013-204877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIBLEY CH, PLASS N, SNOW J, WIGGS EA, BREWER CC, KING KA, ZALEWSKI C, KIM HJ, BISHOP R, HILL S, PAUL SM, KICKER P, PHILLIPS Z, DOLAN JG, WIDEMANN B, JAYAPRAKASH N, PUCINO F, STONE DL, CHAPELLE D, SNYDER C, BUTMAN JA, WESLEY R, GOLDBACH-MANSKY R. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64:2375–86. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON A, VAN DER MEER JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R86–98. doi: 10.1152/ajpregu.00504.2006. [DOI] [PubMed] [Google Scholar]
- TANAKA N, IZAWA K, SAITO MK, SAKUMA M, OSHIMA K, OHARA O, NISHIKOMORI R, MORIMOTO T, KAMBE N, GOLDBACH-MANSKY R, AKSENTIJEVICH I, DE SAINT BASILE G, NEVEN B, VAN GIJN M, FRENKEL J, AROSTEGUI JI, YAGUE J, MERINO R, IBANEZ M, PONTILLO A, TAKADA H, IMAGAWA T, KAWAI T, YASUMI T, NAKAHATA T, HEIKE T. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 2011;63:3625–32. doi: 10.1002/art.30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRELO A, PATEL S, COLMENERO I, GURBINDO D, LENDINEZ F, HERNANDEZ A, LOPEZ-ROBLEDILLO JC, DADBAN A, REQUENA L, PALLER AS. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol. 2010;62:489–95. doi: 10.1016/j.jaad.2009.04.046. [DOI] [PubMed] [Google Scholar]
- TUNCA M, AKAR S, ONEN F, OZDOGAN H, KASAPCOPUR O, YALCINKAYA F, TUTAR E, OZEN S, TOPALOGLU R, YILMAZ E, ARICI M, BAKKALOGLU A, BESBAS N, AKPOLAT T, DINC A, ERKEN E. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 2005;84:1–11. doi: 10.1097/01.md.0000152370.84628.0c. [DOI] [PubMed] [Google Scholar]
- VAN DER HILST JC, BODAR EJ, BARRON KS, FRENKEL J, DRENTH JP, VAN DER MEER JW, SIMON A, INTERNATIONAL HSG. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine (Baltimore) 2008;87:301–10. doi: 10.1097/MD.0b013e318190cfb7. [DOI] [PubMed] [Google Scholar]
- VAN EYCK L, JR, HERSHFIELD MS, POMBAL D, KELLY SJ, GANSON NJ, MOENS L, FRANS G, SCHABALLIE H, DE HERTOGH G, DOOLEY J, BOSSUYT X, WOUTERS C, LISTON A, MEYTS I. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J Allergy Clin Immunol. 2015;135:283–7. e5. doi: 10.1016/j.jaci.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIL SM, MONIES DM, ABOUELHODA M, AL-TASSAN N, AL-DUSERY H, NAIM EA, AL-YOUNES B, SHINWARI J, AL-MOHANNA FA, MEYER BF, AL-MAYOUF S. Association of a mutation in LACC1 with a monogenic form of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2015;67:288–95. doi: 10.1002/art.38877. [DOI] [PubMed] [Google Scholar]
- WISE CA, GILLUM JD, SEIDMAN CE, LINDOR NM, VEILE R, BASHIARDES S, LOVETT M. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11:961–9. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- WISEMAN DH, MAY A, JOLLES S, CONNOR P, POWELL C, HEENEY MM, GIARDINA PJ, KLAASSEN RJ, CHAKRABORTY P, GERAGHTY MT, MAJOR-COOK N, KANNENGIESSER C, THURET I, THOMPSON AA, MARQUES L, HUGHES S, BONNEY DK, BOTTOMLEY SS, FLEMING MD, WYNN RF. A novel syndrome of congenital sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmental delay (SIFD) Blood. 2013;122:112–23. doi: 10.1182/blood-2012-08-439083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Q, LEE GS, BRADY J, DATTA S, KATAN M, SHEIKH A, MARTINS MS, BUNNEY TD, SANTICH BH, MOIR S, KUHNS DB, LONG PRIEL DA, OMBRELLO A, STONE D, OMBRELLO MJ, KHAN J, MILNER JD, KASTNER DL, AKSENTIJEVICH I. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012;91:713–20. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Q, WANG H, SCHWARTZ DM, STOFFELS M, PARK YH, ZHANG Y, YANG D, DEMIRKAYA E, TAKEUCHI M, TSAI WL, LYONS JJ, YU X, OUYANG C, CHEN C, CHIN DT, ZAAL K, CHANDRASEKHARAPPA SC, EPH, YU Z, MULLIKIN JC, HASNI SA, WERTZ IE, OMBRELLO AK, STONE DL, HOFFMANN P, JONES A, BARHAM BK, LEAVIS HL, VAN ROYEN-KERKOF A, SIBLEY C, BATU ED, GUL A, SIEGEL RM, BOEHM M, MILNER JD, OZEN S, GADINA M, CHAE J, LAXER RM, KASTNER DL, AKSENTIJEVICH I. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2015;48(1):67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Q, YANG D, OMBRELLO AK, ZAVIALOV AV, TORO C, ZAVIALOV AV, STONE DL, CHAE JJ, ROSENZWEIG SD, BISHOP K, BARRON KS, KUEHN HS, HOFFMANN P, NEGRO A, TSAI WL, COWEN EW, PEI W, MILNER JD, SILVIN C, HELLER T, CHIN DT, PATRONAS NJ, BARBER JS, LEE CC, WOOD GM, LING A, KELLY SJ, KLEINER DE, MULLIKIN JC, GANSON NJ, KONG HH, HAMBLETON S, CANDOTTI F, QUEZADO MM, CALVO KR, ALAO H, BARHAM BK, JONES A, MESCHIA JF, WORRALL BB, KASNER SE, RICH SS, GOLDBACH-MANSKY R, ABINUN M, CHALOM E, GOTTE AC, PUNARO M, PASCUAL V, VERBSKY JW, TORGERSON TR, SINGER NG, GERSHON TR, OZEN S, KARADAG O, FLEISHER TA, REMMERS EF, BURGESS SM, MOIR SL, GADINA M, SOOD R, HERSHFIELD MS, BOEHM M, KASTNER DL, AKSENTIJEVICH I. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370:911–20. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]