Abstract
Aqueous humor flows out of the eye primarily through the conventional outflow pathway that includes the trabecular meshwork and Schlemm's canal. However, a fraction of aqueous humor passes through an alternative or ‘unconventional’ route that includes the ciliary muscle, supraciliary and suprachoroidal spaces. From there, unconventional outflow may drain through two pathways: a uveoscleral pathway where aqueous drains across the sclera to be resorbed by orbital vessels, and a uveovortex pathway where aqueous humor enters the choroid to drain through the vortex veins. We review the anatomy, physiology and pharmacology of these pathways. We also discuss methods to determine unconventional outflow rate, including direct techniques that use radioactive or fluorescent tracers recovered from tissues in the unconventional pathway and indirect methods that estimate unconventional outflow based on total outflow over a range of pressures. Indirect methods are subject to a number of assumptions and generally give poor agreement with tracer measurements. We review the variety of animal models that have been used to study conventional and unconventional outflow. The mouse appears to be a promising model because it captures several aspects of conventional and unconventional outflow dynamics common to humans, although questions remain regarding the magnitude of unconventional outflow in mice. Finally, we review future directions. There is a clear need to develop improved methods for measuring unconventional outflow in both animals and humans.
Keywords: aqueous humor dynamics, trabecular, uveoscleral, uveovortex, mouse, primate, tracers, indirect
1. Introduction
The pathway of aqueous humor drainage has long been of interest and is of great importance because it provides the fluid resistance that maintains a proper intraocular pressure (IOP). IOP that is too low can impair vision by distorting the retina, cornea, and lens; IOP that is too high can lead to glaucomatous optic neuropathy.
Aqueous humor drainage from the anterior chamber through the trabecular meshwork, Schlemm’s canal, collector channels, aqueous veins, and into the episcleral veins was first proposed by Leber (1873), Schwalbe (1870), and Kneis (1875), and finally demonstrated by Seidel (1921) and Ascher (1942). The latter's observations of clear fluid in aqueous veins established this path as the primary route of aqueous humor outflow from the eye. After exploring aqueous humor drainage in more detail by using tracer molecules, investigators eventually realized that aqueous humor also left the eye through another route, passing through the uvea, the ciliary body and muscle, and into the choroid and sclera. This pathway has been called the uveoscleral, uveovortex, or unconventional pathway to distinguish it from the trabecular pathway, and has been estimated to carry 3 to 82% of the total aqueous humor outflow in different species. This paper will review the historical basis of our understanding of unconventional outflow, its properties and characteristics, how it is measured, and its significance to glaucoma research.
2. Historical Basis of Unconventional Outflow
Leber reported in 1903 that tracers introduced into the anterior chamber passed not only into the conventional trabecular outflow pathway but also were found in the suprachoroidal space (Nesterov 1986). Other early investigators (Nuel and Benoit 1900; Erdmann 1907; Seidel 1921; Kiss 1943) reported that colloidal tracer accumulated outside the conventional outflow pathway, often deep within the peripheral ciliary body, posterior sclera, and choroid after perfusion with these tracers. These studies led investigators to infer the existence of a secondary aqueous humor outflow pathway (Fine 1964) now known as the “unconventional” outflow pathway.
It was not until studies in monkeys by Anders Bill and colleagues in the 1960’s that our functional understanding of unconventional outflow solidified. Bill explored this pathway quantitatively by perfusing radiolabeled molecules of various sizes through the anterior chamber and examining the different pathways by which the tracers left the eye (Bill 1962; Bill 1965; Bill 1966a; Bill 1966c; Bill 1966d). By collecting tracer in the blood, Bill could account for only about 80% of the tracer that left the eye, presumably through the trabecular outflow pathway. The remaining tracer accumulated in the ciliary body, choroid, and sclera. Because tracer accumulated at a constant rate in the sclera and other tissues and because this accumulation was not correlated with the tracer's diffusion coefficient, Bill concluded that there must be a flow from the anterior chamber through the uvea and into the sclera by way of the choroid and suprachoroid.(Bill 1965; Bill and Hellsing 1965; Bill 1966a) Bill referred to this as the “unconventional route” to distinguish it from the trabecular, or “conventional route.”
The definition of unconventional outflow has been expanded to include any of the pathways through which aqueous humor might leave the eye other than through the trabecular pathway, including a corneal route, an iridial route (Fine 1964) and a retinal route (Fowlks et al. 1963). Flow through the corneal and iridial routes has been shown to be negligible (Bill 1977). Flow through the retinal route is generated by the pumping capacity of the retinal pigment epithelium, but is considered to be small as long as the retina remains attached (Pederson and Cantrill 1984). This review will focus on the flow exiting the posterior aspect of the uveal meshwork, passing through the ciliary muscle, and entering the suprachoroidal space.
Bill (1966a; 1966d) found that in cynomolgus and vervet monkeys, the rate of outflow through the unconventional pathway ranged from 25 to 40% of total aqueous humor production. He also noted that the magnitude of this flow changed little when IOP was increased, and concluded that this flow was relatively pressure-insensitive (Bill 1966a). Since the time of these early experiments that demonstrated the unconventional pathway, much effort has been put into determining its flow rate in humans and animals under a variety of experimental conditions. The rate of unconventional outflow is clinically important because it partly determines IOP and it provides a means to reduce IOP for glaucoma therapy.
3. The Anatomy of the Unconventional Outflow Pathway
Because the interstitial spaces of the anterior uvea communicate with the intertrabecular spaces (Fig. 1), a fraction of the aqueous humor outflow that passes into the uveal meshwork can directly enter the interstitial spaces of the ciliary muscle (Henderson 1950). Perfusion studies with microspheres (Inomata et al. 1972) and fluorescein dextran (Tripathi 1977; Lindsey and Weinreb 2002) have demonstrated flow of aqueous humor from this region through the interstitial spaces between the longitudinal ciliary muscle bundles and into the supraciliary and suprachoroidal spaces (Fig. 2). However, where the fluid travels from here has been debated. Bill (Bill 1966a; Bill and Phillips 1971; Bill 1975) and Gabelt and Kaufman (1989) postulate that the fluid seeps through sclera and episclera, and passes into the orbit where it is absorbed by the orbital vasculature (the uveoscleral pathway). Bárány (1967) and others (Pederson et al. 1977; Sherman et al. 1978) have proposed that a significant fraction of this fluid is absorbed osmotically by the choroid and passes into the vortex veins (the uveovortex pathway). As a third potential route, recent studies have proposed drainage into lymphatic vessels located within the ciliary body (the so-called ‘uveolymphatic’ pathway) (Yücel et al. 2009). However, the existence of lymphatic vessels in the eye under normal physiological conditions remains controversial (Schroedl et al. 2014). We will address the evidence for flow through each of these pathways later in this review.
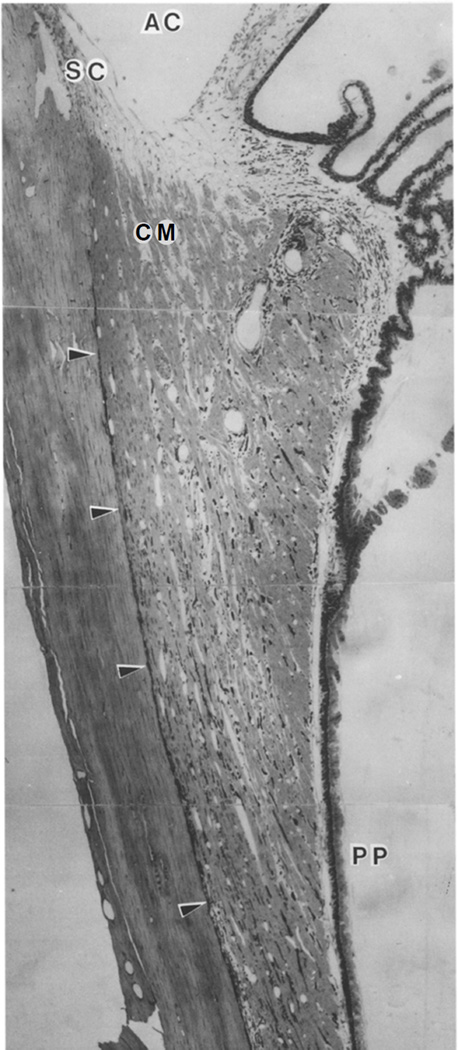
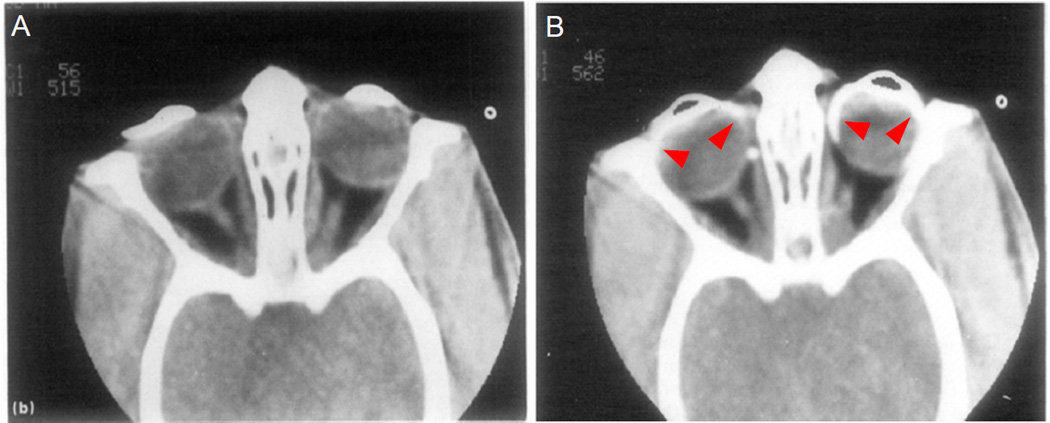
Figure 1.
Meridional section through the uveal tract of a Macaca fascicularis . Arrowheads show supraciliary and suprachoroidal space. Unconventional outflow passes from the anterior chamber (AC), through the most posterior aspects of the uveal meshwork, enters the open spaces between longitudinal aspects of the ciliary muscle (CM) and then enters the suprachoroidal space. SC -- Schlemm’s canal; PP -- pars plana. (Wood et al. 1990)
[Permission Needed]
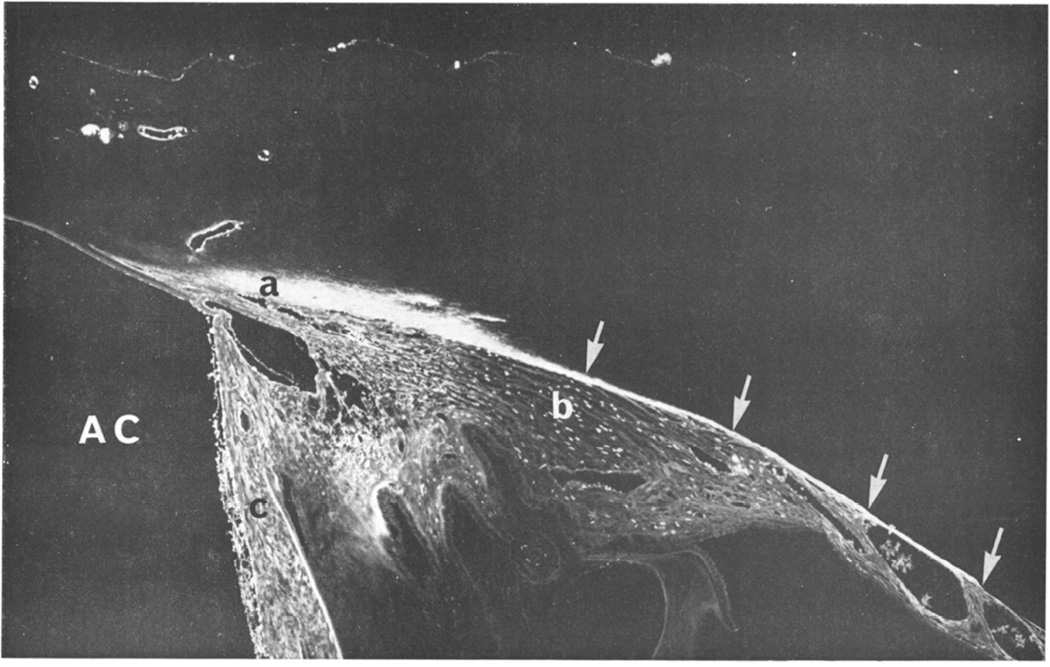
Figure 2.
Light micrograph of fluorescein dextran in the unconventional outflow pathway of a rabbit after intracameral perfusion (AC, anterior chamber). Fluorescence is visible in the conventional aqueous drainage pathway (a), comprising angular meshwork, angular aqueous plexus, and collector channels and aqueous veins, and in the unconventional outflow in the outer aspects of the ciliary body (b) and muscle, and in the suprachoroidal space (arrows). Tracer is also seen in the iris (c), which has been artefactually deflected posteriorly. (Tripathi 1977)
[Permission Needed]
Unconventional outflow, whether through the uveoscleral, uveovortex or uveolymphatic pathway, must pass through the interstitial spaces of the ciliary muscle. Pilocarpine, which causes ciliary muscle contraction and decreases the size of spaces between the muscle bundles, decreases unconventional outflow (Crawford and Kaufman 1987) while atropine, a muscarinic antagonist, relaxes the ciliary muscle and increases unconventional outflow (Bill and Wålinder 1966). This indicates that the ciliary muscle likely represents a significant site of flow resistance along this pathway, and hence ciliary muscle tone can modulate unconventional flow. PGF2α, which increases unconventional outflow, is thought to act by reducing the amount of extracellular matrix between ciliary muscle bundles (Lütjen-Drecoll and Tamm 1988) allowing increased flow through these spaces.
4. Methods of Measurement
The greatest challenge facing studies of unconventional aqueous humor outflow has been measurement of its flow rate. By definition, this pathway encompasses any outflow of aqueous humor that does not pass through the trabecular (conventional) pathway. Unlike the trabecular route, the path of egress is diffuse and difficult to trace, and this complicates measurement of flow through this pathway.
Measurements of unconventional outflow rate have been classified as: (i) direct, tracer-based methods, or (ii) indirect methods. Direct methods are used to estimate both conventional and unconventional outflow from the rate of accumulation of a tracer molecule in ocular tissues and in the blood after introducing the tracer in the anterior chamber. They are believed to be the most accurate, but because they are invasive and may require histologic analysis, they are generally not suitable for use in humans. Indirect methods are used to infer unconventional outflow from the difference between aqueous humor production and aqueous humor outflow through the trabecular pathway, each determined independently. Both methods of estimating unconventional flow will be explored here in more detail along with their underlying assumptions and limitations.
4.1 Direct measurements using tracers
Anders Bill introduced tracer-based methods to determine aqueous humor outflow through both the conventional and unconventional pathways in experimental animals and, to a limited extent, in human eyes (Bill 1962; Bill 1965; Bill and Phillips 1971). His methods still provide the most definitive measurements of aqueous humor outflow through both pathways.
Any tracer introduced into the anterior chamber will be carried away by aqueous humor drainage. However, two characteristics of the tracer can be used to distinguish outflow through the conventional versus unconventional pathway: (i) transit time out of the eye and (ii) filtration based on tracer size. Outflow through the conventional pathway is relatively fast (a minute or less) and fairly insensitive to tracer molecular size, such that the rate of tracer appearance in the blood can be used to determine the flow rate through the conventional outflow pathway. In contrast, tracers draining through the unconventional pathway are retarded or captured as they move through the unconventional outflow pathway such that their transit may take up to two hours depending on tracer size, animal species and dimensions of the eye. Tracer accumulation in the uveal tissues thereby provides a means to estimate the unconventional outflow rate as well as identifying the anatomical route of unconventional outflow (as discussed in section 3).
4.1.1 Tracer-based estimates of conventional outflow: tracer accumulation in the general circulation
Tracers are used to estimate conventional outflow directly by measuring the rate of tracer accumulation in the blood while maintaining a constant tracer concentration in the anterior chamber. Macromolecules of roughly 40 kDa (Toris et al. 1987) or larger (Nilsson 1997) pass quickly through the conventional outflow pathway but pass slowly through or are captured within the unconventional outflow pathway. Under these conditions, the rate of conventional outflow is directly related to the rate of appearance of tracer in the blood.
Tracer concentration in the anterior chamber is maintained constant by using an infusion-withdrawal system that continuously infuses tracer-containing solution (at concentration Co) into the anterior chamber of an anesthetized animal while simultaneously withdrawing at the same rate (Gabelt and Kaufman 1989). This system maintains a constant IOP and a constant tracer concentration in the anterior chamber despite production and drainage of aqueous humor during the perfusion. (Bill 1962; Bill 1965; Bill 1966a; Bill 1966c; Bill 1966d). When the rate of appearance of tracer in the blood, becomes relatively constant, then the conventional outflow rate Qc can be estimated as:
| (1) |
where Vb is the distribution volume of the tracer in the blood that accounts for tracer dilution in the blood after it leaves the eye. Vb can be estimated as M/Cb, after injecting a known quantity, M, of the tracer into the general circulation and measuring its concentration, Cb, in the blood after mixing. For 131I-albumin tracer in cynomolgus monkeys, Bill found that, Vb was the volume equivalent of approximately 7% of the total body weight (Bill 1966a).
Gabelt and Kaufman (1990) used this technique to show that PGF2α does not increase conventional outflow in cynomolgus monkeys despite increasing unconventional outflow in this species. Alternatively, this method may be combined with measurements of aqueous humor production (Qin) to estimate unconventional outflow indirectly as the difference between Qin and Qc.
4.1.2 Tracer-based estimates of unconventional outflow: collection of tracers in ocular tissues
This is the most common method of directly determining unconventional outflow and is considered definitive. The flow rate is calculated from the rate of accumulation of tracers in the tissues of the unconventional pathway after maintaining a constant concentration in the anterior chamber for a fixed time. Typically, a solution with tracer is exchanged with aqueous humor in the anterior chamber of an anesthetized animal and tracer concentration is maintained for 0.5 to 2 hours at constant pressure using an infusion-withdrawal system (Gabelt and Kaufman 1989). At the end of a fixed perfusion time, the anterior chamber is exchanged with a tracer-free solution, and perfused for a short time to remove tracer from the conventional outflow pathway.
The animal is then sacrificed, the eye enucleated and the tissues separated. The mass of the tracer in each tissue (typically, the sclera, choroid, ciliary body, iris and orbital tissue) is then determined by methods specific to the tracer used in the study. The volume of fluid Vi required to move the measured quantity of tracer mass mi, into each tissue is given by Vi= mi/ CAC, where CAC is the concentration of tracer in the anterior chamber and i = l through n, where n is the number of tissues examined (e.g. Table 1). The average unconventional outflow during a time T is then:
| (2) |
Tracers that have been used to study unconventional flow by this method include 131I-γ-globulin (Bill 1966a), 131I-albumin (Bill and Phillips 1971), FITC-dextran (Suguro et al. 1985), and 3H-labeled dextran (Barrie et al. 1985). The tissue distribution of 131I-albumin used to estimate unconventional flow in cynomologus monkeys by Bill (1966a) are given in Table 1. In this example, the total unconventional outflow was 0.46 µl/min, while conventional outflow measured by tracer accumulation in the blood was 0.71 ± 0.09 µl/min, yielding a total outflow rate of 1.17 ± 0.13 µl/min. Thus, roughly 40% of the aqueous outflow passed through the unconventional outflow pathway in this species, typical of other measurements in non-human primates.
Table 1.
Distribution of unconventional outflow in cynomolgus monkey at normal IOP (12 mm Hg) based on distribution of 131I-albumin after 2 hours of perfusion. Uncertainties are standard errors of the mean. (Bill 1966a)
| Tissue | Estimated Volume of AC Fluid Passing into Each Tissue (Vi: µl) |
|---|---|
| Anterior Sclera | 14.5 ± 2.4 |
| Extraocular Tissues | 14.3 ± 5.5 |
| Posterior Sclera | 10.8 ± 3.8 |
| Choroid | 5.1 ± 2.0 |
| Ciliary Body | 4.3 ± 0.5 |
| Iris | 1.2 ± 0.2 |
| Remainder of Eye | 5.1 ± 1.4 |
The distribution of tracer recovered in the various tissues is thought to reflect the anatomical distribution of unconventional outflow. For example, the relatively high tracer content in the anterior sclera suggests that this was a preferential outflow route, as would be expected based on the organization of this tissue (see Fig. 1 and 2). Similarly, the high tracer content in the extraocular tissues suggests that a significant fraction of outflow passed across the sclera, consistent with uveoscleral outflow. In contrast, there was very little tracer in the iris, suggesting negligible iridial outflow. The retinal pathway was not separately assessed in these studies. However, while these measurements clearly indicate tracer accumulation in the various tissues, the tracer distribution does not necessarily reflect the distribution of aqueous humor drainage through those tissues. This is because filtration and diffusion of the tracer affect its transport and may thereby influence tracer accumulation in the various tissues of the unconventional outflow pathway. We will consider these issues further in Section 5.1.
4.1.3 Tracer-based estimates of unconventional outflow: tracer concentration in the vortex veins
Tracers introduced into the anterior chamber also have been used to study the component of unconventional outflow that passes through the suprachoroidal space into the choroidal circulation and vortex veins (the uveovortex pathway). According to this technique, a tracer small enough to pass relatively unimpeded through the vessel walls of the choriocapillaris (e.g. fluorescein) is introduced into the anterior chamber and maintained at constant concentration by the infusion-withdrawal system previously described. One of the four vortex veins is isolated and cannulated. The increased concentration of tracer in the vortex veins, Cvv, relative to that in the general circulation, CB, is used to estimate the uveovortex outflow rate, Quv (Pederson et al. 1977):
| (3) |
where Q is the rate of blood flow out of the cannulated vein and the factor of four accounts for the number of vortex veins. Pederson et al. (1977) found that uveovortex outflow could account for a significant fraction of unconventional outflow (Fig. 3).
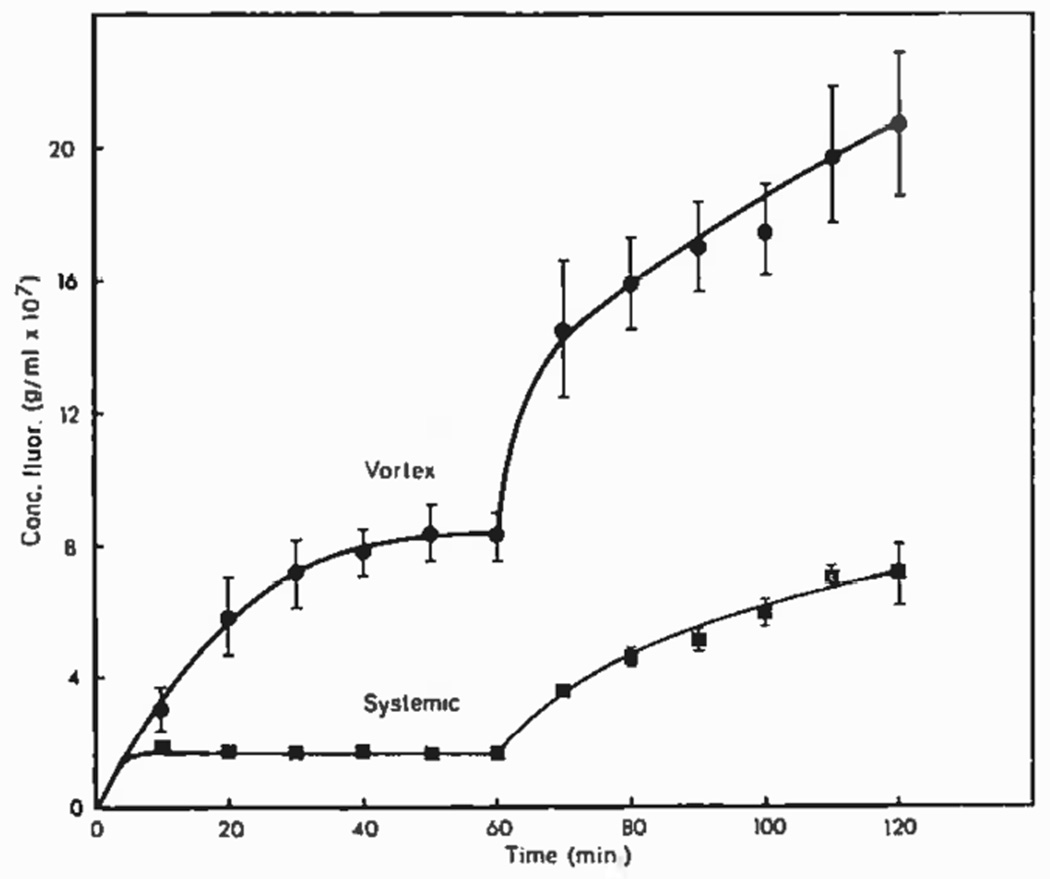
Figure 3.
Fluorescein concentration in the vortex veins and in general circulation in a rhesus monkey after introduction into the anterior chamber. IOP was held at 20 mmHg for 60 minutes and then increased to 32 mmHg. (Pederson et al. 1977).
[Permission Needed]
The size of the tracer molecule is critical for measuring uveovortex outflow. In contrast to small molecular tracers, such as fluorescein, that are able to enter the choroidal vasculature with the aqueous humor flow, large tracers such as albumin are effectively excluded from passing into the choroid (Bill 1962; Bill 1964) and accumulate in the suprachoroidal space. From there, these large tracers diffuse across the sclera and into the orbit. Thus, the pathway taken by tracers the size of albumin or larger likely differs from the pathway taken by aqueous humor and small molecules.
4.1.4 Tracer-based estimates of uveolymphatic outflow: tracer accumulation in lymphatic vessels and lymph nodes
Traditionally, the eye has been considered to be devoid of lymphatics (Streilein 2003). This view has recently been challenged with reports of vessels that express lymphatic markers within the ciliary body (Yücel et al. 2009) and choroid (Koina et al. 2015) of human eyes, although these reports have been met with considerable controversy (Schroedl et al. 2014; Chan-Ling et al. 2015; Heindl et al. 2015). If lymphatics are normally present within the eye, then these vessels may contribute to unconventional outflow.
To measure outflow via the putative uveolymphatic route, Yücel et al. (2009) administered radio-labeled albumin into the anterior chamber of sheep and measured its accumulation in lymph nodes and in cannulated lymphatic vessels (Kim et al. 2011). Tracer accumulated preferentially in the cervical lymph nodes (Yücel et al. 2009), with ~1–2% of tracer passing into the cervical and thoracic lymphatic vessels (Kim et al. 2011). Following intracameral injection, fluorescent tracer nanospheres were found within vessels of the ciliary body that expressed lymphatic markers, suggestive of drainage via these vessels (Yücel et al. 2009). In mice, quantum dots injected unilaterally into the anterior chamber accumulated in ipsilateral lymph nodes in the neck (Tam et al. 2011) and the rate of accumulation was increased by latanoprost (Tam et al. 2013).
When measuring uveolymphatic outflow, it should be recognized that not all tracer recovered within cervical lymphatics had necessarily originated from lymphatic vessels within the eye proper, as some tracer may have entered lymphatic vessels from within the periocular tissues. Albumin used to measure uveolymphatic outflow (Kim et al. 2011) will also follow a uveoscleral route (Bill 1966a) and enter the conjunctiva where lymphatic vessels are plentiful. Consistent with this notion, radiolabelled albumin was measured in the periocular tissues following intracameral injection in sheep (Kim et al. 2011). Once within the periocular space, albumin may enter conjunctival lymphatics that drain into the cervical lymphatics. Quantum dots, while significantly larger than albumin, also accumulate in lymph nodes of mice following intracameral injection (Tam et al. 2011; Tam et al. 2013). However, quantum dots can diffuse through sclera as well (Amrite et al. 2008), although at a rate slower than smaller molecules the size of albumin (Pease et al. 2014).
Currently, there is no clear consensus on the presence of lymphatics in the eye under normal physiological conditions, although lymphatics are recruited during inflammation or if the scleral border is compromised (Schroedl et al. 2014). In light of this lack of consensus and concerns regarding the methods used to measure uveolymphatic outflow, further work is necessary to demonstrate whether uveolymphatic outflow represents a physiologically significant outflow pathway.
4.1.5 Limitations of tracer-based methods
The use of tracers to measure unconventional outflow is based on two fundamental assumptions. First, all tracer that enters a tissue is retained or trapped within the tissue and that no tracer is lost before the measurement. Second, all tracer enters the tissue by the flow of aqueous humor (advection) and not by diffusion.
Retention of tracer
The first assumption will be satisfied if the tracer used is small enough to enter the tissue freely with the aqueous humor but too large to pass through the tissue during the time of the experiment. Toris et al (1987) reported that dextrans of 40 kDa or larger were retained by the tissue whereas smaller dextrans (4 kDa) passed into the circulation or easily crossed the sclera and were lost in the orbit. Bill (1966a) found similarly that albumin (65 kDa) crossed the sclera easily but left the orbit at a low rate, while γ-globulin (150 kDa) was significantly delayed passing through the sclera.
Flow vs diffusion
Tracers can enter a tissue by advection where they are transported at a rate dictated by the velocity of the fluid in which they are carried, or by diffusion where they are transported at a rate dictated by molecular Brownian motion, or by a combination of both advection and diffusion. Tracers that enter a tissue primarily by diffusion therefore cannot be used to make inferences regarding the quantity or velocity of flow. As diffusion becomes more significant for decreasing molecular size, smaller tracers tend to overestimate unconventional outflow based on tracer accumulation within tissue.
Over time t, a tracer will diffuse a distance of roughly , where D is the diffusion coefficient of the tracer, whereas a tracer carried by advection will move an average distance of V t, where V is the average velocity of the fluid. Several consequences of these relationships allow us to evaluate the impact of tracer diffusion on the estimate of unconventional flow. First, because diffusional motion increases with the square root of time while advective motion increases linearly with time, the rate of accumulation of a tracer in a tissue can indicate whether diffusion or advection dominates the transport process. In Bill's study of cynomologus monkeys, tracer accumulation in all unconventional outflow tissues examined after 30 minutes was equivalent to that contained in 13 µl of solution in the anterior chamber, while after 2 hours, it was 55 µl (Bill 1966a). Thus, tracer accumulated roughly linearly over time, which is consistent with an advection-dominated process.
Second, the influence of diffusion on estimating unconventional flow can be explored using tracers of different size that have different diffusion coefficients. If tracers are carried into a tissue primarily by advection, then their rate of accumulation in the tissue should be unaffected by the diffusion coefficient provided that their size is sufficiently small so as not to be excluded from this pathway. Bill (1966a) showed in cynomolgus monkey eyes that roughly the same unconventional outflow rate was found with albumin as with γ-globulin. Pederson and Toris (1987) reported similar findings using 40 and 150 kD dextran.
Third, the diffusion distance, estimated by , relative to the length of the unconventional pathway, can also indicate the importance of diffusion in tracer transport. In large animals, such as monkeys, the length of the unconventional pathway is on the order of millimeters, and the estimated diffusion distance is relatively small, except for very long experiments. For example, the diffusion coefficient of albumin in sclera is D = 1 × 10−7 cm2/sec (Anderson et al. 2008); in a half-hour experiment, a tracer would be expected to diffuse 130 µm, a distance significantly smaller than the length of the unconventional outflow pathway in most species. Pederson and Toris (1987) showed that in cynomolgus monkey eyes, the rate of diffusion was 200 times smaller than the observed rate of tracer movement. However, for smaller eyes, such as those of mice, the unconventional pathway is much shorter than in larger animals and may be comparable to tracer diffusion distances unless very short times are used for tracer delivery (see Section 6.6).
4.2 Indirect estimates of unconventional outflow
The tracer-based methods described in Section 4.1, while accurate, are invasive and thus not generally suitable for use in humans. For this reason, indirect methods have been developed to estimate unconventional outflow. The modified Goldmann equation (Brubaker 2004) relates IOP to aqueous humor inflow (Qin) under steady-state conditions:
| (4) |
where U is the unconventional outflow, c is the conventional outflow facility, and Pe is the episcleral venous pressure. Note that with this definition, c (IOP – Pe) is an estimate of the conventional outflow rate and U is the pressure-independent outflow rate. Note also that U is not necessarily the same as the rate of outflow passing through the unconventional outflow pathway as there may be some pressure-dependence to unconventional outflow (see section 5.2).
If both Qin and c can be measured, then unconventional outflow can be indirectly estimated as the difference between inflow rate and conventional outflow:
| (5) |
Typically, Qin is determined by measuring fluorescein clearance from the anterior chamber and cornea (Brubaker and McLaren 1985). Outflow facility c has been measured by using tonography, in which a small weight is placed on the cornea to elevate IOP and the rate at which IOP returns to baseline is used to determine c (Grant 1950). Alternatively, by measuring IOP and Qin before and after administering a drug (e.g. acetazolamide) that suppresses aqueous humor production, c can be determined by solving Equation (4) at two flow rates and the corresponding IOPs (Yablonski et al. 1985; Hayashi et al. 1989). A value of episcleral venous pressure, Pe, is required to calculate U. This measurement is difficult and values between 8 and 10 mmHg are typically chosen, and the same value is used for all subjects in a trial. More recent evidence suggests that the mean episcleral venous pressure is 6.3 mm Hg in normal subjects (Sit et al. 2011). Importantly, the lower the value used for Pe, the smaller the estimate of U.
Estimates of unconventional flow determined by using the indirect method have varied considerably and, in general, show poor agreement with unconventional outflow measured by direct methods that used tracers. Even in the same study, Toris et al. (2000) reported an unconventional outflow rate in cynomolgus monkeys of 0.14±1.2 µl/min by using indirect methods, but 1.05±0.6 µl/min by using tracers in this same population, almost an order of magnitude greater. Toris et al also noted a lack of correlation between unconventional outflow estimated indirectly and those measured with tracers. Similarly, studies in mice (Millar et al. 2011; Millar et al. 2015) showed poor agreement between tracer-based and indirect estimates of unconventional outflow. In general, measurements with the indirect method are considerably higher than those measured using tracers (Table 2), although measurements by Toris et al. (2000) represent a significant exception. While both direct and indirect methods have been shown to detect changes in response to drug treatment, the absence of any quantitative agreement between the two approaches is troubling and raises questions as to what specifically is being measured by the indirect method.
Table 2.
Studies that reported unconventional outflow measured by the direct method with tracers and by the indirect method. Mean ± standard errors.
| Reference | Drug | Animal | Unconventional Outflow Rate µl/min (Percent of total outflow) |
|
|---|---|---|---|---|
| Indirect Method | Tracer Method | |||
| Toris et al., 2000 | none | Cynomolgus Monkey |
0.14±0.3 (9%) | 1.05±0.26 (70%) |
| Toris, et al., 1995c | Control PGA2 |
Cat | 1.9±0.8 (33%) 4.0±0.5 (59%) |
1.5±0.2 (26%) 2.3±0.3 (34%) |
| Wang, et al., 1999 | Control Epinephrine |
Cat | 2.70±0.75 (42%) 2.04±0.59 (42%) |
1.42±0.48 (22%) 1.23±0.4 (26%) |
| Zhan et al., 1998 | Control Bunazosin |
Rabbit | 0.31±0.12 (13%) 1.04±0.17 (43%) |
0.22±0.03 (9%) 0.31±0.03 (13%) |
| Millar et al, 2011 | none | Mouse male BALB/cJ |
0.029±0.005 (21%) | 0.012±0.003 (9%) |
| Millar et al, 2015 | none | Young mouse BALB/cJ |
0.066±0.022 (37%) |
0.051±0.018* (36%) |
| Millar et al, 2015 | none | Aged mouse BALB/cJ |
0.011±0.007 (8%) |
0.01±0.007* (7%) |
| Millar et al, 2015 | none | Young mouse A/J |
0.12±0.048 (54%) |
0.055±0.13 (28%) |
| Millar et al, 2015 | none | Aged mouse A/J |
0.036±0.031 (25%) |
0.009±0.002 (9%) |
| Millar et al, 2015 | none | Young mouse C3H/HeJ |
0.109±0.028 (71%) |
0.054±0.02* (30%) |
| Millar et al, 2015 | none | Aged mouse C3H/HeJ |
0.024±0.009 (34%) |
0.006±0.003* (9%) |
| Millar et al, 2015 | none | Young mouse C57-BL/6J |
0.08±022 (53%) |
0.044±0.012 (42%) |
| Millar et al, 2015 | none | Aged mouse C57-BL/6J |
0.012±0.008 (14%) |
0.006±0.003 (9%) |
Data corrected from manuscript per communication with authors. Young mice are between 2½-4½ months, while aged mice are between 10–12 months (Millar et al., 2015).
4.2.1 Limitations in the indirect determination of unconventional outflow
The conventional outflow rate is similar to the aqueous humor production rate, and the difference between these (Equation (5)) is, under most circumstances, significantly less than either. Consequently, a relatively small error in either will result in a relatively large error in the estimated unconventional flow. For example, a 10% error in the estimated aqueous humor production rate or conventional outflow rate could result in a 50–100% error in the calculated unconventional outflow.
These considerations indicate that random errors and more importantly, systematic errors, will be magnified by the use of Equation (5). Lim et al. (2008) looked at the effect on estimates of unconventional outflow of using different methods to measure outflow facility (Schiotz tonography, 2-minute pneumatonography, 4-minute pneumatonography and fluorophotometry) and assumptions of episcleral venous pressure between 8 and 11 mm Hg. They found that the unconventional outflow estimated in the same placebo-treated healthy subjects varied from –0.49 to 1.46 µl/min (0%-60% of inflow), depending on the method used. As noted by Brubaker (2004) and others (Becker and Neufeld 2002; Bill 2003; Camras 2003; Kaufman 2003; Yablonski 2003), Equation (5) is subject to a number of assumptions, in particular that conventional outflow facility, unconventional outflow, and episcleral venous pressure are constants that do not change with IOP. These assumptions are, at best, approximations, and in under some conditions, introduce significant systematic errors into the estimate of unconventional outflow, particularly when drugs are applied to the eye. We detail below these systematic errors and their effects on the estimation of unconventional outflow using the indirect method.
Pressure-dependence of outflow facility
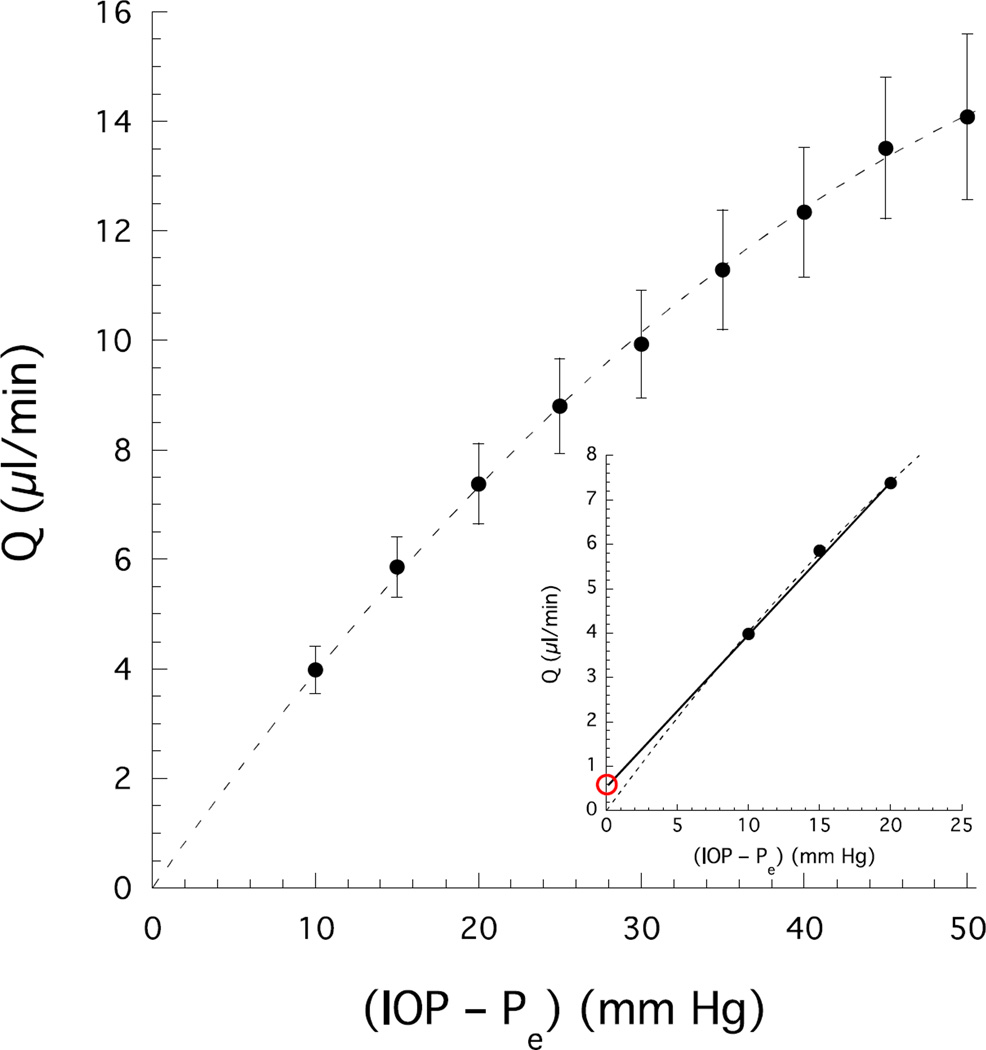
Outflow facility (c) is expressed as a constant in the Goldmann equation, but in fact c decreases as IOP increases (Levene and Hyman 1969; Brubaker 1975), which has been attributed to collapse of Schlemm’s canal (Moses 1979; Van Buskirk 1982). In enucleated eyes, Brubaker (1975) measured a nonlinear relationship between outflow (Q) and IOP (Fig 4) consistent with an IOP-dependent decrease in outflow facility, which is defined Q/(IOP–Pe). Despite a relatively modest decrease in outflow facility with pressure (~1.2% decrease in facility per unit mmHg increase in IOP; Brubaker 1975), non-linearity in the flow-pressure relationship may lead to significant errors in the estimate of unconventional outflow. This is demonstrated in Fig. 4, where the dashed curve represents the best second order polynomial fit to Brubaker’s data and the solid line represents the result of applying Equation (5) at pressures of 10 and 20 mmHg. This line may be extrapolated to the ordinate to yield a non-zero intercept that, according to Equation (5), would be interpreted as U, the pressure-independent or unconventional outflow (with Pe=0 in an enucleated eye). However, in this case, the nonzero intercept results entirely from the non-linearity of the flow-pressure relationship analyzed in terms of an inappropriate linear model prescribed by Equation (5), giving the false appearance of a pressure-independent outflow. For example, a different U would have been determined from a line fitted through Q at 5 and 15 mmHg. Furthermore, the magnitude of the apparent pressure-independent outflow in Fig 4 (~0.5 µL/min) is similar to or larger than that measured in living human eyes (Bill and Phillips 1971). Because a similar non-linear flow-pressure relationship is expected in living eyes, any indirect estimate of unconventional outflow would be subject to large uncertainties. Moreover, because outflow facility decreases with increasing pressure, any indirect estimate of the pressure-independent outflow will tend to overestimate its true value. This may partly explain why the indirect estimates of unconventional outflow are typically greater than those made by using direct tracer-based methods.
Figure 4.
Flow measured in enucleated human eyes (Pe=0) as a function of IOP (uncertainties are standard errors) (Brubaker 1975). The dashed curve is the best fit of Q=b1 IOP + b2 IOP2 to the data. Inset shows flow at low perfusion pressure. The solid line is from Equation (4) with flow measured at pressures of 10 and 20 mmHg. The red circle indicates the apparent unconventional outflow (U) erroneously estimated by using Equation (5). This error arises from the modest pressure-dependence of outflow facility that imposes a non-linearity in the flow-pressure relationship (dashed curved) and is not captured by Equations (4) and (5).
Pressure-insensitivity of unconventional outflow
Bill and others noted a striking characteristic of unconventional outflow, namely its relative insensitivity to the pressure difference that drives the flow (section 5.2). As measured using the indirect method, unconventional outflow is assumed to be entirely independent of IOP. However, direct tracer-based measurements indicate that unconventional outflow is not entirely pressure-independent, although it is relatively pressure-insensitive (Bill 1966a; Becker and Neufeld 2002; Bill 2003; Kaufman 2003). Any pressure-dependent changes in unconventional outflow will be attributed to conventional outflow in Equation (5). This will lead to a moderate underestimate of unconventional outflow and overestimate of conventional outflow as measured using indirect techniques. Kaufman (2003) suggests that the unconventional outflow facility is approximately 5% of total outflow facility in non-human primates. While this may seem small, this is roughly equivalent to 20–50% of total unconventional outflow at physiological IOP, and therefore the pressure-dependent contribution to unconventional outflow cannot be ignored.
This problem may be a particular concern for drug studies if the compounds used increase the pressure dependence of unconventional outflow (e.g. PGF2α (Gabelt and Kaufman 1990; Kaufman 2003)). The assumption of a purely pressure-independent unconventional outflow can greatly confuse interpretation of data from such experiments and may explain why some studies have found that prostaglandins lower IOP primarily by increasing unconventional outflow (Gabelt and Kaufman 1990; Toris et al. 1993; Stjernschantz 2001; Woodward et al. 2010), while other studies suggest that these agents also have a significant effect on conventional outflow (Christiansen et al. 2004; Wan et al. 2007; Lim et al. 2008).
Effect of episcleral venous pressure
The indirect method of estimating unconventional outflow requires an accurate estimate of episcleral venous pressure (Christiansen et al. 2004) and requires that the episcleral pressure be independent of IOP. Studies in monkeys support the latter assumption (Mäepea and Bill 1989), but accurately measuring episcleral venous pressure proves to be very difficult.
Mean reported episcleral venous pressures have ranged from 6.3 mmHg to 11.4 mmHg depending on the method of measurement.(Sit et al. 2011; Sit and McLaren 2011) Because of difficulty in its measurement, some investigators have assumed a single value for episcleral venous pressure for all subjects when calculating unconventional outflow (Toris et al. 2000; Zhao et al. 2010). Errors in episcleral venous pressure directly impact the estimate of unconventional outflow determined using Equation (5). For example, a 1 mmHg error in episcleral venous pressure leads to an error of approximately 0.3 µl/min in unconventional outflow (assuming c=0.3 µl/min/mmHg), which is 25 – 100% of the typical unconventional outflow. Assuming too low a value of episcleral venous pressure in this calculation (Equation (5)) would lead to underestimated unconventional outflow whereas assuming too high a value of episcleral venous pressure would lead to overestimated unconventional outflow
The assumptions inherent in Equations (4) and (5), combined with measurement inaccuracies and systematic errors, lead to considerable uncertainties in indirect estimates of unconventional outflow. Note that while both the direct and indirect methods of calculating unconventional outflow are subject to considerable experimental variability, the systematic errors inherent in the direct method are significantly smaller than those inherent to the indirect method. But unfortunately, there is currently no other method to assess unconventional outflow in humans other than by the indirect methods, and thus they are used despite their limitations. In animals, where unconventional outflow can be directly measured, the lack of agreement between direct and indirect measures casts doubt on the utility of the indirect methods. These issues apply a fortiori in studies on the effects of drugs on unconventional outflow, because drugs could directly affect the pressure dependence of unconventional flow and could alter episcleral venous pressure, thereby leading to erroneous conclusions regarding the effects of these drugs on aqueous humor dynamics. Indirect estimates of unconventional outflow must therefore be interpreted with caution.
5. Physiological Characteristics of Unconventional Outflow
Several features of unconventional outflow are particularly interesting from a physiological perspective. These features include the route of aqueous humor drainage along this pathway, the insensitivity to changes in intraocular pressure, the role of the ciliary muscle, and changes in this pathway after death.
5.1 The final route of unconventional outflow: uveoscleral vs uveovortex flow
Unconventional flow passes through the ciliary muscle and into the suprachoroidal space, but the pathway taken from the suprachoroidal space to exit the eye has been debated. Bill identified this path by tracing the route of radioactive-labeled proteins and other large molecules as they left the eye. These tracers moved through the ciliary muscle, into the suprachoroid space, and accumulated in the sclera and orbit, a route he termed the “uveoscleral” pathway (Bill 1965). This suggested fluid movement across the sclera where it could be collected and drained by the extraocular lymph vessels. Bill calculated the flow rate through this route based on tracer accumulation in these tissues, but he recognized that this method would only give an accurate estimate of unconventional flow if the tracer followed the same path as the aqueous humor and also was not washed away into the general circulation during the course of the experiment.
The use of smaller tracers suggested a somewhat different route. Pederson and co-workers (1977) perfused the anterior chamber of monkeys with fluorescein, a small molecule, and found that a significant fraction of this tracer entered the vortex veins (Fig. 3). The fluorescein concentration in these vessels was somewhat dependent on perfusion pressure, although it was not as pressure-dependent as was conventional outflow. The sensitivity to pressure suggested that movement through this pathway required ultrafiltration of water and small molecules into the uveal capillaries. They suggested that Starling forces were responsible for fluid resorption into the uveal capillaries, with these forces arising from the higher colloidal osmotic pressure in these vessels relative to the uveal interstitial fluid, a possibility first raised by Bárány (1967).
Bill also found tracer in the vortex veins in his early studies of rabbits perfused through the anterior chamber with radio-labeled albumin, but he concluded that less than 1.2% of the tracer within the anterior chamber drained via this route (Bill 1962a). In making this conclusion, Bill assumed that albumin could freely enter the uveal vessels once within the suprachoroidal space, an assumption that is inconsistent with the relatively low protein concentration in suprachoroidal fluid compared to plasma (Emi et al. 1989; Toris et al. 1990). The large size of albumin (67 kDa), as compared to that of fluorescein (0.3 kDa), likely excluded albumin from entering the choriocapillaris and the uveal capillaries, such that albumin would accumulate in the suprachoroidal space, diffuse across the sclera and enter the orbit, thereby explaining Bill’s experimental observations (Yablonski 2002). Larger molecular tracers such as albumin therefore appear to travel along a separate pathway than does aqueous humor and smaller molecular tracers.
Visualization of unconventional outflow in living anesthetized rhesus monkeys by using small tracers has provided indirect evidence for the role of these vessels in unconventional outflow. Butler et al (1984) perfused the anterior chamber with a small radio-opaque contrast agent (789 Da) and created images of this agent by X-ray computed tomography. Despite perfusion for up to 8 hours while the animal was alive, tracer was restricted to the anterior chamber, but immediately after death, tracer was seen migrating from the anterior chamber towards the posterior pole (Fig. 5). This experiment suggests that small molecular tracers that permeate the unconventional pathway are rapidly swept away by circulating blood, but when blood flow ceases soon after death, these tracers accumulate within the tissue.
Figure 5.
Computed tomography oblique sections through the orbital region of an adult rhesus monkey perfused with radio-opaque contrast medium in the anterior chamber. (A) After 1 hour of continuous perfusion while the monkey was alive, contrast medium was confined to the anterior chamber and there was no posterior motion of the medium. Similar images were recorded for up to 8 hours. (B) In the same monkey 35 minutes after death by pentobarbital overdose, contrast medium migrated posteriorly (red arrows). Black voids within the anterior chamber are an imaging artifact. Both images were from the same section plane in the same monkey. The right eye was set to 20 mmHg and left eye to 40 mmHg by using a fluid column. (Butler et al. 1984).
[Permission Needed]
Yablonski (2003) suggested that flow through the uveovortex pathway could be increased by chronic prostaglandin exposure. Prostaglandins have been shown to modify the extracellular matrix of the uvea, and if these modifications also increased the hydraulic permeability of these vessels, then flow through this route would also increase.
Based on the concentrations of fluorescein in the vortex veins, Pedersen et al. (1977) estimated that the uveovortex pathway could account for roughly 10% of aqueous humor outflow in the non-human primate eye. This estimate was similar to that of McMaster and Macri (1968), who found 8% of aqueous outflow to pass through the uveovortex pathway in an arterially-perfused enucleated rabbit eye. These estimates of outflow through the uveovortex pathway are significantly less than the total unconventional flow that has been measured using direct tracer-based techniques in animals (Table 3). In the living eye, unconventional outflow therefore likely drains through both the uveoscleral and uveovortex routes, where the distribution of outflow through each route likely depends on IOP, ciliary muscle tone, and other factors such as pharmacological agents.
Table 3.
Unconventional flow in different species determined by recovering labeled tracers (either collection of tracers in ocular tissues, or collection of tracer in general circulation with a separate measurement of aqueous inflow).
Because of these two parallel pathways, the term uveoscleral flow, which has been used to mean extra-trabecular flow, is misleading and refers to only the portion of unconventional flow that leaves the eye through the sclera. Some have used the term unconventional outflow to refer to the portion of outflow that passes through the uveoscleral route, uveovortex route, or both, and we have used this term in this review, although the term non-trabecular outflow would perhaps be more informative.
5.2 Pressure insensitivity
Bill and others found that as IOP increased between 4 mmHg and 35 mmHg, unconventional flow either remained constant or increased at a much slower rate than did conventional outflow. For example, using tracer-based measurements of unconventional outflow in monkeys, unconventional outflow was 0.44 µl/min at an IOP of 11 mm Hg, but when IOP was increased to 22 mm Hg, unconventional outflow increased only to 0.63 µl/min, less than a 50% increase (Bill 1966a). In contrast, over the same pressure range, conventional outflow increased 5-fold from 0.8 µl/min to 4.18 µl/min (Bill 1966a). Pedersen (1977) and Suguro (1985) also found unconventional outflow to be relatively pressure insensitive. It is important to note that in these studies, unconventional flow was not found to be pressure-independent, only pressure insensitive.
The pressure insensitivity of the unconventional outflow rate seems counterintuitive, since fluid flow requires work be done to overcome viscous drag generated as the fluid passes fixed structures. The energy necessary to do this work is supplied by a gradient in either the hydrostatic pressure or the osmotic pressure of the fluid, or both, as the fluid passes through the tissue. If the hydraulic resistance of the tissue is constant, increasing the pressure gradient should lead to a proportional increase in flow. However, if the resistance is not constant, but increases with pressure, then the flow may appear to be relatively pressure insensitive
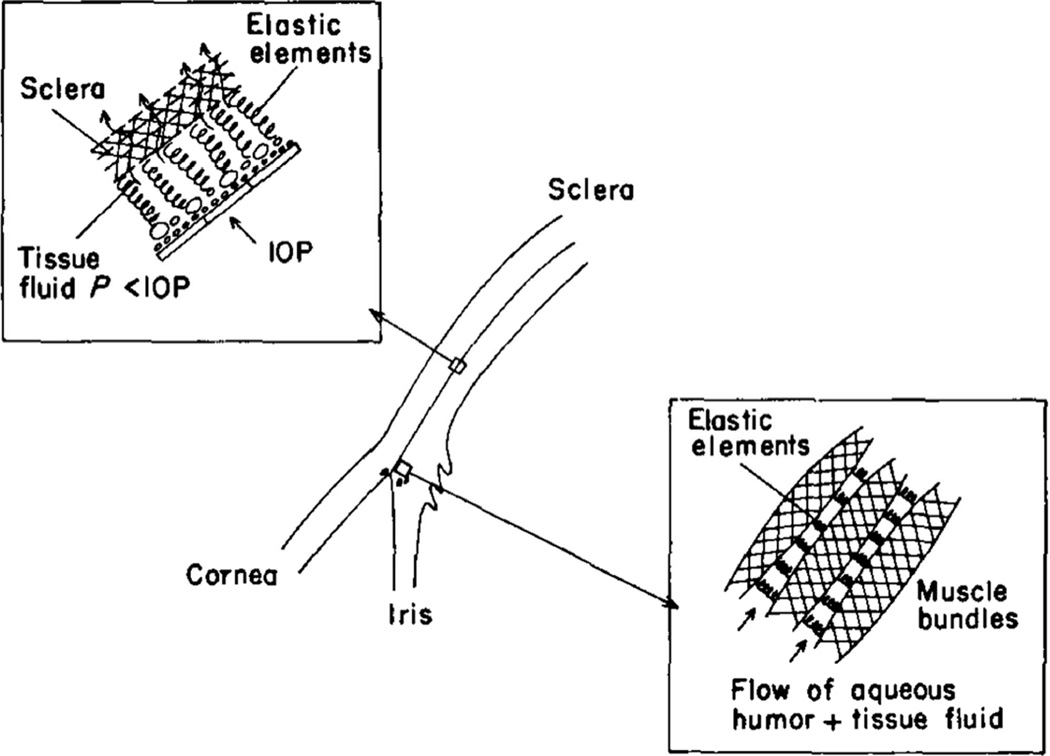
Bill (1977) suggested that the sizes of the interstitial spaces that carry unconventional flow through the ciliary muscle and suprachoroidal spaces are determined by a balance between IOP, that tends to collapse these spaces, and elastic elements and interstitial pressure within the spaces themselves, that tend to keep them open (Fig. 7). As IOP increases, these spaces become smaller, and hence their resistance to flow increases. Bill (1977) referred to this as an “elastic sponge model” because of its similarity to channels in a sponge.
Figure 7.
Aqueous humor from the anterior chamber communicates with tissue fluid between the muscle bundles of the ciliary muscle, the choroid, and the suprachoroidal space. Schematic shows how elastic elements in the ciliary muscle, choroid, and suprachoroid help to keep the interstitial spaces open. (Bill 1977).
[Permission Needed]
After passing through the ciliary muscle and suprachoroidal space, uveoscleral outflow must then traverse the sclera, and transcleral flow is pressure-dependent as it increases in linear proportion to the transcleral pressure gradient (Kleinstein and Fatt 1977). Therefore, in order for the elastic sponge model to explain the pressure-insensitivity of uveoscleral outflow, the resistance to flow through the ciliary muscle and suprachoroidal space must be significantly greater than the resistance to flow across the sclera. However, a large resistance in the ciliary muscle is inconsistent with the observation that the resistance to flow through the sclera is comparable to total unconventional outflow resistance (Fatt and Hedbys 1970; Kleinstein and Fatt 1977; Jackson et al. 2008). Based on our current understanding of ocular physiology, it remains unclear how uveoscleral outflow can exhibit pressure-insensitivity.
Flow through the uveovortex pathway offers a second explanation for the pressure insensitivity of unconventional outflow. When IOP increases, pressure in the uveal capillaries also increases (Mäepea 1992), and consequently, the pressure difference across the capillary wall increases by much less than does a change in IOP. Furthermore, the difference in pressure between IOP and that in the uveal capillaries is minor as compared to the difference in protein osmotic pressure between the aqueous humor and the blood (Pederson et al. 1977; Yablonski 2003) that pulls fluid into the uvea. Thus, changes in IOP would have a minor effect on the total force driving fluid into the choroidal capillaries leaving uveovortex flow relatively insensitive to IOP changes (Johnson 2000).
The modified Goldmann equation (Equation (4)) treats unconventional outflow as a constant, U, that can change depending on the status of the tissues in the unconventional outflow pathways but is independent of IOP. Although this equation has been used for many years, a model that includes the driving forces and facility of outflow through these pathways might better describe aqueous humor dynamics. Modifications that recognize determinants of the unconventional outflows have been proposed by Becker and Neufeld (2002) and discussed by Bill (2003), Kaufman (2003), Yablonski (2003), and Camras (2003) in a series of letters. However, such a model would need to include the effects of pseudofacility (Kaufman 2003), differing driving forces for the uveovortex and uveoscleral outflow pathways (Kaufman 2003; Yablonski 2003), and the increased pressure dependence of unconventional outflow at low IOP (Bill 2003), leading to a somewhat unwieldy expression.
5.3 Role of the ciliary muscle: a principal site of unconventional outflow resistance
The ciliary muscle lies near the entrance of the unconventional outflow pathway, and it is not surprising that its tone greatly influences outflow through this route. Pharmacological or surgical alterations to the ciliary muscle tone cause changes in unconventional outflow. For example, contraction of the ciliary muscle by pilocarpine reduces unconventional outflow by 90% in cynomolgus monkeys (Bill and Wålinder 1966), while relaxation of the ciliary muscle by atropine has the opposite effect (Bill 1967). Prostaglandins tend to relax the ciliary muscle (Poyer et al. 1992), allowing an acute reduction in unconventional outflow resistance, but they also have a slower, more significant hypotensive effect by inducing remodeling of the extracellular matrix within the ciliary muscle (Lütjen-Drecoll and Tamm 1988) (Section 7.1). Pre-treatment with pilocarpine acutely abolishes the effect of prostaglandins on IOP and unconventional outflow in monkeys (Crawford and Kaufman 1987; Nilsson et al. 1989).
Bill (1977) suggested that compression of the ciliary muscle with increasing IOP could increase its flow resistance and thereby contribute to pressure insensitivity of unconventional outflow (Figure 7). When the ciliary muscle is removed by cyclodialysis, most of the resistance it offers is lost (Bill 1966c) and unconventional outflow increases four-fold (Suguro et al. 1985) and becomes pressure dependent (Toris and Pederson 1985). Experiments exploring the role of the ciliary muscle in unconventional outflow have been reviewed by Alm (2009).
5.4 Unconventional outflow in living and post-mortem eyes
Flow through the conventional outflow pathway is pressure-driven and thought to have largely the same characteristics in living and post-mortem eyes (Grant and Trotter 1955; Grant 1963), when corrected for the loss of episcleral venous pressure after death. The unconventional outflow pathway, however, is more complicated. Bill found that there was no correlation between rate of unconventional outflow in the eye of a living animal and that in the contralateral eye from the same animal after death (Bill 1966a). Following death, unconventional outflow was approximately 150% higher than in eyes of living cynomolgus monkeys (Bill 1966a), rabbits (Bill 1966c), and cats (Bill 1966b) , and increased by nearly 400% in vervet monkeys (Bill 1966d). In mice, however, there was no evidence for a difference in unconventional outflow between living and post-mortem (in situ) eyes (Millar et al. 2011). The pressure-insensitivity of unconventional flow in the physiological pressure range suggests that the increase in unconventional outflow after death was not likely caused by the loss of episcleral venous pressure. Unconventional outflow, however, may have increased after death due to loss of ciliary muscle tone and the associated decrease in outflow resistance offered by the ciliary muscle (section 5.3).
6. Characteristic of Unconventional Flow in Different Species
Anatomical (see Henderson (1950) and Tripathi (1974)) or functional differences in the unconventional outflow pathway can affect the drainage of aqueous humor through this route and contribute to differences in unconventional outflow between species. Lower placentals, for example, exhibit a deep ciliary cleft with a relatively undeveloped ciliary muscle that allows open fluid communication to the posterior ciliary body. Primates, in contrast, have a well-developed ciliary muscle without a ciliary cleft that renders a strong dependence of unconventional outflow on ciliary muscle tone and accommodation.
Table 3 shows the unconventional outflow rate and its fraction of total outflow in a variety of species, as measured by tracer techniques. In general, non-human primates have the highest fraction of unconventional outflow. We explore the difference in unconventional outflow between species, focusing on primates and mice because of their utility as models for human eyes and because of considerable discrepancies in literature regarding unconventional flow in mice.
6.1 Human
Only one study (Bill and Phillips 1971) has reported direct measurements of unconventional outflow in living human eyes by using tracer methods. In patients’ eyes destined for enucleation, unconventional outflow was estimated from radioactive albumin tracer accumulation in the posterior scleral shell after injection into the anterior chamber. In 3 eyes, unconventional outflow was estimated to be 0%, 4% and 14% of total outflow from the anterior chamber (an absolute value of unconventional outflow was reported for only one eye, as 0.28 µl/min). As in monkeys, unconventional outflow in humans was reduced by pilocarpine (0% - 3% of total outflow in 3 eyes) and increased by atropine (4% - 27% in 6 eyes; flow rates in 2 eyes were reported as 0.43 and 0.62 µl/min), consistent with the notion developed from studies of monkeys that ciliary muscle tone modulates unconventional flow between muscle bundles (Section 5.3). In histologic samples, albumin tracer was often found in the perivascular space surrounding intrascleral vessels, suggesting that these spaces function as an outflow route, at least for the tracer used.
Indirect estimates (Section 4.2) of unconventional outflow in human have been reported in a number of studies (Table 4). Toris (1999) found that unconventional outflow decreased with age in humans, from 1.52 µl/min (54% of total flow) in young adults to 1.1 µl/min (46%) in seniors, and also found that unconventional outflow was lower in glaucomatous eyes (Toris et al. 2002b), consistent with values in Table 4.
Table 4.
Unconventional outflow in humans as determined by using the indirect method. Qu is unconventional outflow (µl/min) and Qin is inflow rate. All values are population averages. “Fluorophometric” or “tonographic” refers to the method used to measure aqueous humor outflow facility. (LTP –laser trabeculoplasty)
| Condition of Subjects (Method) |
Qu | % Qin | Ref | Notes |
|---|---|---|---|---|
| Normal (tonographic) | 0.8 | 36 | (Townsend and Brubaker 1980) | |
| Normal (tonographic) | 1.06 | 41 | (Coakes and Siah 1984) | Control (Placebo) |
| Young normal [21–23 y] (tonographic) |
−0.38 | 0 | (Mishima et al. 1997) | daytime |
| 1.23 | 85 | nighttime | ||
| Young normal [21–23 y] (fluorophotometric) |
0.57 | 25 | daytime | |
| 0.78 | 54 | nighttime | ||
| Normals (tonographic) | 0.85 – 1.52 |
34 – 62 |
(Brubaker et al. 2001) | Control (Placebo) |
| Normal (fluorophotometric) |
1.5 | 45 | (Wang et al. 2002) | Control (Baseline) |
| 1.1 | 36 | Control (Placebo) | ||
| 1.1 | 39 | Experimental (Baseline) | ||
| Normal (fluorophotometric) |
1.09 | (Toris et al. 2002b) | ||
| Normal [47–76 y] (tonographic) |
0.94 | 38 | (Nau et al. 2013) | daytime |
| 0.07 | 6 | nighttime | ||
| Glaucoma patients before LTP (fluorophotometric) |
1.1 | 78 | (Yablonski et al. 1985) | Control (Baseline) |
| 0.51 | 40 | valign="top"Control (Placebo) | ||
| 0.96 | 64 | Experimental (Baseline) | ||
| Ocular hypertension (fluorophotometric) |
0.60 | 30 | (Hayashi et al. 1989) | |
| Ocular hypertension (fluorophotometric) |
0.66 | (Toris et al. 2002b) | ||
| Ocular hypertension (fluorophotometric) |
1.14 | 46 | (Toris et al. 2004) | Control (Baseline) |
| 0.99 | 48 | Control (Placebo 1) | ||
| 0.86 | 37 | Control (Placebo 2) | ||
| 1.24 | 52 | Experimental (Baseline) | ||
| Ocular hypertension (fluorophotometric) |
0.67 | 25 | (Toris et al. 1995b) | Control (Baseline) |
| 0.62 | 24 | Control (Placebo) | ||
| 0.58 | 23 | Experimental (Baseline) | ||
| Ocular hypertension (fluorophotometric) |
0.35 | 14 | (Toris et al. 1995a) | Control (Baseline) |
| 0.5 | 22 | Control (Placebo) | ||
| 0.12 | 4.8 | Experimental (Baseline) | ||
| Ocular hypertension or glaucoma (tonographic) |
0.64 – 0.96 |
25 – 40 |
(Christiansen et al. 2004) | Varying assumptions on episcleral venous press. |
Considerable variability is seen in the results listed in Table 4, both for normal eyes (0–85% of total outflow was unconventional) as well as for eyes with ocular hypertension or glaucoma (5–78%). Because participants in these studies were untreated controls, such variation is difficult to explain other than as variability in the technique itself. Even within a single study, larger variations in unconventional outflow (5–22%) were reported in the same population (Toris et al. 1995a). Furthermore, most indirect estimates of unconventional outflow are much higher than tracer-based estimates of unconventional outflow (Bill and Phillips 1971), similar to the discrepancies noted in animal studies as described in section 4.2 (Tables 2 and 3).
6.2 Monkeys
Anders Bill and co-workers were the first to measure unconventional outflow in monkeys (Bill 1965; Bill and Hellsing 1965). They demonstrated that unlike the human eye, which has a relatively small fraction of unconventional outflow, in monkeys a significant fraction of aqueous humor passed through the unconventional pathway. In cynomolgus, vervet, and rhesus monkeys, roughly one quarter to one half of aqueous humor outflow passed through the unconventional outflow pathway, while in other species, less than a quarter passed this route (Table 3).
Alm and Nilsson suggested that an age-dependent reduction in unconventional flow may explain the difference between non-human primate and human eyes (Alm and Nilsson 2009). Indeed Gabelt et al. (2003; 2005) reported that unconventional flow in young (3–10 years of age) and middle-aged rhesus monkeys (ages 19–23 years) was twice as high as in older animals (ages 25–29), and they associated this age-related decrease with extracellular matrix accumulation in the ciliary muscle. However, based on tracer studies, the rate they reported for unconventional flow in older monkeys (19.8% of outflow) was still a higher fraction of total outflow than that reported in humans. This also does not explain why the fraction of unconventional flow is much higher in monkeys compared to non-primates, considering that the ciliary musculature is better developed in primates and would seem to pose greater resistance to unconventional outflow relative to that in non-primates.
As noted in Section 5.3, pilocarpine has a large effect on unconventional flow in primates. By contracting the longitudinal fibers of the ciliary muscle, pilocarpine almost completely eliminates unconventional outflow (Fig. 6). In contrast, relaxing the ciliary muscle with atropine increases unconventional outflow in both cynomolgus (Bill 1967) and vervet monkeys (Bill 1969b). The increase in unconventional outflow was relatively modest in cynomolgus monkeys [from 39 to 42% (Bill 1967)] and larger in vervet monkeys [31 to 53% (Bill 1969b)] despite similar dosages. These differences may be attributable to differences in ciliary muscle tone or the reduction in tone caused by general anesthesia or both. For example, if in cynomolgus monkeys, the ciliary muscle is partly contracted by a subthreshold dose of pilocarpine, then atropine has a larger effect on unconventional outflow, increasing from 19 to 56% (Bill 1967). Alternatively, the differences between monkey species may be related to the near absence of a scleral spur in vervet monkeys (Rohen et al. 1967; Bárány 1979).
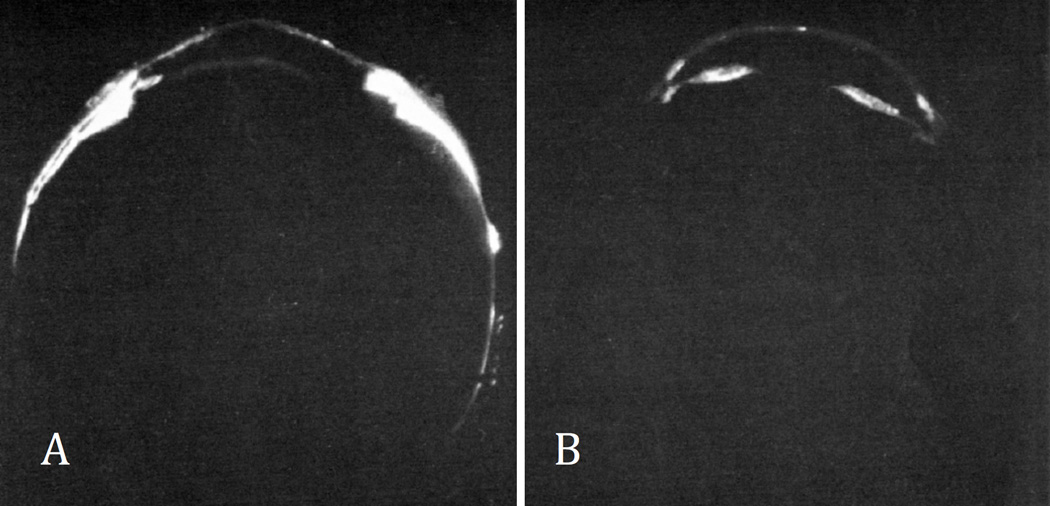
Figure 6.
Effect of atropine on unconventional outflow in cynolmologus monkey eyes with pilocarpine-induced tone in the ciliary muscle. In eyes with atropine and pilocarpine in the anterior chamber (A), radioactively-labeled albumin entered the uvea and sclera to a larger extent than in eyes with pilocarpine only (B). (Bill 1967)
[Permission Needed]
Much of our knowledge about the unconventional outflow comes from these pioneering studies in non-human primates. However, the higher fraction of outflow through this pathway in monkeys than in humans may limit the use of monkeys as a model for evaluating drugs for use in humans. While pilocarpine lowers IOP in humans and has been used as a treatment for glaucoma, its reported effects in monkeys depend on the concentrations used and perhaps also, the anesthetic chosen. In early studies with cynomolgus monkeys anesthetized using pentobarbital (which causes low resting IOP), pilocarpine reduced conventional outflow resistance but paradoxically increased IOP because of its effect on unconventional outflow (Bárány 1962; Bárány 1966; Bill and Wålinder 1966). However, in animals anesthetized by ketamine (which has less effect on resting IOP), pilocarpine decreased IOP, as it does in human eyes (Crawford and Kaufman 1987).
6.3 Rabbit
Unconventional outflow is significantly lower in rabbits as compared to non-human primates (Table 3). This is somewhat puzzling because rabbits possess a deep ciliary cleft and a relatively undeveloped ciliary muscle that should provide a lower resistance to unconventional outflow as compared to primates (Tripathi 1977).
As in primates, unconventional flow in rabbits increases after cyclodialysis (Bill 1966c) or treatment with PGF2α (Goh et al. 1989; Poyer et al. 1992), but not after prostaglandin D2. The decrease in IOP after PGF2α can be inhibited by pilocarpine (Goh et al. 1989). Other studies suggest that unconventional outflow in the rabbit may be increased by rho-kinase inhibitors such as Y-27632 (Honjo et al. 2001) and α1-adrenergic antagonists such as bunazosin (Zhan et al. 1998) and nipradilol (Kanno et al. 1998), the latter possibly involving nitric oxide (Sugiyama et al. 2001).
6.4 Dog
Unconventional outflow in dogs has been documented by perfusion with fluorescent dextran that accumulated in the supraciliary, suprachoroidal, and scleral tissues (Gelatt et al. 1979). As in most other species, pilocarpine reduced unconventional outflow, as evidenced by a reduction in fluorescent dextran labeling posterior to the ciliary body. Perfusion with radiolabelled tracers provided a direct estimate of unconventional outflow that was 15% of total outflow in normotensive beagles but was reduced to 3% in beagles with advanced glaucoma (Barrie et al. 1985), consistent with the reduction in unconventional outflow reported in ocular hypertensive humans (Toris et al. 2002b). Beagles with advanced glaucoma had an accumulation of melanophores and elastic-rich extracellular matrix within the ciliary body musculature visible on histologic examination, and these deposits may have contributed to the elevated unconventional outflow resistance and consequent reduction in unconventional outflow in these animals (Samuelson and Streit 2012).Unconventional outflow increased as IOP was increased from 20 to 50 mmHg, and the pathway became less permeable to microspheres larger than 1 µm at pressures above 20 mmHg (Samuelson et al. 1985).
6.5 Cat
Bill (1966) reported an unconventional outflow of no more than 3% of total outflow in domestic cats, as measured by accumulation of radiolabelled albumin in ocular tissues. However, much higher estimates of unconventional outflow (up to 26%) have been reported by Wang et al. (1999) and Toris et al. (1995c) who measured accumulation of fluorescent dextran in the uvea, sclera and retina. The reason for this large discrepancy remains unclear. Unconventional outflow was increased by prostaglandin A2 in cats (Toris et al. 1995c) but remained relatively unaffected by epinephrine (Wang et al. 1999) based on direct tracer and indirect fluorophotometry methods.
6.6 Mouse
Mice are a useful animal model because of their relatively low cost, ease of use, and short lifespan that makes them ideal for genetic studies. Fluorescent dextran introduced into the anterior chamber of NIH Swiss mice appears in the supraciliary space and choroid within 60 minutes (Lindsey and Weinreb 2002; Bernd et al. 2004) confirming the existence of unconventional outflow in mice (Fig. 8). Using tracers, Millar et al. (2011) directly measured* unconventional outflow in BALB/cJ mice aged between 30 and 42 weeks to be 0.012 µl/min, or approximately 9% of total outflow. With increasing age, however, there appears to be a significant reduction in unconventional outflow in mice (Table 2) (Millar et al. 2015), consistent with the age-related decline in unconventional outflow in humans (Toris et al. 1999) and monkeys (Gabelt et al. 2003).
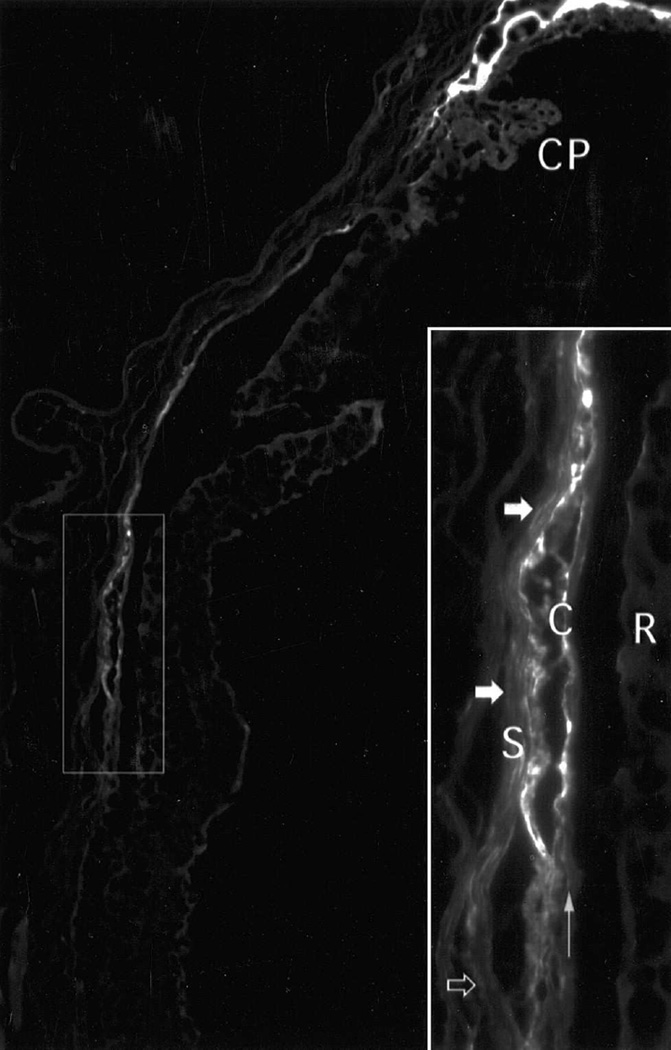
Figure 8.
Tracer decoration of the mouse unconventional outflow pathway 60 minutes after injection of 70 kDa dextran into the anterior chamber. (Lindsey and Weinreb 2002) Fluorescence lined the suprachoroidal space (ciliary processes, CP; choroid ,C; sclera, S). High-magnification inset shows some regions of sclera near the suprachoroidal space contained substantial tracer (closed arrows) and other scleral regions contained minimal tracer (open arrow). Retinal pigment epithelium is indicated by a vertical arrow. Tracer did not enter the retina (R).
[Permission Needed]
Estimates of unconventional outflow using indirect techniques (Section 4.2) in mice have yielded much larger values than those measured using tracers, ranging from 0.029 to 0.157 µl/min, corresponding to 21–83% of total outflow in NIH Swiss White and C57BL/6 mice (Aihara et al. 2003; Crowston et al. 2004; Zhang et al. 2009; Lee et al. 2011; Lei et al. 2011; Millar et al. 2011; Stamer et al. 2011; Boussommier-Calleja et al. 2012). While differences in age or strain may account for some of this variability (Millar et al. 2015), such differences cannot explain why there is such a large discrepancy between direct and indirect estimates of unconventional outflow in mice. Even when measured within the same study, Millar et al. (2011; 2015) reported a roughly 2-fold difference in unconventional outflow as measured by tracers versus that estimated by using the indirect technique. This discrepancy raises concerns regarding the accuracy of indirect estimates of unconventional outflow in mice, particularly considering the difficulty in the measurement itself in this small animal.
The small size of the mouse eye compounds the difficulty of determining unconventional outflow for two reasons. First, the magnitude of the flow rate in mouse eyes is so incredibly small as to push the limits of accuracy of most perfusion systems. Leaks and inaccuracies in the perfusion equipment could introduce significant inaccuracies in the measurement of outflow facility, raising concerns regarding the indirect estimates of unconventional outflow that are based on such measurements. Evaporation from the exposed surface of the perfused eye, for example, could artificially inflate the apparent unconventional outflow rate in indirect studies. Boussommier-Calleja et al. (2013; 2015) have shown that the apparent pressure-independent outflow as measured by perfusion is indistinguishable from zero when enucleated mouse eyes are submerged in a bath of isotonic saline, in contrast to prior studies by the same group who measured a non-zero pressure-independent outflow when the eyes were exposed to room air (Boussommier-Calleja et al., 2012; Stamer et al., 2011; Lei et al., 2011). While fluid would evaporate in any species if the cornea or sclera were not kept fully hydrated, the relative importance of evaporation is potentially much greater in mice because of the larger ocular surface area to volume ratio. While this phenomenon might be of less importance in live animal studies (due to the presence of the extraocular muscles and orbital fat surrounding the sclera), the eyelids are usually kept open during such measurements. Even with hydrating drops, evaporation would tend to increase the osmolarity at the surface of the cornea and draw water from the eye.
Second, whereas in larger eyes, such as that of primates, diffusion does not play a significant role in transporting tracer into tissues around the anterior chamber (Bill 1965; Bill and Hellsing 1965; Bill 1966a; Pederson and Toris 1987), diffusional transport of tracers might be expected to be more significant in smaller eyes such as those of mice and this leads to an overestimate of unconventional outflow when using direct methods. Nevertheless, the distribution of dextrans in the anterior segment of mice was not influenced by tracer molecular weight (Bernd et al. (2004)), consistent with findings in primates (Section 4.1.4). Furthermore, the observed spread of tracer over time through the suprachoroidal space of mice was consistent with bulk flow (Lindsey and Weinreb 2002). These considerations suggest that diffusional transport of tracer may not be a major concern in the measurement of unconventional flow in mice.
The large fraction of flow passing through the unconventional outflow pathway in mice, as estimated by indirect methods, could be seen as an argument against use of mice as a model for human aqueous humor dynamics, where the majority of outflow passes through the trabecular route. However, as discussed in section 4.2.1, unconventional outflow may be overestimated by using indirect methods in mice (Table 2). Furthermore, the observation that pilocarpine reduces IOP in young black Swiss (Avila et al. 2001) and CD1 mice by increasing outflow facility (Li et al. 2014; Overby et al. 2014) argues against the notion that a large quantity of outflow passes through the unconventional pathway in mice (which would be blocked by pilocarpine).
The age-related decline in unconventional outflow observed in multiple mouse strains suggests that older mice having a smaller fraction of unconventional outflow may provide an appropriate model of aqueous humor dynamics in older humans (Millar et al. 2015). If mice are to be accepted as a model for human aqueous humor dynamics, then like humans, the bulk of outflow in mice should pass through the conventional or trabecular outflow pathway. Thus, the accuracy of the reports of high unconventional outflow estimated by using the indirect method should be reexamined and the apparent discrepancy between direct and indirect estimates should be resolved in order to better assess whether mice are an appropriate animal model for aqueous humor dynamics in humans.
7. Clinical Significance
7.1 Drug effects
Cholinergics, adrenergics, and prostagladins can affect the flow of aqueous humor through the unconventional outflow pathway. The principal site of unconventional outflow resistance is the ciliary muscle. Contraction of the ciliary muscle by cholinergics such as pilocarpine decrease the rate of unconventional outflow (Bill and Wålinder 1966) while cylcoplegics such as atropine increase unconventional outflow (Bill 1967; Bill 1969b). However, cholinergics also interfere with accommodation and are no longer used clinically to lower IOP
Adrenergic agents also affect outflow through the unconventional outflow pathway. Bill (Bill 1969a) showed that epinephrine increased unconventional outflow in vervet monkeys, although the mechanism of action was unclear. Alm and Nilsson (Alm and Nilsson 2009) suggested that the effect of epinephrine might be related to relaxation of the ciliary muscle.
The introduction of the prostaglandin PGF2α (Camras et al. 1977) and PGF2α-analogues (e.g. latanoprost , bimatoprost, and travoprost) to the pharmacopeia of glaucoma treatments marked a major change in glaucoma management. Not only is their reduction of IOP additive to most other glaucoma treatments, but the magnitude of their effect made them valuable as ocular hypotensive agent in their own right (Nilsson 1997; Bron et al. 2001). Daily treatment with PGF2α over several days causes a larger decrease in IOP than that from a single application, and the decrease is more marked after multiple dosing (Nilsson 1997). As noted in section 4.2.1, PGF2α increases the pressure-dependency of unconventional outflow (Gabelt and Kaufman 1990; Kaufman 2003). The major effect on unconventional outflow appears to be mediated by remodeling of the extracellular matrix in the ciliary muscle (Ocklind 1998; Sagara et al. 1999; Gaton et al. 2001) that enlarges spaces between ciliary muscle bundles (Fig. 9) and thereby presumably reduces unconventional outflow resistance and increases its pressure-dependency (Lütjen-Drecoll and Tamm 1988).
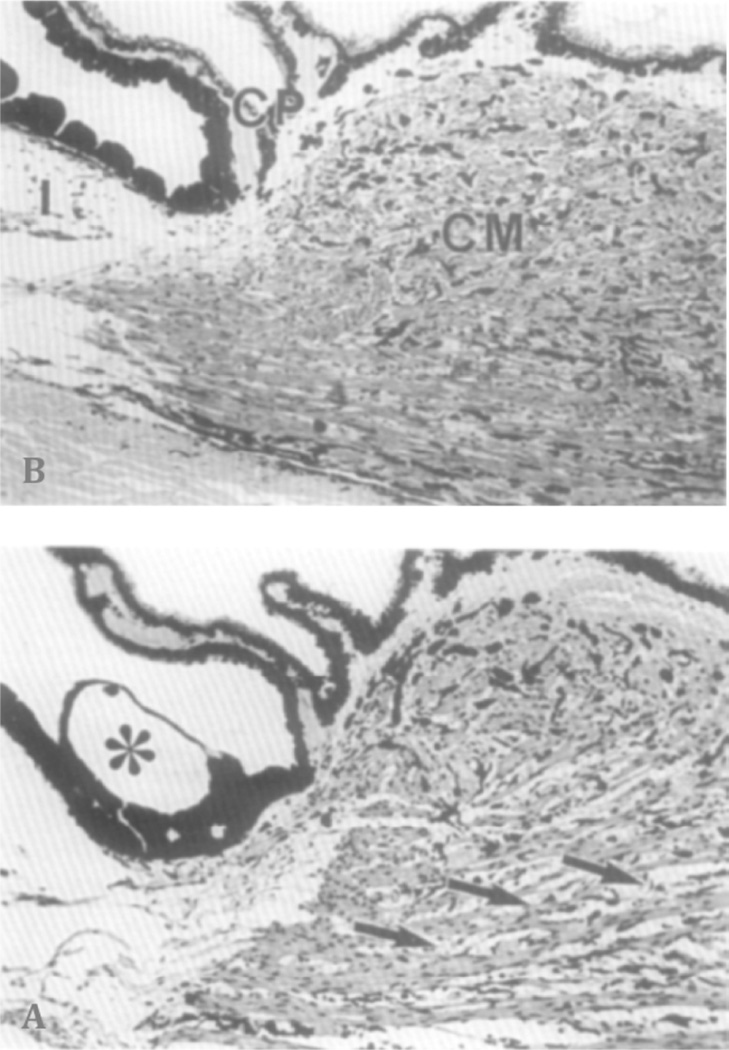
Figure 9.
Sagittal section through the anterior ciliary body. (A) Vehicle-treated control eye. CM, Ciliary muscle; I, iris. CP, ciliary processes. (B) After treatment with PGF2α, for 4 days. Note the enlarged spaces between the thin muscle fiber bundles in the prostaglandin-treated eye (arrows). Asterisk: Greeff’s vesicle (Lütjen-Drecoll and Tamm 1988).
[Permission Needed]
Prostaglandins have an effect somewhat contrary to that of cholinergics; while prostaglandins increase unconventional outflow, cholinergics decrease it while also increasing conventional outflow. For this reason, the combination of prostaglandins and cholinergics would not be expected to be additive in reducing IOP. However, while perhaps not fully additive, combination of the two drugs reduces IOP more effectively than either alone (Fristrom and Nilsson 1993; Toris et al. 2002a). This has led to the suggestion that prostaglandins may also increase conventional outflow (Bahler et al. 2008).
7.2 Surgery
In cyclodialysis, the attachment of the ciliary body to the scleral spur is disrupted, providing unimpeded access of aqueous humor to the suprachoroidal space, bypassing the ciliary muscle. Cyclodialysis is associated with hypotony caused by a decreased aqueous inflow (Chandler and Maumenee 1961) and a greatly increased unconventional outflow (Bill 1966c; Suguro et al. 1985). Cyclodialysis is usually the result of trauma or a surgical complication, although it can be created surgically in desperate circumstances. However, it is not routinely used to treat ocular hypertension because of the very low resulting pressures (7 mmHg or less) and the risk of acute IOP elevation should the cyclodialysis cleft spontaneously close.
While the cyclodialysis procedure is rarely used clinically, the benefits of greatly increased unconventional outflow without decreased aqueous production can be realized by using a suprachoroidal stent to bypass the ciliary muscle (Melamed et al. 2009; Figus et al. 2011; Hoeh et al. 2013; Oatts et al. 2013). These devices have been used successfully to lower IOP, although there have been reports of hypotony and of reactive fibrosis that blocks the bypass pathway (Brandao and Grieshaber 2013). Nonetheless, they offer a potential means of achieving low IOPs that may be of particular benefit to cases of normal tension glaucoma.
7.3 Other clinical issues
The unconventional outflow pathway may be affected by pathologies of the anterior segment. In uveitis, accumulation of cells, protein and debris may block the conventional outflow pathway, and it has been suggested that the unconventional outflow pathway may have developed as an adaption to provide an alternate drainage route for aqueous humor under these circumstances (Alm and Nilsson 2009). Indeed, in uveitis, along with a reduction of aqueous production to roughly one half of its normal rate, there is also a profound increase in unconventional drainage because of a four-fold increase in pressure-dependent unconventional outflow facility (Toris and Pederson 1987; Nilsson 1997; Alm and Nilsson 2009). These changes produce hypotony until the uveitis subsides. It has been suggested that this effect is mediated by PGF2α. (Crawford and Kaufman 1987).
Unconventional outflow may also play a role in the development of uveal effusion, an accumulation of fluid in the uvea that is thought to arise from impaired fluid drainage through the posterior segment and usually associated with abnormal thickening of the sclera, particularly in nanophthalmos (Ward et al. 1988; Jin and Anderson 1990; Lam et al. 2005); uveal effusion can lead to detachment of choroid, ciliary body and retina. If a significant fraction of unconventional outflow passes into the vortex vein, then the proteins in the aqueous humor will be blocked from passing through these vessel walls, and they can only exit the eye by diffusion across the sclera (section 5.1). Thickening of the sclera, such as occurs in nanophthalmos, would reduce this protein diffusion, potentially causing an accumulation of these proteins along the inner surface of the sclera. This in turn would lead to an increase in the local oncotic pressure causing fluid accumulation, as is seen in uveal effusion (Gass 1983; Johnson 2000; Elagouz et al. 2010). This disease is treated by full-thickness sclerectomy to remove the barrier to protein transport.
8. Summary and Future Direction
Anders Bill was the first investigator to explore and characterize the physiologic processes of the unconventional outflow pathways. His pioneering measurement techniques are still the standard for identifying the pathway and estimating the amount of flow through this route. Originally this pathway was a scientific curiosity, but it became clinically important when Camras and Bito found that PGF2α could profoundly redirect flow through this pathway from the conventional pathway, reducing IOP. Our understanding of the unconventional outflow pathway has provided a new approach toward treating elevated IOP in glaucoma patients.
As unconventional outflow is pressure-insensitive under normal conditions, and because at least a fraction of this outflow passes through the uveovortex pathway, we would suggest that the terms “uveoscleral” and “pressure-independent flow” are misleading and can lead to incorrect conclusions about the nature of this route, particularly when considering the effects of drugs. Distinctions between pressure sensitive and anatomical pathways have important ramifications on the methods to be used for measuring and characterizing flow through this route. The more appropriate term for this route of outflow is “unconventional” or “non-trabecular”.
Future animal studies of the unconventional outflow pathway should focus on species whose anatomic and physiologic properties of this route are close to those of humans. The similarity of the mouse outflow pathways to that of the human (Smith et al. 2001; Overby et al. 2014) and particularly the fraction of flow passing through the unconventional outflow pathway, make the mouse an attractive model. However, the method for accurate measurement of flow through the unconventional outflow pathway (the tracer method) is difficult in mice, cumbersome in larger animals, and virtually impossible in humans. Indirect methods to estimate unconventional outflow, while easily used, have serious flaws and the accuracy of such approaches are questionable. Thus, a more robust and accurate method to measure unconventional outflow, both in animals and in living humans, is necessary.
Development of a reliable non-invasive method for accurate measurement of unconventional flow would provide a great advance for characterizing the dynamics of the anterior segment and for developing clinical treatments of glaucoma. One possibility might make use of fluorophotometry. A large macromolecule that would be trapped in or pass very slowly through the unconventional pathway could be introduced into the anterior chamber through the cornea and tracked to the general circulation as an indicator of trabecular flow, while its dilution in the anterior chamber is simultaneously measured to determine aqueous humor inflow. The difference between trabecular flow and inflow rates would be a direct measure of the unconventional outflow rate. Alternatively, tracers could be tracked through the outflow pathways by using high-resolution MRI or OCT.
Anders Bill developed the methods to establish the route and rate of unconventional outflow; our challenge now is to develop methods to measure this flow rate accurately and non-invasively in humans.
Highlights.
aqueous humor drains through an unconventional outflow pathway that has both a uveoscleral and uveovortex component
tracer-based methods are required to reliably measure outflow through these pathways
indirect estimates of unconventional outflow are subject to numerous assumptions and generally give poor agreement with tracer-based methods
the mouse is a promising model for studying aqueous humor outflow
there is a need for improved measurements of unconventional outflow in both animals and humans
Acknowledgments
NIH E019696 (MJ & DRO), NIH EY022359 (DRO), The BrightFocus Foundation (DRO) Research to Prevent Blindness, New York, NY (JWM), and the Mayo Foundation, Rochester, MN (JWM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
During these experiments, the mouse eye was perfused with tracer via the anterior chamber at a rate of 0.5 µL/min, at least 3-fold greater than typical inflow rates in mice.
LITERATURE CITED
- Aihara M, Lindsey JD, et al. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003;44(12):5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- Alm A, Nilsson SFE. Uveoscleral outflow - A review. Experimental Eye Research. 2009;88(4):760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Amrite AC, Edelhauser HF, et al. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- Anderson OA, Jackson TL, et al. Human transscleral albumin permeability and the effect of topographical location and donor age. Invest Ophthalmol Vis Sci. 2008;49(9):4041–4045. doi: 10.1167/iovs.07-1660. [DOI] [PubMed] [Google Scholar]
- Ascher KW. Aqueous Veins. American Journal of Ophthalmology. 1942;25:31. doi: 10.1016/s0002-9394(48)92325-3. [DOI] [PubMed] [Google Scholar]
- Bahler CK, Howell KG, et al. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008;145(1):114–119. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány E. Dissociation of accommodation effects from outflow effects of pilocarpine. In: Paterson G, Miller S, Paterson G, editors. Drug Mechanisms in Glaucoma. London, Churchill: 1966. pp. 275–284. [Google Scholar]
- Bárány E. Pseudofacility and uveoscleral outflow routes. In: Leydhecker W, Karger S, editors. Glaucoma, Tutzing Symposium, Basel. 1967. p. 35. [Google Scholar]
- Bárány EH. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus Ethiops) Investigative Ophthalmology & Visual Science. 1962;1(6):712–727. [PubMed] [Google Scholar]
- Bárány EH. A pharmacologist looks at medical treatment in glaucoma--in retrospect and in prospect. Ophthalmology. 1979;86(1):80–94. doi: 10.1016/s0161-6420(79)35548-8. [DOI] [PubMed] [Google Scholar]
- Barrie KP, Gum GG, et al. Quantitation of uveoscleral outflow in normotensive and glaucomatous beagles by H-3-labeled dextran. American Journal of Veterinary Research. 1985;46(1):84–88. [PubMed] [Google Scholar]
- Becker B, Neufeld AH. Pressure dependence of uveoscleral outflow. J Glaucoma. 2002;11(6):545. doi: 10.1097/00061198-200212000-00019. [DOI] [PubMed] [Google Scholar]
- Bernd AS, Aihara M, et al. Influence of molecular weight on intracameral dextran movement to the posterior segment of the mouse eye. Investigative Ophthalmology & Visual Science. 2004;45(2):480–484. doi: 10.1167/iovs.03-0462. [DOI] [PubMed] [Google Scholar]
- Bill A. The drainage of blood from the uvea and the elimination of aqueous humour in rabbits. Experimental Eye Research. 1962;1(3):200–205. doi: 10.1016/s0014-4835(62)80002-5. [DOI] [PubMed] [Google Scholar]
- Bill A. The drainage of albumin from the uvea. Experimental Eye Research. 1964;3:179–187. doi: 10.1016/s0014-4835(64)80033-6. [DOI] [PubMed] [Google Scholar]
- Bill A. The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irus) with evidence for unconventional routes. Investigative Ophthalmology. 1965;4:911–919. [PubMed] [Google Scholar]
- Bill A. Conventional and uveo-scleral drainage of aqueous humour in the cynomolgus monkey (Macaca irus) at normal and high intraocular pressures. Experimental Eye Research. 1966a;5:45–54. doi: 10.1016/s0014-4835(66)80019-2. [DOI] [PubMed] [Google Scholar]
- Bill A. Formation and drainage of aqueous humour in cats. Experimental Eye Research. 1966b;5(3):185–190. doi: 10.1016/s0014-4835(66)80020-9. [DOI] [PubMed] [Google Scholar]
- Bill A. The routes for bulk drainage of aqueous humor in rabbits with and without cyclodialysis. Documenta Ophthalmologica. 1966c;20:157–169. doi: 10.1007/BF00165414. [DOI] [PubMed] [Google Scholar]
- Bill A. The routes for bulk drainage of aqueous humour in the vervet monkey (Ceropithecus ethiops) Experimental Eye Research. 1966d;5:55–57. doi: 10.1016/s0014-4835(66)80020-9. [DOI] [PubMed] [Google Scholar]
- Bill A. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus) Exp Eye Res. 1967;6(2):120–125. doi: 10.1016/s0014-4835(67)80062-9. [DOI] [PubMed] [Google Scholar]
- Bill A. Early effects of epinephrine on aqueous humor dynamics in vervet monkeys (Cercopithecus ethiops) Exp Eye Res. 1969a;8:35–43. doi: 10.1016/s0014-4835(69)80078-3. [DOI] [PubMed] [Google Scholar]
- Bill A. Effects of atropine on aqueous humor dynamics in the vervet monkey (Cercopithecus ethiops) Exp Eye Res. 1969b;8(3):284–291. doi: 10.1016/s0014-4835(69)80040-0. [DOI] [PubMed] [Google Scholar]
- Bill A. Effects of norepinephrine, isoproterenol, and sympathetic stimulation on aqueous humor dynamics on vervet monkeys. Exp Eye Res. 1970;10:31–46. doi: 10.1016/s0014-4835(70)80006-9. [DOI] [PubMed] [Google Scholar]
- Bill A. Aqueous humor dynamics in monkeys (Macaca irus and Cercopithecus ethiops) Experimental Eye Research. 1971;11(2):195–206. doi: 10.1016/s0014-4835(71)80023-4. [DOI] [PubMed] [Google Scholar]
- Bill A. Blood circulation and fluid dynamics in the eye. Physiological Reviews. 1975;55:383–416. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- Bill A. Basic physiology of the drainage of aqueous humor. In: Bito LZ, Davson H, Fenstermacher JD, editors. The Ocular and Cerebrospinal Fluids. London, New York: San Francisco, Academic Press; 1977. pp. 291–303. [Google Scholar]
- Bill A. Some Thoughts on the Pressure Dependence of Uveoscleral Flow. Journal of Glaucoma. 2003;12(1):88–89. doi: 10.1097/00061198-200302000-00017. [DOI] [PubMed] [Google Scholar]
- Bill A, Hellsing K. Production and drainage of aqueous humor in the cynomolgus monkey (Macaca irus) Investigative Ophthalmology. 1965;4:920–926. [PubMed] [Google Scholar]
- Bill A, Phillips CI. Uveoscleral drainage of aqueous humor in human eyes. Experimental Eye Research. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- Bill A, Wålinder P. The effects of pilocarpine on the dynamics of aqueous humor in a primate (Macaca irus) Invest Ophthalmol. 1966;5(2):170–175. [Google Scholar]
- Boussommier-Calleja A, Bertrand J, et al. Pharmacologic Manipulation of Conventional Outflow Facility in Ex Vivo Mouse Eyes. Investigative Ophthalmology & Visual Science. 2012;53(9):5838–5845. doi: 10.1167/iovs.12-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Li G, et al. Physical Factors Affecting Outflow Facility Measurements in MiceFactors Affecting Facility Measurements in Mice. Investigative Ophthalmology & Visual Science. 2015;56(13):8331–8339. doi: 10.1167/iovs.15-17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Overby DR. The influence of genetic background on conventional outflow facility in mice. Invest Ophthalmol Vis Sci. 2013;54(13):8251–8258. doi: 10.1167/iovs.13-13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao LM, Grieshaber MC. Update on Minimally Invasive Glaucoma Surgery (MIGS) and New Implants. J Ophthalmol. 2013;2013:705915. doi: 10.1155/2013/705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AM, Denis P, et al. Additive IOP-reducing effect of latanoprost in patients insufficiently controlled on timolol. Acta Ophthalmol Scand. 2001;79(3):289–293. doi: 10.1034/j.1600-0420.2001.790316.x. [DOI] [PubMed] [Google Scholar]
- Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Investigative Ophthalmology and Visual Science. 1975;14:286–292. [PubMed] [Google Scholar]
- Brubaker RF. Goldmann's equation and clinical measures of aqueous dynamics. Experimental Eye Research. 2004;78(3):633–637. doi: 10.1016/j.exer.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Brubaker RF, McLaren JW. Uses of fluorophotometry in glaucoma research. Ophthalmology. 1985;92(7):884–890. doi: 10.1016/s0161-6420(85)33939-8. [DOI] [PubMed] [Google Scholar]
- Brubaker RF, Schoff EO, et al. Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics1. American Journal of Ophthalmology. 2001;131(1):19–24. doi: 10.1016/s0002-9394(00)00843-6. [DOI] [PubMed] [Google Scholar]
- Butler JM, Raviola G, et al. Computed tomography of aqueous humour outflow pathways. Exp Eye Res. 1984;39(6):709–719. doi: 10.1016/0014-4835(84)90070-8. [DOI] [PubMed] [Google Scholar]
- Camras CB. Some thoughts on the pressure dependence of uveoscleral flow. J Glaucoma. 2003;12(1):92–93. doi: 10.1097/00061198-200302000-00020. author reply 93–4. [DOI] [PubMed] [Google Scholar]
- Camras CB, Bito LZ, et al. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Investigative Ophthalmology & Visual Science. 1977;16(12):1125–1134. [PubMed] [Google Scholar]
- Chan-Ling T, Koina ME, et al. Author Response: Sufficient Evidence for Lymphatics in the Developing and Adult Human Choroid?LettersIOVS | Month Year | Vol. 56 | No. 10 | page number. Investigative Ophthalmology & Visual Science. 2015;56(11):6711–6713. doi: 10.1167/iovs.15-18011. [DOI] [PubMed] [Google Scholar]
- Chandler PA, Maumenee AE. A major cause of hypotony. Am J Ophthalmol. 1961;52:609–618. doi: 10.1016/0002-9394(61)90145-3. [DOI] [PubMed] [Google Scholar]
- Christiansen GA, Nau CB, et al. Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in patients with ocular hypertension or glaucoma. Ophthalmology. 2004;111(9):1658–1662. doi: 10.1016/j.ophtha.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Coakes RL, Siah PB. Effects of adrenergic drugs on aqueous humour dynamics in the normal human eye. I. Salbutamol. The British Journal of Ophthalmology. 1984;68(6):393–397. doi: 10.1136/bjo.68.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DF, Monro PA. The use of fluorescein-labelled dextrans in investigation of aqueous humour outflow in the rabbit. Exp Eye Res. 1976;23(6):571–585. doi: 10.1016/0014-4835(76)90215-3. [DOI] [PubMed] [Google Scholar]
- Crawford K, Kaufman PL. Pilocarpine antagonizes prostaglandin F2 alpha-induced ocular hypotension in monkeys. Evidence for enhancement of Uveoscleral outflow by prostaglandin F2 alpha. Archives of Ophthamology. 1987;105(8):1112–1116. doi: 10.1001/archopht.1987.01060080114039. [DOI] [PubMed] [Google Scholar]
- Crowston JG, Aihara M, et al. Effect of latanoprost on outflow facility in the mouse. Invest Ophthalmol Vis Sci. 2004;45(7):2240–2245. doi: 10.1167/iovs.03-0990. [DOI] [PubMed] [Google Scholar]
- Elagouz M, Stanescu-Segall D, et al. Uveal effusion syndrome. Surv Ophthalmol. 2010;55(2):134–145. doi: 10.1016/j.survophthal.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Emi K, Pederson JE, et al. Hydrostatic pressure of the suprachoroidal space. Investigative Ophthalmology & Visual Science. 1989;30:233–238. [PubMed] [Google Scholar]
- Erdmann P. Über experimentelles Glaukom nebst Untersuchungen am glaukomatösen Tierauge. Albrecht von Graefes Archiv für Ophthalmologie. 1907;66(2):325–390. [Google Scholar]
- Fatt I, Hedbys BO. Flow of water in the sclera. Experimental Eye Research. 1970;10:243–249. doi: 10.1016/s0014-4835(70)80035-5. [DOI] [PubMed] [Google Scholar]
- Figus M, Lazzeri S, et al. Supraciliary shunt in refractory glaucoma. Br J Ophthalmol. 2011;95(11):1537–1541. doi: 10.1136/bjophthalmol-2011-300308. [DOI] [PubMed] [Google Scholar]
- Fine BS. Observations on the drainage angle in man and rhesus monkey: a concept of the pathogenesis of chronic simple alaucoma: a light and electron Mmcroscopic study. Investigative Ophthalmology & Visual Science. 1964;3(6):609–646. [PubMed] [Google Scholar]
- Fowlks WL, Havener VR, et al. Meridional flow from the cornea ciliaris through the pararetinal zone of the rabbit vitreous. Investigative Ophthalmology. 1963;2:63–71. [PubMed] [Google Scholar]
- Fristrom B, Nilsson SE. Interaction of PhXA41, a new prostaglandin analogue, with pilocarpine. A study on patients with elevated intraocular pressure. Arch Ophthalmol. 1993;111(5):662–665. doi: 10.1001/archopht.1993.01090050096037. [DOI] [PubMed] [Google Scholar]
- Gabelt B, Kaufman P. Prostaglandin F increases uveoscleral outflow inthe cynomolgus monkey. Experimental Eye Research. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- Gabelt BAT, Gottanka J, et al. Aqueous Humor Dynamics and Trabecular Meshwork and Anterior Ciliary Muscle Morphologic Changes with Age in Rhesus Monkeys. Investigative Ophthalmology & Visual Science. 2003;44(5):2118–2125. doi: 10.1167/iovs.02-0569. [DOI] [PubMed] [Google Scholar]
- Gabelt BAT, Okka M, et al. Aqueous Humor Dynamics in Monkeys after Topical R-DOI. Investigative Ophthalmology & Visual Science. 2005;46(12):4691–4696. doi: 10.1167/iovs.05-0647. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. The effect of prostaglandin F2 alpha on trabecular outflow facility in cynomolgus monkeys. Experimental Eye Research. 1990;51(1):87–91. doi: 10.1016/0014-4835(90)90174-s. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gass JD. Uveal effusion syndrome: a new hypothesis concerning pathogenesis and technique of surgical treatment. Transactions of the American Ophthalmological Society. 1983;81:246–260. [PMC free article] [PubMed] [Google Scholar]
- Gaton DD, Sagara T, et al. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F2α-isopropyl ester treatment. Archives of Ophthalmology. 2001;119(8):1165–1170. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- Gelatt KN, Gum GG, et al. Uveoscleral flow of aqueous humor in the normal dog. Am J Vet Res. 1979;40(6):845–848. [PubMed] [Google Scholar]
- Goh Y, Araie M, et al. Effect of topical prostaglandin D2 on the aqueous humor dynamics in rabbits. Graefes Arch Clin Exp Ophthalmol. 1989;227(5):476–481. doi: 10.1007/BF02172902. [DOI] [PubMed] [Google Scholar]
- Goh Y, Oshima T, et al. Mechanism of intraocular pressure reduction observed after topical application of S-1033 in animals. Japanese Journal of Ophthalmology. 1994;38(3):228–235. [Google Scholar]
- Grant W, Trotter R. Tonographic measurments in enucleated eye. Arch Ophthalmol. 1955;53:191–200. doi: 10.1001/archopht.1955.00930010193003. [DOI] [PubMed] [Google Scholar]
- Grant WM. Tonographic method for measuring the facility and rate of aqueous flow in human eyes. Arch. Ophthl. 1950;44:204–214. doi: 10.1001/archopht.1950.00910020209003. [DOI] [PubMed] [Google Scholar]
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yablonski ME, et al. Trabecular outflow facility determined by fluorophotometry in human subjects. Experimental Eye Research. 1989;48:621–625. doi: 10.1016/0014-4835(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Heindl LM, Kaser-Eichberger A, et al. Sufficient Evidence for Lymphatics in the Developing and Adult Human Choroid?LettersIOVS | Month Year | Vol. 56 | No. 10 | page number. Investigative Ophthalmology & Visual Science. 2015;56(11):6709–6710. doi: 10.1167/iovs.15-17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. Principles of Ophthalmology. London, Wm: Heinemann; 1950. [Google Scholar]
- Hoeh H, Ahmed II, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39(3):431–437. doi: 10.1016/j.jcrs.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Honjo M, Inatani M, et al. EFfects of protein kinase inhibitor, ha1077, on intraocular pressure and outflow facility in rabbit eyes. Archives of Ophthalmology. 2001;119(8):1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- Inomata H, Bill A, et al. Unconventional routes of aqueous humor outflow in cynomolgus monkey (Macaca irus) American Journal of Ophthalmology. 1972;73:893. doi: 10.1016/0002-9394(72)90459-x. [DOI] [PubMed] [Google Scholar]
- Jackson TL, Hussain A, et al. Scleral hydraulic conductivity and macromolecular diffusion in patients with uveal effusion syndrome. Invest Ophthalmol Vis Sci. 2008;49(11):5033–5040. doi: 10.1167/iovs.08-1980. [DOI] [PubMed] [Google Scholar]
- Jin JC, Anderson D. Laser and unsutured sclerotomy in nanophthalmos. American Journal of Ophthalmology. 1990;109:575–580. doi: 10.1016/s0002-9394(14)70689-0. [DOI] [PubMed] [Google Scholar]
- Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. Vol. 4. Philadelphia: Saunders; 2000. pp. 2577–2595. [Google Scholar]
- Kanno M, Araie M, et al. Effects of topical nipradilol, a beta-blocking agent with alpha-blocking and nitroglycerin-like activities, on aqueous humor dynamics and fundus circulation. Invest Ophthalmol Vis Sci. 1998;39(5):736–743. [PubMed] [Google Scholar]
- Kaufman PL. Some thoughts on the pressure dependence of uveoscleral flow. J Glaucoma. 2003;12(1):89. doi: 10.1097/00061198-200302000-00018. author reply 93–4. [DOI] [PubMed] [Google Scholar]
- Kim M, Johnston MG, et al. A model to measure lymphatic drainage from the eye. Exp Eye Res. 2011;93(5):586–591. doi: 10.1016/j.exer.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Kiss F. Der Blutkreislauf des Auges. Ophthalmologica. 1943;106(5–6):225–250. [Google Scholar]
- Kleinstein RN, Fatt I. Pressure dependency of trans-sleral flow. Experimental Eye Research. 1977;24:335. doi: 10.1016/0014-4835(77)90146-4. [DOI] [PubMed] [Google Scholar]
- Knies M. Die resorption von blut in der vorderen augenkammer. Archiv für Pathologische Anatomie und Physiologie klinische Medicin. 1875;62:537–553. [Google Scholar]
- Koina ME, Baxter L, et al. Evidence for lymphatics in the developing and adult human choroid. Invest Ophthalmol Vis Sci. 2015;56(2):1310–1327. doi: 10.1167/iovs.14-15705. [DOI] [PubMed] [Google Scholar]
- Lam A, Sambursky RP, et al. Measurement of scleral thickness in uveal effusion syndrome. Am J Ophthalmol. 2005;140(2):329–331. doi: 10.1016/j.ajo.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Leber T. Studien über den Flüssigkeitswechsel im Auge. Albrecht v. Graefes Arch Ophthal. 1873;19:87–106. [Google Scholar]
- Lee YS, Tresguerres M, et al. Regulation of Anterior Chamber Drainage by Bicarbonate-sensitive Soluble Adenylyl Cyclase in the Ciliary Body. J Biol Chem. 2011;286(48):41353–41358. doi: 10.1074/jbc.M111.284679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Overby DR, et al. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011;52(3):1865–1871. doi: 10.1167/iovs.10-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene R, Hyman B. The effect of intraocular pressure on the facility of outflow. Exp Eye Res. 1969;8:116–121. doi: 10.1016/s0014-4835(69)80021-7. [DOI] [PubMed] [Google Scholar]
- Li G, Farsiu S, et al. Pilocarpine-induced dilation of Schlemm's canal and prevention of lumen collapse at elevated intraocular pressures in living mice visualized by OCT. Invest Ophthalmol Vis Sci. 2014;55:3737–3746. doi: 10.1167/iovs.13-13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KS, Nau CB, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008;115(5):790–795. e4. doi: 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JD, Weinreb RN. Identification of the mouse uveoscleral outflow pathway using fluorescent dextran. Invest Ophthalmol Vis Sci. 2002;43(7):2201–2205. [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2a . Experimental Eye Research. 1988;47(5):761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- Mäepea O. Pressures in the anterior ciliary arteries, choroidal veins and choriocapillaris. Experimental Eye Research. 1992;54(5):731–736. doi: 10.1016/0014-4835(92)90028-q. [DOI] [PubMed] [Google Scholar]
- Mäepea O, Bill A. The pressures in the episcleral veins, Schlemm's canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Experimental Eye Research. 1989;49(4):645–663. doi: 10.1016/s0014-4835(89)80060-0. [DOI] [PubMed] [Google Scholar]
- McMaster PRB, Macri FJ. Secondary aqueous humor outflow pathways in the rabbit, cat, and monkey. Archives of Ophthamology. 1968;79:297–303. doi: 10.1001/archopht.1968.03850040299014. [DOI] [PubMed] [Google Scholar]
- Melamed S, Ben Simon GJ, et al. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: a pilot study. Arch Ophthalmol. 2009;127(3):264–269. doi: 10.1001/archophthalmol.2008.611. [DOI] [PubMed] [Google Scholar]
- Millar JC, Clark AF, et al. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011;52(2):685–694. doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- Millar JC, Phan TN, et al. Strain and Age Effects on Aqueous Humor Dynamics in the Mouse. Investigative Ophthalmology & Visual Science. 2015;56(10):5764–5776. doi: 10.1167/iovs.15-16720. [DOI] [PubMed] [Google Scholar]
- Mishima HK, Kiuchi Y, et al. Circadian intraocular pressure management with Latanoprost: Diurnal and nocturnal intraocular pressure reduction and increased uveoscleral outflow. Survey of Ophthalmology 41, Supplement. 1997;2(0):S139–S144. doi: 10.1016/s0039-6257(97)80021-5. [DOI] [PubMed] [Google Scholar]
- Moses RA. Circumferential flow in Schlemm’s canal. American Journal of Ophthalmology. 1979;88:585–591. doi: 10.1016/0002-9394(79)90519-1. [DOI] [PubMed] [Google Scholar]
- Nau CB, Malihi M, et al. Circadian Variation of Aqueous Humor Dynamics in Older Healthy Adults. Invest Ophthalmol Vis Sci. 2013;54:7623–7629. doi: 10.1167/iovs.12-12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov AP. In: Chapter 13: The future for surgery in the glaucomas by increasing uveoscleral outflow. Glaucoma, Cairns JE, editors. Grune & Stratton; 1986. pp. 257–273. [Google Scholar]
- Nilsson SF. The uveoscleral outflow routes. Eye. 1997;11(Pt 2):149–154. doi: 10.1038/eye.1997.43. [DOI] [PubMed] [Google Scholar]
- Nilsson SF, Drecoll E, et al. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Investigative Ophthalmology & Visual Science. 2006;47(9):4042–4049. doi: 10.1167/iovs.05-1627. [DOI] [PubMed] [Google Scholar]
- Nilsson SFE, Samuelsson M, et al. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2a-1-isopropylester in the cynomolgus monkey. Exp Eye Res. 1989;48:707–716. doi: 10.1016/0014-4835(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Nuel J, Benoit F. Des voies d'elimination des liquides intra-oculaires hors de la chambre anterieure et au fond de l’oeil. Arch. d'Opth. 1900;20:161–228. [Google Scholar]
- Oatts JT, Zhang Z, et al. In vitro and in vivo comparison of two suprachoroidal shunts. Invest Ophthalmol Vis Sci. 2013;54(8):5416–5423. doi: 10.1167/iovs.13-11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklind A. Effect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sections. Exp Eye Res. 1998;67(2):179–191. doi: 10.1006/exer.1998.0508. [DOI] [PubMed] [Google Scholar]
- Oka T, Taniguchi T, et al. Aqueous humor dynamics associated with the phorbol ester-induced decrease in intraocular pressure in the rabbit. Jpn J Ophthalmol. 2006;50(6):497–503. doi: 10.1007/s10384-006-0365-6. [DOI] [PubMed] [Google Scholar]
- Overby DR, Bertrand J, et al. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014;55(6):3727–3736. doi: 10.1167/iovs.13-13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease ME, Oglesby EN, et al. Scleral Permeability Varies by Mouse Strain and Is Decreased by Chronic Experimental Glaucoma. Investigative Ophthalmology & Visual Science. 2014;55(4):2564–2573. doi: 10.1167/iovs.13-13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson JE, Cantrill HL. Experimental Retinal Detachment: V. Fluid Movement Through the Retinal Hole. Arch Ophthalmol. 1984;102(1):136–139. doi: 10.1001/archopht.1984.01040030114048. [DOI] [PubMed] [Google Scholar]
- Pederson JE, Gaasterland DE, et al. Uveoscleral aqueous outflow in the rhesus monkey: importance of uveal reabsorption. Investigative Ophthalmology and Visual Science. 1977;16:1008–1017. [PubMed] [Google Scholar]
- Pederson JE, Toris CB. Uveoscleral outflow: diffusion or flow?”. Investigative Ophthalmology & Visual Science. 1987;28(6):1022–1024. [PubMed] [Google Scholar]
- Poyer JF, Gabelt B, et al. The effect of topical PGF(2α) on uveoscleral outflow and outflow facility in the rabbit eye. Experimental Eye Research. 1992;54(2):277–283. doi: 10.1016/s0014-4835(05)90001-8. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Lütjen E, et al. The relationship between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. Albrecht v. Graefes Arch. klin. exp. Ophthal. 1967;172:23–47. doi: 10.1007/BF00577152. [DOI] [PubMed] [Google Scholar]
- Sagara T, Gaton DD, et al. Topical prostaglandin F2α treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Archives of Ophthalmology. 1999;117(6):794–801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- Samuelson D, Streit A. Microanatomy of the anterior uveoscleral outflow pathway in normal and primary open-angle glaucomatous dogs. Vet Ophthalmol. 2012;(15 Suppl 1):47–53. doi: 10.1111/j.1463-5224.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- Samuelson DA, Gum GG, et al. Aqueous outflow in the beagle: unconventional outflow, using different-sized microspheres. Am J Vet Res. 1985;46(1):242–248. [PubMed] [Google Scholar]
- Schroedl F, Kaser-Eichberger A, et al. Consensus Statement on the Immunohistochemical Detection of Ocular Lymphatic Vessels. Investigative Ophthalmology & Visual Science. 2014;55(10):6440–6442. doi: 10.1167/iovs.14-15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe G. Untersuchen über die Lymphbahnen des Auges und ihre Begrenzungen. Arch. Mikrosk. Anat. 1870;6:261–362. [Google Scholar]
- Seidel E. Weitere experimentelle Untersuchungen über die Quelle und den Verlauf der intraokularen Saftströmung. IX Mitteilung. Über den Abfluss des Kammerwassers aus der vorderen AugenKammer. Graefe's Archive for Clinical and Experimental Ophthalmology. 1921;104:357–402. [Google Scholar]
- Sherman SH, Green K, et al. The fate of anterior chamber fluorescein in the monkey eye. I. The anterior chamber outflow pathways. Experimental Eye Research. 1978;27:159–173. doi: 10.1016/0014-4835(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Sit AJ, Ekdawi NS, et al. A novel method for computerized measurement of episcleral venous pressure in humans. Experimental Eye Research. 2011;92(6):537–544. doi: 10.1016/j.exer.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Sit AJ, McLaren JW. Measurement of episcleral venous pressure. Experimental Eye Research. 2011;93(3):291–298. doi: 10.1016/j.exer.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, et al. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001;1:3. doi: 10.1186/1471-213X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, et al. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52(13):9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernschantz JW. From PGF(2alpha)-isopropyl ester to latanoprost: a review of the development of xalatan: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2001;42(6):1134–1145. [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kida T, et al. Involvement of nitric oxide in the ocular hypotensive action of nipradilol. Curr Eye Res. 2001;23(5):346–351. doi: 10.1076/ceyr.23.5.346.5438. [DOI] [PubMed] [Google Scholar]
- Suguro K, Toris CB, et al. Uveoscleral outflow following cyclodialysis in the monkey eye using a fluorescent tracer. Investigative Ophthalmology & Visual Science. 1985;26(6):810–813. [PubMed] [Google Scholar]
- Takashima Y, Taniguchi T, et al. Ocular hypotensive mechanism of intravitreally injected brain natriuretic peptide in rabbit. Invest Ophthalmol Vis Sci. 1996;37(13):2671–2677. [PubMed] [Google Scholar]
- Tam AL, Gupta N, et al. Quantum dots trace lymphatic drainage from the mouse eye. Nanotechnology. 2011;22(42):425101. doi: 10.1088/0957-4484/22/42/425101. [DOI] [PubMed] [Google Scholar]
- Tam ALC, Gupta N, et al. Latanoprost Stimulates Ocular Lymphatic Drainage: An In Vivo Nanotracer Study. Translational Vision Science & Technology. 2013;2(5):3. doi: 10.1167/tvst.2.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toris CB. Aqueous humor dynamics I. In: Civan MM, editor. Current topics in membranes. The Eye’s Aqueous Humor. Vol. 62. 2008. pp. 193–229. [Google Scholar]
- Toris CB, Alm A, et al. Latanoprost and cholinergic agonists in combination. Survey of Ophthalmology. 2002a;(47 Suppl 1):S141–S147. doi: 10.1016/s0039-6257(02)00309-0. [DOI] [PubMed] [Google Scholar]
- Toris CB, Camras CB, et al. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993;100(9):1297–1304. doi: 10.1016/s0161-6420(93)31484-3. [DOI] [PubMed] [Google Scholar]
- Toris CB, Gleason ML, et al. Effects of brimonidine on aqueous humor dynamics in human eyes. Archives of Ophthalmology. 1995a;113(12):1514–1517. doi: 10.1001/archopht.1995.01100120044006. [DOI] [PubMed] [Google Scholar]
- Toris CB, Gregerson DS, et al. Uveoscleral outflow using different-sized fluorescent tracers in normal and inflamed eyes. Experimental Eye Research. 1987;45(4):525–532. doi: 10.1016/s0014-4835(87)80063-5. [DOI] [PubMed] [Google Scholar]
- Toris CB, Koepsell SA, et al. Aqueous humor dynamics in ocular hypertensive patients. Journal of Glaucoma. 2002b;11(3):253–258. doi: 10.1097/00061198-200206000-00015. [DOI] [PubMed] [Google Scholar]
- Toris CB, Pederson JE. Effect of intraocular pressure on uveoscleral outflow following cyclodialysis in the monkey eye. Investigative Ophthalmology & Visual Science. 1985;26:1745–1749. [PubMed] [Google Scholar]
- Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Investigative Ophthalmology & Visual Science. 1987;28(3):477–481. [PubMed] [Google Scholar]
- Toris CB, Pederson JE, et al. Extravascular albumin concentration of the uvea. Investigative Ophthalmology & Visual Science. 1990;31(1):43–53. [PubMed] [Google Scholar]
- Toris CB, Tafoya ME, et al. Effects of apraclonidine on aqueous humor dynamics in human eyes. Ophthalmology. 1995b;102(3):456–461. doi: 10.1016/s0161-6420(95)31000-7. [DOI] [PubMed] [Google Scholar]
- Toris CB, Yablonski ME, et al. Aqueous humor dynamics in the aging human eye. American Journal of Ophthalmology. 1999;127(4):407–412. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- Toris CB, Yablonski ME, et al. Prostaglandin A2 increases uveoscleral outflow and trabecular outflow facility in the cat. Experimental Eye Research. 1995c;61(6):649–657. doi: 10.1016/s0014-4835(05)80015-6. [DOI] [PubMed] [Google Scholar]
- Toris CB, Zhan G, et al. Increase in outflow facility with unoprostone treatment in ocular hypertensive patients. Archives of Ophthalmology. 2004;122(12):1782–1787. doi: 10.1001/archopht.122.12.1782. [DOI] [PubMed] [Google Scholar]
- Toris CB, Zhan GL, et al. Aqueous humor dynamics in monkeys with laser-induced glaucoma. Journal of Ocular Pharmacology & Therapeutics. 2000;16(1):19–27. doi: 10.1089/jop.2000.16.19. [DOI] [PubMed] [Google Scholar]
- Townsend DJ, Brubaker RF. Immediate effect of epinephrine on aqueous formation in the normal human eye as measured by fluorophotometry. Investigative Ophthalmology & Visual Science. 1980;19(3):256–266. [PubMed] [Google Scholar]
- Tripathi RC. Ch 3: Comparative physiology and anatomy of the aqueous outflow pathway. In: Davson H, Graham LT, editors. The Eye. Comparative Physiology. 5: London, England; New York, NY: Academic Press; 1974. pp. 163–356. [Google Scholar]
- Tripathi RC. Uveoscleral drainage of aqueous humour. Experimental Eye Research. 1977;(25 suppl):305–308. doi: 10.1016/s0014-4835(77)80026-2. [DOI] [PubMed] [Google Scholar]
- Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Investigative Ophthalmology and Visual Science. 1982;22:625–632. [PubMed] [Google Scholar]
- Wan Z, Woodward DF, et al. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48(9):4107–4115. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Hayashi M, et al. Effects of multiple dosing of epinephrine on aqueous humor dynamics in human eyes. Journal of Ocular Pharmacology & Therapeutics. 2002;18(1):53–63. doi: 10.1089/108076802317233216. [DOI] [PubMed] [Google Scholar]
- Wang YL, Toris CB, et al. Effects of topical epinephrine on aqueous humor dynamics in the cat. Experimental Eye Research. 1999;68(4):439–445. doi: 10.1006/exer.1998.0623. [DOI] [PubMed] [Google Scholar]
- Ward RC, Gragoudas ES, et al. Abnormal scleral findings in uveal effusion syndrome. American Journal of Ophthalmology. 1988;106:139–146. doi: 10.1016/0002-9394(88)90825-2. [DOI] [PubMed] [Google Scholar]
- Wood RL, Koseki T, et al. Structural analysis of poential barriers to bulk-flow exchanges between uvea and sclera in eyes of Macaque monkeys. Cell Tissue Research. 1990;260:459–468. doi: 10.1007/BF00297225. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH-P, et al. Bimatoprost Effects on Aqueous Humor Dynamics in Monkeys. Journal of Ophthalmology. 2010 doi: 10.1155/2010/926192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonski ME. Letter to the Editor. Journal of Glaucoma. 2002;12(1):90–92. doi: 10.1097/00061198-200302000-00019. [DOI] [PubMed] [Google Scholar]
- Yablonski ME. Some thoughts on the pressure dependence of uveoscleral flow. J Glaucoma. 2003;12(1):90–92. doi: 10.1097/00061198-200302000-00019. author reply 93–4. [DOI] [PubMed] [Google Scholar]
- Yablonski ME, Cook DJ, et al. A fluorophotometric study of the effect of argon laser trabeculoplasty on aqueous humor dynamics. American Journal of Ophthalmology. 1985;99:579–582. doi: 10.1016/s0002-9394(14)77963-2. [DOI] [PubMed] [Google Scholar]
- Yücel YH, Johnston MG, et al. Identification of lymphatics in the ciliary body of the human eye: a novel “uveolymphatic” outflow pathway. Exp Eye Res. 2009;89(5):810–819. doi: 10.1016/j.exer.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Zhan GL, Lee PY, et al. Time dependent effects of sympathetic denervation on aqueous humor dynamics and choroidal blood flow in rabbits. Current Eye Research. 2002;25(2):99–105. doi: 10.1076/ceyr.25.2.99.10161. [DOI] [PubMed] [Google Scholar]
- Zhan GL, Toris CB, et al. Bunazosin reduces intraocular pressure in rabbits by increasing uveoscleral outflow. Journal of Ocular Pharmacology & Therapeutics. 1998;14(3):217–228. doi: 10.1089/jop.1998.14.217. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davidson BR, et al. Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Invest Ophthalmol Vis Sci. 2009;50(2):765–770. doi: 10.1167/iovs.08-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Hejkal JJ, et al. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Investigative Ophthalmology & Visual Science. 2010;51(6):3145–3151. doi: 10.1167/iovs.09-4415. [DOI] [PubMed] [Google Scholar]