Abstract
We evaluated the adherence and acceptability of a vaginal ring containing dapivirine, maraviroc, or both drugs for 28 days during a Phase I placebo-controlled trial in 48 HIV-negative sexually abstinent U.S. women aged 18 to 40. Adherence was assessed weekly by clinical interview and computer-assisted self-interviewing; acceptability assessment occurred at the last product-use visit. Study retention was 98% (47/48); 94% (45/48) reported being fully adherent with ring use during the 28-day period. Two participants experienced the ring partially coming out. Analysis was blinded and behavioral data were combined across study groups. Most women reported being very comfortable having the ring in their vagina; 44% preferred continuous use, whereas 51% had no preference compared to episodic use. Although a range of minor ring concerns were expressed, few were actually experienced. High adherence to and acceptability of this vaginal ring in this Phase I trial contributes to its promise as a sustained mechanism for multidrug vaginal microbicide delivery.
Keywords: HIV prevention, pre-exposure prophylaxis, microbicides, adherence, acceptability, vaginal ring
Introduction
Recent biomedical developments for HIV pre-exposure prophylaxis (PrEP) have highlighted both successes and challenges with daily or peri/pre-coital use of antiretroviral (ARV)-based prevention methods (1,2). Several topical and oral effectiveness PrEP trials yielded divergent results, mainly attributed to varying levels of product adherence within and across trials. These results highlight that ARV-based prevention approaches are highly dependent on correct dosing to achieve adequate protection (3). These approaches have aptly been coined “bio-behavioral,” as individuals have to adjust their behavior to incorporate correct use of these products into their lives and the technologies have to be developed to fit with people’s lives and behaviors (4,5).
Worldwide, women continue to be disproportionately affected by HIV (6) thus the search for novel, acceptable female-initiated methods remain an important priority. Microbicide vaginal rings can offer sustained single or multidrug topical delivery that can simplify use and improve product effectiveness. Rings offer several advantages over the applicator-and-gel approach, such as continuous use for one to several months and coital independence of product application (7); thus rings have the potential to increase product adherence, acceptability, and effectiveness. Previous studies in Africa have shown overall high acceptability and high reported adherence to vaginal rings for HIV prevention (8–11). Two Phase III trials are ongoing in Africa, testing the safety and effectiveness of a vaginal ring containing 25 mg of dapivirine (DPV), a non-nucleoside reverse transcriptase inhibitor (12,13).
In Europe, the United States, and most recently in South Africa, vaginal rings are currently used for contraception (14). Rings are also used for treatment of symptoms related to menopause. Studies have repeatedly shown high acceptability among women who use NuvaRing®, a combined hormonal contraceptive ring, both in the United States and abroad (15–17). In a European study, sexual comfort was reportedly high and 15% of women and 29% of partners felt the ring during intercourse at least occasionally (15). In one comparative study between NuvaRing and oral contraceptives with young women in the United States, the ring’s overall acceptability was good (65%) however, more women thought that the ring, compared with the pill, interfered with sex and fewer partners liked the ring (18). In contrast, in another United States-based comparative study, women preferred the vaginal ring to both the contraceptive patch and oral contraceptives (5). Thus, in preparation for the introduction of a vaginal ring as a novel HIV prevention method, it is critical to understand the acceptability of various product attributes as well as users’ experiences and skills needed for correct and consistent use in a range of settings.
MTN-013/IPM 026 was the first trial of a multidrug silicone elastomer vaginal ring with DPV and maraviroc (MVC, a CCR5 co-receptor antagonist). We evaluated the adherence and acceptability of vaginal rings containing DPV alone, MVC alone, DPV and MVC, or placebo during this 28-day safety and pharmacokinetics study conducted at three sites in the United States. Specifically, we sought to identify key product and use attributes as well as contextual issues that were affecting vaginal ring adherence and acceptability in this population.
Methods
Study Sample, Design, and Procedures
This multisite, double-blind, randomized, placebo-controlled Phase I safety and pharmacokinetics trial was conducted with 48 HIV-negative sexually abstinent women, at the University of Pittsburgh, PA, the University of Alabama at Birmingham, and the Fenway Institute, Boston, MA from September 2011 to September 2012 (ClinicalTrials.gov identifier: NCT01363037) (19). The four-arm study evaluated vaginal rings containing 25 mg DPV plus 100 mg MVC, 25 mg DPV only, 100 mg MVC only, or a placebo ring. The ring was intended for 1 month of use. Women were randomized 1:1:1:1 into one of the four study groups and asked to use their assigned ring continuously for 28 days. All study rings looked identical to preserve blinding and consisted of an off-white, flexible silicone elastomer matrix ring with a 56 mm outer diameter and a 7.7 mm cross-sectional diameter. All sites received Institutional Review Board approval before implementation. More details about primary endpoints, results, study procedures, recruitment, and eligibility criteria are described elsewhere (19). Vaginal ring instructions and demonstration were provided by clinicians at the enrollment visit before initial insertion, and participants received a handout that they brought home with them1. All participants inserted the ring themselves at the clinic, and were asked to keep the ring in for 28 days. However, the instruction pamphlet provided instructions on removal and reinsertion should it be necessary. The clinical staff was requested to remove the ring at the day 28 clinic visit and did so for almost all participants, unless the ring was removed or expelled before the visit.
Briefly, women were eligible for the study if they were aged aged 18 to 40, HIV negative, in general good health, used an effective method of contraception, had regular menstrual cycles (with at least 21 days between menses for those not on progestin-only contraceptives) and had a normal Pap test in the past 12 months. Major exclusion criteria included a known adverse reaction to silicone or any other components of the study products, recreational injection drug use in the past 12 months, clinically apparent gynecological abnormalities, or severe pelvic organ prolapse. Women were asked to avoid inserting non-study vaginal products including tampons or other objects into the vagina and to remain sexually abstinent during the study for safety reasons and to ensure integrity of the genital samples collected for pharmacokinetic analyses. Sexual abstinence was defined as not engaging in receptive vaginal, oral, or anal intercourse.
Measures
After obtaining informed consent and confirming trial eligibility, behavioral assessments, including sexual activity and vaginal practices, were administered at enrollment and weekly thereafter, during the study product-use period (days 7, 14, 21, and 28) via computer-assisted self-interviewing (CASI) to ensure maximum confidentiality of responses. Ring adherence (defined as ring never fully out of the vagina), ring removals, partial and full ring expulsion, and reasons for those were assessed by CASI and clinical interviews through Case Report Forms (at weekly follow-up visits on days 7, 14, 21, and 28) as described in Table I. Overall acceptability of the ring, concerns about the ring, and use attributes and experiences were assessed via CASI at baseline and/or day 28 (see Table I). A semi-structured interview (SSI), conducted by an experienced and study-trained interviewer and lasting for approximately 30 minutes, was administered immediately after CASI on day 28, and participants were asked in greater depth about ring physical attributes, their experience using the ring, changes in feelings about the ring over time, concerns about wearing the ring for 28 days, and recommendations about the ring for future use. Interviewers took handwritten notes during the SSI, then typed and expanded their notes immediately after completing each SSI. In this manuscript, indented, italicized text indicates summaries of responses provided by participants as noted by interviewers during the SSI, paraphrased as close as possible to what participants actually stated, italicized text with quote marks (" ") indicates actual verbatim responses captured in interviewer notes.
Table I.
Ring adherence and acceptability measures
| Adherence | Acceptability | ||||
|---|---|---|---|---|---|
| Data collection mode |
Concerns | Overall | Use attributes and experiences |
Physical attributes |
|
| CASI, CRF | CASI, SSI | CASI, SSI | CASI, SSI | SSI | |
| Topics | Ring removals, partial and full ring expulsions, and reasons why | Usage, side effects, health, hygiene, social dis/approval. | Like/dislike | Comfort (overall emotional, genitourinary), ease of use (insertion, removal), ring awareness and use regimen preference (continuous, episodic, removal during menses) | Size, shape, color, feel, and texture. |
| Timing of assessment | Days 7, 14, 21, 28 | Days 0, 28 | Day 28 | Day 28 | Day 28 |
Adherence was assessed at each follow-up visit by CASI and CRF. Acceptability, assessed by CASI, included a list of initial concerns about the ring, at baseline before ring insertion (day 0). The survey of concerns included 11 statements with check boxes adapted from prior ring studies (9, 19), with a mark all that apply instruction, and the additional option to specify any other concern. Alternatively, participants could check a box at the end of the list indicating that they had no concerns. A longer CASI, administered at product use end visit (day 28), re-assessed the same list of concerns experienced at any time during the study asked how concerns about the ring had changed (increased, decreased or stayed the same) and assessed overall like, use attributes, and ring experiences. Acceptability was also explored by SSI at day 28.
CASI: computer-assisted self-interview; CRF: Case Report Forms used for clinical interviews; SSI: semi-structured interview.
Analysis
Descriptive statistics (mean, median, and percentages) are presented for demographic, behavioral, adherence, and acceptability measures for all enrolled participants. Ring concerns, assessed at baseline and day 28 are presented graphically, as are summaries of participant experiences with the ring and the degree to which the experiences bothered them. The McNemar test and exact p-values were used to compare the proportion of participants who expressed concerns about the product at baseline and at day 28. Two qualitative analysts (who were not the SSI interviewers) independently reviewed expanded SSI notes for quality and comprehensibility and conducted data analysis using NVivo (version 9.0, Burlington, MA). Analysis was performed after developing a codebook and establishing inter-coder reliability (ICR) at ≥ 80% using NVivo, with a set of 14 primary codes. During establishment of ICR, all discrepancies were discussed and resolved by consensus between the two analysts. The average ICR among the two coders was 92%. The codebook was adapted from a previous ring study (20) and included codes derived from the questions in the qualitative interview and codes that emerged thematically from an initial reading of the textual data. The codebook loosely followed a conceptual framework for understanding microbicide acceptability, which includes a time continuum (before using the ring in the study, experience actually using the ring, and likely use of the ring in the future) as well as attributes of the user, the product, the relationships and the broader social context (20). Coded textual SSI data were summarized by key topics and presented thematically to support and illustrate CASI findings.
Results
A total of 48 women were enrolled in the study and provided follow-up data through the product-use end visit (day 28); 47 participants (98%) completed the study (through the post-product termination visit). For the SSI, a total of 45 women were interviewed across three study sites: Boston (N=8), Pittsburgh (N=23), and Birmingham (N=14) at the day 28 visit. Because there was no difference in adherence or acceptability by study group, an expected finding given that the rings were identical except for the drugs involved (or absent), behavioral data were combined across study groups (data not shown). As shown in Table II, participants had a mean age of 29.6 years (standard deviation 6.2), 81% were unmarried, 75% had post-secondary school education, 50% were white, 83% defined themselves as heterosexual, 65% currently had a primary sex partner, and 67% were sexually active in the past 3 months. Three women reported ever injecting drugs, 2 reported lifetime transactional sex, and 17 (35%) had a history of sexually transmitted infection. All participants had used vaginal products in their lifetime, including tampons (96%), personal lubricants (56%), vaginal medication (50%), douching (44%), contraceptive vaginal ring (21%), or spermicides (21%) and 77% had ever used condoms.
Table II.
MTN-013/IPM026 participants background characteristics
| Characteristic | Category | N=48 | % |
|---|---|---|---|
| Site (retention) | Pittsburgh (retained) | 24 (23) | 50 (96) |
| Birmingham (retained) | 16 (16) | 33(100) | |
| Boston (retained) | 8 (8) | 17(100) | |
| Age | Median, mean (min-max) | 29.0, 29.6 (20–40) | |
| Married | Yes | 9 | 19 |
| No | 39 | 81 | |
| Earn an income | Yes | 35 | 73 |
| No | 13 | 27 | |
| Highest level of education | Secondary, complete | 12 | 25 |
| Attended college or university | 36 | 75 | |
| Latina or Hispanic descent | Yes | 3 | 6 |
| No | 45 | 94 | |
| Race | Black only | 18 | 38 |
| White only | 24 | 50 | |
| Other | 6 | 13 | |
| Past 3 months any recreational drug (not injection) | Yes | 4 | 8 |
| No | 44 | 92 | |
| Lifetime injection drug use | Yes | 3 | 6 |
| No | 45 | 94 | |
| Lifetime transactional sex | Yes | 2 | 4 |
| No | 46 | 96 | |
| Lifetime STI | Yes | 17 | 35 |
| No | 31 | 65 | |
| Sexual orientation | Lesbian/gay/homosexual | 3 | 6 |
| Straight/heterosexual | 40 | 83 | |
| Bisexual | 5 | 10 | |
| Has currently a primary sex partner* | Yes | 31 | 65 |
| No | 17 | 35 | |
| Menses through day 28 visit | Yes | 38 | 5.5, 5.7 |
| Median, mean (min-max) days | 79 | 2.5–9.3 | |
| Past 3-month sexual activity | Vaginal sex (with or without a condom) | 27 | 56 |
| Anal sex (with or without a condom) | 4 | 8 | |
| Receiving oral sex | 21 | 44 | |
| Giving oral sex | 22 | 46 | |
| Finger sex | 16 | 33 | |
| Nonpenetrative sex | 11 | 23 | |
| Inserting a sex toy | 3 | 6 | |
| Other (tantric sex) | 1 | 2 | |
| Ever used the following | Male/female condom | 37 | 77.1 |
| Vaginal products: | Contraceptive vaginal ring, cervical barrier or menstrual cup | 13 | 27.1 |
| Spermicidal sponge, cream, or jelly | 10 | 20.8 | |
| Douche or other personal hygiene product | 21 | 43.8 | |
| Tampons | 46 | 95.8 | |
| Personal or sexual lubricant | 27 | 56.3 | |
| Vaginal medication | 24 | 50 | |
One participant at the Pittsburgh site withdrew for personal reasons after day 29.
(*) Primary sex partner was defined as a person the participant has sex with on a regular basis or who she consider to be her main partner. This individual may be a lover, spouse, boyfriend, or girlfriend. Among the 13 participants with vaginal device experience, 9 had a history of contraceptive ring use only, 3 had experience with cervical barriers or menstrual cups only, and 1 had both. Finger sex was defined as “When you or a partner inserts finger(s) into your vagina”; non-penetrative sex was defined as: “When you have any kind of sex with a man or woman, without having something inserted inside of you (e.g. rubbing each other or mutual masturbation”. STI: sexually transmitted infection.
Adherence
Overall, participants reported following protocol requirements, with 43 of 48 participants (90%) reporting no sex, three participants (6%) reporting non-penetrative sex, and one participant giving oral sex. One participant reported vaginal sex, as she had considered withdrawing from the study; ultimately, she ended up staying until completion (see below).
Self-reported ring adherence was very high: 45 women (94%) reported never having the ring fully out during the 28-day ring use period. Two women reported partial expulsions (and were per our definition classified as adherers). Three women fully removed the ring; of these, one also reported two episodes of partial expulsion during urination and bowel movement. None of the removals lasted more than 36 hours (Table III).
Table III.
Ring adherence and cases of partial and full expulsions during the 28 days of product use
| Ring Expulsion Status | N=48 | % | Duration | Explanation | Action Taken |
|---|---|---|---|---|---|
| Never out for 28 days* | 43 | 90 | NA | NA | |
| Partially out only* | 2 | 4 | NA | No explanation provided | |
| Ring fully out only (removals) | 2 | 4 | |||
| 36 hours | Participant had a new job and wanted to discontinue the study. She had vaginal sex then removed the ring. | Reinserted at the clinic after continuation in the study was deemed possible. | |||
| 24 hours | Protocol required removal because of candidiasis diagnosis | Reinserted at the clinic per clinical instructions. | |||
| Ring fully out and partially out | 1 | 2 | 1 minute | Participant removed the ring once to inspect discoloration on the ring. Participant also described two partial expulsions (slippage) during urination and bowel movement. She removed the ring, rinsed it, and reinserted it immediately "with no problems." | Self-reinserted |
(*) These participants were classified as ring “adherent. NA: not applicable.”
Ring Concerns
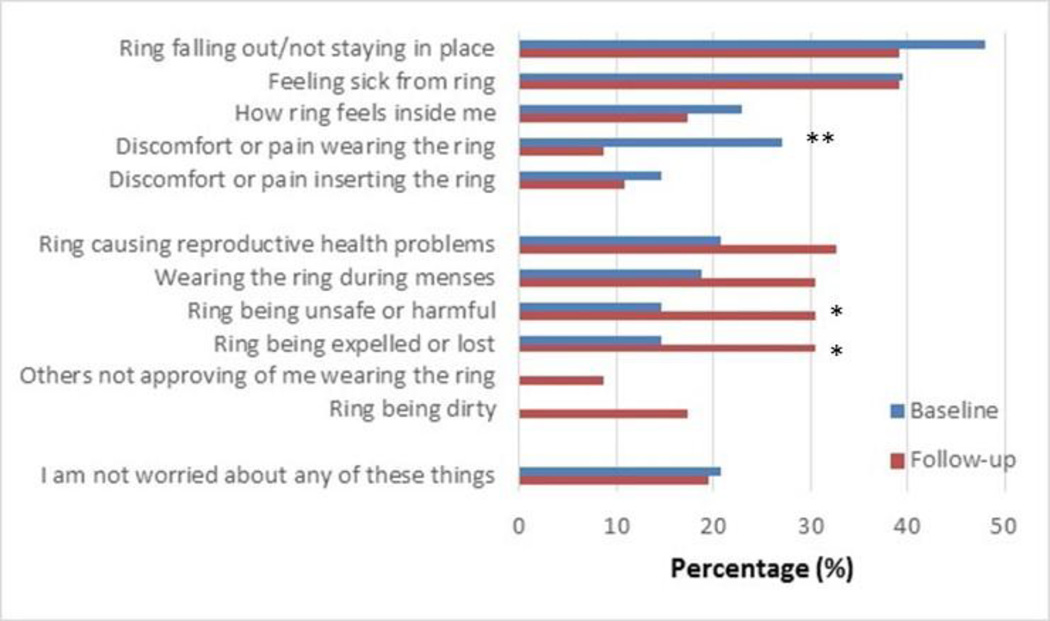
About 20% of participants (10/48) had no initial worries or concerns about the ring at baseline, before initiating use. Of those, five reported no concerns at follow-up, four reported at least one concern at follow-up, and one did not complete a follow-up acceptability interview. At day 28, about the same number (9/46) reported having had no concerns during the study (see Figure 1). A majority (33/46; 72%) had some concerns at both baseline and during follow-up, whereas 11% (5/46) reported no concerns at both assessments. The four participants (8%) who had indicated no concerns at baseline but reported concerns at follow-up mentioned the ring being dirty (n=1); feeling sick from wearing the ring (n=1); and the ring causing infection, infertility, or other reproductive health problems (n=2). During the SSI, these four participants indicated that these were only minor worries or that the worries were transient. Overall, participants’ concerns about immediate physical effects decreased. Per CASI, worries about discomfort or pain wearing the ring significantly decreased between baseline and day 28 (26% vs. 9%, McNemar test p<0.01). However, concerns about harmful health effects and expulsion increased: more participants were concerned about the ring being unsafe or harmful during follow-up (30%) than at baseline (15%; McNemar test p=0.04). More also had concerns about the ring being expelled or lost during follow-up (30%) than at baseline (15%; McNemar test p=0.04). New and mostly minor concerns emerged during follow-up in a small proportion of participants: the ring being dirty (18%) and partners not approving of ring use (9%). These new concerns were discussed during the SSI; for example, participants reported that their partners’ issues were mainly related to being worried about her safety and her experiencing side effects. For dirtiness, one participant explained her concerns about hygiene:
I did not like that the ring was "dirty" from being in there for so long. Or at least I felt it was dirty. I felt like I wanted to rinse the ring out. Nothing particular about the ring made me feel that way, I just have a high standard of cleanliness. (Age 24)
Figure 1. Concerns about the ring at baseline and during the study. N=48.
Initial ring concerns were assessed before ring insertion at baseline, and at the day 28 follow-up visit, for concerns during the study (N=48). The McNemar test was used to compare the proportion reporting concerns at baseline and follow-up. “Others not approving of me wearing the ring” was explained as “my significant other, partner or family members.” All four participants who marked this response at follow-up were referring to their male partner, as clarified during the SSI. (*) p=0.04; (**) p<0.01
Several concerns came and went, and participants often stated that ultimately they were happy with the ring. One participant said that at times, she was worried about not feeling the ring internally and would check her bed in the morning to make sure it had not fallen out. But ultimately she stopped worrying about it. She said, “the product is so good you can’t feel it.” (Age 25)
Another similarly reported,
After inserting the ring I didn’t feel it. Then I started to wonder if it was in right. I started to feel anxious about the ring, not sure of the side effects after I inserted it. Even though I didn’t feel the ring while I was wearing it I was still anxious about the ring, and wondering if it was still in. (Age 31)
Initial concerns with the ring’s size and appearance also mostly disappeared after use:
When I opened the package it was a bit intimidating. All I thought was, “How am I going to put this in there?” When I saw the ring it was bigger than I thought. After I inserted it, I felt accomplished, even more so when I couldn’t feel it and I didn’t have to deal with it. (Age 36)
Ring Experience and Acceptability
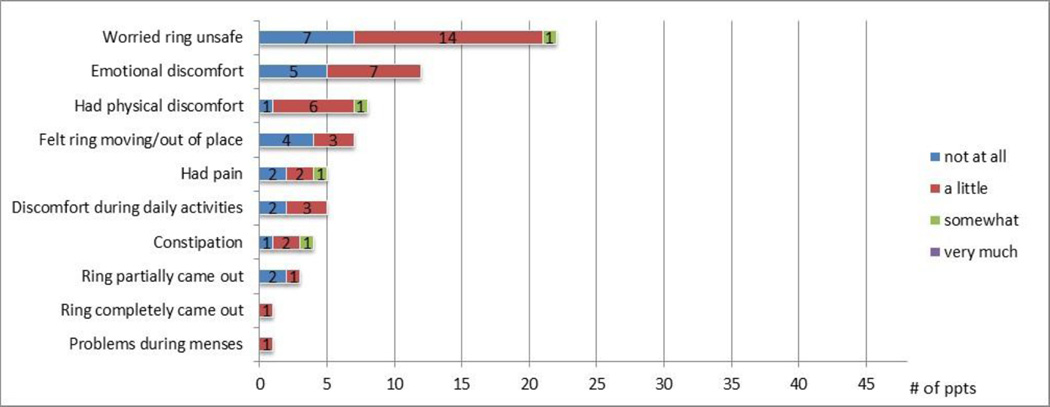
At day 28, participants were asked by CASI about ring-related negative emotions experienced during the study: 49% reported having been worried about the safety of the ring or that the ring may be harmful (22/45) and 27% reported having had emotional discomfort such as worries, fears, guilt, or any other unpleasant feelings (12/45). However, most participants reported that these feelings were of low intensity (Figure 2).
Figure 2. Ring-related negative experiences during the study by degree of discomfort, among those reporting any problem.
Assessed by CASI at the product use end visit (day 28 visit) among those who completed the acceptability survey (N=45). The denominator pertaining to problems during menses was answered by 40 participants (5 among those who did not have menses reported “not applicable”). Participants were first asked if they ever experienced any specific ring-related problem during the study (yes/no). If yes, they were then asked how much they were bothered by it on a 4-point Likert scale. (Combined responses to the two questions are presented.) Note: the answer “very much” was never selected.
Overall, when focusing on actual ring use experience participants’ responses were positive: per CASI, 100% (46/46) reported that they liked or very much liked the ring, 100% also reported that it was easy or very easy to use, and 93% (42/45) were comfortable or very comfortable with having the ring in their vagina. At the end of product use, women were asked how much they liked the ring now, compared with when they started the study; 37% (17/46) liked the ring more than when they started, and no one liked it less. Two participants (4%) said they would prefer not to wear the ring daily, whereas 52% (24/46) had no preference and 43% (20/46) preferred daily use.
Per CASI, 87% (39/45) said that the ring was easy or very easy to insert (note: per protocol, staff removed the ring). For those who experienced some difficulties with insertion, most instances were initial difficulties with getting the ring into a “figure 8” shape as reported during the SSI. Several participants also mentioned during the SSI that ring insertion was easier than anticipated, but initially they were intimidated or worried that they had done it incorrectly.
When asked about difficulties or negative experiences using the ring by CASI, a few participants reported infrequent discomfort or pain (once or twice) during the study, and with low intensity; the most frequent level of inconvenience was “a little”, and no one reported being bothered “very much” (Figure 2). One participant reported a minor problem with the ring during her menses, described as more cramping.
Overall, 61% (28/46) had no preference about the ring during menses and 17% (8/46) said they would prefer not to wear the ring during menses. Among the 38 participants who menstruated while using the ring, 66% (25) had no preference, 21% (8) preferred not to wear the ring, and 13% (5) preferred wearing the ring during menses. Of those who menstruated, 37% (14/38) indicated at day 28 that they had some worries about wearing the ring during menses compared with none among those who did not menstruate (Fisher’s exact test p=0.08). Menstruation was not associated with the following outcomes: overall ring acceptability, preference for daily versus episodic use, or likelihood of daily use in the future. In other subgroup analyses, we found no association between age (20 to 29 vs. 30 to 40 years old) and having a history of vaginal device use (contraceptive ring, cervical barriers or menstrual cup; N=13) or a history of douching (N=21) and the same acceptability and preference outcomes (data not shown).
Five women experienced ring discoloration where the ring became more yellow. None raised this as a problem. One participant mentioned the discoloration during her SSI, which she believed was due to wearing the ring during menstruation. She said she would prefer to avoid wearing the ring during menses, and said she would not want to wear the ring more than 28 days because it “would seem dirty” (Age 22). This participant also reported removing the ring herself to examine the discoloration and had two partial expulsions (Table II). During the SSI, another participant wondered whether the ring would discolor because of menses or because of the presence of the DPV medication. The main issues relative to menses discussed in the SSI were the inconvenience of not using tampons per protocol requirement (this was for the pharmacokinetic and safety endpoints, as tampon use is not contraindicated with the vaginal ring). Two other participants, similarly to the one mentioned above, had experienced their menses and in the SSI said they would prefer to remove the ring during their menses because it felt dirty. Although opinions were divided on keeping versus removing the ring during menses, one woman suggested an alternative: changing the ring after her periods, for cleanliness reasons. A few women attributed a variety of menstrual changes to the ring (e.g. longer or shorter menses, more or less cramping, change in thickness or volume of menses).
By CASI, 10 participants reported that their vagina felt wetter while wearing the ring, and none indicated it was bothering them; no one reported that their vagina was drier. During the SSI, the most common complaints were about increased vaginal discharge or discomfort as one participant reported, “It seemed like I was a bit wetter, like not a whole lot and not enough to matter but enough to notice.“ (Age 23). Another participant said she found that the ring made her vagina feel tighter and more sensitive in a good way, and she was able to orgasm without penetration (Age 29).
Regarding hypothetical preference for use: continuous (as experienced in the study) versus episodic (defined as not wearing the ring every day), opinions expressed during the SSI were divided, as highlighted below. One participant had no problem with continuous use as long as she could not feel the ring, which was her experience during the study. She said using a product like this is a “no brainer” (Age 25). Another said:
I would feel more protected from HIV with continuous use. There is also less to worry about with continuous use. Sometimes sex happens spontaneously. Therefore episodic use would not be ideal. I felt comfortable with the 28-day time period with the exception of the time that I had my period. It would be better if I were permitted to use tampons.” (Age 20)
However, another stated:
If it was episodic it would be “pretty awesome.” I personally wouldn’t be worried of any sort of irritation, if the ring was used episodically. Continuous use just seems more prone to infection even though a doctor says it is okay. Having the ring in for so long just seems unnatural…because it seems that since it is “foreign” your body should reject the ring even though the ring is manufactured to not be recognized as a foreign object. (Age 35)
Another woman also preferred episodic use, with the option to remove the ring during her periods and when having sex: “I felt like it was unclean during my period and I imagine it would be uncomfortable for both of us during sex.” (Age 24)
Product Physical Attributes
Opinions about ring physical attributes were primarily probed during the SSI (see Table I). When asked about what they did not like about the ring, although several women (N=14) indicated that there was nothing they actively disliked about it, 19 said that they felt intimidated by, and disliked, the ring’s size (overall diameter) and thickness (rod diameter). Four participants said that the thickness of the ring made it challenging to insert. One participant said, “The ring was a bit frightening because it was big. The thickness was scary. I had a little difficulty inserting the ring for the first time.” (Age 24)
Once the ring was inserted, nearly all of these women said that they were pleasantly surprised that the ring was not noticeable. As this participant said:
I didn’t like the size and the thickness of the ring. When I first saw the ring I was intimidated by the size. I thought I would feel it since it was so big and bulky. The thickness of the ring made me think I would have problems inserting the ring since it looked to be too big. I did not have problems with inserting it…After I inserted the ring I didn’t feel it, so I felt relieved that I didn’t feel it and it didn’t hurt me since it was so big. While wearing the ring I didn’t feel it so I totally forgot it was even in there and I didn’t worry about it. (Age 27)
One participant did not like that she could not feel the ring, and one said that not feeling it was both a positive and negative quality of the ring; she liked not noticing it was inserted but this also contributed to worry about potential unnoticed problems:
I liked least the fact that you could not feel the ring…. Not feeling the ring can be a good thing and it can be a bad thing. During the study, I checked it a lot to see if it was there. I didn't need to check it for positioning; I just wanted to check if it was there. (Age 20)
In the SSI, participants had recommendations about the ring’s physical attributes: regarding color, 26 preferred the white color, because it is simple, plain, or looked “medical grade.” Some had concern that a dye may affect health. Nine said the ring is not seen, so color does not matter, whereas 10 preferred another color (e.g., purple, pink, blue, multicolor, glow-in-the-dark, “fun colors”) to make the ring look more fun, “less sterile,” more natural, or more “feminine”. Notably, one participant thought it would be interesting if the ring changed color as the medicine is released to know if it was used correctly (Age 27). About a third (18) of the participants stated that they would prefer the ring to be thinner, and a few (4) would prefer the ring to be more flexible.
Discussion
This is the first study of a combination microbicide vaginal ring intended for monthly use. Healthy, sexually abstinent U.S. women used the ring for 28 days and found it acceptable; adherence was very high and minimal removals or expulsions were reported. Consistent and continuous use of the medicated ring will likely be important to ensure that drug levels do not fall below the threshold for protection against HIV acquisition. Indeed, in this trial, the decline in drug levels of DPV and MVC were both greater than 10-fold by 3 days post-use for all rings, and tissue DPV levels were below the lower limit of quantification by day 3 (19). This emphasizes the need for high adherence to continuous ring use in order to maintain sufficient drug levels for HIV inhibition.
Overall, participants’ experience with the ring was mostly positive, and although many women were initially intimidated by the ring, most women were pleasantly surprised by their experience with the ring after they initiated use. Women reported very few problems with the ring and were very positive about the product. The attribute that participants liked most about the ring was being able to forget about it. Being worry free and able to “forget it” were salient characteristics of other topical dosage forms as well (21). The least liked attributes were related to the size and thickness of the ring, which resulted in minor insertion problems for some participants. This was also reported in a previous study of this placebo silicone ring in sub-Saharan Africa (20). Different rings are being developed with different thicknesses, sizes, polymer composition, and duration of use (22). It will be important to assess users’ reactions, preferences, and ease of use across these different types of rings. The appearance of the ring could be a deterrent for some and should be addressed in educational messages that highlight the importance of trying before deciding if one likes it or not. Indeed, very few problems were reported after insertion, insertion itself got easier with practice, and for many, concerns about the ring decreased or disappeared with time. Concerns that were more frequently reported during the follow-up period were typically transient, and those that emerged were reportedly minor.
Most participants had their menses during the study, yet very few reported problems using the ring during menses, no ring removals were due to menses, and about half of the participants stated no preference regarding wearing the ring during their menses or not. Some minor concerns around ring use during menses were raised during the SSI, including the importance of being able to use tampons with the ring, fear of dirtiness, and possible changes in menstrual patterns. Larger ongoing trials may help to ascertain whether ring use has any effect on menses. Several concerns were reported at baseline, but very few of the concerns actually materialized. As reported in another study, many concerns decreased over time and with use of the ring, such as skills-related concerns (20). However, new concerns increased or emerged during the study, most prominently concerns about harm and safety of the product. These may be related to being in a blinded trial and testing investigational products, along with experiencing complex and sometimes invasive study procedures for safety and pharmacokinetic assessments. Dirtiness of the ring also emerged as a concern at study follow-up and may be grounded in the actual experience of keeping a device in the vagina continuously for 1 month. Hygiene concerns are important to investigate and are closely monitored in ongoing Phase III ring trials, as they may play a role in women’s decision-making about ring use.
There are several limitations to this study. First, the study was conducted with a small sample of low-risk women during a Phase I trial. Exploratory subgroup analyses were underpowered because of a small sample size and could have detected only a very large effect size. However, much of the qualitative information we collected provided useful insight into ring acceptability and experiences. MTN-013/IPM 026 was brief, with use required only for 28 continuous days, so participants did not have the opportunity to have extended experience wearing the ring during menses. Menses-related concerns and removals related to menses were mentioned in a sub-Saharan African study with a placebo ring (11), and menses-related issues are carefully monitored in current Phase III ring trials. Participants were also required to not use tampons and had numerous biological samples collected.
These protocol requirements may also affect women’s perceptions about the cleanliness and safety of the ring. Acceptability and adherence in this sample may not be generalizable to other populations or geographical settings. Indeed, women were asked to be sexually abstinent, so the high acceptability reported here is among abstinent ring users. The importance of acceptability in the context of sexual intercourse cannot be determined in this study. Further, most participants had a history of tampon use, and in other settings, insertion of vaginal products may be less common. Higher product adherence in the United States as compared with Africa was reported for daily vaginal gel and oral tablet in a multisite open-label prevention trial (23), so it is not clear if the high adherence experienced in this study will translate to other settings. Current Phase III trials of the vaginal ring in Africa are using drug biomarkers to assess ring adherence and will provide evidence in the near future as to whether high ring adherence holds its promise with long-term use, sexual activity, and in different populations (12,13).
Many of the problems or concerns reported here can be addressed with appropriate counseling, similar to the recommendations provided in a study of the contraceptive ring among young U.S. women (24). Addressing initial concerns early and proactively may facilitate ring uptake in the future and decrease worries about ring use. First ring insertion in the clinic is another way of overcoming initial fears with an unfamiliar product. Other methods such as the female condom (25) and the diaphragm had facilitated acceptance through initial insertion at the clinic to overcome barriers with use and to assess user skills (26).
In conclusion, socio-behavioral findings about the vaginal ring in this Phase I study suggest that this method holds strong promise as a sustained, user-controlled, reversible approach for multidrug vaginal microbicide delivery.
Acknowledgments
sources of support:
We would like to acknowledge the women who participated in this study and to extend special thanks to the clinical research sites’ teams in Pittsburgh, PA, Birmingham, AL, and Boston, MA. The full MTN-013/IPM 026 study team can be viewed at http://www.mtnstopshiv.org/studies/2241. The study was designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The International Partnership for Microbicides (IPM), IND sponsor, supplied the products used in this study (dapivirine vaginal ring [VR], maraviroc VR, maraviroc/dapivirine VR, placebo VR).
Footnotes
The link to the vaginal ring use instructions sheet provided to participants is http://www.mtnstopshiv.org/sites/default/files/attachments/Section%209%20-Study%20Product%20Considerations%20-%20Version%201.0%2002Sept11.pdf (section 9.1).
REFERENCES
- 1.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26(7):F13–F19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 2.Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annual review of medicine. 2013;64:219. doi: 10.1146/annurev-med-050911-163701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrix CW, Andrade A, Kashuba A, et al. Tenofovir-Emtricitabine directly observed dosing: 100% Adherence Concentrations (HPTN 066). Paper presented at 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston. [Google Scholar]
- 4.Amico K. Adherence to preexposure chemoprophylaxis: the behavioral bridge from efficacy to effectiveness. Curr Opin HIV AIDS. 2012;7:542–548. doi: 10.1097/COH.0b013e3283582d4a. [DOI] [PubMed] [Google Scholar]
- 5.Creinin MD, Meyn LA, Borgatta L, et al. Multicenter Comparison of the Contraceptive Ring and Patch: A Randomized Controlled Trial. Gynecol Oncol. 2008;111(2, Part 1):267–277. doi: 10.1097/01.AOG.0000298338.58511.d1. 210.1097/1001.AOG.0000298338.0000258511.d0000298331. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. [Google Scholar]
- 7.Friend D. Intravaginal rings: controlled release systems for contraception and prevention of transmission of sexually transmitted infections. Drug Deliv Transl Res. 2011;1(3):185–193. doi: 10.1007/s13346-011-0024-4. [DOI] [PubMed] [Google Scholar]
- 8.Woodsong C, Montgomery E, Masenga G, et al. Safety and acceptability of vaginal ring as microbicide delivery method in African women. Paper presented at Microbicides; May 23–26, 2010; Pittsburg PA. 2010. [Google Scholar]
- 9.van der Straten A, Woodsong C, ET M, Nel A. High adherence and acceptability of a monthly Dapivirine vaginal ring for HIV prevention in Africa. Paper presented at International Microbicides Conference; Sydney, Australia. 2012. [Google Scholar]
- 10.Nel A, Kamupira M, Woodsong C, van der Straten A, ET M, van Niekerk N. Safety, acceptability, and pharmacokinetics assessment (adherence of monthly Dapivirine Vaginal Microbicide Rings (Ring-004) for HIV prevention. Paper presented at Conference on Retrovirus and Opportunistic Infections; Seattle. 2012. [Google Scholar]
- 11.Montgomery E, van der Straten A, Cheng H, et al. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;16(7):1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- 12.Microbicides Trial Network; 2012. Phase III trial of dapivirine ring begins in Africa: ASPIRE testing new HIV prevention approach for women. http://www.mtnstopshiv.org/node/4546. [Google Scholar]
- 13.International Partnership for Microbicides, Inc; 2012. First efficacy trial of a microbicide ring to prevent HIV is underway: the ring study to assess IPM’s monthly ARV ring for women. http://www.ipmglobal.org/publications/first-efficacy-trial-microbicide-ring-prevent-hiv-underway. [Google Scholar]
- 14.NuvaRing. Registration In South Africa, MSD Pharmaceuticals. 2012 [Google Scholar]
- 15.Novak A, de la Loge C, Abetz L, van der Meulen EA. The combined contraceptive vaginal ring, NuvaRing: an international study of user acceptability. Contraception. 2003 Mar;67(3):187–194. doi: 10.1016/s0010-7824(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 16.Brache V, Faundes A. Contraceptive vaginal rings: a review. Contraception. 2010;82(5):418–427. doi: 10.1016/j.contraception.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Brache V, Payán LJ, Faundes A. Current status of contraceptive vaginal rings. Contraception 3// 2013;87(3):264–272. doi: 10.1016/j.contraception.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Stewart FH, Brown BA, Raine TR, Weitz TA, Harper CC. Adolescent and young women's experience with the vaginal ring and oral contraceptive pills. J Pediatr Adolesc Gynecol. 2007 Dec;20(6):345–351. doi: 10.1016/j.jpag.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen B, Panther L, Marzinke MA, et al. Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: a Double-Blind Randomized Trial. Journal of Acquired Immune Deficiency Syndromes. 2015 May 28; doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Straten A, Montgomery E, Cheng H, et al. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in African women. AIDS Behav. 2012;16(7):1775–1786. doi: 10.1007/s10461-012-0215-0. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg JJ, Rosen RK, Bregman DE, et al. "Set it and forget it": women's perceptions and opinions of long-acting topical vaginal gels. AIDS Behav. 2014 May;18(5):862–870. doi: 10.1007/s10461-013-0652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malcolm R, Fetherston S, McCoy C, Boyd P, Major I. Vaginal rings for delivery of HIV microbicides. International Journal of Women's Health. 2012 Nov 20;4(1):595–605. doi: 10.2147/IJWH.S36282. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minnis AM, van der Straten A, Salee P, Hendrix CW. Pre-exposure Prophylaxis Adherence Measured by Plasma Drug Level in MTN-001: Comparison Between Vaginal Gel and Oral Tablets in Two Geographic Regions. AIDS Behav. 2015 May 13; doi: 10.1007/s10461-015-1081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein LB, Sokal-Gutierrez K, Ivey SL, Raine T, Auerswald C. Adolescent experiences with the vaginal ring. J Adolesc Health. 2008 Jul;43(1):64–70. doi: 10.1016/j.jadohealth.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witte SS, El-Bassel N, Gilbert L, Wu E, Chang M, Hill J. Promoting female condom use to heterosexual couples: findings from a randomized clinical trial. Perspect Sex Reprod Health. 2006 Sep;38(3):148–154. doi: 10.1363/psrh.38.148.06. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery E, Blanchard K, Cheng H, et al. Diaphragm and lubricant gel acceptance, skills and patterns of use among women in an effectiveness trial in Southern Africa. Eur J Contracept Reprod Health Care. 2009;14(6):410–419. doi: 10.3109/13625180903215430. [DOI] [PubMed] [Google Scholar]