Abstract
Membrane fusion during herpesvirus entry into host cells is a complex process where multiple glycoproteins interact to relay the triggering signal from a receptor-binding protein to the conserved fusogen gB through the conserved heterodimer gH/gL. Crystal structures of individual glycoproteins are available, yet high-order “supercomplexes” have been elusive. Recent structures of complexes between gH/gL from human cytomegalovirus or Epstein-Barr virus and the receptor-binding proteins that form at early stages of herpesviral entry highlighted mechanisms that control tropism and revealed dynamic intermediate complexes containing gH/gL that may directly participate in membrane deformation and juxtaposition. Determining how the triggering signal reaches the fusogen gB represents the next frontier in structural biology of herpesvirus entry.
Introduction
To enter host cells, enveloped viruses must fuse their envelopes with the host plasma membrane or the membrane of an endocytic vesicle. The high kinetic barrier of this process is overcome by specialized viral surface glycoproteins called viral fusogens that are triggered once the virus arrives at the right cell and/or the right compartment such as an endosome. In most enveloped viruses, the viral fusogen receives the triggering signal from the cell, either by binding a receptor or a co-receptor or by sensing the acidic pH of the intra-cellular compartment. In some cases, the fusogen instead receives the triggering signal from another viral protein that itself directly interacts with the trigger. Yet, membrane fusion during the entry of herpesviruses is even more complex because it requires multiple viral glycoproteins and diverse host receptors.
Herpesviruses are double-stranded DNA, enveloped viruses that infect nearly all vertebrates and even bivalves. These viruses are divided into three subfamilies, alpha-, beta-, and gammaherpesviruses. Eight human herpesviruses establish lifelong latent infections and cause a range of ailments from skin lesions and ocular diseases to encephalitis, cancers, congenital infections, and disseminated disease in the immunocompromised people, for example organ transplant recipients or AIDS patients. Like other enveloped viruses, herpesviruses penetrate cells by enabling the fusion of the viral envelope and cellular membrane, which can occur at the endosomal or plasma membrane, depending on the identity of the herpesvirus and its target cell. In herpesviruses, membrane fusion requires three conserved glycoproteins gB, gH, and gL plus various non-conserved glycoproteins specific to individual herpesviruses [1]. Such remarkable complexity begs the question of how several glycoproteins work together to accomplish membrane fusion.
Structural studies of herpesvirus entry glycoproteins have been invaluable in elucidating their respective functions and mechanisms in membrane fusion. Crystal structures have not only illustrated the basis for receptor specificity, but have also revealed receptor-induced conformational changes in receptor-binding glycoproteins and helped to pinpoint gB as the viral fusogen and gH/gL heterodimer as an activator of gB. Current model [2] posits that herpesvirus glycoproteins orchestrate membrane fusion through a sequential activation process termed cascade [3] whereby a herpesviral receptor-binding protein binds its cognate receptor, somehow transmits a signal to gH/gL that, in turn, activates the gB (Fig. 1). However, complexes between gH/gL and the receptor-binding proteins or gB have been elusive, and how the triggering signal initiated by binding of a receptor is transmitted to gH/gL and subsequently to gB remains unclear. This review focuses on recently published electron microscopy (EM) reconstructions of gH/gL-containing supercomplexes that form at early stages of herpesviral entry, the insights gleaned from these studies, and remaining questions.
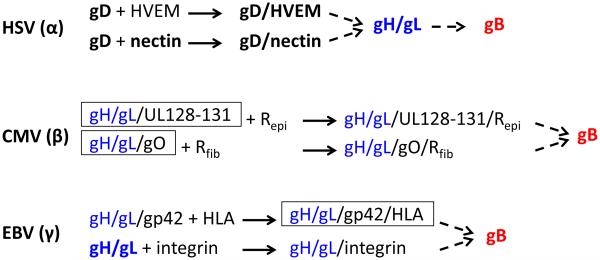
Fig. 1. Fusogenic cascade in prototypes of three subfamilies of herpesviruses.
This diagram is limited to proteins that are essential for membrane fusion during viral entry. The conserved gH/gL (blue) and gB (red) components are colored. Known or presumed stable binding events are shown as solid arrows. Triggering events that may involve only transient binding are shown as dashed arrows. Proteins and complexes, for which crystal structures are known, are shown in bold. Note that only postfusion structures are available for gB. Complexes recently reconstructed by EM, which are the focus of this review, are boxed. Rfib and Repi are postulated CMV receptors in fibroblasts and epithelial cells, respectively.
The complex choreography of herpesvirus membrane fusion
Entry of herpesviruses into targets cells is regulated by interactions with a number of cellular receptors that mediate attachment, initiate signaling cascades, or trigger virus internalization. However, cell penetration requires the presence of an entry receptor on the cell surface. Many herpesviruses enter more than one cell type and engage structurally unrelated entry receptors. The ability of herpesviruses to enter multiple cell types is also reflected in the variety of viral glycoproteins used to bind receptors [1]. In some herpesviruses, the receptor-binding function belongs to a non-conserved protein while in other herpesviruses, receptor binds the conserved gH/gL complex directly (Fig. 1). Herpes simplex virus (HSV-1) uses a non-conserved receptor-binding protein, gD, to engage one of its three cellular entry receptors: nectin-1, a cell adhesion molecule found on cell junctions on epithelial cells and neurons; a herpesvirus entry mediator (HVEM) found on lymphocytes [4-6]; or a non-protein receptor 3-O-modifed heparan sulfate [7]. The Epstein-Barr virus (EBV), a gammaherpesvirus, employs glycoprotein gp42 to bind MHC Class II HLA molecule on B cells [8, 9] but uses gH/gL to directly bind integrins αVβ5, αVβ6, or αVβ8 on epithelial cells [10, 11]. Entry of the cytomegalovirus (CMV), a betaherpesvirus, into fibroblasts depends on the presence of the trimeric gH/gL/gO complex [12, 13] whereas entry into both epithelial and endothelial cells requires a pentameric complex formed by gH/gL, UL128, UL130, and UL131 [14, 15], but the respective receptors have not yet been identified.
Binding of a cellular entry receptor by a viral glycoprotein culminates in triggering of the conserved herpesviral fusogen gB. The crystal structures of gB from HSV-1 [16], CMV [17, 18] and EBV [19] share many similarities with the structures of vesicular stomatitis virus glycoprotein G [20] and baculovirus gp64 [21], and all three belong to the recently formed class III of viral fusogens [22]. Since then, a number of structural and mutagenesis studies have firmly established gB as a viral fusogen responsible for mediating virus and host membrane fusion during viral entry [23-25].
Unlike the majority of viral fusogens, gB does not work alone and always requires the gH/gL heterodimer that lacks an apparent functional counterpart in other enveloped viruses. Although some herpesviruses, such as Kaposi’s sarcoma herpesvirus (KSHV), use gH/gL to bind cellular receptors [26], the universal requirement of gH/gL for entry indicates a more conserved function, currently thought to be activation of gB [27-30]. The apparent structural plasticity of the gH/gL homologs suggested that gH/gL functions as an adaptor that transmits the triggering signals from various non-conserved inputs to the highly conserved fusion protein gB and activates it [31]. Thus, regardless of the initial trigger, fusogenic cascades in all herpesviruses appear to converge on the gH/gL complex (Fig. 1). How gH/gL interacts with receptor-binding glycoproteins or receptors and how it transmits activating signal to gB has remained a mystery.
gH/gL homologs are either boot or cylinder-shaped
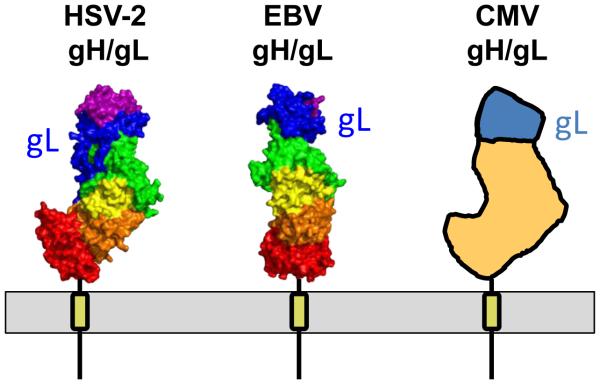
gH has a large ectodomain and a single C-terminal transmembrane anchor while gL lacks a transmembrane region. The two proteins form a stable 1:1 complex. The extracellular portion of the HSV-2 gH/gL forms an unusual boot-shaped complex [27] (Fig. 2). gH is composed of individual domains H1A, H1B, H2A, H2B, and H3 domains, from N to C terminus. gL is “sandwiched” between domains H1A and H1B and interacts extensively with both, illustrating why gH and gL are always found as a complex. A similar architecture has been observed in gH/gL structure from another alphaherpesvirus, Varicella zoster virus (VZV) [30]. Given the conservation of gH/gL in sequence and function, structures of its homologs would be expected to have similar architecture. Surprisingly, EBV gH/gL has a cylindrical, elongated shape in which all domains are aligned in the same plane [32] (Fig. 2). The distinct shapes of two gH/gL complexes, boot vs. cylinder, arise from differences in relative domain orientations, especially at the H1B/gL and H2A/H2B interfaces, demonstrating a certain structural plasticity. Although the crystal structure of CMV gH/gL has not yet been determined, EM reconstructions show boot-shaped particles [33] (Fig. 2), which means that the structure of CMV gH/gL resembles HSV gH/gL rather than EBV gH/gL despite low sequence identity, ~18%, among the three gH homologs. The structure of a C-terminal fragment of the gH ectodomain of pseudorabies virus (PRV), an alphaherpesvirus, bound to an Fab has also been determined [34]. Puzzlingly, the domain orientations in PRV gHC resemble those in EBV gH rather than those in more closely related HSV-1 or VZV gH. However, this structure lacks the N-terminal portion of gH and the entire gL, which, in addition to the bound Fab, could have affected its conformation. A more conclusive analysis awaits the structure of the entire PRV gH/gL.
Fig. 2. Structural similarities and differences between gH/gL complexes from HSV, EBV, and CMV.
Crystal structures of gH/gL from HSV-2 gH/gL (PDB: 3M1C) [27] and EBV gH/gL (PDB: 3PHF) [32] are shown in surface representation and colored by domain as described earlier [31]. gL is shown in blue and labeled. Figure was made using Pymol (http://www.pymol.org). Schematic depiction of CMV gH/gL structure is based on EM reconstruction of gH/gL-containing complexes and the observation that its structure resembles HSV-2 gH/gL [33]. Approximate location of CMV gL is shown. Membrane and transmembrane anchors are shown schematically.
Sequence and structure conservation in gH increases from N to C terminus such that the heterodimer can be divided into i. the variable, virus-specific N-terminal module that may bind a respective receptor-binding protein or a receptor, depending on the virus and ii. the conserved C-terminal module required for a common fusion task, perhaps, interaction with gB. Structures of gH/gL complexes from HCMV and EBV bound to their cognate receptors, described in subsequent sections, support the former conclusion while locations of neutralizing epitopes are consistent with the latter [27, 30, 33, 35]. On the surface of the viral envelope or cell, gH/gL is probably standing on its “toe” to have more of its surface available for potential interactions (Fig. 2).
Unlike non-covalently associated gH/gL from HSV or EBV, HCMV gH/gL is a heterodimer covalently linked by a disulfide bond between residues gH-C95 and gL-C47 [33]. Why are HCMV gH/gL covalently linked? Despite limited sequence similarity, both HSV and EBV gL share a core chemokine fold [31, 36], interact extensively with their gH partners and form very stable complexes. By contrast, CMV gL does not share obvious homology with either HSV or EBV gL, and neither its sequence nor predicted secondary structure resembles chemokines. It is possible that CMV gH/gL heterodimer lacks extensive non-covalent interactions and requires a covalent bond for stability. Indeed, the disulfide is essential for gH/gL complex formation because mutation of either cysteine has been shown to prevent secretion of the soluble gH/gL variant [33].
Unexpectedly, the soluble gH/gL heterodimer further dimerizes into species referred to as gH/gL homodimer because gL-C144 on one heterodimer forms a disulfide with gL-C144 on another heterodimer resulting in a two gH/gL complexes interact at the boot top [33]. This homodimer is unlikely to form on the cellular surface or on viral envelope because the architecture of the gH/gL homodimer is inconsistent with the location of the membrane anchor and is more likely to be an artifact of having an exposed gL-C144 and soluble heterodimer (no binding partners or the membrane). The same cysteine gL-C144 forms disulfide with gO-C351 and UL128-C162, as described in the next section.
CMV gH/gL forms three distinct complexes
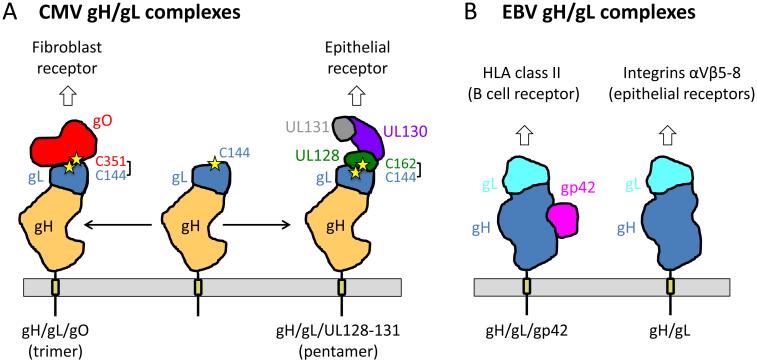
In addition to forming the gH/gL heterodimer, the gH and gL of HCMV participate in two additional kinds of complexes: a trimer of gH, gL and gO; and a pentameric glycoprotein complex formed by gH/gL, UL128, UL130, and UL131 (Fig. 3A). Both the trimer and the pentamer are more abundant on the virus surface than gH/gL [37], with the pentamer being the main target of the neutralizing humoral response to CMV infection in epithelial/endothelial cells [38, 39]. While gH/gL and gB are sufficient to mediate cell-cell fusion in cells transduced with recombinant adenoviruses [40], CMV entry into both epithelial and endothelial cells requires the gH/gL/UL128-131 pentamer [14, 15]. Overexpression of the pentamer on cell surface interferes with CMV entry into epithelial cells but not fibroblasts, which suggests that these cells have a pentamer-specific receptor [41]. By contrast, CMV entry into fibroblasts requires the trimer [12], which probably binds a fibroblast-specific receptor.
Fig. 3. CMV and EBV have mutually exclusive gH/gL-containing complexes.
(A) CMV gH/gL/gO (trimer) and gH/gL/UL128-131 (trimer) determine viral tropism for fibroblasts and epithelial cells, respectively. The binding sites for gO and UL128 on gL overlap as each forms a disulfide with the same cysteine on gL, C144. Cysteines forming this disulfide are shown as stars and labeled. Schematic depiction of CMV gH/gL complexes is based on their EM reconstructions [33]. Approximate location of CMV gL is shown. The shape and the location of gO, UL128, UL130, and UL131 are approximate. Membrane and transmembrane anchors are shown schematically. (B) EBV gH/gL/gp42 and gH/gL determine viral tropism for B cells and epithelial cells, respectively. The binding sites for gp42 and integrins αVβ6-8 overlap. Schematic depiction of EBV gH/gL structure is based on its crystal structure [32]. Location and shape of gp42 is based on EM reconstruction of gp42/gH/gL/HLA complex [46]. Membrane and transmembrane anchors are shown schematically.
CMV gH/gL/gO and the gH/gL/UL128-131 are mutually exclusive complexes
Ciferri and colleagues have recently elucidated the architecture and the nature of interactions that shape CMV gH/gL complexes by producing soluble forms of gH/gL, trimer, and pentamer and characterizing them using mass spectrometry, EM, and mutagenesis [33]. gH/gL maintains similar boot-like conformation in all three complexes, while both gO and UL128/130/131 bind to overlapping regions at the top of the gH/gL boot and form protrusions (Fig. 3A). Membrane-distal placement of these ligands within the trimer or the pentamer puts them in an excellent position to engage receptors. Mass spectrometry analysis of the trimer and the pentamer has revealed that both gO and UL128 form a disulfide bond with the same cysteine on gL, gL-C144, using either gO-C351 or UL128-C162. This means that gO and UL128-131 form mutually exclusive complexes with gH/gL, which explains why trimer levels increase when expression of UL proteins is suppressed [37].
How important the disulfide bonds between gL and UL128 or gL and gO are for the stability of pentamer or trimer remains unclear. When gL-C144 or UL128-C162 were replaced with serines, the higher complexes still formed and, in the case of the pentamer, were shown to retain the same overall structure [33]. (Of note, gO-C351 mutation reduced gO expression, which resulted in expression of gH/gL but not the trimer.) Despite no obvious changes in the gross structure of the pentamer, when both UL128-C162 and gL-C144 were mutated, the pentamer was unable to mediate receptor interference of CMV entry into epithelial cells or support cell fusion of transduced epithelial cells. Thus, the double cysteine mutation probably reduced interaction of the pentamer with a receptor necessary for entry into epithelial cells and their fusion. Mutating both cysteines was important for this phenotype because single cysteine mutants only partially interfered with CMV infection and had no obvious effect on cell-cell fusion. The receptor-binding site may be located in the vicinity of the disulfide and could include residues from gL as well as UL proteins. If so, the disulfide bond between gL and UL128 could help maintain specific conformation necessary for receptor binding.
The alternative use of the same binding site on gL may provide a means of controlling tropism such that viruses with abundant amount of pentamer are better at entering epithelial and endothelial cells whereas viruses with low amounts of pentamer but high amounts of trimer enter fibroblasts. Recent work suggests that CMV protein UL148, which localizes to the endoplasmic reticulum, may act as a tropism switch by controlling the pentamer-to-trimer ratio by an unknown mechanism [42].
Somewhat surprisingly, infection of both fibroblasts and epithelial cells has been found to correlate with the abundance of the trimer, leading to the conclusion that while the pentamer and the trimer provide respective receptor binding functions in epithelial cells and fibroblasts, only the trimer can provide the conserved herpesvirus gH/gL entry function, such as activating gB, in all cell types [43]. Why only the trimer but not the pentamer could activate gB despite similar overall architecture is unclear, and whether the trimer has fundamentally different properties remains to be seen. Finally, whether gH/gL heterodimer itself is functional during CMV cell entry given its low levels in the virus [37] is unclear.
Two gH/gL complexes determine EBV tropism
EBV entry into B cells requires viral glycoprotein gp42, which binds human leukocyte antigen (HLA) class II receptors on B cells [8, 9]. gp42 forms a stable complex with gH/gL and binds HLA as a part of this complex (Fig. 3B). In contrast, entry into epithelial cells, another target of EBV, does not require gp42 and is instead inhibited by it. This is because epithelial cell entry requires a direct interaction between gH/gL and αvβ5, αvβ6, and αvβ8 integrins on epithelial cells (Fig. 3B). The gp42-binding site within gH/gL overlaps the binding site for integrins, and thus gp42 inhibits entry into epithelial cells by blocking integrin binding. Thus, EBV carries two different kinds of gH/gL complexes: a heterodimer gH/gL and a heterotrimer gH/gL/gp42 that are mutually exclusive for epithelial or B cell entry [44] (Fig. 3B). The relative amounts of each complex in the virion determine EBV tropism. EBV virions produced in B cells have low amounts of gp42/gH/gL complex because they interact with HLA molecules in the ER and get degraded. These gH/gL-rich virions infect epithelial cells whereas EBV virions produced in epithelial cells, which lack HLA, are rich in gp42/gH/gL complex and are infectious for B cells. This mechanism of tropism switching has been proposed to function to maintain persistent infection in the infected host [45].
The EBV B cell entry complex has a dynamic, jack-knife shaped structure
The structure of a soluble variant of the gp42/gH/gL/HLA complex, referred to as the B cell entry complex or triggering complex, was recently reconstructed to 29-Å resolution using negative-stain EM [46]. The unambiguous fit of the crystal structures of gp42/HLA and gH/gL into the EM maps yielded models of the complexes and clarified how gp42 and gH/gL interact. Earlier studies showed that gp42 is divided into a C-terminal C-type lectin domain (CTLD) that engages HLA [9] and an unstructured N-terminus that binds gH/gL [47]. Although the N-terminus of gp42 provides the majority of binding energy between gp42 and gH/gL [46], EM reconstruction showed that a hydrophobic pocket within the CTLD [48] also interacts with gH/gL. Mutations within the hydrophobic pocket in CTLD and its predicted contact region within gH/gL disrupted B cell fusion [46, 48, 49], which showed that both sides of the predicted gp42/gH/gL interface are important. The hydrophobic pocket undergoes small conformational changes upon HLA binding [9, 48], and while contributing only a small amount of binding energy to the gp42-gH/gL interaction, could participate in transmitting triggering signal from gp42 upon HLA binding.
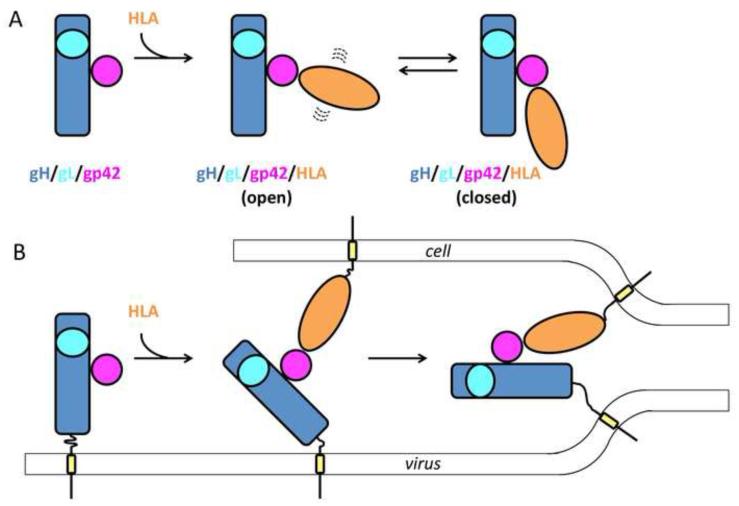
The soluble gp42/gH/gL/HLA complex is a ~170-Å-long structure that has a V/Y shape with the arms formed by gH/gL and gp42/HLA, respectively. Half of the purified soluble complexes adopted a well-defined closed conformation, in which the two arms are oriented parallel to each other (Fig. 4A). The other half adopted a heterogeneous open conformation, in which the two arms are oriented at an angle relative to each other, which varied from 40 to 155 degrees (Fig. 4A). Due to this conformational flexibility, the gp42/gH/gL/HLA complex resembles a jack knife. The heterogeneity of the open conformation observed in EM reconstructions suggests that the gp42/gH/gL/HLA complex has a dynamic structure that samples conformational space and that such hinge-like motion is an intrinsic property of the complex (Fig. 4A).
Fig. 4. A diagram illustrating the dynamic nature of the EBV B cell entry complex and its potential direct role in membrane fusion.
(A) EM reconstructions show that a soluble variant of the gH/gL/gp42/HLA complex has a dynamic structure that exists in heterogeneous open or a fixed closed conformations [46]. Proteins are shown schematically, but their relative orientations are based on EM reconstructions. (B) A model of how conformational flexibility of the gH/gL/gp42/HLA complex could facilitate fusion by deforming and bringing the apposed membranes into proximity. Membrane and transmembrane anchors are shown schematically.
The closed conformation appears important for fusion. PEGylation of gp42 through the introduction of cysteine mutations within the loop at the junction of the hydrophobic pocket and HLA-binding site blocked fusion [46]. Although PEGylation did not affect binding of either HLA or gH/gL, most of the gp42/gH/gL/HLA complexes adopted an open rather than the closed conformation. PEGylation thus shifted the equilibrium towards the open conformation. Given the concomitant block in fusion, the ability of the gp42/gH/gL/HLA complexes to adopt the closed conformation is likely a necessary step in B cell fusion. C-termini of gH and the HLA in the EM model map to one end of the complex, distal from the hinge, and are within 70 Å from each other in the closed conformation but farther away in the open conformation. Prior to fusion, gH/gL and HLA would be anchored in the opposing membranes, viral and host (Fig. 4B). Therefore, when the gp42/gH/gL/HLA complex is formed, it most likely adopts the open conformation (Fig. 4B). By converting into a closed state, the triggering complex could draw the viral and cellular membranes into proximity (Fig. 4B). The architecture of the triggering complex imposes constraints on the membrane and suggests that the formation of the B cell entry complex not only brings the two membranes together but may also deform the viral and/or cellular membranes.
Incidentally, V/Y shaped structures appearing to bridge the opposing membranes prior to fusion were noted in cryo-electron tomography reconstructions of HSV-1 caught in the process of cell entry [50]. Such V/Y shaped structures may be commonly formed at early stages of entry. Although the composition of such structures is unknown, it is tempting to think that the V/Y shape may be a common architecture of gH/gL-containing triggering supercomplexes.
Conclusions
The unique component of herpesvirus fusion machinery, gH/gL is emerging as a complex of multiple functions: a scaffold for presenting receptor-binding proteins, an activator for gB, and a membrane manipulator. Recent EM reconstructions of complexes between gH/gL and the receptor-binding proteins that form at early stages of herpesviral entry have highlighted mechanisms that control tropism and have revealed dynamic intermediate complexes containing gH/gL that may directly participate in membrane deformation. Yet, many questions regarding the complex fusion mechanism of herpesviruses remain unsolved.
The EM-based model of the EBV B-cell triggering complex is the first snapshot of an intermediate stage in EBV entry and highlights the potential for the complex to bring the two membranes into proximity and possibly result in deformation of viral or cellular or both membranes. Whether formation of a dynamic, jack-knife structure is a common feature of the triggering complexes in herpesviruses remains to be determined.
Formation of the mutually exclusive gH/gL-containing complexes appears to be a common strategy for controlling tropism in CMV, a beta-herpesvirus, and EBV, a gammaherpesvirus. Yet, alphaherpesviruses HSV-1 and HSV-2 use a single receptor-binding protein, gD, that can directly bind multiple unrelated receptors. Incidentally, while CMV and EBV gH/gL form stable complexes with their cognate receptor-binding proteins, HSV gD/gH/gL complexes, with or without receptors, have not yet been captured. The reasons for such divergence are not yet clear; perhaps, additional components, for example co-receptors, such as recently described integrins that bind HSV gH/gL [51], are necessary to stabilize the triggering gD/gH/gL/receptor complexes in HSV. Alternatively, triggering complexes of HSV may be transient. Ultimately, the key unanswered question is how the triggering signal from gH/gL reaches the fusogen gB. Biologically relevant gH/gL/gB complexes have not yet been captured for any herpesvirus. Characterization of these and other transient glycoprotein complexes is the next frontier in structural biology of herpesvirus entry.
Highlights.
HCMV and EBV control tropism by making mutually exclusive gH/gL complexes Receptor-binding proteins bind the variable N-terminal region of the gH/gL complex The EBV B-cell triggering complex has a dynamic jack-knife structure gH/gL supercomplexes may facilitate membrane deformation and juxtaposition
Acknowledgements
Author acknowledges the funding support of the NIH grant 1R21AI107171 and the Investigators in Pathogenesis award from Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Author declares no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
** of outstanding interest
* of special interest
- 1.Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci. 2008;65(11):1653–68. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg RJ, et al. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4(5):800–32. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atanasiu D, et al. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol. 2010;84(23):12292–9. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6(5):401–10. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 5.Carfi A, et al. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8(1):169–79. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 6.Di Giovine P, et al. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS pathogens. 2011;7(9):e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiwari V, et al. Soluble 3-O-sulfated heparan sulfate can trigger herpes simplex virus type 1 entry into resistant Chinese hamster ovary (CHO-K1) cells. J Gen Virol. 2007;88:1075–9. doi: 10.1099/vir.0.82476-0. Pt 4. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, et al. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71(6):4657–62. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullen MM, et al. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol Cell. 2002;9(2):375–385. doi: 10.1016/s1097-2765(02)00465-3. [DOI] [PubMed] [Google Scholar]
- 10.Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by alphavbeta5 in addition to alphavbeta6 and alphavbeta8, and integrin binding triggers a conformational change in glycoproteins gHgL. Journal of virology. 2011;85(24):13214–23. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20464–9. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanarsdall AL, Chase MC, Johnson DC. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. Journal of virology. 2011;85(22):11638–45. doi: 10.1128/JVI.05659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wille PT, et al. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. Journal of virology. 2010;84(5):2585–96. doi: 10.1128/JVI.02249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryckman BJ, et al. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80(2):710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102(50):18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldwein EE, et al. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 17.Burke HG, Heldwein EE. Crystal Structure of the Human Cytomegalovirus Glycoprotein B. PLoS Pathog. 2015;11(10):e1005227. doi: 10.1371/journal.ppat.1005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandramouli S, et al. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat Commun. 2015;6:8176. doi: 10.1038/ncomms9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A. 2009;106(8):2880–5. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roche S, et al. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313(5784):187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 21.Kadlec J, et al. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat Struct Mol Biol. 2008;15(10):1024–30. doi: 10.1038/nsmb.1484. [DOI] [PubMed] [Google Scholar]
- 22.Baquero E, Albertini AA, Gaudin Y. Recent mechanistic and structural insights on class III viral fusion glycoproteins. Curr Opin Struct Biol. 2015;33:52–60. doi: 10.1016/j.sbi.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Hannah BP, et al. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J Virol. 2007;81(9):4858–4865. doi: 10.1128/JVI.02755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, et al. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology. 2013;435(2):239–49. doi: 10.1016/j.virol.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backovic M, et al. Characterization of EBV gB indicates properties of both class I and class II viral fusion proteins. Virology. 2007;368(1):102–113. doi: 10.1016/j.virol.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn AS, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus. Nature medicine. 2012;18(6):961–6. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdary TK, et al. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol. 2010;17(7):882–8. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atanasiu D, et al. Bimolecular complementation defines functional regions of Herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol. 2010;84(8):3825–34. doi: 10.1128/JVI.02687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Jardetzky TS, Longnecker R. The large groove found in the gH/gL structure is an important functional domain for Epstein-Barr virus fusion. J Virol. 2013;87(7):3620–7. doi: 10.1128/JVI.03245-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing Y, et al. A site of varicella-zoster virus vulnerability identified by structural studies of neutralizing antibodies bound to the glycoprotein complex gHgL. Proc Natl Acad Sci U S A. 2015;112(19):6056–61. doi: 10.1073/pnas.1501176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stampfer SD, Heldwein EE. Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry. Current opinion in virology. 2012 doi: 10.1016/j.coviro.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuura H, et al. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(52):22641–6. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Ciferri C, et al. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A. 2015;112(6):1767–72. doi: 10.1073/pnas.1424818112. This paper reports negative-stain EM reconstructions of HCMV gH/gL/gO and gH/gL/UL128-131 complexes, shows that these complexes are mutually exclusive, and provides a mechanistic explanation for this. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backovic M, et al. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(52):22635–40. doi: 10.1073/pnas.1011507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciferri C, et al. Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies. PLoS Pathog. 2015;11(10):e1005230. doi: 10.1371/journal.ppat.1005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malkowska M, et al. Alphaherpesvirinae and Gammaherpesvirinae glycoprotein L and CMV UL130 originate from chemokines. Virol J. 201310:1. doi: 10.1186/1743-422X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Zhou M, et al. Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope. J Virol. 2013;87(17):9680–90. doi: 10.1128/JVI.01167-13. This paper shows that the relative amounts of the trimer and the pentamer vary among different CMV strains and that these differences correlate with tropism for fibroblasts and epithelial cells, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fouts AE, et al. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol. 2012;86(13):7444–7. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freed DC, et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc Natl Acad Sci U S A. 2013;110(51):E4997–5005. doi: 10.1073/pnas.1316517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanarsdall AL, et al. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. Journal of virology. 2008;82(23):11837–50. doi: 10.1128/JVI.01623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryckman BJ, Chase MC, Johnson DC. HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14118–23. doi: 10.1073/pnas.0804365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Li G, et al. A viral regulator of glycoprotein complexes contributes to human cytomegalovirus cell tropism. Proc Natl Acad Sci U S A. 2015;112(14):4471–6. doi: 10.1073/pnas.1419875112. This paper reports the discovery of a tropism switch in human cytomegalovirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Zhou M, Lanchy JM, Ryckman BJ. Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism. J Virol. 2015;89(17):8999–9009. doi: 10.1128/JVI.01325-15. This paper presents evidence supporting the conclusion that CMV entry into both epithelial cells and fibroblasts correlates with the surface amount of the trimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, et al. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72(7):5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chesnokova LS, Jiang R, Hutt-Fletcher LM. Viral Entry. Curr Top Microbiol Immunol. 2015;391:221–35. doi: 10.1007/978-3-319-22834-1_7. [DOI] [PubMed] [Google Scholar]
- 46**.Sathiyamoorthy K, et al. Assembly and architecture of the EBV B cell entry triggering complex. PLoS Pathog. 2014;10(8):e1004309. doi: 10.1371/journal.ppat.1004309. This report visualizes the structure of the gp42/gH/gL/HLA complex by negative-stain EM and characterize a novel interaction interface between gp42 and gH/gL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirschner AN, et al. Binding site interactions between Epstein-Barr virus fusion proteins gp42 and gH/gL reveal a peptide that inhibits both epithelial and B-cell membrane fusion. J Virol. 2007;81(17):9216–9229. doi: 10.1128/JVI.00575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirschner AN, et al. Structure of Epstein-Barr Virus glycoprotein 42 suggests a mechanism for triggering receptor-activated virus entry. Structure. 2009:17. doi: 10.1016/j.str.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva AL, et al. Mutational analyses of Epstein-Barr virus glycoprotein 42 reveal functional domains not involved in receptor binding but required for membrane fusion. J Virol. 2004;78(11):5946–56. doi: 10.1128/JVI.78.11.5946-5956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maurer UE, Sodeik B, Grunewald K. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10559–64. doi: 10.1073/pnas.0801674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gianni T, et al. alphavbeta6- and alphavbeta8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9(12):e1003806. doi: 10.1371/journal.ppat.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]