Abstract
Personal prescription drug importation occurs in the United States because of the high cost of U.S. medicines and lower cost of foreign equivalents. Importation carries a risk of exposure to counterfeit (i.e., falsified, fraudulent), adulterated, and substandard drugs. Inadequate health insurance may increase the risk of importation. We use inverse probability weighted marginal structural models and data on 87,494 individuals from the 2011-2013 National Health Interview Survey to estimate the marginal association between no health insurance and importation within U.S. subpopulations. The marginal prevalence difference [95% confidence limits] for those without (prevalence = 0.031) versus those with health insurance was 0.016 [0.011, 0.021]. The prevalence difference was higher among persons who were Hispanic, born in Latin America, Russia, or Europe, traveled to developing countries, and did not use the Internet to fill prescriptions or to find health information. Health insurance coverage may effectively reduce importation, especially among particular subpopulations.
Keywords: prescription drugs, counterfeit drugs, health insurance, drug importation, health services accessibility
Introduction
Because of the exorbitant cost of domestic prescription drugs and lower cost of equivalent products from foreign countries, personal prescription drug importation remains a major public health concern in the United States (Ellison, 2013; Hartman, Martin, Benson, & Catlin, 2013; Kanavos, Ferrario, Vandoros, & Anderson, 2013; Morgan & Kennedy, 2010). High out-of-pocket prescription drug costs motivate U.S. consumers to import similar prescription drugs for personal use from Canada and other countries by ordering them from Internet sources or carrying them across borders (Cunningham & Carrier, 2014; IMS Institute for Healthcare Informatics 2011, 2012). While such practices may improve access to medications and reduce costs for patients, they are potentially hazardous. Foreign medications are more likely to be counterfeit (i.e., falsified, fraudulent), adulterated, or otherwise substandard than those sold domestically (Mackey & Liang, 2013; Palumbo, Mullins, Slagle, & Rizer, 2007; Rudolf & Bernstein, 2004; Woo, Wolfgang, & Batista, 2008). Prescription drugs with compromised integrity could result in patients receiving a different drug than the one purchased, a harmful or inert substance, or a wrong dose of the correct drug (Liang, 2006). Health insurance, with or without a prescription drug benefit, can potentially serve as a cost-effective intervention to reduce importation by consumers (Sommers & Oellerich, 2013). Specifically, providing health insurance to uninsured individuals most likely to import could decrease out-of-pocket drug costs and subsequently reduce importation, especially if the insurance provided has a prescription drug benefit (Baicker et al., 2013; Finkelstein et al., 2012). Unfortunately, little scientific evidence is available that quantifies the effect of health insurance on importation and whether this effect is greatest among individuals at highest risk of importing.
Population-based complex survey data, such as the National Health Interview Survey, can be used to estimate the effect of health insurance on drug importation and assess whether this effect varies across subpopulations in the United States. Documenting whether the effect varies by population can identify subgroups most likely to benefit from providing insurance. However, estimating and testing the heterogeneity of an effect across U.S. populations not conditional on other factors besides subpopulation while accounting for potential confounders has rarely been done using complex survey data despite the need for such unconditional estimates when performing cost-effectiveness analyses (Brumback, Bouldin, Zheng, Cannell, & Andresen, 2010). Such unconditional estimates are needed to most accurately predict the costs and health benefits of intervening on a population in the presence of a noncollapsible effect measure or a potential confounder that is also an effect modifier, especially if information on potential confounders will not be routinely available when interventions are implemented (Greenland, 1996; Hernán & Robins, 2015; Miettinen & Cook, 1981). The aforementioned estimation and testing has rarely been done likely due in part to a lack of clarity regarding methods for obtaining the effect estimates of interest. In 2010, Brumback et al. (2010) outlined model-based standardization methods to obtain the desired effect estimates of interest using inverse probability weighted (IPW) marginal structural models (MSMs). However, examples that demonstrate the use of these IPW methods in the published literature remain infrequent (Cannell et al., 2011; Do, Wang, & Elliott, 2013; Hall et al., 2013).
The primary objective of this study was to use IPW MSMs fit using complex survey data to estimate the effect of health insurance on personal prescription drug importation across subpopulations of the United States defined by potentially important characteristics (National Association of Boards of Pharmacy, 2015a; U.S. Food and Drug Administration, 2013; Zullo, Dore, & Galárraga, 2015). A secondary study objective was to provide a published application of the techniques outlined by Brumback et al. (2010) to encourage greater use of these important methods for complex survey data in the applied literature.
Conceptual Model for the Role of Health Insurance in Personal Prescription Drug Importation
If individuals decide to import a prescription drug, there are two primary pathways by which they may do so. The first is that they or their agent (e.g., a relative, friend, or acquaintance) may physically travel outside the United States, purchase the prescription drugs, and physically transport the drugs across national borders. The second is that they or their agent may order a drug product through Internet websites that mail the product to U.S. consumers from foreign countries. Individuals who decide to travel outside the United States may cross national borders into Mexico or Canada using ground or air transportation. Alternatively, they may use air transportation to travel outside North America and obtain medicines from countries like India, Thailand, Brazil, China, or the Philippines. Individuals who order drugs through Internet web-sites are often purchasing products from many of the same aforementioned countries (Examining the implications, 2004).
Since much of prescription drug importation occurs through online purchasing instead of travel, the economic decision to purchase foreign medications can be distilled down to three key elements of purchasing goods using the Internet: convenience (ease of purchasing), price, and access (pervasiveness; Borenstein & Saloner, 2001). Under this general framework, basic economic theory would suggest that individuals maximize a utility function that directly includes health (intrinsically beneficial) as well as health care and medicines, which are purchased for their transitive effect on health. As articulated by Benito Arrunada (2004), “The purchase of medicines via Internet, as with other types of electronic commerce, can be beneficial for consumers in terms of service and, less clearly, prices.” Regarding service and convenience, consumers may value the greater range of purchasing hours or hours of operation, the ability to substitute user travel with mail service and greater ease of engaging in comparative shopping, and the potentially wider variety of products available. E-Commerce is probably most often used when patients can estimate demand in advance, such as with chronic illnesses. Conditions that are easy for the patient to self-identify (i.e., obesity, balding, erectile dysfunction, etc.), that have been previously diagnosed, and that may result in embarrassment when speaking about them to a physician or requesting drugs for them at the pharmacy are also suited to online purchasing. Drugs that are well known to patients and historically not covered by health insurance (e.g., sildenafil) are hypothesized to be purchased frequently online, though the exact classes and drugs imported remain empirically unknown to researchers and regulators. Other consumer considerations in the decision to purchase online may be (a) a lack of direct contact between patient and provider, (b) inadequate or total lack of clinical review by a physician or a pharmacist, and (c) perceived risk of exposure to unapproved, contaminated, false, or expired products.
Therefore, despite other secondary considerations, the decision to import via purchasing medications online is essentially a matter of two primary considerations—cost and convenience. Health insurance with a pharmacy benefit reduces the real price of covered medications and thus strongly influences the cost of medications to an individual consumer (Briesacher et al., 2011; Roebuck & Liberman, 2009). Conversely, not having health insurance would make generics and other less expensive alternatives, like personally imported medications, more salient and preferred. As such, health insurance likely in turn influences the decision to purchase medications online, which is consistent with prior, more general work on health care utilization (Arrow, 1963; Grossman, 1972). The purchase of medications online often results in importation of medications from foreign countries (National Association of Boards of Pharmacy, 2015b). Thus, our a priori hypothesis is that not having any health insurance coverage will increase the frequency of personal prescription drug importation from foreign countries.
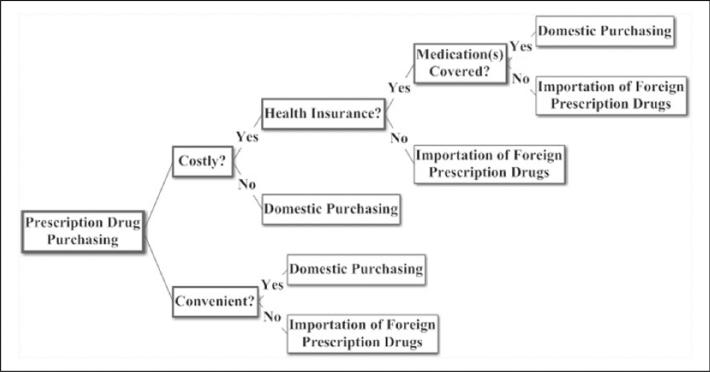
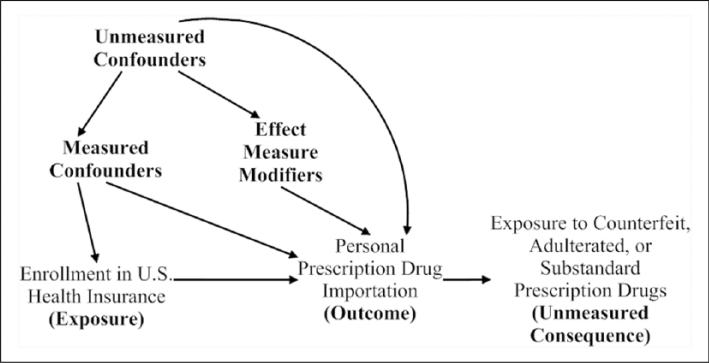
The conceptual model underlying our study is depicted by Figure 1, which is a representation of the decision to import medications based on health economic theory, whereby consumers attempt to maximize a utility function. Although the decisions are not truly binary, Figure 1 simplifies the large number of possibilities into key two-way choices where a significant difference in probability exists between alternative options. Figure 2 characterizes how we operationalized our Figure 1 conceptual model and guided the data analysis. Figure 2 is a directed acyclic graph, which represents our a priori subject-matter knowledge and assumptions regarding the relationship between health insurance and personal prescription drug importation. As is standard in directed acyclic graphs, nodes correspond to measured or unmeasured variables, directed arrows represent causation, and all common causes of any two or more variables have been included (Hernán, Hernandez-Diaz, Werler, & Mitchell, 2002).
Figure 1.
Conceptual framework for the economic decision to import prescription drugs for personal use.
Figure 2.
Operationalization of conceptual framework using a directed acyclic graph for the relationship between health insurance and drug importation, National Health Interview Survey 2011-2013.
As part of examining the role of insurance, we further wanted to determine whether the hypothesized relationship between health insurance and drug importation would be more pronounced among populations at highest risk of importation in part due to their greater access to foreign medicines, increased opportunities to import, and lower likelihood of having access to health insurance (National Association of Boards of Pharmacy, 2015a; U.S. Food and Drug Administration, 2013; Zullo et al., 2015). Such populations include Hispanics; persons not born in the United States; persons without transportation; persons who travel outside the United States; and persons who have looked up health information, scheduled a medical appointment, previously filled a prescription, or engaged in chat groups to learn about health on the Internet. Gender was also evaluated as a potential effect modifier because of males being at a higher risk for importation than females. First, while many of the drugs imported are likely common generic and brand medications used to treat chronic conditions (e.g., atorvastatin for hyperlipidemia), there exist anecdotal reports that some of the drugs imported are “lifestyle” drugs predominately used by men (Importation of prescription drugs, 2004). Examples of these drugs include sildenafil for erectile dysfunction and oxymetholone, which is an anabolic steroid intended for treatment of anemias but is often used by male bodybuilders, athletes, and others who wish to build muscle and improve physical appearance (Brower, 1993; Fejos, Neumajer, Beni, & Jankovics, 2014; Graham et al., 2009). Second, published literature suggests that males are more likely to be risk takers than females; examples include driving a car dangerously, having unprotected sexual intercourse with many partners, and taking illicit drugs (Pawlowski, Atwal, & Dunbar, 2008). Third, men are less likely to have health insurance coverage than women (Kaiser Family Foundation, 2013; Sandman, Simantov, & An, 2000).
New Contribution
To the best of our knowledge, this study is the first to examine the role of U.S. health insurance in personal prescription drug importation. Prior empirical literature on importation is scant due to the difficulty of obtaining person-level data. Furthermore, little work has been done to suggest effective interventions to reduce potentially harmful drug importation. Our use of recent and nationally representative U.S. data in combination with underused but valuable analytic methods (i.e., MSMs) to examine health insurance as a potential intervention target to reduce importation is a novel contribution to the literature.
Method
Study Population
We analyzed data from the 2011-2013 National Health Interview Survey (NHIS; http://www.cdc.gov/NCHS/NHIS.htm), a nationally representative in-person survey conducted annually by trained personnel of the National Center for Health Statistics (NCHS, 2012, 2013, 2014). The NHIS is a cross-sectional survey intended to “monitor the health of the U.S. population” and designed to represent the civilian noninstitutionalized population residing in the United States (Adams, Martinez, Vickerie, & Kirzinger, 2011; NCHS, 2012, 2013, 2014).
Participants were selected using multistage cluster sampling of households from across the country. In the first stage, the United States was divided into geographically defined areas called primary sampling units, which were then grouped into strata using sociodemographic characteristics of the area. In the second stage, specific geographic area segments were sampled from within each primary sampling unit and further subdivided into clusters containing a small number of housing units. One adult (≥18 years old) was randomly selected from each household for a detailed interview on health and other behaviors (NCHS, 2012, 2013, 2014).
Black, Hispanic, and Asian populations, particularly adults aged 65 years and older, were oversampled to provide more precise estimates for these growing populations (NCHS, 2012, 2013, 2014). Survey sampling weights provided by the NCHS were used to account for this oversampling and produce estimates representative of the noninstitutionalized adult U.S. population. We combined the 2011-2013 NHIS data sets to increase the number of respondents in the sample and the precision of our estimates (NCHS, 2012, 2013, 2014). Overlap was negligible in terms of the participants who completed the questionnaire between 2011 and 2013. The sample was restricted from 102,096 U.S. adults to the 87,494 individuals with complete information on the exposure, outcome, potential effect measure modifiers, and potential confounders. This study used deidentified publicly available data, was not deemed human subjects research, and therefore did not require the approval of an institutional review board.
Exposure
The primary exposure, health insurance status, was a binary indicator of having no health insurance coverage versus any coverage in the previous 12 months. Any health insurance included public only, private only, or both public and private insurance. Public insurance was defined per NCHS documentation as coverage by Medicaid, Medicare, other government-sponsored programs (e.g., state- or county-level medically indigent adult programs), or a military health plan such as TRICARE, Veterans Affairs (VA), or Civilian Health and Medical Program of the Department of Veterans Affairs (CHAMP-VA; NCHS, 2012, 2013, 2014). Respondents with only catastrophic medical insurance, dental insurance, or who were covered only by the Indian Health Service were categorized as having no health insurance coverage (NCHS, 2012, 2013, 2014). In secondary analyses, a multilevel categorical variable for none, private only, public only, and both private and public insurance was created. Adults with unknown insurance status (primary analysis unweighted N = 357; 0.4%) were excluded from the analytic sample.
Given that the aforementioned health insurance variables do not distinctly capture whether an individual has prescription drug coverage, an additional secondary analysis was performed using a binary indicator for not having prescription drug coverage versus having any prescription drug coverage. However, this indicator was only available among those who have a private insurance plan exclusively or in combination with public insurance. Among adults with a private plan, those with unknown drug coverage status (unweighted N = 2,768; 5.1%) were excluded from the secondary analytic sample.
Effect Measure Modifiers
The effect modifiers of interest were binary and categorical indicators. The binary indicators included sex, international travel, looked up health information on the Internet, filled prescription on the Internet, scheduled medical appointment on the Internet, used online chat groups to learn about health, and transportation. The categorical indicators were geographic region of birth and race/ethnicity. Due to the design of the survey question by NCHS, international travel included travel from 1995 to 2013 and excluded travel to Europe (particular countries unspecified by NCHS), Japan, Australia, New Zealand, and Canada, thus implicitly emphasizing travel to low- and middle-income countries. Adults with unknown values for any of the effect modifiers (primary analysis unweighted N = 2,521; 2.5%) were excluded from the analytic sample.
Potential Confounders
Covariates that were considered to be potential confounders and their specifications were selected based on our conceptual model (Figure 1), the directed acyclic graph (Figure 2), and the previous literature (Zullo et al., 2015). Covariates included current marital status, education, comorbidity burden, household income-to-national poverty threshold ratio, age, U.S. citizenship status, region of residence in the United States, history of health insurance coverage, trouble finding a health care provider, recent and past employment status (last week, past 12 months), number of family members in poor health, number of family members with work limitations due to health, personal health status, and worry about paying medical bills if a person got sick or had an accident.
We categorized age as 18 to 44, 45 to 64, or ≥65 years. Household income- to-national poverty threshold ratio is an indicator of the severity of poverty (U.S. Census Bureau, 2011). We categorized this household poverty ratio as poor (<1), near poor (1 to <2), or not poor (≥2; NCHS, 2012, 2013, 2014). A modified Charlson Comorbidity Index was calculated with values ranging from 0 to 17 to measure comorbidity burden (Charlson, Pompei, Ales, & MacKenzie, 1987). The following conditions were used to calculate the Charlson Comorbidity Index: myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, liver disease, diabetes, cancer, and renal disease. Cancer and renal disease were each given a weight of two. For each respondent, the total number of comorbidities was calculated and then based on the distribution of responses, categorized into 0, 1 to 3, and 4 to 15 conditions. Highest educational attainment was categorized as less than high school (never attended to 12th grade, no diploma), high school completion (high school graduate or GED/GED equivalent), some college, and completed college or graduate/professional degree (associate degree to doctoral degree). Adults with unknown (primary analysis unweighted N = 1,702; 1.7%) or indeterminate (primary analysis unweighted N = 10,005; 10.3%) values for any of the covariates were excluded from the analytic sample.
Outcome
Our study outcome was a self-reported binary indicator of whether a given individual imported one or more prescription drugs for personal use from a foreign country. This outcome was derived from the survey question: “During the past 12 months, are any of the following true for you? . . . You bought prescription drugs from another country to save money” (Adams, Kirzinger, & Martinez, 2012). The structure of the outcome variable, which focused the response on importation due to monetary savings, was appropriate since economic considerations are accepted as the primary motivation for multiple health-related behaviors, including importation (Becker, 1976; Bloch, Bush, & Campbell, 1993). All individuals who completed the Sample Adult Core Questionnaire were asked about importation, not just those who were taking prescription drugs. Adults with unknown importation behavior (primary analysis unweighted N = 17; 0.0%) were excluded from the analytic sample.
Statistical Analysis and Modeling
Overview of Marginal Structural Models
MSMs are a class of models that can be used to consistently estimate marginal quantities of interest via inverse probability weighting (Hernán, Brumback, & Robins, 2000; Robins, Hernán, & Brumback, 2000). In the context of observational complex survey data, MSMs represent a model-based standardization technique that can be used to correct for confounding due to measured covariates while accounting for the complex survey design and also estimating and testing heterogeneity of exposure effects across subgroups (Brumback et al., 2010; Rothman, Greenland, & Lash, 2008; Sato & Matsuyama, 2003). In comparison with alternative model-based standardization approaches, MSMs are most useful when the analyst is more confident that he or she can correctly specify a model for the exposure than for the outcome (Bieler, Brown, Williams, & Brogan, 2010; Korn & Graubard, 1999; Norton et al., 2014; Rothman et al., 2008). In the presence of effect modification by a potential confounder where the effect modification is not accounted for, MSMs are advantageous because other standardization methods may produce standard errors that are too small whereas MSMs produce conservative standard errors (Rothman et al., 2008).
Next, we generally describe how to fit an MSM to estimate a prevalence difference using complex survey-weighted data that accounts for potential confounding and examines effect modification. For a single binary exposure (X), one binary effect modifier (M), one binary potential confounder (Z), a binary observed outcome (Y), and a binary potential outcome (Yx; i.e., the outcome under an intervention to set the exposure variable X to level x), an inverse probability-of-exposure weight can be estimated for each participant in the survey-weighted study population. The numerator of the inverse probability-of-exposure weight is the probability that a person in the survey-weighted study population received the exposure that they were observed to have conditional on the effect measure modifier. The denominator of the inverse probability-of-exposure weight is the probability that a person in the survey-weighted study population received the exposure that they were observed to have conditional on the effect measure modifier and potential confounder. The aforementioned numerator and denominator can be obtained from fitting the logistic regression models shown in Equations (1) and (2) in the survey-weighted study population.
| (1) |
| (2) |
The aforementioned inverse probability-of-exposure weights are then multiplied by the survey weights to yield a combined weight for each study participant. The combined weights are used to fit the MSM linear regression model shown in Equation (3) to estimate the desired prevalence differences.
| (3) |
Analytic Approach
Since the NHIS uses a complex stratified multistage sampling scheme, the NCHS provides survey weights for each single year of data that are to be used in all analyses. This ensures that the analytic sample for a given year represents the source population of all noninstitutionalized adults in the United States during that year of the survey. Per NCHS documentation, the 1-year survey weights provided by NCHS for each adult were divided by the number of years of data that we pooled; therefore, we divided the 1-year survey weights by 3, since data were pooled from 2011, 2012, and 2013 (NCHS, 2012, 2013, 2014). This division by 3 was done to ensure that the sample size in the 3-year survey-weighted data matched the sample size of the source population between 2011 and 2013 (NCHS, 2012, 2013, 2014). The 1-year survey weights were used to examine participants’ characteristics during a single survey year, while the 3-year survey weights were used for all other subsequent analyses.
Percentages for participant characteristics were estimated for the survey-weighted population by survey year. The unweighted pooled data were then reweighted as a function of combined weights that were the product of two terms: (a) the aforementioned 3-year survey weights and (b) inverse probability-of-exposure weights. The inverse probability-of-exposure weights standardized the 3-year survey-weighted population to account for potential confounding of the relationship between a given health insurance measure and importation. Prevalences and prevalence differences of importation by health insurance that accounted for potential confounders were obtained based on frequency distributions or linear regression models estimated in the combined weighted population. Differences rather than ratios of the prevalence were the quantities of interest given that differences more readily quantify the public health impact of providing health insurance and are more useful for cost-effectiveness analyses.
Each subject had a combined weight that was a product of their 3-year survey and inverse probability-of-exposure weight. When the exposure was binary, the numerator and denominator of the exposure weights were obtained from binary logistic regression models fit in the survey-weighted sample similar to those shown in Equations (1) and (2). When the exposure had more than two levels, multinomial logistic regression models were fit in the survey-weighted sample. When not examining effect modification, the logistic regression models for the numerator were estimated without predictors while the denominator was estimated as a function of all potential confounders. When examining effect modification, the logistic regression models for the numerator were estimated as a function of all the effect modifiers of interest while the denominator was estimated as a function of all the effect modifiers and potential confounders of interest. All exposure weights appeared to be well behaved (see Appendix A, which describes the distribution of the MSM weights; Cole & Hernán, 2008).
Prevalence differences were estimated from various linear regression models fit in the combined weighted data similar to the model shown in Equation (3). The first set of linear regression models included solely terms for the health insurance measure of interest. The second set of linear regression models included terms for the health insurance measure, terms for the effect measure modifier of interest, and product terms between the health insurance measure and the effect measure modifier of interest. Effect measure modifiers were included in the second set of regression models one at a time.
F tests were used to assess heterogeneity across levels of the effect measure modifier of interest. Statistical significance was based on a two-sided type 1 error of 0.05. The aforementioned F tests as well as point estimates and 95% confidence limits were generated using SAS PROC SURVEYREG and the method of Taylor linearization, which provides asymptotically conservative estimates (see Appendix B, which provides example SAS code used to fit the MSMs outlined above to assess overall and subgroup effects in complex survey data; Brumback et al., 2010). For comparison, all analyses based on frequency distributions and linear regression models were completed using solely the 3-year survey-weighted data. Furthermore, additional sensitivity analyses were performed to assess the potential for selection bias due to study exclusions. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of Subjects and Marginal Prevalence
As shown in Table 1, the unweighted sample for the analysis contained 87,494 individuals, which after survey-weighting, represented 199,980,565 U.S. adults. The distributions of covariates were similar across all survey years, supporting our decision to merge data across years. The majority of subjects in the survey-weighted 2011-2013 population were married U.S. citizens who had at least some college education and were not poor. Almost 50% of respondents were not worried about paying medical bills if they became sick or had an accident and nearly 78% had health insurance coverage that was comparable with 1 year prior to the interview. There were 1,752 individuals who reported buying prescription drugs from another country, which resulted in a population-level survey-weighted frequency of 3,791,839 individuals or 1.9% of the adult U.S. population.
Table 1.
Characteristics of Survey-Weighted Study Population by Survey Year Among U.S. Adults, National Health Interview Survey 2011-2013.
| 2011 | 2012 | 2013 | 2011-2013 | |
|---|---|---|---|---|
| Unweighted | N = 28,332 | N = 29,486 | N = 29,676 | N = 87,494 |
| Survey-weighted | N = 198,149,357 | N = 199,753,078 | N = 202,039,261 | N = 199,980,565 |
| n (%)a | n (%)a | n (%)a | n (%)b | |
| Health insurancec | ||||
| None | 41,313,770 (20.8) | 44,052,160 (22.1) | 42,627,082 (21.1) | 42,664,337 (21.3) |
| Public | 38,502,201 (19.4) | 39,816,914 (19.9) | 42,163,727 (20.9) | 40,160,947 (20.1) |
| Private | 102,973,107 (52.0) | 100,124,931 (50.1) | 101,145,001 (50.1) | 101,414,346 (50.7) |
| Public and private | 15,360,279 (7.8) | 15,759,073 (7.9) | 16,103,451 (8.0) | 15,740,934 (7.9) |
| Imported foreign prescription drugs | ||||
| Yes | 4,223,624 (2.1) | 3,946,802 (2.0) | 3,205,092 (1.6) | 3,791,839 (1.9) |
| No | 193,925,733 (97.9) | 195,806,276 (98.0) | 198,834,169 (98.4) | 196,188,726 (98.1) |
| Geographic region of birth | ||||
| Asia | 7,753,790 (3.9) | 7,787,236 (3.9) | 8,338,309 (4.1) | 7,959,778 (4.0) |
| Africa, Middle East | 2,501,194 (1.3) | 2,200,345 (1.1) | 2,578,983 (1.3) | 2,426,841 (1.2) |
| Europe, Russia | 4,619,444 (2.3) | 4,288,311 (2.1) | 4,688,443 (2.3) | 4,532,066 (2.3) |
| Latin America | 17,743,155 (9.0) | 19,327,447 (9.7) | 19,416,264 (9.6) | 18,828,955 (9.4) |
| United States | 165,531,774 (83.5) | 166,149,739 (83.2) | 167,017,262 (82.7) | 166,232,925 (83.1) |
| Marital status | ||||
| Married | 106,956,104 (54.0) | 106,556,792 (53.3) | 107,710,931 (53.3) | 107,074,609 (53.5) |
| Not married | 91,193,253 (46.0) | 93,196,286 (46.7) | 94,328,330 (46.7) | 92,905,956 (46.5) |
| Gender | ||||
| Female | 101,650,000 (51.3) | 102,534,216 (51.3) | 104,193,346 (51.6) | 102,792,521 (51.4) |
| Male | 96,499,357 (48.7) | 97,218,862 (48.7) | 97,845,915 (48.4) | 97,188,045 (48.6) |
| Highest level of school completed | ||||
| Less than high school | 27,746,959 (14.0) | 27,596,331 (13.8) | 26,995,596 (13.4) | 27,446,295 (13.7) |
| High school | 51,878,544 (26.2) | 50,641,062 (25.4) | 51,674,176 (25.6) | 51,397,927 (25.7) |
| Some college | 40,109,764 (20.2) | 41,299,078 (20.7) | 40,214,322 (19.9) | 40,541,055 (20.3) |
| Colleged | 78,414,090 (39.6) | 80,216,607 (40.2) | 83,155,167 (41.2) | 80,595,288 (40.3) |
| Age, years | ||||
| 18-44 | 97,633,395 (49.3) | 97,456,761 (48.8) | 97536794 (48.3) | 97,542,317 (48.8) |
| 45-64 | 69,757,240 (35.2) | 70,223,627 (35.2) | 70368222 (34.8) | 70,116,363 (35.1) |
| ≥65e | 30,758,722 (15.5) | 32,072,690 (16.1) | 34134245 (16.9) | 32,321,886 (16.2) |
| U.S. citizen | ||||
| Yes | 182,765,378 (92.2) | 183,940,069 (92.1) | 185,615,895 (91.9) | 184,107,114 (92.1) |
| No | 15,383,979 (7.8) | 15,813,009 (7.9) | 16,423,366 (8.1) | 15,873,451 (7.9) |
| Region of residence in the United States | ||||
| Northeast | 34,987,175 (17.7) | 35,357,761 (17.7) | 34,962,865 (17.3) | 35,102,600 (17.6) |
| Midwest | 45,900,098 (23.2) | 45,780,043 (22.9) | 45,404,590 (22.5) | 45,694,910 (22.8) |
| South | 71,299,011 (36.0) | 73,082,320 (36.6) | 75,113,711 (37.2) | 73,165,014 (36.6) |
| West | 45,963,073 (23.2) | 45,532,954 (22.8) | 46,558,095 (23.0) | 46,018,041 (23.0) |
| Household income-to-poverty ratio | ||||
| Poor | 28,124,313 (14.2) | 28,869,381 (14.5) | 28,259,727 (14.0) | 28,417,807 (14.2) |
| Near poor | 35,557,118 (17.9) | 36,596,902 (18.3) | 37,161,153 (18.4) | 36,438,391 (18.2) |
| Not poor | 134,467,926 (67.9) | 134,286,795 (67.2) | 136,618,381 (67.6) | 135,124,367 (67.6) |
| Employment statusc | ||||
| Job last week | 121,442,107 (61.3) | 124,792,126 (62.5) | 124,767,036 (61.8) | 123,667,090 (61.8) |
| Job past 12 months | 14,523,159 (7.3) | 13,839,057 (6.9) | 12,988,156 (6.4) | 13,783,457 (6.9) |
| No job past 12 months | 51,850,868 (26.2) | 50,410,254 (25.2) | 52,544,677 (26.0) | 51,601,933 (25.8) |
| Never worked | 10,333,223 (5.2) | 10,711,641 (5.4) | 11,739,392 (5.8) | 10,928,085 (5.5) |
| Reported personal health status | ||||
| Excellent/very good | 120,908,976 (61.0) | 121,658,160 (60.9) | 123,315,123 (61.0) | 121,960,753 (61.0) |
| Good | 51,341,770 (25.9) | 53,040,999 (26.6) | 52,252,617 (25.9) | 52,211,795 (26.1) |
| Fair/poor | 25,898,611 (13.1) | 25,053,919 (12.5) | 26,471,521 (13.1) | 25,808,017 (12.9) |
| Charlson Comorbidity Indexe | ||||
| None | 94,406,887 (47.6) | 96,847,123 (48.5) | 97,312,049 (48.2) | 96,188,686 (48.1) |
| 1-3 | 90,577,093 (45.7) | 90,552,768 (45.3) | 90,786,533 (44.9) | 90,638,798 (45.3) |
| 4-15 | 13,165,377 (6.6) | 12,353,187 (6.2) | 13,940,679 (6.9) | 13,153,081 (6.6) |
| Number of family members in poor health | ||||
| 0 | 187,644,082 (94.7) | 188,421,979 (94.3) | 190,744,657 (94.4) | 188,936,906 (94.5) |
| 1 | 8,926,184 (4.5) | 9,738,811 (4.9) | 9,725,445 (4.8) | 9,463,480 (4.7) |
| 2 to 7 | 1,579,091 (0.8) | 1,592,288 (0.8) | 1,569,159 (0.8) | 1,580,179 (0.8) |
| Family member with work limitations due to health | ||||
| Yes | 38,831,476 (19.6) | 37,929,079 (19.0) | 38,910,383 (19.3) | 38,556,979 (19.3) |
| No | 159,317,881 (80.4) | 161,823,999 (81.0) | 163,128,878 (80.7) | 161,423,586 (80.7) |
| Trouble finding a doctor | ||||
| Yes | 6,477,028 (3.3) | 5,679,674 (2.8) | 5,238,864 (2.6) | 5,798,522 (2.9) |
| No | 191,672,329 (96.7) | 194,073,404 (97.2) | 196,800,397 (97.4) | 194,182,043 (97.1) |
| Health insurance coverage compared to 1 year ago | ||||
| Better | 15560495 (7.9) | 16,282,912 (8.2) | 14,385,901 (7.1) | 15,409,769 (7.7) |
| Worse | 33857457 (17.1) | 27,090,655 (13.6) | 28,052,371 (13.9) | 29,666,828 (14.8) |
| Same | 148731405 (75.1) | 156,379,511 (78.3) | 159,600,989 (79.0) | 154,903,968 (77.5) |
| Race and ethnicity | ||||
| Hispanic | 27,791,575 (14.0) | 30,126,066 (15.1) | 30,484,038 (15.1) | 29,467,226 (14.7) |
| Non-Hispanic White | 135,715,788 (68.5) | 134,751,278 (67.5) | 134,847,026 (66.7) | 135,104,697 (67.6) |
| Non-Hispanic Black | 23,458,344 (11.8) | 23,257,868 (11.6) | 24,253,675 (12.0) | 23,656,629 (11.8) |
| Non-Hispanic Asian | 9,564,468 (4.8) | 10,175,013 (5.1) | 10,858,690 (5.4) | 10,199,390 (5.1) |
| Non-Hispanic Otherf | 1,619,182 (0.8) | 1,442,853 (0.7) | 1,595,832 (0.8) | 1,552,622 (0.8) |
| Worried about paying medical bills if get sick or have accident | ||||
| Very | 38,623,048 (19.5) | 38,502,037 (19.3) | 38,089,825 (18.9) | 38,404,970 (19.2) |
| Somewhat | 62,988,771 (31.8) | 62,477,286 (31.3) | 62,943,830 (31.2) | 62,803,296 (31.4) |
| Not at all | 96,537,538 (48.7) | 98,773,755 (49.4) | 101,005,606 (50.0) | 98,772,300 (49.4) |
Note.
Estimates were survey-weighted using the known 1-year weights for the National Health Interview Survey provided by the National Center for Health Statistics.
Estimates were survey-weighted using the 3-year survey weights.
Categories are mutually exclusive.
Completed college, graduate degree, or professional degree.
Includes myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, liver disease, diabetes, cancer, and renal disease.
All other race includes American Indians, Alaskan Natives, Native Hawaiians, and other Pacific Islanders.
Marginal Prevalence Difference
Based on Table 2, survey-weighted prevalences of importation were higher among those who did not have health insurance compared with those with insurance. Similarly, those who did not have a prescription drug benefit as part of their private insurance plan also had an elevated prevalence compared with those that did have a prescription drug benefit. For all examined health insurance measures, the prevalence differences estimated by the IPW survey-weighted model were attenuated compared with the survey-weighted prevalence differences, but qualitative inference based on the point estimates remained the same. Furthermore, all attenuated estimates were statistically significant, except for the “none” versus “private” comparison. In addition, the prevalence difference comparing “none” versus “private” was substantially smaller than the prevalence differences comparing “none” versus “public” and “none” versus “public and private.”
Table 2.
Survey-Weighted and Correcteda Prevalence and Prevalence Differences for Personal Prescription Drug Importation by Insurance Status Among Survey-Weighted Population, National Health Interview Survey 2011-2013b.
| P | 95% CL | PD | 95% CL | IPW P | 95% CL | IPW PD | 95% CL | |
|---|---|---|---|---|---|---|---|---|
| Health insurance | ||||||||
| Uninsuredc | 0.040 | 0.037, 0.043 | 0.027 | 0.023, 0.030 | 0.031 | 0.026, 0.036 | 0.016 | 0.011, 0.021 |
| Insured | 0.013 | 0.012, 0.014 | 0 | — | 0.014 | 0.013, 0.016 | 0 | — |
| Health insuranced,e | ||||||||
| Nonec | 0.040 | 0.037, 0.043 | 0 | — | 0.028 | 0.024, 0.032 | 0 | — |
| Public | 0.013 | 0.011, 0.015 | 0.026 | 0.022, 0.030 | 0.012 | 0.009, 0.016 | 0.015 | 0.010, 0.021 |
| Private | 0.012 | 0.011, 0.013 | 0.027 | 0.024, 0.031 | 0.023 | 0.007, 0.039 | 0.005 | −0.011, 0.021 |
| Public and private | 0.014 | 0.011, 0.017 | 0.026 | 0.021, 0.031 | 0.011 | 0.002, 0.019 | 0.017 | 0.007, 0.027 |
| Private health plan prescription drug benefitf | ||||||||
| No | 0.019 | 0.015, 0.023 | 0.008 | 0.004, 0.011 | 0.016 | 0.013, 0.020 | 0.005 | 0.001, 0.009 |
| Yes | 0.011 | 0.010, 0.012 | 0 | — | 0.011 | 0.010, 0.012 | 0 | — |
Note. P = prevalence; PD = prevalence difference; CL = confidence limits; IPW = inverse probability weighted.
Accounts for marital status, education, comorbidity burden, family income-to-poverty ratio, age, U.S. citizenship status, region of residence, history of health insurance coverage, trouble finding a health care provider, recent and past employment status, number of family members in poor health, number of family members with work limitations due to health, personal health status, and worry about paying medical bills if got sick or had an accident.
Estimates were survey-weighted using the 3-year weights.
No insurance includes respondents who either reported having no insurance or only having catastrophic medical insurance, dental insurance, or who were covered only by the Indian Health Service.
Categories are mutually exclusive and a subset of health insurance; public insurance includes coverage by Medicaid, Medicare, other government-sponsored programs, or a military health plan such as the Department of Defense TRICARE, Veterans Affairs (VA), or Civilian Health and Medical Program of the Department of Veterans Affairs health insurance (CHAMP-VA).
Comparisons are none versus public, none versus private, and none versus public and private.
Denominator is any person with a private health insurance plan; individuals can have either private insurance or both private and public insurance coverage.
Effect Modification of Any Health Insurance
As shown in Table 3, prior to standardization using IPW, prevalence differences were heterogeneous by sex, travel outside the United States, use of online chat groups to learn about health, race/ethnicity, and global geographic region of birth. After standardization to correct for potential confounding, prevalence differences were heterogeneous by travel outside the United States, filling a prescription on the Internet, race/ethnicity, and geographic region of birth. Specifically, prevalence differences were higher among persons who were Hispanic; born in Latin America, Russia, or Europe; traveled to a developing country; and did not use the Internet to fill prescriptions or to look up health information.
Table 3.
Assessing Effect Measure Modification of the Survey-Weighted and Correcteda Prevalence Difference for Personal Prescription Drug Importation by no Health Insurance Versus any Health Insurance, National Health Interview Survey, 2011-2013b.
| Effect measure modifier | No insurance Nc,d | Insurance Nc | PD | 95% CL | IPW PD | 95% CL |
|---|---|---|---|---|---|---|
| Sex* | ||||||
| Male | 22,125,731 | 75,062,313 | 0.022 | 0.017, 0.028 | 0.016 | 0.008, 0.024 |
| Female | 20,538,606 | 82,253,915 | 0.032 | 0.027, 0.037 | 0.016 | 0.011, 0.022 |
| Traveled outside United States** | ||||||
| Yes | 13,358,855 | 55,281,930 | 0.049 | 0.042, 0.056 | 0.028 | 0.018, 0.038 |
| No | 29,305,483 | 102,034,298 | 0.018 | 0.014, 0.022 | 0.011 | 0.007, 0.016 |
| Looked up health information on Internet | ||||||
| Yes | 17,725,729 | 76,686,103 | 0.026 | 0.020, 0.031 | 0.012 | 0.005, 0.019 |
| No | 24,938,608 | 80,630,125 | 0.029 | 0.024, 0.033 | 0.02 | 0.014, 0.026 |
| Filled a prescription on Internet* | ||||||
| Yes | 1,676,827 | 13,244,085 | 0.049 | 0.021, 0.077 | 0 | –0.017, 0.017 |
| No | 40,987,510 | 144,072,143 | 0.027 | 0.023, 0.030 | 0.018 | 0.013, 0.023 |
| Scheduled medical appointment on Internet | ||||||
| Yes | 1,288,439 | 9,614,350 | 0.045 | 0.020, 0.070 | 0.02 | –0.010, 0.052 |
| No | 41,375,898 | 147,701,878 | 0.027 | 0.023, 0.030 | 0.016 | 0.012, 0.021 |
| Used online chat groups to learn about health | ||||||
| Yes | 1,585,895 | 5,432,874 | 0.051 | 0.028, 0.073 | 0.028 | 0.008, 0.048 |
| No | 41,078,442 | 151,883,354 | 0.026 | 0.022, 0.030 | 0.016 | 0.011, 0.021 |
| Race and ethnicity*** | ||||||
| Hispanic | 11,645,066 | 17,822,160 | 0.062 | 0.053, 0.071 | 0.041 | 0.027, 0.055 |
| Non-Hispanic White | 22,640,663 | 112,464,035 | 0.007 | 0.003, 0.012 | 0.001 | –0.002, 0.005 |
| Non-Hispanic Black | 5,816,507 | 17,840,122 | 0.008 | 0.002, 0.015 | 0.01 | –0.004, 0.026 |
| Non-Hispanic Asian | 1,954,666 | 8,244,724 | 0.03 | 0.009, 0.051 | 0.002 | –0.015, 0.020 |
| Non-Hispanic Othere | 607,435 | 945,188 | 0.023 | 0.000, 0.046 | 0.012 | –0.003, 0.028 |
| Geographic region of birth** | ||||||
| Asia | 15,84,797 | 6,374,981 | 0.035 | 0.009, 0.061 | 0 | –0.022, 0.020 |
| Africa, Middle East | 635,342 | 1,791,498 | 0.053 | –0.003, 0.110 | 0.015 | –0.020, 0.051 |
| Europe, Russia | 785,058 | 3,747,008 | 0.03 | –0.012, 0.072 | 0.023 | –0.027, 0.073 |
| Latin America | 8,680,855 | 10,148,101 | 0.066 | 0.054, 0.078 | 0.042 | 0.022, 0.061 |
| United States | 30,978,286 | 135,254,639 | 0.009 | 0.006, 0.012 | 0.003 | –0.000, 0.007 |
| Had transportation | ||||||
| Yes | 41,633,050 | 154,562,830 | 0.027 | 0.023, 0.030 | 0.016 | 0.012, 0.021 |
| No | 1,031,287 | 2,753,398 | 0.028 | 0.007, 0.049 | 0.007 | –0.014, 0.028 |
Note. PD = prevalence difference; CL = confidence limits; IPW = inverse probability weighted.
Corrected for marital status, education, comorbidity burden, family income-to-poverty ratio, age, U.S. citizenship status, region of residence, history of health insurance coverage, trouble finding a health care provider, recent and past employment status, number of family members in poor health, number of family members with work limitations due to health, personal health status, and degree to which person would worry about paying medical bills if became sick or had an accident.
Estimates were survey-weighted using the 3-year weights.
Survey-weighted number of persons in sample.
No insurance includes respondents who either reported having no insurance or only having catastrophic medical insurance, dental insurance, or who were covered only by the Indian Health Service.
All other race includes American Indians, Alaskan Natives, Native Hawaiians, and other Pacific Islanders.
p < .05.
p < .01.
p < .001 for test of effect measure modification.
Effect Modification of Insurance Types and Prescription Drug Insurance
As indicated by the IPW survey-weighted results (see Appendix C), there was evidence for modification of the effect of health insurance type, specifically modification of none versus private, none versus public, and none versus both public and private by global geographic birth region and race/ethnicity. The prevalence differences for the aforementioned comparisons were higher among Hispanics and non-Hispanic Asians. The prevalence differences were also higher among individuals born in Latin America, Europe, Russia, or Asia. When no prescription drug benefit was the health insurance measure of interest, the prevalence difference was heterogeneous by filling a prescription on the Internet as indicated by the IPW survey-weighted results (see Appendix D). Specifically, the prevalence difference was higher among those who filled a prescription online.
Discussion
We fit marginal structural survey-and-exposure–weighted linear regression models using 2011-2013 NHIS data to estimate the effect of various health insurance measures on personal prescription drug importation and examined whether this effect varied by subpopulation. This method was selected to obtain marginal (unconditional) effects for the entire noninstitutionalized adult U.S. population as well as within specific U.S. subgroups defined by potential effect measure modifiers. Such marginal effects are of primary interest for cost-effectiveness analyses to guide health policy decisions and could be incorporated into work estimating the value of expanding health insurance to the uninsured in important subgroups (Choudhry, Patrick, Antman, Avorn, & Shrank, 2008; Franks, Muennig, & Gold, 2005; Muennig, Franks, & Gold, 2005).
We found evidence that provision of any type of health insurance may be an effective intervention to reduce prescription drug importation in the United States. Furthermore, we observed that providing any type of health insurance may be most effective among those who are Hispanic; born in Latin America, Russia, or Europe; travel to developing countries outside the United States; and do not use the Internet to fill prescriptions or look up health information. Therefore, policy makers should consider targeting the aforementioned subpopulations of U.S. residents when continuing to expand health insurance as part of the Affordable Care Act. Analyses examining the relationship between type of insurance and importation suggest that public and combination public–private insurance may be more effective at reducing importation than private insurance. Prior evidence suggests that public insurance is more effective at reducing out-of-pocket costs than private insurance and thus, may be more effective at reducing importation (Ku & Broaddus, 2008). Combining supplemental private insurance with public may cover any remaining gaps to further reduce out-of-pocket costs, as is the case with Medicare Supplement Insurance (Medigap) policies that cover copayments, coinsurances, and deductibles not covered by fee-for-service Medicare. Furthermore, the addition of a prescription drug benefit to a private insurance plan provides a small, but significant, reduction in importation.
Our finding that the prevalence difference was higher among those who did not use the Internet to fill prescriptions suggests that Internet use to fill prescriptions may be a proxy for socioeconomic status or degree of marginalization. Specifically, those who do not use the Internet may be of a lower socioeconomic status or more marginalized than those who do. Lower socioeconomic status or marginalized groups may have fewer resources to acquire domestic medications in the absence of health insurance.
Studies have empirically demonstrated that health insurance has several beneficial effects, including reducing financial strain, increasing utilization of health services, and improving the identification of illnesses (Baicker et al., 2013; Finkelstein et al., 2012). The potential reduction in importation is an important additional benefit of expanding health insurance that is especially valued by U.S. regulatory agencies attempting to avoid bioterrorism attacks and compromised patient safety (Courtney, Bond, & Maher, 2014; Elbe, Roemer-Mahler, & Long, 2015). The aforementioned pleiotropic effects of health insurance interventions can make such interventions particularly cost-effective. Furthermore, the Affordable Care Act of 2010 makes health insurance a feasible intervention through the individual mandate, subsidies to purchase insurance, and the expansion of Medicaid by states (Sheils & Haught, 2011).
Lowering the price of prescription drugs among those who would otherwise import is an alternative potential intervention to reduce drug importation. However, identifying individuals who import is extremely difficult because importation is illegal and poorly documented, making this alternative intervention infeasible (Kesselheim & Choudhry, 2008). Although it might be ideal to significantly lower the cost of prescription drugs to all U.S. consumers (i.e., the entire U.S. market), there are no known mechanisms to do so. The introduction of new and very effective hepatitis C drugs (e.g., sofosbuvir, boceprevir, telaprevir) is an example (McCarthy, 2015). Even in the face of tremendous public scrutiny over the cost of the drugs, manufacturers did not begin to lower prices until sufficient competition entered the market. Despite the lower prices, the drugs remained extremely expensive because they were all branded products. Furthermore, the government has almost no mechanisms by which to lower the price of prescription drugs (Holt, 2003). Public insurance offered by the government, such as Medicare and Medicaid insurance, is one of the few ways that government can lower the out-of-pocket cost of drugs to consumers.
While health and prescription drug insurance may be effective interventions for reducing importation, the effectiveness of such interventions may be limited by coverage restrictions on drugs. Restricted coverage results in high patient out-of-pocket costs for patent-protected brand-name drugs without suitable generic alternatives (e.g., the aforementioned new hepatitis C treatments, sofosbuvir, telaprevir, and boceprevir; Brennan & Shrank, 2014). Such costs may serve as strong motivation for consumers to continue to import drugs despite having health and prescription drug insurance. Therefore, providing health insurance and prescription drug coverage does not guarantee affordable access to drugs, especially those on higher formulary tiers, but providing insurance and prescription drug coverage still may be an effective intervention to reduce importation.
The integrity of drugs varies by country and global region. While products from Canada and parts of Europe are likely safe, products from low- and middle-income countries may not be safe as these countries have weaker enforcement of counterfeiting laws and weaker regulation of pharmaceutical dissemination (Kesselheim & Choudhry, 2008; U.S. Food and Drug Administration, 2011). If global country or region of birth predicts the place from which individuals are importing, then expanding health insurance coverage for U.S. residents originating from low- and middle-income countries may be the most cost-effective way to minimize domestic exposure to drugs with compromised integrity.
There were several limitations to our study. First, the cross-sectional design limits causal inference. Second, we were unable to address the effect of insurance on reducing exposure to counterfeit, adulterated, and substandard drugs because the NHIS did not have a direct or indirect measure of exposure to such drugs. We were also unable to verify whether purchasing drugs from foreign countries and intercountry shipment of drugs (i.e., true importation) were perfectly synonymous. Third, there may be unmeasured confounders that bias the effect estimates. Based on theory and previous empirical work, we believe that we have controlled for the strongest confounders given the rich set of covariates available, but we acknowledge that the possibility of unmeasured confounding (i.e., omitted variable bias or “unobservables” in the economics par-lance) always exists and could explain observed heterogeneity of prevalence differences across strata of effect measure modifiers (if the degree and direction of unmeasured confounding bias varied by the level of the effect measure modifiers; Gunasekara, Carter, & Blakely, 2008). Technology access, utilization, and preferences (e.g., for newer technologies) may be one such unmeasured confounder. Fourth, our approach requires consistency and we must assume positivity and correct model specification (Cole & Hernán, 2008). Fifth, exclusion of individuals with missing information may have induced selection bias. However, this selection bias may be negligible since accounting for selection bias related to the major study exclusions using inverse probability weights did not change inference (results not shown; Hernán, Hernandez-Diaz, & Robins, 2004). Sixth, we must assume that the survey-weighted population accurately represents the U.S. populations of interest. Seventh, as is the case with all self-reported data, variables are subject to measurement error due to inaccurate recall and the tendency toward responses that are more socially desirable. This measurement error may have further resulted in biased effect estimates. Eighth, we could not examine the association between insurance and importation among individuals with a high need for expensive prescription drugs given that necessary information on prescription drug dispensing was not available in the NHIS data, though it would be interesting to examine the aforementioned association in future work. Last, because of the broad nature of the outcome survey question, we were unable to identify the specific modes by which participants imported and thus unable to assess whether the role of health insurance varied by mode of importation. Determining the mode would be important if medications obtained by traveling across U.S. borders or to other countries were more or less likely to be of compromised integrity than medications obtained through online purchasing.
Our study had several strengths. Given the scant empirical literature that precluded us from comparing our findings with prior studies, we are among the first studies to examine the role of health insurance in personal prescription drug importation. This scant literature is in large part due to the fact that information on the purchase of foreign drug products has historically been difficult to obtain. As such, our study can serve as a model for similar research on personal importation in the United States and other countries to establish an informative literature base. The large, representative U.S. sample is another study strength because it supported a more precise analysis.
Conclusions
In conclusion, we observed that the prevalence of drug importation was higher among those who did not have health insurance compared with those with insurance. Health insurance is a potentially effective intervention target for reducing personal prescription drug importation, and based on our empirical findings, this effectiveness likely varies across subpopulations in the United States. Future research should (a) use prospective rather than cross-sectional data to examine the role of health insurance in personal prescription drug importation, (b) attempt to understand whether the risk of exposure to drugs with compromised integrity differs by mode of importation, (c) assess the role of health insurance by mode of importation, and (d) assess for differences in effect modification by mode of importation.
Acknowledgments
The authors would like to thank the participants of the National Health Interview Survey and Dr. Babette Brumback for expert advice.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a National Institutes of Health grant (5R24HD041020-14) to the Brown University Population Studies and Training Center and an Agency for Healthcare Research and Quality grant (5K12HS022998-02) to the Brown University Center for Evidence-based Medicine. The research was additionally funded by a Nora Kahn Piore Award from the Brown University School of Public Health and a summer studies grant from the Brown University Population Studies and Training Center.
Appendix A
Distribution of the Marginal Structural Model Weights
Not Examining Effect Modification
When not examining effect modification, the mean (standard deviation) of the stabilized weights for any health insurance was 1.02 (0.99) with a minimum of 0.08 and maximum of 75.50. The mean (standard deviation) of the stabilized weights for the multilevel categorization of health insurance (i.e., none, public, private, public, and private) was 1.08 (3.94) with a minimum of 0.013 and a maximum of 340.54. The mean (standard deviation) of the stabilized weights for prescription drug benefit among those with a private health insurance plan was 1.00 (0.18) with a minimum of 0.28 and maximum of 2.37.
Examining Effect Modification
When examining effect modification, the mean (standard deviation) of the stabilized weights for any health insurance was 1.02 (1.03) with a minimum of 0.08 and maximum of 78.71. The mean (standard deviation) of the stabilized weights for the multilevel categorization of health insurance (i.e., none, public, private, public, and private) was 1.08 (3.14) with a minimum of 0.025 and a maximum of 208.49. The mean (standard deviation) of the stabilized weights for prescription drug benefit among those with a private health insurance plan was 0.99 (0.17) with a minimum of 0.28 and maximum of 2.31.
Appendix B
SAS Code That Demonstrates the Complex Survey Marginal Structural Model for Examining Effect Modification
In this appendix, we provide SAS code to fit the complex survey marginal structural model described in the text when the exposure is a binary indicator of having any health insurance. The original format of NHIS data files containing one record per adult is appropriate to fit the models. The pooled 2011-2013 data set is called NHISCOMB. The records in the data set must be sorted by the person-level unique identifiers, which must be preserved in the variable sequence “hhx fmx fpx.”
The SAS code shown below is organized as follows. First, we use PROC SURVEYLOGISTIC to fit two models (analogous to Equations 1 and 2 in the main text) for the probability of not having health insurance. Inverse probability-of-exposure weights were obtained by taking a ratio of a function of the predicted probabilities outputted from the aforementioned two models. Second, we use an SAS data step to calculate the combined weights for each person as the product of the inverse probability-of-exposure weights and the survey weights provided by NCHS. Last, we use PROC SURVEYREG to fit the final weighted linear regression model (analogous to Equation 3 in the main text) that estimates the causal parameter of interest and its conservative standard error.
The outcome variable in Models 1 and 2 is a dichotomous variable “insurance” indicating whether the person lacked health insurance (insurance=1) or had health insurance (insurance=0). The “event” option specifies the event category for the binary response model, ensuring PROC LOGISTIC models the probability that the outcome variable is 1. Hence, Models 1 and 2 model the probability of not having health insurance. The “weight” statement names the variable containing the survey sampling weights (surveyweight). The “param=ref” option specifies reference cell coding. The “class” statement names the classification variables to be used in the model. Model 1 includes as regressors the effect modifiers: sex travel hit1a hit2a hit3a hit5a hiscodi3 geobrth regionbr ahcdlyr5. Model 2 includes the variables in Model 1 plus the following potential confounders: r_maritl educ1 Charlson rat_cat3 age_p citizenp region ahicomp aprvtryr wrklyr4 fhstatpr fwklimyn aworpay phstat.
For each model, we output a new data file (option “out=” in PROC SURVEYLOGISTIC) that contains, for each person, the original unique identifier variables plus the predicted probabilities from the model (option “p=”). As an example, the first PROC SURVEY LOGISTIC creates the data set num with its predicted probabilities as the variable “p_num”.
In the subsequent data step, we merge the three output files into the file combinedw_data that contains the predicted probabilities from the two survey logistic models. We then compute the stabilized inverse probability-of-exposure weights from Models 1 and 2. For a person who did not have health insurance, the stabilized inverse probability-of-exposure weight was a ratio of the predicted probabilities from Models 1 and 2 [p_num/p_den]. For a person who did have health insurance, the stabilized inverse probability-of-exposure weight was a ratio of the predicted probabilities subtracted from 1 [(1-p_num)/(1-p_den)]. Then we take the product of the exposure weights and NCHS-provided survey weights to obtain the “combinedweight”.
Finally, we call PROC SURVEYREG to fit a weighted linear regression model. The outcome variable in this model is a dichotomous variable “importation” indicating whether the person imported prescription drugs from non-U.S. countries (importation=1) or did not import (importation=0). The predictors in the model were the indicator of health insurance (insurance), an indicator for the effect measure modifiers, sex, coded as 1=male and 0=female, and a product term between the insurance and sex indicators.
Use of the “strata” and “cluster” statements in the weighted linear regression model specifies the variables for the sampling strata and the clusters nested within those strata. Both are necessary to obtain correct standard errors, confidence intervals, and p values. The “solution clparm” options produce confidence limits for the parameter estimates. The confidence limits will be asymptotically conservative. We fit the model using the combined weights by specifying the variable “combinedweight” in the “weight” statement. The “estimate” statement allows for the comparisons of interest and calculation of the prevalence differences.
/*MODEL 1*/proc surveylogistic data=NHISCOMB; weight surveyweight; class regionbr hiscodi3/param=ref; model insurance (event=‘1’) = sex travel hit1a hit2a hit3a hit5a hiscodi3 geobrth regionbr ahcdlyr5; output out=num (keep=hhx fmx fpx p_num) p=p_num; run; proc sort data=NHISCOMB; by hhx fmx fpx; run; proc sort data=num; by hhx fmx fpx; run; /*MODEL 2*/ proc surveylogistic data=NHISCOMB; weight surveyweight; class region wrklyr4 regionbr hiscodi3 educ1 Charlson rat_cat3 age_p fhstatpr ahicomp aworpay phstat/param=ref; model insurance (event=‘1’) = sex travel hit1a hit2a hit3a hit5a hiscodi3 geobrth regionbr ahcdlyr5 r_maritl educ1 Charlson rat_cat3 age_p citizenp region ahicomp aprvtryr wrklyr4 fhstatpr fwklimyn aworpay phstat; output out=den (keep=hhx fmx fpx p_den) p=p_den; run; proc sort data=den; by hhx fmx fpx; run; data combinedw_data; merge NHISCOMB p_den p_num; by hhx fmx fpx; if insurance=1 then ipw=p_num/p_den if insurance=0 then ipw=(1-p_num)/(1-p_den); combinedweight=ipw*surveyweight; run; proc surveyreg data= combinedw_data; class sex insurance; strata strat_p; cluster psu_p; model importation=sex insurance insurance*sex /solution clparm; weight combinedweight; estimate ‘Uninsured v. Insured Among Men’ insurance -1 1 sex*insurance 0 0 -1 1; estimate ‘Uninsured v. Insured Among Women’ insurance -1 1 sex*insurance -1 1 0 0; run;
Appendix C
Assessing Effect Measure Modification of the Survey-Weighted and Correcteda Prevalence Difference for Personal Prescription Drug Importation by Type of Health Insurance, National Health Interview Survey, 20ll-20l3b.
| Insurance typec | Effect measure modifier | Nd | PD | 95% CL | IPW PD | 95% CL |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Nonee | Male | 22,125,731 | 0 | — | 0 | — |
| Female | 20,538,606 | 0 | — | 0 | — | |
| Publicf | Male | 17,287,874 | 0.022 | 0.016, 0.028 | 0.017 | 0.008, 0.025 |
| Female | 22,873,074 | 0.031 | 0.026, 0.036 | 0.022 | 0.012, 0.031 | |
| Private | Male | 50,620,382 | 0.023 | 0.017, 0.028 | 0.008 | –0.005, 0.022 |
| Female | 50,793,965 | 0.033 | 0.027, 0.038 | 0.011 | –0.011, 0.034 | |
| Bothg | Male | 7,154,058 | 0.021 | 0.014, 0.028 | 0.017 | 0.001, 0.033 |
| Female | 8,586,876 | 0.031 | 0.025, 0.037 | 0.023 | 0.010, 0.036 | |
| Traveled outside United States | ||||||
| Nonee | Yes | 13,358,855 | 0 | — | 0 | — |
| No | 29,305,483 | 0 | — | 0 | — | |
| Publicf | Yes | 9,626,934 | 0.042 | 0.033, 0.051 | 0.028 | 0.016, 0.041 |
| No | 30,534,014 | 0.017 | 0.013, 0.021 | 0.013 | 0.005, 0.021 | |
| Private | Yes | 41,410,483 | 0.050 | 0.043, 0.048 | 0.014 | –0.013, 0.042 |
| No | 60,003,864 | 0.019 | 0.015, 0.023 | 0.011 | –0.001, 0.023 | |
| Bothg | Yes | 4,244,513 | 0.048 | 0.038, 0.058 | 0.026 | –0.002, 0.054 |
| No | 11,496,421 | 0.015 | 0.010, 0.020 | 0.017 | 0.009, 0.025 | |
| Looked up health information on Internet | ||||||
| Nonee | Yes | 17,725,729 | 0 | — | 0 | — |
| No | 24,938,608 | 0 | — | 0 | — | |
| Publicf | Yes | 13,318,142 | 0.025 | 0.018, 0.032 | 0.017 | 0.009, 0.024 |
| No | 26,842,805 | 0.026 | 0.021, 0.032 | 0.021 | 0.012, 0.031 | |
| Private | Yes | 57,952,796 | 0.026 | 0.020, 0.032 | 0.011 | 0.005, 0.018 |
| No | 43,461,551 | 0.030 | 0.025, 0.035 | 0.006 | –0.021, 0.034 | |
| Bothg | Yes | 5,415,165 | 0.022 | 0.013, 0.030 | 0.012 | –0.006, 0.031 |
| No | 10,325,769 | 0.028 | 0.022, 0.033 | 0.025 | 0.014, 0.037 | |
| Filled a prescription on Internet | ||||||
| Nonee | Yes | 1,676,827 | 0 | — | 0 | — |
| No | 40,987,510 | 0 | — | 0 | — | |
| Publicf | Yes | 2,017,994 | 0.050 | 0.020, 0.081 | 0.009 | –0.012, 0.031 |
| No | 38,142,953 | 0.025 | 0.021, 0.029 | 0.020 | 0.013, 0.026 | |
| Private | Yes | 9,759,991 | 0.049 | 0.021, 0.077 | 0.003 | –0.014, 0.021 |
| No | 91,654,355 | 0.028 | 0.024, 0.031 | 0.010 | –0.004, 0.025 | |
| Bothg | Yes | 1,466,099 | 0.048 | 0.014, 0.082 | 0.021 | 0.002, 0.040 |
| No | 14,274,835 | 0.026 | 0.022, 0.031 | 0.020 | 0.009, 0.031 | |
| Scheduled medical appointment on Internet | ||||||
| Nonee | Yes | 1,288,439 | 0 | — | 0 | — |
| No | 41,375,898 | 0 | — | 0 | — | |
| Publicf | Yes | 1,233,660 | 0.045 | 0.017, 0.072 | 0.019 | –0.002, 0.041 |
| No | 38,927,288 | 0.026 | 0.022, 0.030 | 0.019 | 0.013, 0.026 | |
| Private | Yes | 7,761,212 | 0.045 | 0.019, 0.070 | 0.013 | –0.006, 0.032 |
| No | 93,653,135 | 0.027 | 0.024, 0.031 | 0.009 | –0.004, 0.024 | |
| Bothg | Yes | 619,479 | 0.051 | 0.021, 0.081 | 0.034 | 0.015, 0.052 |
| No | 15,121,455 | 0.025 | 0.021, 0.030 | 0.019 | 0.009, 0.030 | |
| Used Online Chat Groups to Learn About Health | ||||||
| Nonee | Yes | 1,585,895 | 0 | — | 0 | — |
| No | 41,078,442 | 0 | — | 0 | — | |
| Publicf | Yes | 1,203,506 | 0.051 | 0.023, 0.078 | 0.034 | 0.007, 0.061 |
| No | 38,957,441 | 0.025 | 0.021, 0.029 | 0.019 | 0.012, 0.025 | |
| Private | Yes | 3,855,486 | 0.053 | 0.029, 0.076 | 0.033 | 0.012, 0.055 |
| No | 97,558,861 | 0.026 | 0.023, 0.030 | 0.009 | –0.005, 0.023 | |
| Bothg | Yes | 373,882 | 0.030 | –0.011, 0.072 | 0.046 | 0.023, 0.068 |
| No | 15,367,052 | 0.025 | 0.021, 0.030 | 0.019 | 0.009, 0.030 | |
| Race and ethnicity*** | ||||||
| Nonee | Hispanic | 11,645,066 | 0 | — | 0 | — |
| Non-Hispanic White | 22,640,663 | 0 | — | 0 | — | |
| Non-Hispanic Black | 5,816,507 | 0 | — | 0 | — | |
| Non-Hispanic Asian | 1,954,666 | 0 | — | 0 | — | |
| Non-Hispanic, Otherh | 607,435 | 0 | — | 0 | — | |
| Publicf | Hispanic | 6,430,643 | 0.054 | 0.043, 0.065 | 0.030 | 0.012, 0.049 |
| Non-Hispanic White | 24,604,313 | 0.008 | 0.004, 0.013 | 0.009 | 0.001, 0.018 | |
| Non-Hispanic Black | 6,919,527 | 0.010 | 0.003, 0.016 | 0.010 | –0.001, 0.023 | |
| Non-Hispanic Asian | 1,862,228 | 0.038 | 0.014, 0.062 | 0.021 | 0.001, 0.040 | |
| Non-Hispanic, Otherh | 344,236 | 0.025 | 0.002, 0.048 | 0.013 | 0.000, 0.026 | |
| Private | Hispanic | 10,814,294 | 0.066 | 0.056, 0.075 | 0.042 | 0.028, 0.056 |
| Non-Hispanic White | 74,275,749 | 0.008 | 0.004, 0.012 | –0.006 | –0.025, 0.012 | |
| Non-Hispanic Black | 9,775,884 | 0.008 | 0.002, 0.015 | 0.010 | –0.001, 0.022 | |
| Non-Hispanic Asian | 6,050,305 | 0.027 | 0.006, 0.048 | –0.000 | –0.019, 0.018 | |
| Non-Hispanic, Otherh | 498,115 | 0.021 | –0.002, 0.045 | 0.009 | –0.005, 0.023 | |
| Bothg | Hispanic | 577,223 | 0.087 | 0.075, 0.100 | 0.078 | 0.068, 0.089 |
| Non-Hispanic White | 13,583,973 | 0.003 | –0.001, 0.008 | 0.004 | –0.007, 0.016 | |
| Non-Hispanic Black | 1,144,711 | 0.005 | –0.004, 0.014 | –0.008 | –0.049, 0.032 | |
| Non-Hispanic Asian | 332,191 | 0.046 | 0.024, 0.068 | 0.029 | 0.013, 0.046 | |
| Non-Hispanic, Otherh | 102,837 | 0.025 | 0.002, 0.048 | 0.013 | 0.000, 0.026 | |
| Geographic region of birth*** | ||||||
| Nonee | Asia | 1,584,797 | 0 | — | 0 | — |
| Africa, Middle East | 635,342 | 0 | — | 0 | — | |
| Europe, Russia | 785,058 | 0 | — | 0 | — | |
| Latin America | 8,680,855 | 0 | - | 0 | - | |
| United States | 30,978,286 | 0 | - | 0 | - | |
| Publicf | Asia | 1,555,822 | 0.046 | 0.017, 0.075 | 0.022 | –0.000, 0.045 |
| Africa, Middle East | 469,124 | 0.062 | 0.000, 0.124 | 0.017 | –0.028, 0.062 | |
| Europe, Russia | 910,305 | 0.039 | –0.004, 0.083 | 0.046 | –0.015, 0.108 | |
| Latin America | 4,094,484 | 0.056 | 0.041, 0.071 | 0.033 | 0.008, 0.059 | |
| United States | 33,131,213 | 0.010 | 0.006, 0.013 | 0.009 | 0.002, 0.015 | |
| Private | Asia | 4,586,059 | 0.030 | 0.004, 0.057 | –0.004 | –0.027, 0.018 |
| Africa, Middle East | 1,263,463 | 0.052 | –0.004, 0.109 | –0.001 | –0.039, 0.035 | |
| Europe, Russia | 2,473,654 | 0.031 | –0.012, 0.074 | –0.076 | –0.264, 0.110 | |
| Latin America | 5,773,790 | 0.071 | 0.058, 0.084 | 0.041 | 0.018, 0.063 | |
| United States | 87,317,380 | 0.009 | 0.006, 0.012 | –0.000 | –0.014, 0.013 | |
| Bothg | Asia | 233,100 | 0.056 | 0.029, 0.084 | 0.034 | 0.015, 0.053 |
| Africa, Middle East | 58,912 | –0.006 | –0.136, 0.124 | –0.187 | –0.629, 0.255 | |
| Europe, Russia | 363,049 | 0.001 | –0.058, 0.060 | 0.045 | –0.016, 0.106 | |
| Latin America | 279,826 | 0.095 | 0.074, 0.115 | 0.092 | 0.077, 0.108 | |
| United States | 14,806,047 | 0.006 | 0.001, 0.010 | 0.005 | –0.004, 0.015 | |
| Had transportation | ||||||
| Nonee | Yes | 41,633,050 | 0 | — | 0 | — |
| No | 1,031,287 | 0 | — | 0 | — | |
| Publicf | Yes | 38,269,912 | 0.026 | 0.022, 0.030 | 0.019 | 0.013, 0.026 |
| No | 1,891,035 | 0.031 | 0.010, 0.052 | 0.015 | –0.005, 0.036 | |
| Private | Yes | 100,777,096 | 0.027 | 0.024, 0.031 | 0.009 | –0.003, 0.023 |
| No | 637,250 | 0.021 | –0.010, 0.053 | 0.021 | –0.001, 0.043 | |
| Bothg | Yes | 15,515,822 | 0.026 | 0.021, 0.030 | 0.020 | 0.010, 0.031 |
| No | 225,112 | 0.027 | –0.004, 0.060 | 0.026 | 0.004, 0.047 | |
Note. PD = Prevalence difference; CL= confidence limits; IPW = inverse probability weighted.
Corrected for marital status, education, comorbidity burden, family income-to-poverty ratio, age, U.S. citizenship status, region of residence, history of health insurance coverage, trouble finding a health care provider, recent and past employment status, number of family members in poor health, number of family members with work limitations due to health, personal health status, and degree to which person would worry about paying medical bills if became sick or had an accident.
Estimates were survey-weighted using the 3-year weights.
Comparisons are none versus public, none versus private, and none versus both; categories are mutually exclusive.
Survey-weighted number of persons in sample.
No insurance includes respondents who either reported having no insurance or only having catastrophic medical insurance, dental insurance, or who were covered only by the Indian Health Service.
Public insurance includes coverage by Medicaid, Medicare, other government-sponsored programs, or a military health plan such as the Department of Defense TRICARE, Veterans Affairs (VA), or Civilian Health and Medical Program of the Department of Veterans Affairs health insurance (CHAMP-VA).
Both represents individuals who have both public and private insurance coverage.
All other race includes American Indians, Alaskan Natives, Native Hawaiians, and other Pacific Islanders.
* p < .05.
** p < .01.
p < .001 for test of effect measure modification.
Appendix D
Assessing Effect Measure Modification of the Survey-Weighted and Correcteda Prevalence Difference for Personal Prescription Drug Importation by Prescription Drug Benefit Among Those With a Private Insurance Health Plan, National Health Interview Survey, 2011-2013b.
| Effect measure modifier | No insurance Nc,d | Insurance Nc | PD | 95% CL | IPW PD | 95% CL |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 8,414,995 | 48,539,275 | 0.005 | –0.000, 0.012 | 0.004 | –0.002, 0.010 |
| Female | 8,013,583 | 47,058,391 | 0.01 | 0.004, 0.016 | 0.006 | 0.001, 0.012 |
| Traveled outside United States | ||||||
| Yes | 5,681,109 | 38,124,990 | 0.013 | 0.003, 0.023 | 0.01 | 0.000, 0.019 |
| No | 10,747,470 | 57,472,677 | 0.006 | 0.002, 0.010 | 0.004 | 0.000, 0.007 |
| Looked up health information on Internet | ||||||
| Yes | 8,266,007 | 52,834,164 | 0.011 | 0.004, 0.018 | 0.008 | 0.001, 0.016 |
| No | 8,162,572 | 42,763,502 | 0.005 | 0.001, 0.010 | 0.002 | –0.001, 0.006 |
| Filled a prescription on Internet* | ||||||
| Yes | 1,566,426 | 9,312,872 | 0.032 | 0.009, 0.056 | 0.029 | 0.005, 0.053 |
| No | 14,862,152 | 86,284,794 | 0.005 | 0.001, 0.009 | 0.002 | –0.000, 0.006 |
| Scheduled medical appointment on Internet | ||||||
| Yes | 1,017,106 | 7,136,385 | –0.005 | –0.018, 0.008 | –0.005 | –0.019, 0.007 |
| No | 15,411,472 | 88,461,282 | 0.009 | 0.004, 0.013 | 0.006 | 0.002, 0.010 |
| Used online chat groups to learn about health | ||||||
| Yes | 504,434 | 3,576,201 | 0.020 | –0.011, 0.053 | 0.010 | –0.021, 0.042 |
| No | 15,924,144 | 92,021,465 | 0.007 | 0.003, 0.012 | 0.005 | 0.001, 0.009 |
| Race and ethnicity | ||||||
| Hispanic | 1,400,812 | 9,543,531 | 0.002 | –0.012, 0.017 | –0.001 | –0.014, 0.012 |
| Non-Hispanic White | 12,864,430 | 71,181,004 | 0.009 | 0.004, 0.014 | 0.006 | 0.001, 0.011 |
| Non-Hispanic Black | 1,333,771 | 9,053,767 | 0.003 | –0.003, 0.011 | 0.001 | –0.004, 0.008 |
| Non-Hispanic Asian | 729,340 | 5,362,448 | 0.017 | –0.009, 0.044 | 0.014 | –0.011, 0.040 |
| Non-Hispanic Othere | 100,225 | 456,917 | –0.005 | –0.012, 0.002 | –0.005 | –0.012, 0.002 |
| Geographic region of birth | ||||||
| Asia | 543,998 | 4,046,746 | 0.025 | –0.011, 0.062 | 0.021 | –0.013, 0.055 |
| Africa, Middle East | 130,109 | 1,119,310 | 0.001 | –0.043, 0.045 | –0.011 | –0.042, 0.018 |
| Europe, Russia | 411,515 | 2,280,882 | 0.060 | 0.000, 0.119 | 0.038 | –0.011, 0.088 |
| Latin America | 803,862 | 5,001,612 | 0.004 | –0.018, 0.027 | –0.000 | –0.020, 0.020 |
| United States | 14,539,094 | 83,149,116 | 0.006 | 0.002, 0.010 | 0.004 | 0.000, 0.008 |
| Had transportation | ||||||
| Yes | 16,272,048 | 94,960,019 | 0.008 | 0.004, 0.012 | 0.005 | 0.001, 0.009 |
| No | 156,530 | 637,647 | –0.013 | –0.051, 0.024 | –0.019 | –0.051, 0.013 |
Note. PD = prevalence difference; CL= confidence limits; IPW = inverse probability weighted.
Corrected for marital status, education, comorbidity burden, family income-to-poverty ratio, age, U.S. citizenship status, region of residence, history of health insurance coverage, trouble finding a health care provider, recent and past employment status, number of family members in poor health, number of family members with work limitations due to health, personal health status, and degree to which person would worry about paying medical bills if became sick or had an accident.
Estimates were survey-weighted using the 3-year weights.
Survey-weighted number of persons in sample.
No insurance includes respondents who either reported having no insurance or only having catastrophic medical insurance, dental insurance, or who were covered only by the Indian Health Service.
All other race includes American Indians, Alaskan Natives, Native Hawaiians, and other Pacific Islanders.
p < .05.
** p < .01.
*** p < .001 for test of effect measure modification.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adams PF, Kirzinger WK, Martinez ME. Vital Health Statistics Series 10, No. 256. U.S. Department of Health and Human Services; Hyattsville, MD: Dec, 2012. Summary health statistics for U.S. adults: National Health Interview Survey, 2011. [Google Scholar]
- Adams PF, Martinez ME, Vickerie JL, Kirzinger WK. Vital Health Statistics Series 10, No. 251. U.S. Department of Health and Human Services; Hyattsville, MD: Dec, 2011. Summary health statistics for the U.S. population: National Health Interview Survey, 2010. [PubMed] [Google Scholar]
- Arrow KJ. Uncertainty and the welfare economics of medical care. American Economic Review. 1963;53:941–973. [Google Scholar]
- Arrunada B. Quality safeguards and regulation of online pharmacies. Health Economics. 2004;13:329–344. doi: 10.1002/hec.827. doi:10.1002/hec.827. [DOI] [PubMed] [Google Scholar]
- Baicker K, Taubman SL, Allen HL, Bernstein M, Gruber JH, Newhouse JP, Smith J. The Oregon experiment—Effects of Medicaid on clinical outcomes. New England Journal of Medicine. 2013;368:1713–1722. doi: 10.1056/NEJMsa1212321. doi:10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker GS. The economic approach to human behavior. University of Chicago Press; Chicago, IL: 1976. [Google Scholar]
- Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. American Journal of Epidemiology. 2010;171:618–623. doi: 10.1093/aje/kwp440. doi:10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- Bloch PH, Bush RF, Campbell L. Consumer “accomplices” in product counterfeiting: A demand side investigation. Journal of Consumer Marketing. 1993;10(4):27–36. doi:10.1108/07363769310047374. [Google Scholar]
- Borenstein S, Saloner G. Economics and electronic commerce. Journal of Economic Perspectives. 2001;15(1):3–12. [Google Scholar]
- Brennan T, Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014;312:593–594. doi: 10.1001/jama.2014.8897. doi:10.1001/jama.2014.8897. [DOI] [PubMed] [Google Scholar]
- Briesacher BA, Zhao Y, Madden JM, Zhang F, Adams AS, Tjia J, Soumerai SB. Medicare part D and changes in prescription drug use and cost burden: National estimates for the Medicare population, 2000 to 2007. Medical Care. 2011;49:834–841. doi: 10.1097/MLR.0b013e3182162afb. doi:10.1097/MLR.0b013e3182162afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroids. Psychiatric Clinics of North America. 1993;16:97–103. [PubMed] [Google Scholar]
- Brumback BA, Bouldin ED, Zheng HW, Cannell MB, Andresen EM. Testing and estimating model-adjusted effect-measure modification using marginal structural models and complex survey data. American Journal of Epidemiology. 2010;172:1085–1091. doi: 10.1093/aje/kwq244. doi:10.1093/aje/kwq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Brumback BA, Bouldin ED, Hess J, Wood DL, Sloyer PJ, Andresen EM. Age group differences in healthcare access for people with disabilities: Are young adults at increased risk? Journal of Adolescent Health. 2011;49:219–221. doi: 10.1016/j.jadohealth.2010.11.251. doi:10.1016/j.jadohealth.2010.11.251. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Patrick AR, Antman EM, Avorn J, Shrank WH. Cost-effectiveness of providing full drug coverage to increase medication adherence in post-myocardial infarction Medicare beneficiaries. Circulation. 2008;117:1261–1268. doi: 10.1161/CIRCULATIONAHA.107.735605. doi:10.1161/CIRCULATIONAHA.107.735605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. American Journal of Epidemiology. 2008;168:656–664. doi: 10.1093/aje/kwn164. doi:10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney B, Bond KC, Maher C. Regulatory underpinnings of Global Health security: FDA’s roles in preventing, detecting, and responding to global health threats. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2014;12:239–246. doi: 10.1089/bsp.2014.0046. doi:10.1089/bsp.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham P, Carrier E. Trends in the financial burden of medical care for nonelderly adults with diabetes, 2001 to 2009. American Journal of Managed Care. 2014;20:135–142. [PubMed] [Google Scholar]
- Do DP, Wang L, Elliott MR. Investigating the relationship between neighborhood poverty and mortality risk: A marginal structural modeling approach. Social Science & Medicine. 2013;91:58–66. doi: 10.1016/j.socscimed.2013.03.003. doi:10.1016/j.socscimed.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S, Roemer-Mahler A, Long C. Medical countermeasures for national security: A new government role in the pharmaceuticalization of society. Social Science & Medicine. 2015;131:263–271. doi: 10.1016/j.socscimed.2014.04.035. doi:10.1016/j.socscimed.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Ellison K. H.R. 3715—113th Congress: Personal Drug Importation Fairness Act of 2013. 2013 Retrieved from https://www.govtrack.us/congress/bills/113/hr3715.
- Examining the implications of drug importation: Hearing before the Committee on the Judiciary, United States Senate; 108th Cong., 2nd session; 2004. [Google Scholar]
- Fejos I, Neumajer G, Beni S, Jankovics P. Qualitative and quantitative analysis of PDE-5 inhibitors in counterfeit medicines and dietary supplements by HPLC-UV using sildenafil as a sole reference. Journal of Pharmaceutical and Biomedical Analysis. 2014;98:327–333. doi: 10.1016/j.jpba.2014.06.010. doi:10.1016/j.jpba.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Finkelstein A, Taubman S, Wright B, Bernstein M, Gruber J, Newhouse JP, Baicker K. The Oregon Health Insurance Experiment: Evidence from the First Year. Quarterly Journal of Economics. 2012;127:1057–1106. doi: 10.1093/qje/qjs020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P, Muennig P, Gold M. Is expanding Medicare coverage cost-effective? BMC Health Services Research. 2005;5, doi: 10.1186/1472-6963-5-23. Article 23. doi:10.1186/1472-6963-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MR, Ryan P, Baker JS, Davies B, Thomas NE, Cooper SM, Kicman AT. Counterfeiting in performance- and image-enhancing drugs. Drug Testing and Analysis. 2009;1:135–142. doi: 10.1002/dta.30. doi:10.1002/dta.30. [DOI] [PubMed] [Google Scholar]
- Greenland S. Absence of confounding does not correspond to collapsibility of the rate ratio or rate difference. Epidemiology. 1996;7:498–501. [PubMed] [Google Scholar]
- Grossman M. On the concept of health capital and the demand for health. Journal of Political Economy. 1972;80:223–255. [Google Scholar]
- Gunasekara FI, Carter K, Blakely T. Glossary for econometrics and epidemiology. Journal of Epidemiology & Community Health. 2008;62:858–861. doi: 10.1136/jech.2008.077461. doi:10.1136/jech.2008.077461. [DOI] [PubMed] [Google Scholar]
- Hall AG, Schumacher JR, Cannell MB, Berry JB, Schiaffino M, Park S. Tobacco use in Florida: Comparisons between adults living with and without disabilities. Disability and Health Journal. 2013;6:213–219. doi: 10.1016/j.dhjo.2013.01.008. doi:10.1016/j.dhjo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Hartman M, Martin AB, Benson J, Catlin A. National health spending in 2011: Overall growth remains low, but some payers and services show signs of acceleration. Health Affairs (Millwood) 2013;32:87–99. doi: 10.1377/hlthaff.2012.1206. doi:10.1377/hlthaff.2012.1206. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. American Journal of Epidemiology. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Robins JM. Causal inference. CRC Press; Boca Raton, FL: 2015. [Google Scholar]
- Holt MA. International prescription drug cost containment strategies and suggestions for reform in the United States. Boston College International and Comparative Law Review. 2003;26:325–354. [Importation of prescription drugs: Hearing before the Committee on Health, Education, Labor, and Pensions, United States Senate, 108th Cong., 2nd session, on examining prescription drug reimportation, focusing on efforts to reduce drug costs, patient safety concerns, recent state action, fraudulent and counterfeit drugs, an international comparison of rising prescription drug expenditures, and S. 2328, to amend the Federal Food, Drug, and Cosmetic Act with respect to the importation of prescription drugs (2004).] [Google Scholar]
- IMS Institute for Healthcare Informatics The use of medicines in the United States: Review of 2010. 2011 Retrieved from https://www.imshealth.com/files/web/IMSH%20Institute/Reports/The%20Use%20of%20Medicines%20in%20the%20United%20States%202010/Use_of_Meds_in_the_U.S._Review_of_2010.pdf.
- IMS Institute for Healthcare Informatics The use of medicines in the United States: Review of 2011. 2012 Retrieved from http://www.imshealth.com/files/web/IMSH%20Institute/Reports/The%20Use%20of%20Medicines%20in%20the%20United%20States%202011/IHII_Medicines_in_U.S_Report_2011.pdf.
- Kaiser Family Foundation Distribution of nonelderly uninsured adults by gender, United States. State health facts. 2013 Retrieved from http://kff.org/uninsured/state-indicator/distribution-by-gender-2/
- Kanavos P, Ferrario A, Vandoros S, Anderson GF. Higher US branded drug prices and spending compared to other countries may stem partly from quick uptake of new drugs. Health Affairs (Millwood) 2013;32:753–761. doi: 10.1377/hlthaff.2012.0920. doi:10.1377/hlthaff.2012.0920. [DOI] [PubMed] [Google Scholar]
- Kesselheim AS, Choudhry NK. The international pharmaceutical market as a source of low-cost prescription drugs for U.S. patients. Annals of Internal Medicine. 2008;148:614–619. doi: 10.7326/0003-4819-148-8-200804150-00006. [DOI] [PubMed] [Google Scholar]
- Korn EL, Graubard BI. Analysis of health surveys. Wiley; New York, NY: 1999. [Google Scholar]
- Ku L, Broaddus M. Public and private health insurance: Stacking up the costs. Health Affairs (Millwood) 2008;27:w318–327. doi: 10.1377/hlthaff.27.4.w318. doi:10.1377/hlthaff.27.4.w318. [DOI] [PubMed] [Google Scholar]
- Liang BA. Fade to black: Importation and counterfeit drugs. American Journal of Law & Medicine. 2006;32:279–323. doi: 10.1177/009885880603200207. [DOI] [PubMed] [Google Scholar]
- Mackey TK, Liang BA. Improving global health governance to combat counterfeit medicines: A proposal for a UNODC-WHO-Interpol trilateral mechanism. BMC Medicine. 2013;11 doi: 10.1186/1741-7015-11-233. article 233. doi:10.1186/1741-7015-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. New drug for hepatitis C contributes to 13% rise in spending on prescription drugs in US. BMJ. 2015;350:h2055. doi: 10.1136/bmj.h2055. doi:10.1136/bmj.h2055. [DOI] [PubMed] [Google Scholar]
- Miettinen OS, Cook EF. Confounding: Essence and detection. American Journal of Epidemiology. 1981;114:593–603. doi: 10.1093/oxfordjournals.aje.a113225. [DOI] [PubMed] [Google Scholar]
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Commonwealth Fund Issue Brief. 2010;89:1–12. [PubMed] [Google Scholar]
- Muennig P, Franks P, Gold M. The cost effectiveness of health insurance. American Journal of Preventive Medicine. 2005;28:59–64. doi: 10.1016/j.amepre.2004.09.005. doi:10.1016/j.amepre.2004.09.005. [DOI] [PubMed] [Google Scholar]
- National Association of Boards of Pharmacy Buying medicine online: What are sites listed as not recommended doing wrong? 2015a Retrieved from http://www.nabp.net/programs/consumer-protection/buying-medicine-online/
- National Association of Boards of Pharmacy . Internet Drug Outlet Identification Program: July Progress Report for State and Federal Regulators. Author; Mount Prospect, IL: 2015b. [Google Scholar]
- National Center for Health Statistics . Survey description, National Health Interview Survey, 2011. Author; Hyattsville, MD: 2012. [Google Scholar]
- National Center for Health Statistics . Survey description, National Health Interview Survey, 2012. Author; Hyattsville, MD: 2013. [Google Scholar]
- National Center for Health Statistics . Survey description, National Health Interview Survey, 2013. Author; Hyattsville, MD: 2014. [Google Scholar]
- Norton EC, Carroll NW, Miller MM, Coyne K, Wang JJ, Kleinman LC. Computing risk ratios from data with complex survey design. Health Services and Outcomes Research Methodology. 2014;14:3–14. doi:10.1007/s10742-014-0114-0. [Google Scholar]
- Palumbo FB, Mullins CD, Slagle AF, Rizer J. Policy implications of drug importation. Clinical Therapeutics. 2007;29:2758–2767. doi: 10.1016/j.clinthera.2007.12.029. doi:10.1016/j.clinthera.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Pawlowski B, Atwal R, Dunbar RIM. Sex differences in everyday risk-taking behavior in humans. Evolutionary Psychology. 2008;6:29–42. [Google Scholar]
- Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Roebuck MC, Liberman JN. Impact of pharmacy benefit design on prescription drug utilization: A fixed effects analysis of plan sponsor data. Health Services Research. 2009;44:988–1009. doi: 10.1111/j.1475-6773.2008.00943.x. doi:10.1111/j.1475-6773.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Wolters Kluwer Health; Philadelphia, PA: 2008. [Google Scholar]
- Rudolf PM, Bernstein IB. Counterfeit drugs. New England Journal of Medicine. 2004;350:1384–1386. doi: 10.1056/NEJMp038231. doi:10.1056/NEJMp038231. [DOI] [PubMed] [Google Scholar]
- Sandman D, Simantov E, An C. Out of touch: American men and the health care system. Commonwealth Fund Men’s and Women’s Health Survey Findings. 2000 Mar; Retrieved from http://www.commonwealthfund.org/~/media/files/publications/fund-report/2000/mar/out-of-touch-american-men-and-the-health-care-system/sandman_outoftouch_374-pdf.pdf.
- Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14:680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. doi:10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- Sheils JF, Haught R. Without the individual mandate, the Affordable Care Act would still cover 23 million; premiums would rise less than predicted. Health Affairs (Millwood) 2011;30:2177–2185. doi: 10.1377/hlthaff.2011.0708. doi:10.1377/hlthaff.2011.0708. [DOI] [PubMed] [Google Scholar]
- Sommers BD, Oellerich D. The poverty-reducing effect of Medicaid. Journal of Health Economics. 2013;32:816–832. doi: 10.1016/j.jhealeco.2013.06.005. doi:10.1016/j.jhealeco.2013.06.005. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau Poverty thresholds for 2011 by size of family and number of related children under 18 years. 2011 Retrieved from https://www.census.gov/hhes/www/poverty/data/threshld/
- U.S. Food and Drug Administration Counterfeit drugs questions and answers. 2011 Retrieved from http://www.fda.gov/Drugs/DrugSafety/ucm169898.htm.
- U.S. Food and Drug Administration BeSafeRx: Know your online pharmacy. For health professionals. 2013 Retrieved from http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/BuyingMedicinesOvertheInternet/BeSafeRxKnowYourOnlinePharmacy/ucm292982.htm.
- Woo J, Wolfgang S, Batista H. The effect of globalization of drug manufacturing, production, and sourcing and challenges for American drug safety. Clinical Pharmacology & Therapeutics. 2008;83:494–497. doi: 10.1038/sj.clpt.6100493. doi:10.1038/sj.clpt.6100493. [DOI] [PubMed] [Google Scholar]
- Zullo AR, Dore DD, Galárraga O. Development and validation of an index to predict personal prescription drug importation by United States adults. Journal of Pharmaceutical Health Services Research. 2015;6:33–41. doi: 10.1111/jphs.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]