Abstract
Stimulant use disorders are associated with deficits in striatal dopamine receptor availability, abnormalities in mesocorticolimbic resting-state functional connectivity (RSFC), and impulsivity. In methamphetamine-dependent research participants, impulsivity is correlated negatively with striatal D2-type receptor availability, and mesocorticolimbic RSFC is stronger than in controls. The extent to which these features of methamphetamine dependence are interrelated, however, is unknown. This question was addressed in two studies. In Study 1, 19 methamphetamine-dependent and 26 healthy control subjects underwent [18F]fallypride positron emission tomography to measure ventral striatal dopamine D2-type receptor availability, indexed by binding potential (BPND), and functional magnetic resonance imaging (fMRI) to assess mesocorticolimbic RSFC, using a midbrain seed. In Study 2, an independent sample of 20 methamphetamine-dependent and 18 control subjects completed the Barratt Impulsiveness Scale in addition to fMRI. Study 1 showed a significant group by ventral striatal BPND interaction effect on RSFC, reflecting a negative relationship between ventral striatal BPND and RSFC between midbrain and striatum, orbitofrontal cortex, and insula in methamphetamine-dependent participants but a positive relationship in the control group. In Study 2, an interaction of group with RSFC on impulsivity was observed. Methamphetamine-dependent participants users exhibited a positive relationship of midbrain RSFC to the left ventral striatum with cognitive impulsivity, whereas a negative relationship was observed in healthy controls. The results indicate that ventral striatal D2-type receptor signaling may affect system-level activity within the mesocorticolimbic system, providing a functional link that may help explain high impulsivity in methamphetamine-dependent individuals.
Keywords: resting state functional connectivity, D2 dopamine receptors, mesocorticolimbic system, drug abuse, methamphetamine, midbrain, striatum
Introduction
Chronic stimulant exposure can produce profound and long-lasting changes in the brain, affecting dopaminergic markers and associated brain function and behavior (1-4). Low striatal D2-type receptor availability is associated with impulsivity and enhanced escalation of cocaine self-administration in rats (5), and can predict failure of behavioral treatment in stimulant users (6, 7). D2-type receptor deficits also may contribute to the behavioral phenotypes that accompany addiction, as evidenced by negative association of striatal D2-type receptor availability with impulsivity (2) and temporal discounting of rewards (8) in methamphetamine-dependent subjects. Thus, dysfunction in dopamine signaling may promote the initiation as well as the maintenance of addiction.
Stimulant-induced impairments in reward-driven behavior are associated with aberrant signaling within the mesocorticolimbic dopamine system in animals (9, 10). Consistent with these findings is the observation that methamphetamine-dependent subjects have stronger resting-state functional connectivity (RSFC) of the midbrain to terminal field regions of the mesocorticolimbic system than control subjects (4). Midbrain RSFC also is related to impairments in prefrontal cortical function during reward-related, risky decision-making in methamphetamine-dependent individuals (4).
Stimulant-induced dopaminergic neurotransmission produces neuroplastic changes in the ventral striatum, including alterations in glutamatergic transmission (11), synaptic plasticity, and dendritic spine morphology (11-16). Such neural adaptations and associated reorganization of dopaminergic brain networks are thought to underlie deficits in inhibitory control and impulsivity linked with addiction (17). Accordingly, dopamine D2-type receptor deficits in the ventral striatum may contribute to abnormal signaling within the mesocortical system and impulsive behavior in methamphetamine-dependent individuals.
The goal of this study was to investigate the potential links between ventral striatal dopamine D2-type receptor availability, intrinsic midbrain activity, and measures of impulsivity in methamphetamine-dependent subjects. In one study, the relationship between ventral striatal dopamine D2-type receptor availability and midbrain RSFC was examined in methamphetamine-dependent and healthy control subjects. On the basis of findings that methamphetamine-dependent subjects exhibit lower D2-type receptor availability throughout the striatum (2) and greater RSFC between midbrain and striatum than healthy controls (4), it was expected that methamphetamine-dependent participants would exhibit a negative relationship between ventral striatal D2-type receptor availability and RSFC of midbrain to striatum. In the second study, the relationship of midbrain RSFC to self-reported impulsivity was evaluated. As impulsivity is negatively related to ventral striatal D2-type receptor availability in methamphetamine-dependent subjects (2), it was hypothesized that the strength of RSFC between the midbrain and the ventral striatum would be positively related to impulsivity in methamphetamine-dependent subjects studied here.
Methods
Eighty-two participants (44 healthy control and 39 methamphetamine-dependent subjects) received a complete explanation of the procedures to be used, and provided written informed consent, as approved by the UCLA Institutional Review Board. Methamphetamine dependence was determined by the Structured Clinical Inventory for DSM-IV-TR (18) or the Mini-International Neuropsychiatric Interview (MINI) (19). In Study 1, 26 healthy control subjects and 19 methamphetamine-dependent subjects took part in positron emission tomography (PET) as well as resting-state fMRI. The healthy controls completed the imaging procedures as part of a large range of assessments while participating in the UCLA Consortium for Neuropsychiatric Phenomics (CNP; www.phenomics.ucla.edu) (20). In Study 2, an independent sample of 18 healthy controls and 20 methamphetamine-dependent subjects took part in resting-state fMRI and provided self-report measures of impulsivity. Exclusion criteria, determined by physical examination, medical history, and laboratory blood tests, were systemic, neurological, cardiovascular, or pulmonary disease, or head trauma with loss of consciousness. Current Axis-I diagnoses other than nicotine dependence (any group) and methamphetamine dependence (methamphetamine user groups) were also exclusionary.
Measure of impulsivity: Barratt Impulsiveness Scale
Self-report data were collected using the Barratt Impulsiveness Scale (BIS-11) (21). A 2-factor model was implemented to determine scores for cognitive and behavioral impulsivity, on the basis of a psychometric evaluation of the measure (22). According to the model, cognitive impulsivity reflects difficulties in attentional control, concentration, careful and deliberate thinking, and planning; whereas behavioral impulsivity reflects impulsive action, racing thoughts, and frequent changes in employment and residences.
fMRI acquisition
Functional magnetic resonance imaging (fMRI) was performed on a 3-T Siemens Trio MRI system, with 152 resting-state T2*-weighted, echoplanar images (EPI) acquired (slice thickness = 4 mm; 34 slices; TR = 2 s; TE = 30 ms; flip angle = 90°; matrix = 64 × 64; fov = 200 mm). Resting-state images were acquired for 5 min, while participants viewed a black screen. High-resolution, T2-weighted, matched-bandwidth (MBW) and T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scans were also acquired. The orientation for these scans was oblique axial to maximize brain coverage and to optimize signal from ventromedial PFC.
PET imaging acquisition
Dopamine D2-type (D2 and D3) receptor availability, measured as binding potential (BPND), was assessed using [18F]fallypride, a radioligand with high affinity for dopamine D2-type receptors (23). PET scanning was performed on a Philips Gemini Tru Flight PET/CT tomograph in 3D mode (Philips Electronics NV, Netherlands) (resolution = 5.0 mm × 4.8 mm, full width at half maximum (FWHM)). Images of 90 slices were obtained with a 2×2×2-mm3 voxel size. A CT-transmission scan was performed to obtain data for measured attenuation correction. Dynamic emission data were acquired after bolus injection of the radiotracer (~5 mCi ± 10%), specific activity ≥ 1 Ci/μmol) in two scanning blocks of 80-min each, as 1-min frames. To reduce discomfort and radiation exposure to the bladder wall, participants were allowed a short break between scanning blocks to void. Data were reconstructed using the 3D row action maximum likelihood algorithm (3D-RAMLA). Scatter and random corrections were applied.
Resting-state fMRI image processing
Image analysis was performed using FSL 5.0.2.1 (www.fmrib.ox.ac.uk/fsl). The images were realigned to compensate for motion (24), and high-pass temporal filtering (100 s) was applied. Data were skull-stripped and spatially smoothed (5-mm FWHM Gaussian kernel). Images were further pre-processed to include additional nuisance regressors: average signal of cerebrospinal fluid and white-matter, and two metrics of motion-related artifact, specifically frame-wise displacement and a combination of the temporal derivative of the time series and root-mean-squared variance over all voxels (25). Global signal regression was not applied. The EPI images were registered to the MBW image, then to the high-resolution MPRAGE image, and finally into standard Montreal Neurological Institute space, using a 12-parameter affine transformation and nonlinear registration using FMRIB's nonlinear image registration tool (FNIRT) (26). A 9-mm spherical midbrain ROI was created at the coordinates (MNI: x = 0, y = −15, z = 9) from a study examining differences in midbrain RSFC between methamphetamine users and controls (4). The mean time series across all voxels within the midbrain seed from pre-processed images were used as covariates in separate whole-brain, voxelwise resting-state correlation analyses.
PET image processing
Reconstructed [18F]fallypride PET data (2 blocks; 1-min × 80-frames) were averaged into 16 frames, each consisting of the average of 10-min of dynamic data. PET images were corrected for motion (24), then co-registered to the corresponding structural MRI (27). Volumes of interest (VOI) for the dorsal and ventral striatum were anatomically-defined using the FSL software package (28). The cerebellum VOI was drawn manually, as a bilateral region in MNI-152 space and transformed into native space for each participant's MRI scan.
VOI-based time-activity data were then extracted from PET images and imported into PMOD (PMOD 3.1, Zurich) for kinetic modeling. Time-activity curves were fit using the simplified reference tissue model (SRTM) (29). The cerebellum was selected as the reference region because it has a negligible concentration of D2-type receptors (30). A volume-weighted average of k2′, estimated from high-activity regions (caudate and putamen), was computed. Time-activity curves were refit using SRTM2 (31) applying the computed k2′ values to the VOIs. BPND, which is an index of receptor availability, was then calculated as BPND = R1*k2′/k2a – 1, where R1 = K1/K1′ is the ratio of tracer-delivery parameters from plasma to tissues in the target region and reference region, and k2a is the single-compartment rate constant for transfer from the target-region tissue compartment to plasma.
Analysis of the relationship between midbrain RSFC and dopamine D2-type receptor BPND
A test of group differences in the association between midbrain RSFC and ventral striatal BPND (group × BPND interaction) was conducted with a whole-brain voxelwise analyses of midbrain RSFC. Ventral striatal BPND (left and right regions combined) was used as a covariate of interest. Post-hoc analyses were conducted to test the strength and direction of the relationship between midbrain RSFC and ventral striatal BPND for each group. For within- and between-group mixed-effects analyses, all whole-brain fMRI statistics were corrected for multiple comparisons by using cluster-correction with voxel height threshold of Z > 2.3 and cluster significance of P < 0.05. Because there are age- and sex-related effects on brain function and striatal dopamine receptor availability (32-35), all analyses included age, which was mean centered and sex as covariates of no interest.
Analysis of the relationship between midbrain to ventral striatum RSFC and impulsivity
Following the observation of a significant group × ventral striatal BPND interaction with midbrain RSFC to left (but not right) ventral striatum, an anatomically-defined VOI using the Harvard-Oxford subcortical atlas was created for the left ventral striatum. The VOI of the left ventral striatum was used to extract average parameter estimates (β-values) from the midbrain RSFC contrast maps, which corresponds to the strength of functional connectivity with the midbrain. A general linear model was used to examine the relationship between connectivity values and self-report impulsivity using SPSS version 21. Connectivity values were entered as an independent variable in ANCOVA, which included and tested the main effects of age, sex, and group and the interaction between group and RSFC on impulsivity measures. The outcome measures of BIS cognitive and behavioral impulsivity scores were analyzed separately.
Results
Participant characteristics
Study 1 included 26 healthy controls (14 men, 12 women, 29.00 ± 8.45 years old) and 19 methamphetamine users (10 men, 9 women, 31.69 ± 8.71 years old). On average, individuals in the Methamphetamine Group used methamphetamine for 12.32 ± 6.74 years and on 26.0 ± 6.25 days in the 30 days preceding study entry. The Methamphetamine and Control Groups did not significantly differ in age, sex or the frequency of alcohol use (p's > 0.05), but they did differ in the frequency of marijuana use (t = 3.95, p = 0.003) (Table 1). Because methamphetamine-dependent individuals were more likely to be tobacco cigarette smokers (Chi-Square = 42.99, p < 0.001), the relationships between cigarette use and ventral striatal BPND, midbrain RSFC, and self-report impulsivity were examined. There were no significant relationships between the outcome measures and smoking status, cigarettes per day and the number of days of cigarette use in the 30 days preceding study entry.
Table 1.
Characteristics of Research Participants
| Study 1 (PET and RSFC) | Study 2 (RSFC and BIS-11) | |||
|---|---|---|---|---|
| Control Group (n=26) | Methamphetamine Group (n=19) | Control Group (n=18) | Methamphetamine Group (n=20) | |
| Age (years)b | 29.0 ± 8.45 | 30.95 ± 8.17 | 38.9 ± 9.63 | 37.0 ± 9.64 |
| Sex (female/male) | 12/14 | 9/10 | 5/13 | 7/13 |
| Alcohol Use | ||||
| Days used (in past 30) | 5.39 ± 6.70 | 5.37 ± 7.88 | 3.50 ± 5.43 | 3.45 ± 6.89 |
| Marijuana Use | ||||
| Days used (in past 30)ab | 0.08 ± 0.27 | 10.11 ± 13 | 0.11 ± 0.47 | 2.70 ± 7.26 |
| Tobacco Use (# smokers)a | 0 | 19 | 5 | 18 |
| Days smoked (in past 30) | 0 | 21.2 ± 2.54 | 13.9 ± 14.95 | 18.5 ± 12.50 |
| Methamphetamine Use | ||||
| Days used (in past last 30) | 26.0 ± 6.25 | 22.4 ± 7.37 | ||
| Years of heavy use | 12.3 ± 6.74 | 6.82 ± 4.91 | ||
Data shown are means ± SEM, where appropriate.
Significant differences between the groups by Student's t-test (p = 0.003).
Significant differences between the methamphetamine groups by Student's t-test (p= 0.04 and p = 0.03).
Study 2 included an independent sample of 18 healthy controls (13 men, 5 women, 38.94 ± 9.63 years old) and 20 methamphetamine-dependent subjects (13 men, 7 women, 37.00 ± 9.64 years old). The Methamphetamine and Control Groups did not significantly differ in age, sex, or frequency of alcohol, marijuana, or cigarette use in the 30 days preceding study entry. On average, individuals in the Methamphetamine Group used methamphetamine for 6.82 ± 4.91 years and on 22.39 ± 7.37 days in the 30 days preceding study entry (Table 1). The two Methamphetamine groups did not differ significantly in sex distribution, smoking status, or frequency of methamphetamine or alcohol use, but they did differ in age (p = 0.04) and frequency of marijuana use (p = 0.03).
RSFC and relationship to ventral striatal BPND
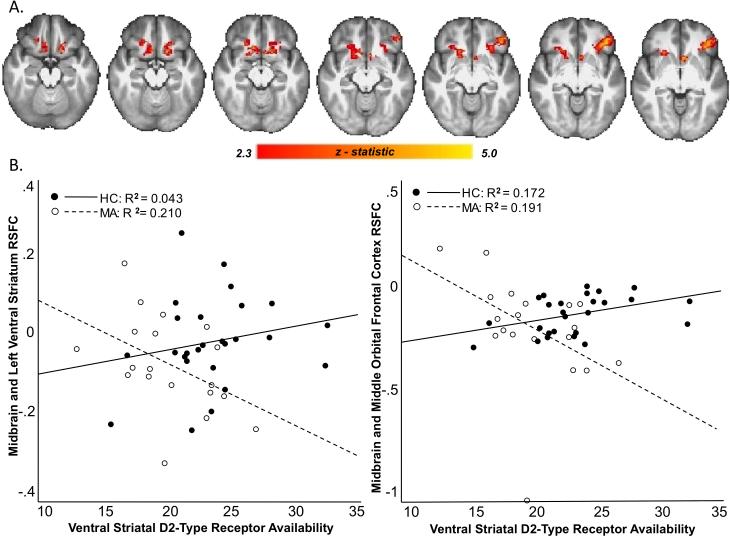
Methamphetamine-dependent subjects had lower ventral striatal BPND than controls (t = 2.862, df = 43, p = 0.006), and Levene's test indicated that there was equal variance between the groups (F=.05, p = 0.824). Relative to healthy controls, methamphetamine-dependent subjects exhibited stronger RSFC between the midbrain and caudate, putamen, insula and medial prefrontal cortex (p < 0.05, whole-brain corrected). There was a significant group by ventral striatal BPND interaction on RSFC between midbrain and orbital frontal cortex, putamen, ventral striatum, caudate, and insula (p < 0.05, whole-brain corrected) (Fig. 1). Results remained significant after age and sex were removed as nuisance covariates. Post hoc analyses showed that the interaction was driven by a significant negative relationship between ventral striatal BPND and midbrain RSFC to orbital frontal cortex, putamen, ventral striatum, caudate and insula in the Methamphetamine Group with no significant relationship in the Control Group (p < 0.05, whole-brain corrected).
Figure 1. Relationship between Midbrain RSFC and Ventral Striatal BPND.
A. Brain regions where the relationship between resting-state connectivity with the midbrain seed and ventral striatal BPND varied by group. Connectivity maps show a group by ventral striatal BPND interaction on RSFC of Midbrain with ventral and dorsal striatum, caudate, orbital frontal cortex, and insula (p < 0.05, whole-brain corrected). Sequential slices from Z of 27 to Z of 33 are shown. B. Scatter plots show the relationship between midbrain RSFC and ventral striatal receptor availability in the two groups (• HC and ○ MA).
Relationship between midbrain RSFC with left ventral striatum and impulsivity
As in a prior report (36), which included 15 of the 20 participants in study 2, the Methamphetamine group exhibited stronger midbrain RSFC with striatum, amygdala, hippocampus and medial orbital frontal cortex than the Control Group. The groups differed significantly in cognitive impulsivity (t = 2.331, df = 44, p = 0.012) and behavioral impulsivity (t = 1.71, df = 44, p = 0.04), with methamphetamine users reporting greater impulsivity than controls. There was a main effect of midbrain RSFC to left ventral striatum on cognitive impulsivity (t = − 2.79, p = 0.009). The relationship between cognitive impulsivity and RSFC between midbrain and left nucleus ventral striatum differed by group (t = 2.55 p = 0.016), with the Methamphetamine Group showing a positive relationship (r = .272, p = 0.10) and the Control Group showing a negative relationship (r = −.588, p = 0.017) (Fig. 2). There was no main effect of RSFC between midbrain and left ventral striatum on behavioral impulsivity (t = 5.44, p = 0.176) and no interaction between group and RSFC between midbrain and ventral striatum (t = −1.629, p = 0.113). Levene's test for equality of variance showed no significant group differences in variance in measures of cognitive impulsivity (F=.002, p = 0.965), behavioral impulsivity (F= 2.582, p= 0.117), or in RSFC between midbrain and ventral striatum (F= .753, p= 0.391). The results were not affected by removing age and sex as nuisance covariates.
Figure 2. Relationship between Midbrain and Ventral Striatal RSFC and Self-report Cognitive Impulsivity Scores.
Scatter plots show the relationship between self-report cognitive impulsivity on the Barratt Impulsiveness Scale and RSFC of midbrain with the left ventral striatum in the two groups (• HC and ○ MA). Slopes of the two plots differ significantly (group by RSFC interaction on cognitive impulsivity: p = 0.016). The RSFC measure was associated with DA receptor availability in an independent sample.
Discussion
This study provides support for the hypothesis that dopaminergic transmission is a common neurobiological factor influencing addiction and impulsivity (37). The results extend findings of heightened mesocorticolimbic RSFC (4) and an inverse relationship between striatal D2-type receptor availability and impulsivity in methamphetamine-dependent individuals (2). Observations that intrinsic mesocorticolimbic signaling, as indicated by the strength of midbrain RSFC with dopaminergic terminal field areas, is negatively related to ventral striatal D2-type receptor availability and positively related to impulsivity in methamphetamine-dependent subjects, suggests that striatal D2-type receptor signaling is linked to impulsivity by functional interaction of the midbrain with the ventral striatum.
Although striatal D2-type receptor deficits have been observed in studies of various addictions (37), their influence on network connectivity has not been examined. Connections between the midbrain ventral tegmental area and the nucleus accumbens contribute to facilitation of appetitive behaviors in addiction (13). Dopamine release in the nucleus accumbens activates a subset of medium spiny neurons that have reciprocal GABAergic inhibitory projections with the midbrain ventral tegmental area (38). Low ventral striatal D2-type receptor availability coupled with reduced dopamine release relative to healthy controls (7, 39), may attenuate the GABAergic inhibitory feedback that regulates dopamine neuronal activity (40), resulting in stronger RSFC of the midbrain with its terminal fields. This is a plausible explanation of how ventral striatal D2-type receptor signaling may upregulate midbrain activity in methamphetamine-dependent individuals.
Notably, pharmacological manipulations that augment dopaminergic function can alter RSFC within the mesocorticolimbic system. For example, methylphenidate administration, which would augment striatal dopaminergic activity, increases connectivity in corticolimbic and corticocortical circuits but reduces connectivity between the ventral striatum and putamen in cocaine users (41). In healthy control subjects, connectivity between the midbrain and default mode network is greater in participants receiving L-dopa than those receiving placebo or haloperidol (42). Another study shows that amphetamine-induced striatal dopamine release was positively associated with connectivity within the cortico-striatal-thalamic network in control subjects and in recreational amphetamine users (average of 40 occasions of use), but striatal D2-type BPND was not associated with changes in striatal RSFC in either group (43). These results are consistent with the lack of correlation between striatal BPND and RSFC in healthy controls reported here. However, the absence of a relationship in recreational amphetamine users despite the negative association of striatal D2-type BPND with mesocorticolimbic RSFC in methamphetamine-dependent individuals may reflect a difference between occasional and prolonged stimulant abuse.
Signaling through dopamine D2-type receptors, especially in the striatum, has been identified as a key factor in impulsive behavior (37). In this regard, both low D2-type receptor availability in the ventral striatum (44, 45) and lesions to the nucleus accumbens core increase impulsive choices in rodents (46). Moreover, local administration of aripiprazole, a D2/3 partial agonist, in the nucleus accumbens, reduces impulsive behavior (Besson et al, 2010). Similarly, self-reports of impulsivity are negatively related to ventral striatal dopamine D2-type of receptor availability in human methamphetamine users (2) and positively related to RSFC between the ventral striatum and prefrontal cortex in cocaine users (47). We extend these previous studies by suggesting that ventral striatal D2-type receptor availability contributes to the signaling between the midbrain and ventral striatum and this signaling is associated with self-reports of impulsivity.
This study focused on behavioral and cognitive dimensions of impulsivity (22). Behavioral impulsivity refers to impulsive action or the failure to suppress prepotent responses, whereas cognitive impulsivity reflects impulsive choices or decisions (48). Although methamphetamine-dependent individuals show deficits in motor response inhibition (49-51) and greater behavioral impulsivity as measured by self-report in this study, methamphetamine-dependent and control groups did not differ in how behavioral impulsivity is related to midbrain RSFC with the ventral striatum. This negative finding is in line with reports that provide limited evidence for involvement of the ventral striatum in response inhibition (see review (52)). Perhaps it is relevant that motor-response inhibition, as measured in the Stop Signal Task, is associated with dorsal but not ventral striatal D2-type receptor availability (53, 54).
Cognitive impulsivity is measured using tasks that require mental control, the ability to shift mental set, self-monitoring, or reasoning (48). Performance on tasks that tap into cognitive impulsivity, such as risky decision-making tasks, has been linked to ventral striatal function. For example, ventral striatal activation increases during the selection of high-reward but risky options on the Wheel of Fortune Task (55) and the Risk Taking Task (56). The group difference in the relationship between the midbrain RSFC with ventral striatum and cognitive impulsivity observed here is in line with previous observations of greater ventral striatal activity during risky decision-making in methamphetamine-dependent individuals relative to controls (4) and the negative relationship between ventral striatal D2-type receptor availability and task performance in healthy controls (57).
Although abnormalities in midbrain RSFC associated with addiction have been observed before (4), and a contribution of low striatal D2-type receptor availability to impulsivity has been suggested (37), this study provides a potential mechanism by which these addiction-related phenotypes are linked. These data suggest that chronic methamphetamine use leads to cognitive impulsivity, at least in part, by augmenting connectivity of mesocorticolimbic structures, presumably due to stimulant-induced loss of striatal D2-type receptors. The firing of dopaminergic neurons codes for the magnitude and probability of the incentive-value of a stimulus (58), and signaling through the mesolimbic system promotes motivated drug-seeking behavior (59, 60). Stronger connectivity between the midbrain and ventral striatum in methamphetamine-dependent individuals, therefore, may promote bias toward rewarding stimuli, thereby enhancing impulsive drug use.
This report has some limitations. The fact that all of the healthy control subjects in Study 1 were nonsmokers precludes the possibility to control for smoking status. However, consistent with our findings, methamphetamine-dependent individuals in studies that did control for smoking status exhibited lower receptor availability (2) and higher midbrain RSFC (4) than controls. Moreover, there were no significant relationships between cigarette-use variables with brain and impulsivity measures in this study. Still, because smoking affects D2-type receptor BPND (61, 62) and RSFC of executive control networks (63), future research will be needed to separate the effects of smoking from those of methamphetamine on the association between striatal D2-type receptor BPND and midbrain RSFC. Also because the entry criteria for this study excluded individuals with dependence on substances other than methamphetamine for the Methamphetamine group and nicotine for both groups, our sample may not be representative of methamphetamine-dependent individuals and limits the generalizability of findings. However, years of methamphetamine use was positively related to ventral striatal BPND and negatively related to cognitive impulsivity. Although it would be tempting to speculate that the link between ventral striatal D2-type receptor and cognitive impulsivity is mediated by midbrain RSFC, lack of overlap in all measures across all subjects precluded a formal test of mediation. Moreover, the results presented here are based on correlations, which may be affected by factors that were not considered. One such factor is striatal volume, which is positively correlated with striatal D2-type receptor availability in methamphetamine-dependent individuals (64). In addition, non-dopaminergic neurotransmission may affect the relationships under study. For example, GABAergic projections from the ventral striatum to midbrain may affect dopaminergic signaling and the strength of connectivity between these regions. Finally, there are limitations associated with the use of [18F]fallypride as a radiotracer. Although [18F]fallypride has high affinity for dopamine D2-type receptors (65), it does not distinguish between D2 and D3 receptor subtypes, and does not resolve binding to receptors in different compartments, such as pre- vs. post- synaptic receptors on different cellular elements in a region. Despite these limitations, the results presented go beyond previous observations of low D2-type receptor availability and heightened mesocorticolimbic RSFC in methamphetamine dependence to advance a more comprehensive approach in understanding the neural substrates of impulsivity in addiction.
Table 2.
Brain regions that exhibited differences between groups in the relationship between midbrain RSFC and ventral striatal BPND
| Brain region | x | y | z | Z statistic |
|---|---|---|---|---|
| Group by Ventral Striatal BPND Interaction on Midbrain RSFC | ||||
| Lateral OFC/Inferior frontal gyrs (L) | −42 | 36 | -8 | 4.26 |
| Ventral Striatum (L) | -6 | 14 | -6 | 3.96 |
| Middle OFC/Subcallosal Cortex (L) | -16 | 16 | -16 | 3.93 |
| Middle OFC/Subcallosal Cortex (R) | 12 | 20 | -14 | 3.92 |
| Putamen (R) | 18 | 12 | -10 | 2.97 |
| Insula (L) | -28 | 22 | -12 | 2.95 |
| Caudate (L) | -12 | 22 | 0 | 2.47 |
Z-statistic maps were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05.
x, y, z reflect coordinates for peak voxel or for other local maxima in MNI space.
Regions are presented in order of the Z-statistic.
L or R refers to left or right hemisphere.
Acknowledgments
The research described here was funded in part by NIH grants P20 DA022539, R01 DA020726 (EDL), NIDA R01DA027633 (RAR), K23DA027734 (ACD), R21DA034928 (ACD), UL1TR000124 (UCLA CTSA), the Consortium for Neuropsychiatric Phenomics (NIH Roadmap for Medical Research grants UL1-DE019580 and RL1DA024853), and endowments from the Thomas P. and Katherine P. Pike Chair in Addiction Studies, and the Marjorie Greene Trust. None of the sponsors were involved with the design, collection, analysis or interpretation of data, writing the manuscript or the decision to submit the manuscript for publications.
Footnotes
Disclosure/Conflict of Interest
The investigators have no conflicts of interest or financial disclosures to report.
References
- 1.Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, et al. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012 Apr 25;32(17):5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009 Nov 25;29(47):14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2014 Nov 4; doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014 Jul 1;71(7):812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011 Feb 24;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011 Jun;168(6):634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012 Sep;17(9):918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, et al. Low Dopamine D2/D3 Receptor Availability is Associated with Steep Discounting of Delayed Rewards in Methamphetamine Dependence. Int J Neuropsychopharmacol. 2015 Jan 20; doi: 10.1093/ijnp/pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006 Nov;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 10.Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008 Jul;33(8):1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997 Oct 3;278(5335):58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 13.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008 Oct 12;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001 Mar 1;39(3):257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997 Nov 1;17(21):8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JB. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IP) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 20.Bilder RM, Sabb FW, Parker DS, Kalar D, Chu WW, Fox J, et al. Cognitive ontologies for neuropsychiatric phenomics research. Cogn Neuropsychiatry. 2009;14(4-5):419–450. doi: 10.1080/13546800902787180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995 Nov;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol Assess. 2013 Jun;25(2):631–642. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol. 1995 Apr;22(3):283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002 Oct;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 25.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2011 Feb 1;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson J, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report. 2007 [Google Scholar]
- 27.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001 Jun;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 28.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011 Jun 1;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996 Dec;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 30.Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, Murali D, et al. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010 May;30(5):994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Carson RE. Noise Reduction in the Simplified Reference Tissue Model for Neuroreceptor Functional Imaging. J Cereb Blood Flow Metab. 2002;22(12):1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- 32.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. The American journal of psychiatry. 1998 Jun;155(6):768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- 33.Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front Neuroendocrinol. 2010 Apr;32(2):227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grady CL, Grigg O, Ng C. Age differences in default and reward networks during processing of personally relevant information. Neuropsychologia. 2012 Jun;50(7):1682–1697. doi: 10.1016/j.neuropsychologia.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The modulation of BOLD variability between cognitive states varies by age and processing speed. Cereb Cortex. 2012 Mar;23(3):684–693. doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky Decision-making: Prefrontal Function and Mesocorticolimbic Resting-state Connectivity in Methamphetamine Users. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.399. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014 Jan;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993 Jan-Apr;18(1):75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997 Apr 24;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 40.Rahman S, McBride WJ. Feedback control of mesolimbic somatodendritic dopamine release in rat brain. J Neurochem. 2000 Feb;74(2):684–692. doi: 10.1046/j.1471-4159.2000.740684.x. [DOI] [PubMed] [Google Scholar]
- 41.Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of Methylphenidate on Resting-State Functional Connectivity of the Mesocorticolimbic Dopamine Pathways in Cocaine Addiction. JAMA Psychiatry. 2013 Jun;26:1–11. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, et al. Dopamine-dependent architecture of corticosubcortical network connectivity. Cereb Cortex. 2012 Jul;23(7):1509–1516. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- 43.Schrantee A, Ferguson B, Stoffers D, Booij J, Rombouts S, Reneman L. Effects of dexamphetamine-induced dopamine release on resting-state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging Behav. 2015 Jul 7; doi: 10.1007/s11682-015-9419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007 Mar 2;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jupp B, Caprioli D, Saigal N, Reverte I, Shrestha S, Cumming P, et al. Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur J Neurosci. 2013 May;37(9):1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- 46.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001 Jun 29;292(5526):2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 47.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015 Jun 1;72(6):584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 48.White JL, Moffitt TE, Caspi A, Bartusch DJ, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. J Abnorm Psychol. 1994 May;103(2):192–205. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- 49.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2012 Jan;38(2):259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005 Aug 1;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, et al. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011 Mar 30;31(13):4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010 Dec;92(4):533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012 May 23;32(21):7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, et al. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci. 2015 Apr 15;35(15):5990–5997. doi: 10.1523/JNEUROSCI.4850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Matthews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004 Sep 15;15(13):2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- 57.Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, et al. Risk-Taking Behavior: Dopamine D2/D3 Receptors, Feedback, and Frontolimbic Activity. Cereb Cortex. 2013 Aug 21; doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005 Mar 11;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 59.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002 Oct 10;36(2):229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 60.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992 May;13(5):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 61.Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, et al. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol. 2012 Aug;15(7):989–994. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008 Apr;165(4):507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 63.Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014 May;71(5):523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED. Gray-matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Mol Psychiatry. 2015 Jun;20(6):764–771. doi: 10.1038/mp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002 Dec 1;46(3):170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]