Abstract
Rationale
Cigarette smoke exposure is associated with an increased risk of the acute respiratory distress syndrome (ARDS); however, the mechanisms underlying this relationship remain largely unknown.
Objective
To assess pathways of lung injury and inflammation in smokers and nonsmokers with and without lipopolysaccharide (LPS) inhalation using established biomarkers.
Methods
We measured plasma and bronchoalveolar lavage (BAL) biomarkers of inflammation and lung injury in smokers and non-smokers in 2 distinct cohorts of healthy volunteers, one unstimulated (n=20) and one undergoing 50 µg LPS inhalation (n=30).
Measurements and Main Results
After LPS inhalation, cigarette smokers had increased alveolar capillary membrane permeability as measured by BAL total protein, compared to nonsmokers (median: 274 vs 208 µg/mL, p = 0.04). Smokers had exaggerated inflammation compared to nonsmokers, with increased BAL interleukin-1β (p = 0.002), neutrophils (p = 0.02), plasma interleukin-8 (p = 0.003), and plasma matrix metalloproteinase-8 (p = 0.006). Alveolar epithelial injury after LPS was more severe in smokers than non-smokers, with increased plasma (p = 0.04) and decreased BAL (p = 0.02) surfactant protein D. Finally, smokers had decreased BAL vascular endothelial growth factor (VEGF) (p < 0.0001) with increased soluble VEGF receptor-1 (p = 0.0001).
Conclusions
Cigarette smoke exposure may predispose to ARDS through an abnormal response to a “second hit,” with increased alveolar-capillary membrane permeability, exaggerated inflammation, increased epithelial injury and endothelial dysfunction. LPS inhalation may serve as a useful experimental model for evaluation of the acute pulmonary effects of existing and new tobacco products.
Keywords: Acute Respiratory Distress Syndrome, Tobacco and the Lung
Introduction
While many harmful effects of tobacco have been known for decades, cigarette smoke exposure has only recently been identified as a risk factor for the acute respiratory distress syndrome (ARDS). Over the past several years, studies in a variety of populations have found an increased risk of ARDS amongst smokers. In critically ill blunt trauma patients, active and passive cigarette smoke exposure have been associated with an increased risk of ARDS;1 likewise, smoking is associated with an increased risk of ARDS in non-pulmonary sepsis2 and following blood transfusion,3 as well as with primary graft dysfunction,4 a form of ARDS that occurs within 72 hours of lung transplant. Furthermore, cigarette smokers who develop ARDS do so with fewer comorbidities and at a younger age,5 suggesting that smokers may be more prone to developing ARDS with a lower severity of illness. However, the mechanisms underlying the relationship between cigarette smoke and ARDS remain poorly characterized.
Potential mechanisms by which cigarette smoking may predispose patients to develop ARDS have largely been extrapolated from other experimental settings. Cigarette smoke has numerous acute effects on the lung, several of which are implicated in ARDS pathogenesis, including alveolar inflammation,6 increased alveolar epithelial permeability,7 increased pulmonary endothelial permeability8 and platelet dysfunction.9 In a recent study of ex vivo human lungs rejected for organ transplantation, donor smoking was associated with increased pulmonary edema, with evidence of inflammation and epithelial injury in bronchoalveolar lavage (BAL), while heavy smoking was associated with impaired alveolar fluid clearance.10 Another study found that cigarette smokers with ARDS had higher edema fluid to plasma protein ratios, reflecting increased alveolar capillary barrier permeability.11 Additional human clinical studies with direct relevance to ARDS are needed to obtain a better understanding of the mechanisms through which cigarette smoke predisposes patients to ARDS, with the ultimate goal of developing new preventative and therapeutic strategies for this frequently fatal syndrome as well as identifying biomarkers that could become the basis for regulation of existing and new tobacco products.
In this study, we compared the association of cigarette smoke exposure with plasma and BAL biomarkers of lung injury and inflammation, chosen a priori, in two groups to (1) test if cigarette smoke increases alveolar capillary membrane permeability in response to a “second hit”, and (2) study mechanistic biomarkers that may help explain differences in alveolar-capillary permeability between smokers and non-smokers. We hypothesized that cigarette smoke exposure primes the lung to develop increased alveolar capillary membrane permeability and ultimately ARDS through an exaggerated inflammatory and injurious response to a “second-hit,” which we modeled with inhaled lipopolysaccharide (LPS). Some of the results of this study have been previously reported in the form of an abstract.
Methods
Subjects
Samples from two previously enrolled cohorts were analyzed in this study. The first cohort was comprised of healthy outpatients enrolled in an elective bronchoscopy study at the University of Colorado.6 Subjects, who had no history of cardiac, lung, liver or renal dysfunction, underwent bronchoscopy with BAL. Details of the bronchoscopy are available in the online supplement. We utilized BAL samples from 20 healthy subjects without history of alcohol use disorders. Of these 20 subjects, 10 were active smokers by self-report. The study was approved by the University of Colorado Institutional Review Board. All subjects provided consent for participation, including for the use of samples in future studies.
The second cohort consisted of healthy non-alcoholic volunteers enrolled in a study at Queen’s University, Belfast, United Kingdom.12 This study was originally designed to assess the anti-inflammatory effects of simvastatin in humans exposed to LPS. Of the 30 enrolled subjects, 9 were active smokers by self-report. Subjects were randomized to either simvastatin or placebo for 4 days and then exposed to 50 µg inhaled LPS (Escherichia coli serotype O26:B6; Sigma Chemicals, Poole, Dorset, UK). Bronchoscopy with BAL was performed 6 hours after LPS inhalation. Details of the LPS inhalation and bronchoscopy are available in the online supplement. Plasma was obtained both prior to LPS administration and 24 hours afterwards. After the conclusion of this study, four additional smokers were enrolled at Queen’s University, Belfast; while recruitment was similar to the initial 30 Belfast subjects, these 4 subjects did not receive statins or placebo. Additionally, these 4 subjects underwent 2 bronchoscopies – first, without LPS stimulation (with plasma drawn before the procedure and 24 hours later), and then 4 weeks later, LPS inhalation followed by bronchoscopy with BAL 6 hours later (with plasma drawn as per the prior studies). The study was approved by the local research ethics committee. All subjects provided consent for participation, including for the use of samples in future studies.
Measurements
Total Protein
Total protein was measured in BAL samples only via Bradford assay (Bio-Rad Laboratories, Hercules, California).
Inflammatory Biomarkers
Interleukin (IL)-8 and IL-1β were measured in plasma and BAL by enzyme linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). Matrix metalloproteinases (MMP)-1, 2, 3, 7, 8, 9 were measured in LPS stimulated plasma by cytometric bead array (R&D systems). MMP-8 only was measured in baseline plasma (ELISA, R&D Systems). Polymorphonuclear neutrophils (PMN) were measured in BAL only by manual count.
Surfactant Protein D
Surfactant protein D (SP-D) was measured in plasma and BAL using ELISA (Yamasa Corporation, Tokyo, Japan).
Vascular Endothelial Growth Factor & Receptor
Vascular Endothelial Growth Factor (VEGF) was measured in plasma and BAL using ELISA (R&D systems). Soluble VEGF receptor-1 (sVEGFr-1) was measured in BAL alone using ELISA (R&D systems).
Statistical Analysis
Normally distributed variables were compared using a student’s t test and displayed as mean ± SD. Non-normally distributed variables were compared using the Mann-Whitney U-test and displayed as median with interquartile range (IQR). In the Belfast cohort, linear regression was performed to assess whether the differences in biomarkers between smokers and non-smokers were independent of statin exposure. Regression with an interaction term was used to compare the mean difference in each biomarker between smokers and nonsmokers at baseline and after LPS inhalation; this analysis formally tests the hypothesis that the association between LPS administration and the biomarker of interest differs in smokers as compared to non-smokers. Interaction was adjusted for age as a possible confounder. Log transformation was used as needed to fulfill all assumptions required for linear regression testing. A p value ≤ 0.05 was considered statistically significant. Statistical analyses were performed with STATA 13.1 (StataCorp LP, College Station, TX).
RESULTS
Demographics
All subjects were healthy without comorbidities. There was no difference in sex between cohorts. The Colorado cohort was older than the Belfast cohort (mean age 40 vs 26 years, p < 0.0001). There was no significant difference in age or gender distribution between smokers and non-smokers, both within each cohort and including all subjects. The additional 4 Belfast subjects did not differ in age (26 vs. 26 years, p = 0.95) or gender distribution (25% vs. 47% male, p = 0.41) from the initial Belfast cohort.
Alveolar Capillary Membrane Permeability
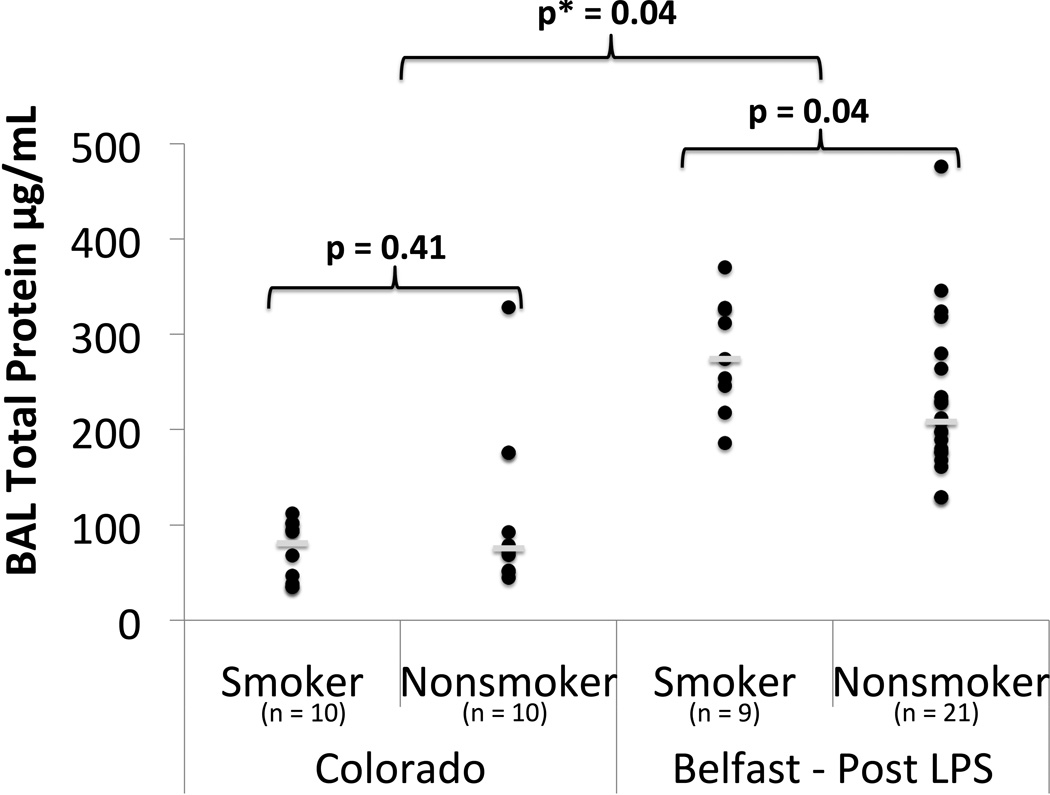
Alveolar capillary membrane permeability was assessed by BAL total protein. In unstimulated BAL from the Colorado cohort, there was no significant difference in total protein between non-smokers and smokers (Figure 1, Supplement Table S1). Following LPS inhalation in the Belfast cohort, smokers had increased total protein compared to non-smokers (median: 274 vs. 208 µg/mL, p = 0.04) (Figure 1). Linear regression demonstrated an interaction between cigarette smoking and LPS (p= 0.04), indicating that cigarette smokers develop exaggerated alveolar capillary membrane permeability compared to non-smokers in response to inhaled LPS.
Figure 1.
BAL Total protein in smokers vs nonsmokers in unstimulated state (Colorado) and 6 hours post 50 µg LPS inhalation (Belfast). ● = individual data points. __ = median value. p = p value from Mann Whitney U test. p* = p value from linear regression with interaction testing between LPS and smoking for log transformed BAL total protein.
Inflammatory Biomarkers in Plasma
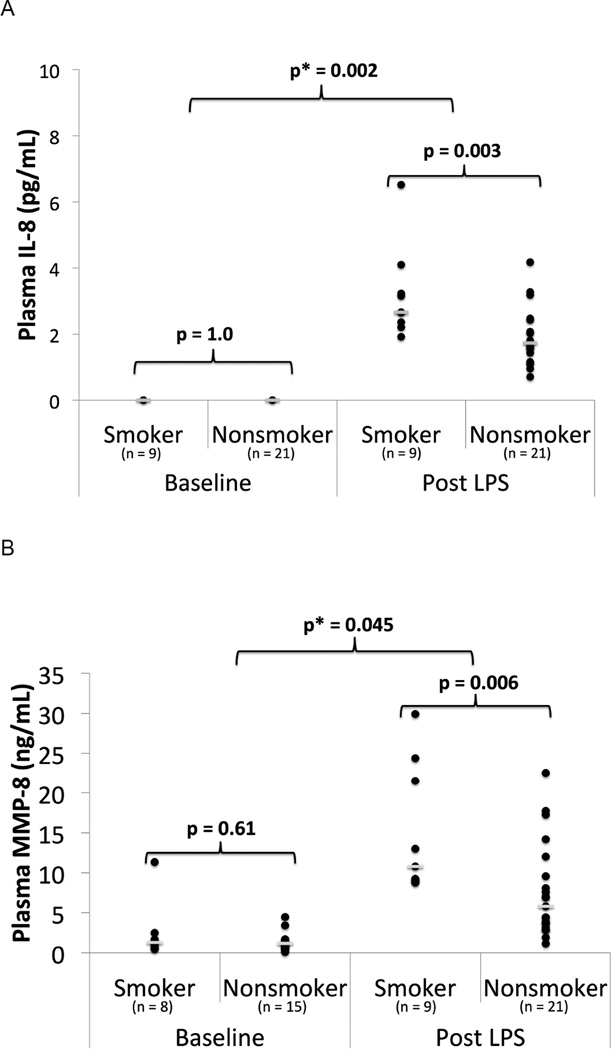
In the Belfast cohort prior to LPS inhalation, there was no significant difference in plasma IL-8, IL-1β or MMP-8 in smokers compared to non-smokers. After LPS, plasma IL-8 and MMP-8 increased in both smokers and non-smokers; however, levels were higher in smokers than in non-smokers (median IL-8 level 3 vs 2 pg/mL p=0.003; median MMP-8 level 11 vs 6 ng/mL, p=0.006) (Figure 2, Table S2). There were no significant associations between smoking and plasma IL-1β or MMPs-1,2,3,7, or 9. Linear regression showed statistically significant interactions between cigarette smoking and LPS when examining plasma MMP-8 (p = 0.045) and IL-8 (p = 0.002).
Figure 2.
Inflammatory Biomarkers in Plasma in smokers vs nonsmokers from the Belfast cohort at baseline and 24 hours post 50 µg LPS inhalation. A) Plasma IL-8 B) Plasma MMP-8. ● = individual data points. __ = median value. p = p value from Mann Whitney U test. p* = p value from linear regression with interaction testing between LPS and smoking for the biomarker of interest.
Inflammatory Biomarkers in BAL
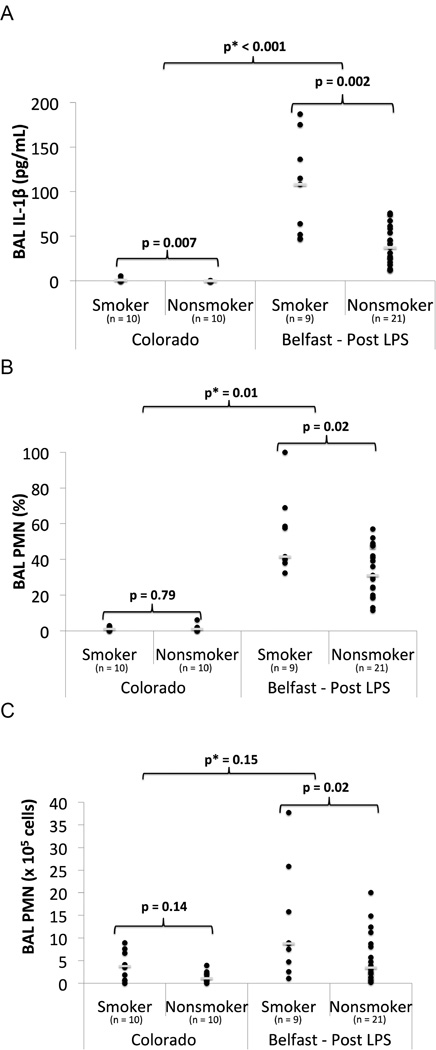
In unstimulated BAL from the Colorado cohort, there was no significant difference in PMN’s between smokers and non-smokers. IL-1β was higher in smokers compared to non-smokers (median: 1 vs. 0 pg/mL, p = 0.007) (Figure 3, Table S1). In the Belfast cohort after LPS inhalation, cigarette smokers had increased BAL IL-1β (median: 108 vs. 37 pg/mL, p = 0.002), percent PMNs (median: 42% vs. 31%, p = 0.02), and total PMNs (median: 9 vs. 3×105 cells, p = 0.02) compared to non-smokers (Figure 3, Table S1). BAL IL-8 levels after LPS were similar between smokers and non-smokers. Linear regression identified significant interactions between cigarette smoking and LPS for IL-1β (p<0.001) and percentage PMNs (p=0.01), indicating that the response of these markers to LPS was significantly different in smokers compared with non-smokers.
Figure 3.
Inflammatory Biomarkers in BAL Fluid of smokers vs nonsmokers in unstimulated state (Colorado) and 6 hours post 50 µg LPS inhalation (Belfast). A) BAL IL-1β, B) BAL PMN (%), C) BAL PMN (× 105 cells). ● = individual data points. __ = median value. p = p value from Mann Whitney U test. p* = p value from linear regression with interaction testing between LPS and smoking for the biomarker of interest.
SP-D in Plasma
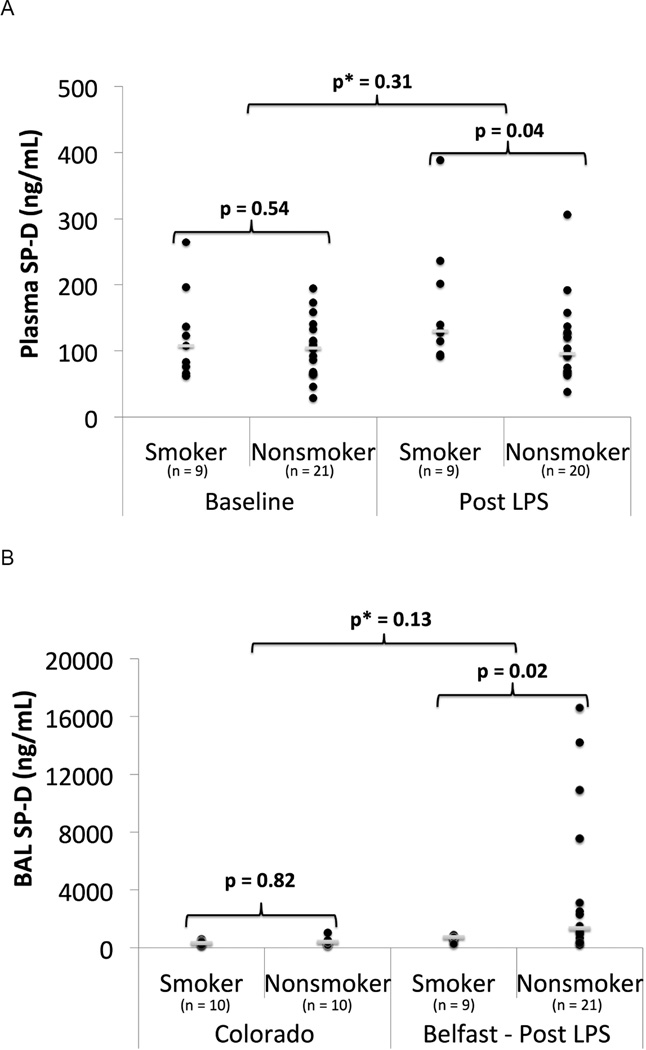
In the Belfast cohort at baseline, there was no significant difference in plasma SP-D between smokers and non-smokers. After LPS inhalation, plasma SP-D was higher (median 130 vs 96 ng/mL, p=0.04) in smokers than in non-smokers (Figure 4, Table S2). However, linear regression did not detect an interaction between LPS and smoking for plasma SP-D (p=0.31).
Figure 4.
SP-D in smokers vs nonsmokers. A) Plasma SP-D from the Belfast cohort at baseline and 24 hours post 50 µg LPS inhalation. B) BAL SP-D in smokers vs nonsmokers in unstimulated state (Colorado) and 6 hours post 50 µg LPS inhalation (Belfast). ● = individual data points. __ = median value. p = p value from Mann Whitney U test. p* = p value from linear regression with interaction testing between LPS and smoking for plasma SP-D and log transformed BAL SP-D.
SP-D in BAL
In unstimulated BAL from the Colorado cohort, there was no significant difference in SP-D between smokers and non-smokers (Figure 4, Table S1). In the Belfast cohort following LPS inhalation, BAL SP-D was lower (median: 724 vs. 1347 ng/mL, p = 0.02) in smokers compared to non-smokers (Figure 4). Linear regression demonstrated no interaction between cigarette smoking and LPS for BAL SP-D (p=0.13).
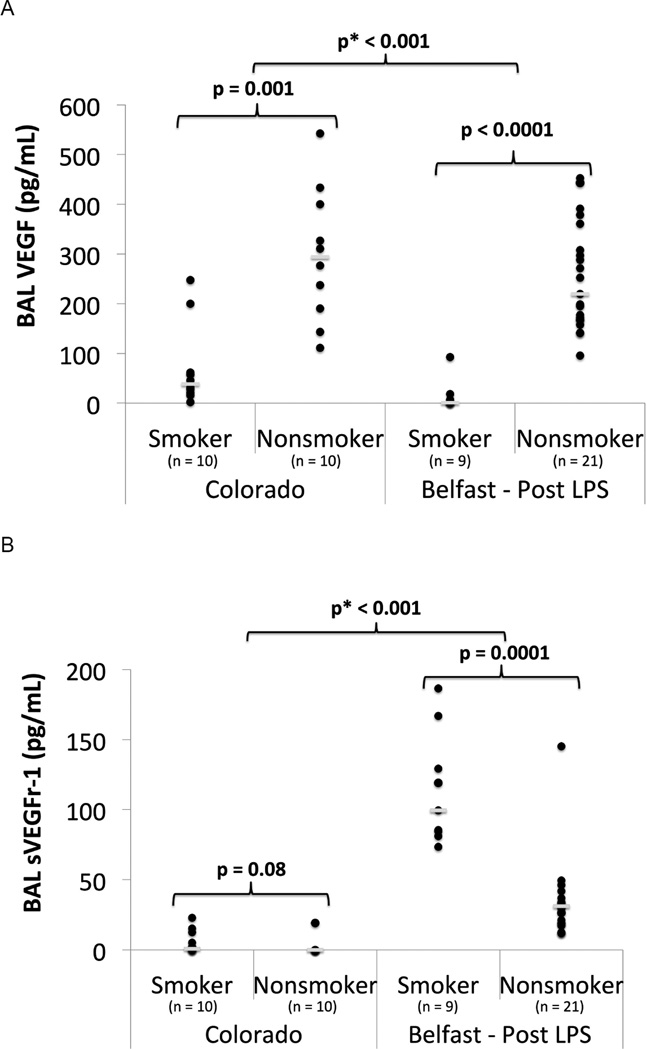
VEGF & Receptor in Plasma and BAL
There was no significant difference in plasma VEGF levels between smokers and non-smokers either at baseline or following LPS inhalation. In unstimulated BAL from the Colorado cohort, cigarette smoking was associated with decreased BAL VEGF (p = 0.001) and a trend toward increased sVEGFR-1 (p = 0.08) (Figure 5, Table S1). After LPS inhalation in the Belfast cohort, cigarette smoke was associated with decreased VEGF (median: 1 vs. 219 pg/mL, p < 0.0001) and increased sVEGFR-1 (median: 99 vs. 31 pg/mL, p= 0.0001) in the BAL (Figure 5). A test for interaction between cigarette smoke and LPS exposure was significant for both VEGF (p < 0.001) and sVEGFr-1 (p < 0.001), indicating that these biomarkers respond differently to LPS in smokers versus nonsmokers.
Figure 5.
BAL VEGF & sVEGFR-1 in smokers vs nonsmokers in unstimulated state (Colorado) and 6 hours post 50 µg LPS inhalation (Belfast). A) BAL VEGF B) BAL sVEGFR-1. ● = individual data points. __ = median value. p = p value from Mann Whitney U test. p* = p value from linear regression with interaction testing between LPS and smoking for log transformed VEGF and sVEGFR-1.
Effect of Statins & Age on Analyses
Since some subjects in the Belfast cohort were randomized to a statin, linear regression was used to determine whether findings were independent of statin exposure. This analysis confirmed that all findings were independent of statin use (Tables S3 & S5). Additionally, since the Belfast and Colorado cohorts differed in age, we adjusted for age when performing interaction testing between the cohorts, and all findings were independent of age and statin use (Tables S4 & S6).
Cohort Comparison
To ensure that differences identified above were due to smoking status and not unmeasured differences in patients enrolled by the two sites, we enrolled a separate small cohort of smokers who underwent bronchoscopy both with and without LPS exposure, as described in the Methods. Baseline levels of BAL biomarkers in these subjects did not significantly differ when compared to smokers from our Colorado cohort, with the exception of sVEGRr-1, which was elevated compared to our Colorado cohort (median: 21 vs. 1 pg/mL p = 0.01).
Next, we compared BAL biomarkers from the 4 additional Belfast subjects’ first bronchoscopy (baseline) to those obtained during their second bronchoscopy (post-LPS). From baseline to post-LPS, these subjects showed dramatic increases in BAL IL-1β, IL-8, and total protein with less dramatic changes in SP-D, VEGFr-1 and VEGF levels (Figure S1, Table S7). This pattern of changes closely mirrored those we observed in comparing smokers from our Colorado cohort to our Belfast cohort.
Discussion
This study indicates that healthy cigarette smokers have an altered response to inhaled LPS that is remarkably similar to the pattern of findings in patients with ARDS, including altered alveolar-capillary permeability to protein, along with acute inflammation and lung epithelial cell injury. The finding that smokers are more prone than nonsmokers to the development of alveolar-capillary barrier dysfunction in the setting of an inflammatory stimulus provides a key potential explanation for the epidemiologic links between smoking and the development of human ARDS.
Increased alveolar-capillary membrane permeability is the fundamental pathophysiologic hallmark of ARDS. The disruption of the alveolar-capillary membrane, due to endothelial and/or alveolar epithelial injury, results in the influx of protein-rich edema fluid into the alveolar space. ARDS patients have elevated total protein levels in edema fluid and BAL compared to those with cardiogenic edema.13,14 Furthermore, increased BAL total protein levels in ARDS patients have been associated with poor outcomes.15 In this study, cigarette smokers developed increased alveolar-capillary membrane permeability to protein after LPS inhalation compared to non-smokers, suggesting that smokers may be more prone to developing increased barrier permeability in the presence of a “second hit,” such as pneumonia or sepsis.
The remaining biomarkers in this study were selected a priori based on previous studies in ARDS patients to determine whether the mechanisms they represent may mediate the relationship between cigarette smoke exposure and ARDS. In humans, BAL IL-1β peaks at ARDS onset16 and remains elevated in non-survivors.17 Similarly, IL-8 has been identified as an important mediator of lung injury in ARDS18 and is predictive of ARDS onset and associated with clinical outcomes.19 IL-1β and IL-8 are critical to the endothelial adhesion and recruitment of neutrophils into the lung airspaces, and neutrophils play a key role in the dysregulated inflammation implicated in ARDS pathogenesis.20 Neutrophils act through a variety of mechanisms, including the formation of neutrophil-extracellular traps and the release of reactive oxygen species and proteinases, including MMPs, which participate in basement membrane breakdown and are elevated in ARDS patients21 and predictive of outcomes.22
Prior studies suggest that cigarette smoke is associated with alveolar inflammation. In healthy volunteers, cigarette smoke has been associated with increased alveolar macrophages and BAL pro-inflammatory cytokines such as IL-1β,6 although the association with IL-8 is more controversial.6,23 Furthermore, in a prior study, healthy smokers had increased BAL IL-1β and PMNs but not IL-8 compared to non-smokers after LPS inhalation,24 although no interaction testing was performed. In our study, cigarette smokers had a statistically significant exaggerated inflammatory response, as determined by interaction testing, compared to non-smokers. This finding implies that smokers respond to an inflammatory stimulus differently than non-smokers, and this exaggerated inflammatory response may contribute to the increased alveolar-capillary membrane permeability that was observed in our study. Given the importance of inflammation to ARDS pathogenesis, this exaggerated inflammatory response may play a significant role in increasing the risk of ARDS in cigarette smokers.
SP-D is a biomarker of type II epithelial cell injury. Plasma SP-D is increased in patients with ARDS25 and associated with increased mortality and fewer ventilator free days.26 Decreased BAL SP-D is associated with increased mortality in ARDS patients.25 Low tidal volume ventilation attenuated the rise of plasma SP-D, indicating that alveolar epithelial injury is fundamental to ARDS pathogenesis.26 In our study, smokers had more severe alveolar epithelial injury, as measured by SP-D, than non-smokers after LPS inhalation. Similar changes have also been observed in the setting of critical illness, as donor smoking was associated with decreased BAL SP-D in ex vivo human lungs rejected for organ transplantation.10 Taken together, these findings indicate that cigarette smoking is associated with early alveolar epithelial injury that may only be identifiable in the setting of a “second hit”. Furthermore, given the importance of the alveolar epithelium to alveolar-capillary membrane integrity, this finding may in part explain both the increased barrier permeability after LPS inhalation and the increased risk of ARDS in smokers.
VEGF is important to endothelial cell survival, regulating permeability and angiogenesis. In prior studies, BAL VEGF is decreased in healthy smokers,6 although plasma VEGF is unchanged.27 These effects of smoking on VEGF are thought to play a key mechanistic role in a variety of diseases, such as COPD.28 In ARDS, patients have elevated plasma VEGF,29 and plasma from ARDS patients increases permeability across endothelial cell monolayers, an effect prevented by the addition of VEGF inhibitors,29 suggesting a role for VEGF in ARDS pathogenesis. In BAL, decreased VEGF has been associated with ARDS, while a rise is associated with ARDS recovery,30 although a different study reported equivalent levels in hydrostatic and permeability edema.31 Soluble VEGFr-1 inhibits VEGF, and is increased in alveolar fluid from ARDS patients.32 Our study not only confirms baseline VEGF homeostatic abnormalities in the lungs of smokers, but also implies that smokers have a different response to LPS than non-smokers with regards to VEGF and its natural inhibitor. As this pattern of abnormalities is similar to that of ARDS patients, the abnormal VEGF homeostasis in smokers may promote ARDS in the setting of a less severe stimulus, echoing clinical observations.5
This study has several strengths. By studying healthy volunteers using an experimental model of ARDS, we were able to test the effect of cigarette smoke on pathways of interest without the comorbidities and confounders typical of cohort studies. Furthermore, since chronic alcohol use is associated with an increased risk of ARDS,33 and chronic alcohol use and cigarette smoking often coexist, the exclusion of chronic alcohol users from our study eliminates a key confounder. A second strength of this study was interaction testing between cigarette smoke and LPS. While prior studies have noted a variety of differences between smokers and non-smokers, our study design and use of interaction testing found that the response of cigarette smokers to LPS is statistically different compared to non-smokers. Finally, results from the 4 additional smokers that underwent bronchoscopy both before and after LPS supported the findings that we identified in smokers in our two cohorts, although the small sample size of this group does limit statistical validation.
This study also has limitations. First, cigarette smoking history was obtained by self-report. Prior studies suggest that biomarkers of cigarette smoke exposure provide a more accurate assessment of true exposure in critically ill populations.34 However, since subjects in this study were healthy young volunteers, both self-reported smoking status and alcohol use should be accurate.35,36 Second, modest sample size limited our power to detect differences in biomarkers between smokers and nonsmokers. However, even with this sample size, the magnitude of the differences in biomarkers between smokers and nonsmokers in this study was large and statistically significant, supporting our initial hypothesis. Third, some patients in the Belfast cohort did receive a statin. Although we adjusted for statin use with linear regression and identified no substantive effects on our main analyses, future studies should consider eliminating this potential confounder. Finally, due to the logistical difficulties of enrolling large numbers of subjects in a study with multiple bronchoscopy procedures, we used two cohorts to compare BAL biomarkers. Although there are potential differences between these two cohorts, we used linear regression to adjust for age and statin use, and all subjects were young and without major comorbidities. Furthermore, BAL biomarkers from the Colorado cohort were similar to those in several prior studies of healthy smokers and non-smokers,37–39 supporting our decision to use this cohort as a baseline comparison group. Lastly, BAL biomarkers prior to LPS exposure in the 4 additional Belfast smokers that underwent bronchoscopy both before and after LPS did not differ significantly when compared to the Colorado cohort with the exception of BAL sVEGFr-1. In all smokers, unstimulated BAL sVEGFr-1 levels were near the minimum detectable level of our assay. In the Colorado cohort, 5 of 10 smokers had undetectable levels. BAL sVEGFr-1 in the 5 subjects with detectable levels did not differ from unstimulated levels in the additional 4 Belfast subjects. These findings suggest that our decision to compare two different cohorts was reasonable but do not completely exclude the possibility of additional unmeasured confounders.
In conclusion, this study suggests that cigarette smoke primes the lungs to develop ARDS by promoting an abnormal response to a “second hit,” with increased alveolar-membrane capillary permeability, exaggerated inflammation, alveolar epithelial injury and endothelial dysfunction. The identification of key mechanisms that may predispose smokers to the development of ARDS lays the foundation for preventative strategies that could be beneficial in smokers clinically at risk for ARDS or therapeutic strategies in smokers with ARDS. In addition, since the pattern of biomarker abnormalities in the LPS model mirrors that in ARDS and we observed smoking-associated changes in these biomarkers, LPS inhalation may serve as a novel model for the identification of acute pulmonary toxicities of existing and new tobacco products, including e-cigarettes. The use of this model and the identification of biomarkers of harm associated with tobacco products may inform the risk evaluation and regulation of tobacco products. Although further research is needed to validate our findings in critically ill subjects, this study fills an important gap in our understanding of the mechanistic relationship between cigarette smoke exposure and ARDS.
Supplementary Material
What is the key question
How does cigarette smoke exposure predispose patients to develop ARDS?
What is the bottom line
After lipopolysaccharide exposure, smokers have exaggerated alveolar-capillary barrier permeability, inflammation and epithelial injury, suggesting that priming of these pathways may contribute to the increased risk of ARDS observed in smokers.
Why read on
To our knowledge, this study is the first to use a human experimental model to focus on directly investigating the mechanisms through which cigarette smoke exposure predisposes patients to develop ARDS.
Acknowledgments
SUPPORTED BY: Northern Ireland R&D Office; UK Intensive Care Society (DFM); NHLBI HL51856 (MAM), HL110969 (CSC), HL126345-01 (FM) and R24 AA019661 (ELB). Some research reported in this publication was supported by grant number 1P50CA180890 from the National Cancer Institute and Food and Drug Administration Center for Tobacco Products (CSC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
REFERENCES
- 1.Calfee CS, Matthay MA, Eisner MD, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. American journal of respiratory and critical care medicine. 2011;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calfee CS, Matthay MA, Kangelaris KN, et al. Cigarette Smoke Exposure and the Acute Respiratory Distress Syndrome. Critical care medicine. 2015 doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. American journal of respiratory and critical care medicine. 2013;187(5):527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh SJ, Zhuo H, Benowitz NL, et al. Prevalence and impact of active and passive cigarette smoking in acute respiratory distress syndrome. Critical care medicine. 2014;42(9):2058–2068. doi: 10.1097/CCM.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnham EL, Kovacs EJ, Davis CS. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. American journal of physiology Lung cellular and molecular physiology. 2013;304(12):L873–L882. doi: 10.1152/ajplung.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones JG, Minty BD, Lawler P, et al. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1(8159):66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 8.Lu Q, Sakhatskyy P, Grinnell K, et al. Cigarette smoke causes lung vascular barrier dysfunction via oxidative stress-mediated inhibition of RhoA and focal adhesion kinase. American journal of physiology Lung cellular and molecular physiology. 2011;301(6):L847–L857. doi: 10.1152/ajplung.00178.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Lee JW, Wickersham N, et al. Donor smoking is associated with pulmonary edema, inflammation and epithelial dysfunction in ex vivo human donor lungs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(10):2295–2302. doi: 10.1111/ajt.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;163(6):1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 12.Shyamsundar M, McKeown ST, O'Kane CM, et al. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. American journal of respiratory and critical care medicine. 2009;179(12):1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holter JF, Weiland JE, Pacht ER, et al. Protein permeability in the adult respiratory distress syndrome. Loss of size selectivity of the alveolar epithelium. The Journal of clinical investigation. 1986;78(6):1513–1522. doi: 10.1172/JCI112743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprung CL, Rackow EC, Fein IA, et al. The spectrum of pulmonary edema: differentiation of cardiogenic, intermediate, and noncardiogenic forms of pulmonary edema. The American review of respiratory disease. 1981;124(6):718–722. doi: 10.1164/arrd.1981.124.6.718. [DOI] [PubMed] [Google Scholar]
- 15.Clark JG, Milberg JA, Steinberg KP, et al. Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Annals of internal medicine. 1995;122(1):17–23. doi: 10.7326/0003-4819-122-1-199501010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;164(10 Pt 1):1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 17.Meduri GU, Kohler G, Headley S, et al. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 18.Folkesson HG, Matthay MA, Hebert CA, et al. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. The Journal of clinical investigation. 1995;96(1):107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Critical care medicine. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-2. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal A, Baker CS, Evans TW, et al. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. The European respiratory journal. 2000;15(5):895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 21.Fligiel SE, Standiford T, Fligiel HM, et al. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Human pathology. 2006;37(4):422–430. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Kong MY, Li Y, Oster R, et al. Early elevation of matrix metalloproteinase-8 and-9 in pediatric ARDS is associated with an increased risk of prolonged mechanical ventilation. PloS one. 2011;6(8):e22596. doi: 10.1371/journal.pone.0022596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mio T, Romberger DJ, Thompson AB, et al. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. American journal of respiratory and critical care medicine. 1997;155(5):1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 24.Wesselius LJ, Nelson ME, Bailey K, et al. Rapid lung cytokine accumulation and neutrophil recruitment after lipopolysaccharide inhalation by cigarette smokers and nonsmokers. The Journal of laboratory and clinical medicine. 1997;129(1):106–114. doi: 10.1016/s0022-2143(97)90167-0. [DOI] [PubMed] [Google Scholar]
- 25.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. American journal of respiratory and critical care medicine. 1999;160(6):1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 26.Eisner MD, Parsons P, Matthay MA, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58(11):983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt-Lucke C, Belgore F, Reinhold D, et al. Soluble vascular endothelial growth factor, soluble VEGF receptor Flt-1 and endothelial function in healthy smokers. International journal of cardiology. 2005;100(2):207–212. doi: 10.1016/j.ijcard.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Kasahara Y, Tuder RM, Cool CD, et al. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. American journal of respiratory and critical care medicine. 2001;163(3 Pt 1):737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 29.Thickett DR, Armstrong L, Christie SJ, et al. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;164(9):1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 30.Thickett DR, Armstrong L, Millar AB. A role for vascular endothelial growth factor in acute and resolving lung injury. American journal of respiratory and critical care medicine. 2002;166(10):1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 31.Ware LB, Kaner RJ, Crystal RG, et al. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. The European respiratory journal. 2005;26(1):101–105. doi: 10.1183/09031936.05.00106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins GD, Roberts J, McAuley DF, et al. Regulation of vascular endothelial growth factor bioactivity in patients with acute lung injury. Thorax. 2005;60(2):153–158. doi: 10.1136/thx.2004.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA : the journal of the American Medical Association. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 34.Hsieh SJ, Ware LB, Eisner MD, et al. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Critical care medicine. 2011;39(1):40–45. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons JS, Wills TA, Emery NN, et al. Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addictive behaviors. 2015;50:205–212. doi: 10.1016/j.addbeh.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soulakova JN, Hartman AM, Liu B, et al. Reliability of adult self-reported smoking history: data from the tobacco use supplement to the current population survey 2002–2003 cohort. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2012;14(8):952–960. doi: 10.1093/ntr/ntr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuschner WG, D'Alessandro A, Wong H, et al. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. The European respiratory journal. 1996;9(10):1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 38.McCrea KA, Ensor JE, Nall K, et al. Altered cytokine regulation in the lungs of cigarette smokers. American journal of respiratory and critical care medicine. 1994;150(3):696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 39.Merchant RK, Schwartz DA, Helmers RA, et al. Bronchoalveolar lavage cellularity. The distribution in normal volunteers. The American review of respiratory disease. 1992;146(2):448–453. doi: 10.1164/ajrccm/146.2.448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.