Abstract
Introduction
Regional variability in interstitial fluid pressure confounds use of intramuscular pressure measurement to assess muscle force. It is hypothesized that interstitial flow is dependent on intramuscular pressure. The goal of this study was to assess the feasibility of using fluorescent microspheres to evaluate movement of interstitial fluid in skeletal muscle.
Methods
Two diameters of fluorescent microspheres were injected into the rat tibialis anterior during both static (n=6) and passively lengthened (10% strain) experimental conditions (n=6). Microsphere dispersion was evaluated using confocal imaging of transverse muscle sections.
Results
Confocal microscopy fluorescent microspheres tracked interstitial fluid while not penetrating the muscle fiber. When compared to the static condition, significantly greater dispersion (P=0.003) was seen with passively lengthened conditions (17±9% vs 31±7%, respectively). Dispersion did not differ for the 2 microsphere sizes (P=0.811).
Discussion
Fluorescent microspheres track movement of interstitial fluid, and dispersion is dependent on passive lengthening.
Keywords: interstitial fluid, interstitial space, intramuscular pressure, confocal microscopy, passive lengthening, tibialis anterior
INTRODUCTION
Muscle weakness is common in the progression of many diseases and disorders. The weakness can be apparent, as in neuromuscular transmission disorders1 where the decrease in force production is due to a lack of innervation as opposed to an issue with skeletal muscle properties. Alternatively, it can be a true weakness, as in muscle atrophy, with a decline in force production attributed to decreased muscle cross sectional area2,3. Muscle strength is, therefore, often evaluated clinically to diagnose and monitor many diseases4,5. The use of an ordinal grading system to assess muscle strength dates back to the early 1900s. Today, the most commonly used manual muscle testing grading system is that developed by the committee of the Medical Research Council (MRC) using a scale from 0 (no contraction) to 5 (normal strength)6.
Quantitative techniques have been developed and are available to evaluate muscle strength in patients to diagnose and monitor diseases. These techniques include handheld dynamometers7, electromyography (EMG)8, EMG-informed neuromusculoskeletal modeling9,10, and buckle transducers11-13. Although these tools can be useful in a clinical examination, the grading systems and quantitative methods still have some downfalls. EMG-informed neuromusculoskeletal models rely on indirect validation methods, such as joint moments9,10. Moreover, these models have never been directly validated with muscle forces measured from in vivo human trials due to a lack of measurement techniques. One limitation with dynamometry and the MRC grading scale is these techniques evaluate a group of muscles as opposed to a single muscle. Also limiting, the buckle transducer yields a direct measure of force, however the assessment is highly invasive, and measurements on a tendon with more than one distal insertion will not yield the force production of a single muscle.
Intramuscular pressure (IMP) is correlated with muscle force and therefore may be used as a surrogate measurement of force development in individual muscles14. However, there is regional variability in IMP15-18 that is likely a reflection of interstitial fluid mechanics within the muscle. The connective tissue acts as a heterogeneous structural matrix, and when the tissue deforms with passive lengthening, movement of the interstitial fluid is induced. It is likely that the connective tissue within skeletal muscle presents an anatomical boundary that constrains interstitial fluid movement and thus creates pressure gradients that contribute to the regional variability in IMP measurements. Force and IMP increase as a muscle is passively lengthened, in part due to the elastic nature of these connective tissues19. Regional pressure variability can therefore be investigated using passive lengthening in muscle because of the pressure-length relationship.
This study was undertaken to develop a novel technique to evaluate interstitial fluid movement in skeletal muscle by injecting fluorescent microspheres. Improved understanding of the fluid mechanics within skeletal muscle will improve IMP measurements so that it can be used as a tool for assessing force. The use of fluorescent microspheres has become a common technique in investigating regional organ perfusion since it was initially introduced by Glenny et al. in 199320. Fluorescent microspheres have also been used to identify injection sites in rats21 and illuminate flow through the lymphatic system22. The size of the fluorescent microspheres was an important consideration in the study; large spheres could cause mechanical interference in the interstitial space, but they would be easiest to visualize compared to smaller diameters. We hypothesized that dispersion of fluorescent microspheres would be dependent on passive lengthening, and there would be no significant effect of microsphere diameter on dispersion.
METHODS
Animals
Six adult male, skeletally mature, Sprague Dawley rats (~300 g), were acquired from Charles River Laboratories. Rats were group housed and maintained on a 12 hour light-dark schedule under specific pathogen-free conditions with unlimited access to food and water. All rats were given at least 2 days to acclimate to the facility prior to experimentation. During the experiment, rats were anesthetized with an intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) and euthanized following tissue harvest. Harvested tibialis anterior (TA) muscles were stored at −80°C until further analysis. All protocols were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic, in compliance with National Institute of Health guidelines.
Fluorescent microsphere injection
Two diameters of fluorescent microspheres, 0.1 μm Fluorescent Sky Blue (Spherotech FP-00570-2, Lake Forest, IL) and 0.2 μm Fluoresbrite Yellow Green (Poly Science, Inc. 21636, Warrington, PA) were used to evaluate the fluid dispersion's dependence on fluorescent microsphere size. We identified an acceptable range of diameters for the fluorescent microspheres by taking into consideration the hypothesized interstitial space and past perfusion studies. The final diameters were determined by the availability from manufacturers. Fluorescent microspheres were prepared in a 1:10 volume solution in phosphate buffered saline before injection. The fluorescent microsphere solution was injected at room temperature into the mid-belly of the rat TA using a Hamilton syringe with a 30G needle. The injection rate was approximately 2 μL/sec for the 10 μL solution. The muscle was stabilized by minutien pins, and the injection site was marked by placing a pin adjacent to the tissue.
Experimental design
In each animal, 2 experimental conditions were used to examine interstitial fluid dispersion within the right and left TA muscles: static (n=6) and passively lengthened (n=6). Use of the right versus left limb was alternated between static and passively lengthened conditions across animals. The knee and ankle were fixed at 90°, a common lower limb position used in rats23,24. The distance from origin to insertion of the distal tendon of the TA was measured with digital calipers. This reference position was defined as the in vivo resting length. Olesen et al. showed the optimal length in the calf muscles of rats to be at lengths just slightly longer than the reference length23. The muscle was excised, and the subsequent experimental steps varied depending on the condition. For the static condition, the muscle was stretched manually and pinned on cork with 10% strain before injecting fluorescent microspheres <1 minute after pinning to assess the control baseline dispersion due to the injection pressure alone. For the passively lengthened condition, fluorescent microspheres were injected at the in vivo resting length, and then the muscle was subjected to 10% strain before pinning on cork (dispersion influenced by the combined effects of injection pressure plus IMP). Since the force (or pressure)-length curve is fairly constant until it begins to increase beyond optimal length, only positive strain was evaluated for this initial study. In both conditions, the muscle was frozen in melting isopentane cooled by liquid nitrogen <1 minute after microsphere injections.
Tissue sample preparation
Each sample was transected at the location of the injection site to form 2 subsets for sectioning, proximal and distal. Samples were sectioned (20 μm transverse) in a stereological fashion moving away from the injection site. Each muscle section was labeled with wheat germ agglutinin conjugated with Alexa Fluor 594 (Molecular Probes W11262; 1 mg/mL) following previously described methods25 in order to visualize the interstitial space.
Confocal microscopy and image analysis
A confocal microscopy system (Nikon Instruments Inc., Melville NY) equipped with Argon (488 nm) and solid state (405 and 561 nm) lasers capable of simultaneous multi-label fluorescence imaging was used to evaluate the axial dispersion of fluorescent microspheres. A 20× objective lens (Plan Fluor, 0.75NA) was used to image the sections containing fluorescent microspheres. All imaging and quantification were done by an investigator blinded to the experimental condition. Imaging began at the injection site and progressed using an iterative method until the last section with fluorescent microspheres in the interstitial space was identified for the proximal and distal subsets. The samples from a single subset were evaluated for fluorescent microspheres, starting with a section at the midpoint between the injection site and end of the subset. The presence or absence of fluorescent microspheres determined if evaluation would continue on the group of samples closest to the injection site or on the opposite group toward the end of the subset.
The distal and proximal dispersion range of the fluorescent microspheres was defined by the section with the last visible fluorescent microsphere in the interstitial space. The distance from the injection site was calculated for each subset of muscle and combined to determine the overall dispersion of fluorescent microspheres as a percent of the in vivo length (Figure 1). Confocal images were saved separately for each fluorescence channel using Nikon C1 software as 8-bit grayscale-TIFF files. All images were pseudo-colored and merged in NIS-Elements software (Nikon Instruments Inc., Melville NY) for analysis.
Figure 1.
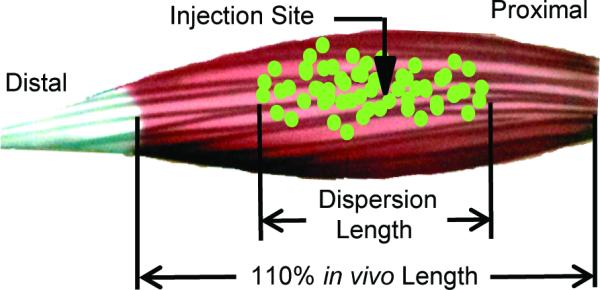
The distance from the injection site to the last section with fluorescent microspheres visible in the interstitial space is calculated for each muscle to determine the dispersion length as a percent of the in vivo length. The time of injection was varied to evaluate 2 groups, static and passively lengthened, each with a strain of 10% (110% in vivo length).
Each section was evaluated for the presence of: 1) 0.1 μm (Sky Blue); 2) 0.2 μm (Yellow Green); or 3) both fluorescent microspheres in 8 of the samples. These data were used to determine whether there was dependence on fluorescent microsphere diameter for the overall axial dispersion by performing statistics on the totals in 1 and 2 (as described above). Equal dispersion was defined in a muscle section when both diameters of fluorescent microspheres were present.
Statistical Analysis
All data were analyzed using JMP (JMP version 10.0; SAS Institute Inc., Cary NC). The 2 hypotheses, the effect of experimental condition and the effect of fluorescent microsphere on axial dispersion, were evaluated using a 2-way ANOVA. Unless otherwise specified, all data are reported as mean ± standard error. Significance was accepted at the α<0.05 level.
RESULTS
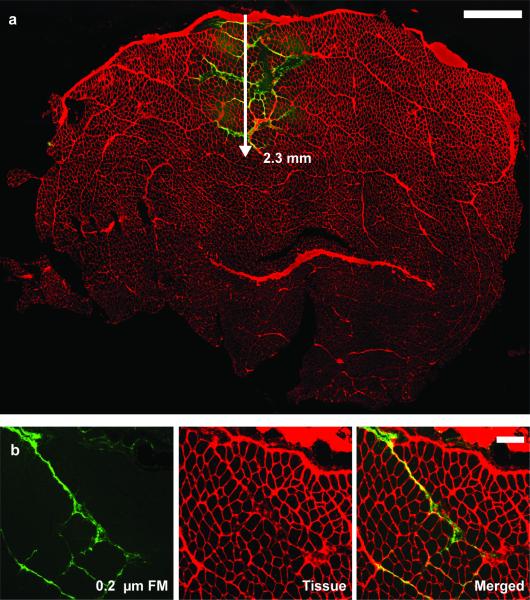
In total, 12 rat TA muscles were used to understand the fluid flow in skeletal muscle following a 10 μL injection of fluorescent microspheres. A dependence on passive lengthening and fluorescent diameter was evaluated. The injection site was clearly evident, and the dispersion of the fluorescent microspheres could be readily visualized (Figure 2a). Fluorescent microspheres stayed in the interstitial space and did not penetrate the muscle fibers (Figure 2). Fluorescent microspheres were most abundant in the larger interstitial spaces between fascicles, but they could also be seen in the much smaller interstitial space between muscle fibers (Figure 3). There was not a significant interaction between lengthening conditions and microsphere size (P=0.979).
Figure 2.
a) Representative TA muscle cross-section at the injection site with the fluorescent microspheres (FM) (green) in the extracellular space (red). Fluorescent microspheres dispersed at a depth of 2.3 mm into the tissue, remaining in the interstitial space. Scale is 1 mm. b) Transverse section of TA taken about 5 mm away from the injection site. The 0.2 μm fluorescent microspheres (green) stay within the interstitial space (red) and do not penetrate the muscle fiber. Scale bar is 200 μm.
Figure 3.
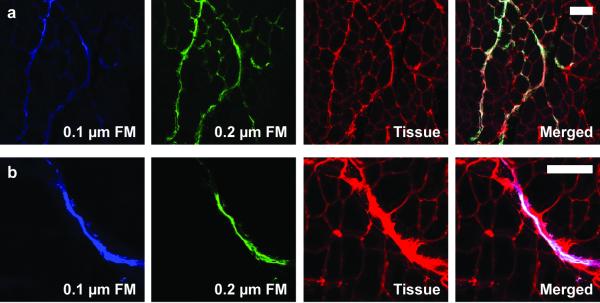
Representative transverse sections of the TA muscle with fluorescent microspheres (FM) of 2 sizes (0.1 μm in blue and 0.2 μm in yellow-green) in the extracellular space (red) at varying distances from the injection site. Dispersion of the 2 microsphere diameters is similar at both a) the injection site and b) 4.4 mm from the injection site. Statistical analysis of sections yielded no significant difference between the 2 diameters (main effect, P=0.811). Scale bars are 100 μm.
Effect of passive lengthening
Passive lengthening of the rat TA increased the dispersion of fluorescent microspheres (main effect, P=0.003; Figure 4). Recall for the static condition, the sample was pinned with 10% strain and injected after it was secured in place. Dispersion of microspheres in the axial direction for the static condition was 17 ± 9%. For the samples in the passively lengthened condition, injected and then secured in place, the axial dispersion was 31 ± 7%. Data were normally distributed.
Figure 4.
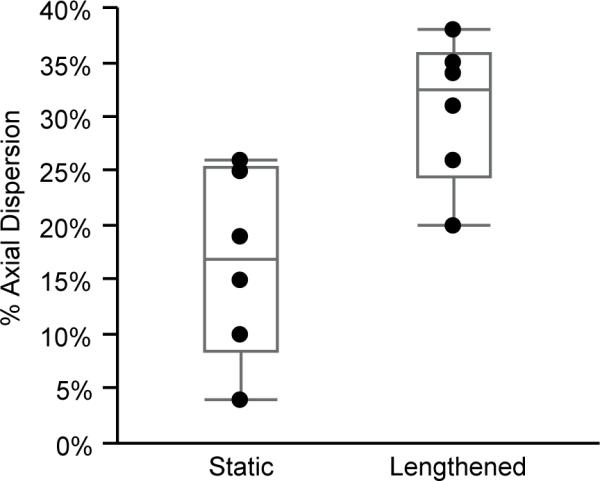
The 0.2 μm fluorescent microsphere dispersion in the static (n=6) and passively lengthened (n=6) conditions. There was a significant difference (main effect, P=0.003) of the fluorescent microsphere dispersion in the passively lengthened group compared to the static group. Gray lines represent the quartiles.
Diameter of fluorescent microspheres
There was no effect of microsphere diameter on dispersion under either static or passively lengthened conditions. In the majority of sections imaged (78 ± 5%), dispersion was equivalent for the 2 diameters of fluorescent microspheres. Both microsphere diameters were seen near the injection site (Figure 3a) and distal from the injection (Figure 3b). Statistical analysis of sections yielded no significant difference between the 2 diameters (main effect, P=0.811). Thus, dispersion of the 2 fluorescent microspheres was not dependent on diameter.
DISCUSSION
This study introduced a novel technique of using fluorescent microspheres to evaluate intramuscular interstitial fluid movement. Our findings support the hypothesis that dispersion of fluorescent microspheres will vary with passive lengthening and that there is no dependence on diameter for fluorescent microsphere dispersion. Typically, flow studies are used to evaluate perfusion through vessels26,27 as opposed to flow in the interstitium. Past studies involving fluid dynamics of interstitial tissue evaluated changes in chemical composition after exertion or altered gravity conditions to assess movement of fluid from extracellular to/from intracellular compartments28-31, whereas the use of fluorescent microspheres offers direct observation in the interstitial space.
Our results confirm that the dispersion of fluorescent microspheres follows interstitial fluid movement. Dispersion was greater during the passively lengthened condition, which is an indication of increased interstitial fluid movement. When a muscle contracts or is passively lengthened, there is deformation of the fibers and connective tissue32. This deformation causes fluid movement, which we could track spatially with the injected fluorescent microspheres. The variations in IMP may result from regional variations in the interstitial fluid, and this technique provides a way to investigate such fluid movement with skeletal muscles.
There was no effect of fluorescent microsphere diameter on their dispersion, although it can be affected by osmotic pressure driven by mass. The mass is a function of radius cubed, and our 2 sizes of fluorescent microspheres differed by a factor of 2, resulting in an 8-fold increase in mass. We expected larger particles to experience greater osmotic effects and thus result in greater overall dispersion. However, since there was no difference in dispersion for the 2 diameters, the effect of osmotic pressure is assumed to be minimal. The effects of diffusion were minimized in the experimental design by freezing the tissue <1 min after microsphere injections.
This study demonstrates a method to assess fluid movement in skeletal muscle, though limitations were present. We assumed the dispersion of fluorescent microspheres was dependent only on fluid flow. However, charge interaction, spatial interference, and injection pressure effects may also have played a role in the dispersion. The use of uncharged polystyrene microspheres eliminated concerns of charge interactions with the surrounding tissue reducing dispersion. The dispersion of particles may have been due to a primary mechanism of mechanical interaction with surrounding tissue rather than a secondary mechanism of deformed tissue initiating interstitial fluid movement, which in turn would cause the displacement. By evaluating 2 diameters we were able to show that larger microspheres dispersed the same distance as the smaller diameter microspheres, which indicates little spatial interference in the interstitial space. Additionally, the larger of the 2 diameters, 0.2 μm, was an order of magnitude below the interstitial space (a range of 2-40 μm) we observed in the confocal images. Thus, we assumed little mechanical/spatial interference was present to alter the flow of the particles together with the interstitial fluid. The effect of hydrostatic pressure from the injection was accounted for by using the static condition as a baseline control. The larger dispersion in the passively lengthened condition was due entirely to bulk fluid flow initiated by increased IMP from tissue deformation. Additional concern arose with regard to varied injection pressure of fluorescent microspheres; localized pressure from the injection was likely to alter the dispersion. In an attempt to decrease variance from these effects, the injection was performed with the same volume and rate for all samples.
The primary motivation of this study was to provide a proof-of-concept and investigate if fluorescent microspheres could be used to track fluid movement within the interstitial space of skeletal muscle. Our confirmed hypothesis (dispersion of fluorescent microspheres has a dependence on passive length change) yields support to pursue more in-depth studies into the relationship between fluid movement and pressure variations within skeletal muscle. In future studies, an automated syringe should be used for injections to ensure that a constant injection rate is achieved. The effects of injection volume were not evaluated; increasing the volume greatly could change the hydrodynamics within the muscle tissue. Previously reported concentration and volume values could be determined from a study using fluorescent microspheres to identify gene transfer injection sites in skeletal muscle of rats for any future experiments21. Future studies can use 0.2 μm fluorescent microspheres since no mechanical interference with the tissue was seen, and the larger diameter is easier to locate in the interstitial space. Additional studies evaluating a larger variation in microsphere diameter (e.g. 0.2 and 2 μm) would also be beneficial. The static and passively lengthened conditions could be controlled with the use of an apparatus to ensure that any displacement of the fluorescent microspheres is due to deformations during lengthening at a controlled rate. Additional perturbations of length changes should be evaluated for better comparison to previous IMP studies. These perturbations should include both lengthening and shortening of the muscle. In addition, the use of an external pressure cuff would help to evaluate any relationship between pressure and microsphere dispersion.
There is still much to learn about how the interstitial space changes when the muscle contracts and causes deformations to tissues to induce fluid flow. This study introduced a technique that would allow the interstitial space to be studied through injection of fluorescent microspheres. This technique has the potential to be employed for evaluating changes in extracellular space that would expand the knowledge of the basic interactions in skeletal muscle. Gaining a better understanding of interstitial fluid movement would help improve and define appropriate applications of the IMP sensor in the clinic. Increased knowledge regarding pressure variation within muscle (identified by uniform dispersion of microspheres) will help to enhance measurements of IMP used to assess force in a single muscle. The IMP sensor will aid physicians in diagnosing and monitoring disease conditions that affect muscle strength and improve surgical procedures with outcomes that rely on knowledge of individual muscle force, such as tendon transfer surgeries.
Acknowledgement
This research was supported by grants from the National Institute of Health R01-HD31467 (KRK) and T32-HL105355 (SMG). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1255833 (LQE) and the Mayo Graduate School. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
ABREVIATIONS
- EMG
electromyography
- FM
fluorescent microsphere
- IMP
intramuscular pressure
- MRC
Muscle Research Council
- TA
tibialis anterior
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interests.
REFERENCES
- 1.Shelton GD. Myasthenia gravis and disorders of neuromuscular transmission. The Veterinary Clinics of North America Small Animal Practice. 2002;32(1):189–206, vii. doi: 10.1016/s0195-5616(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 2.Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Archiv - European Journal of Physiology. 1997;434(3):246–253. doi: 10.1007/s004240050392. [DOI] [PubMed] [Google Scholar]
- 3.Phillips SK, Woledge RC, Bruce SA, Young A, Levy D, Yeo A, et al. A study of force and cross-sectional area of adductor pollicis muscle in female hip fracture patients. Journal of the American Geriatrics Society. 1998;46(8):999–1002. doi: 10.1111/j.1532-5415.1998.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 4.Ali NA, O'Brien JM, Jr., Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. American Journal of Respiratory and Critical Care Medicine. 2008;178(3):261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 5.Barry DT, Gordon KE, Hinton GG. Acoustic and surface EMG diagnosis of pediatric muscle disease. Muscle & Nerve. 1990;13(4):286–290. doi: 10.1002/mus.880130403. [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, Boes CJ, Mulder D, Millikan C, Windebank AJ, Dyck PJB, et al. History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. Journal of the Peripheral Nervous System. 2005;10(2):158–173. doi: 10.1111/j.1085-9489.2005.0010206.x. [DOI] [PubMed] [Google Scholar]
- 7.Agre JC, Magness JL, Hull SZ, Wright KC, Baxter TL, Patterson R, et al. Strength testing with a portable dynamometer: reliability for upper and lower extremities. Archives of Physical Medicine & Rehabilitation. 1987;68(7):454–458. [PubMed] [Google Scholar]
- 8.Mills KR. The basics of electromyography. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(Suppl 2):ii32–35. doi: 10.1136/jnnp.2005.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. Journal of Biomechanics. 2003;36(6):765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 10.Sartori M, Farina D, Lloyd DG. Hybrid neuromusculoskeletal modeling to best track joint moments using a balance between muscle excitations derived from electromyograms and optimization. Journal of Biomechanics. 2014;47(15):3613–3621. doi: 10.1016/j.jbiomech.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Fukashiro S, Komi PV, Jarvinen M, Miyahita M. In-vivo Achilles tendon loading during jumping in humans. European Journal of Applied Physiology. 1995;71(5):453–458. doi: 10.1007/BF00635880. [DOI] [PubMed] [Google Scholar]
- 12.Herzog W, Leonard TR, Renaud JM, Wallace J, Chaki G, Bornemisza S. Force-length properties and functional demands of cat gastrocnemius, soleus and plantaris muscles. Journal of Biomechanics. 1992;25(11):1329–1335. doi: 10.1016/0021-9290(92)90288-c. [DOI] [PubMed] [Google Scholar]
- 13.Komi PV, Salonen M, Jarvinen M, Kokko O. In vivo registration of Achilles tendon forces in man. I. Methodological development. International Journal of Sports Medicine. 1987;8(Suppl 1):3–8. doi: 10.1055/s-2008-1025697. [DOI] [PubMed] [Google Scholar]
- 14.Winters TM, Sepulveda GS, Cottler PS, Kaufman KR, Lieber RL, Ward SR. Correlation between isometric force and intramuscular pressure in rabbit tibialis anterior muscle with an intact anterior compartment. Muscle & Nerve. 2009;40(1):79–85. doi: 10.1002/mus.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis J, Kaufman KR, Lieber RL. Correlation between active and passive isometric force and intramuscular pressure in the isolated rabbit tibialis anterior muscle. Journal of Biomechanics. 2003;36(4):505–512. doi: 10.1016/s0021-9290(02)00430-x. [DOI] [PubMed] [Google Scholar]
- 16.Nakhostine M, Styf JR, van Leuven S, Hargens AR, Gershuni DH. Intramuscular pressure varies with depth. The tibialis anterior muscle studied in 12 volunteers. Acta Orthopaedica Scandinavica. 1993;64(3):377–381. doi: 10.3109/17453679308993649. [DOI] [PubMed] [Google Scholar]
- 17.Sadamoto T, Bonde-Petersen F, Suzuki Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. European Journal of Applied Physiology. 1983;51(3):395–408. doi: 10.1007/BF00429076. [DOI] [PubMed] [Google Scholar]
- 18.Sejersted OM, Hargens AR, Kardel KR, Bloom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. Journal of Applied Physiology. 1984;56:287–295. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. Journal of Biomechanics. 2008;41(2):465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glenny RW, Bernard S, Brinkley M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. Journal of Applied Physiology (1985) 1993;74(5):2585–2597. doi: 10.1152/jappl.1993.74.5.2585. [DOI] [PubMed] [Google Scholar]
- 21.Flynn MA, Vodovotz Y, Kornowski R, Epstein S, Gordon D, Keiser JA. Use of fluorescent microspheres to localize in vivo gene transfer injection sites. Biotechniques. 2000;28(3):470–472. doi: 10.2144/00283st04. [DOI] [PubMed] [Google Scholar]
- 22.Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schonbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. The FASEB Journal. 2001;15(10):1711–1717. doi: 10.1096/fj.01-0067com. [DOI] [PubMed] [Google Scholar]
- 23.Olesen AT, Jensen BR, Uhlendorf TL, Cohen RW, Baan GC, Maas H. Muscle-specific changes in length-force characteristics of the calf muscles in the spastic Han-Wistar rat. Journal of Applied Physiology. 2014;117(9):989–997. doi: 10.1152/japplphysiol.00587.2014. [DOI] [PubMed] [Google Scholar]
- 24.Peixinho CC, Martins NSD, de Oliveira LF, Machado JC. Reliability of measurements of rat lateral gastrocnemius architectural parameters obtained from ultrasound biomicroscopic images. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0087691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Experimental Gerontology. 2013;48(9):881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y, Mohning KM, Hradil VP, Wessale JL, Segreti JA, Nuss ME, et al. Evaluation of tissue perfusion in a rat model of hind-limb muscle ischemia using dynamic contrast-enhanced magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2002;16(3):277–283. doi: 10.1002/jmri.10169. [DOI] [PubMed] [Google Scholar]
- 27.Rezaee M, Yeung AC, Altman P, Lubbe D, Takeshi S, Schwartz RS, et al. Evaluation of the percutaneous intramyocardial injection for local myocardial treatment. Catheterization and Cardiovascular Interventions. 2001;53:271–276. doi: 10.1002/ccd.1164. [DOI] [PubMed] [Google Scholar]
- 28.Hargens AR, Tipton CM, Gollnick PD, Mubarak SJ, Tucker BJ, Akeson WH. Fluid shifts and muscle function in humans during acute simulated weightlessness. Journal of Applied Physiology. 1983;54(4):1003–1009. doi: 10.1152/jappl.1983.54.4.1003. [DOI] [PubMed] [Google Scholar]
- 29.Sjogaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. The American Journal of Physiology. 1985;248(2 Pt 2):R190–196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- 30.Sjogaard G, Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1982;243(3):R271–R280. doi: 10.1152/ajpregu.1982.243.3.R271. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y, Schoeller DA, Nakamura E, Morimoto T, Kimura M, Oda S. Extracellular water may mask actual muscle atrophy during aging. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2010;65(5):510–516. doi: 10.1093/gerona/glq001. [DOI] [PubMed] [Google Scholar]
- 32.Styf J. Compartment Syndromes: Diagnosis, Treatment, and Complications. CRC Press; Boca Raton, FL: 2004. [Google Scholar]