Abstract
Stimulated largely by the availability of new technology, biomedical research at the molecular-level and chemical-based control approaches arguably dominate the field of infectious diseases. Along with this, the proximate view of disease etiology predominates to the exclusion of the ultimate, evolutionary biology-based, causation perspective. Yet, historically and up to today, research in evolutionary biology has provided much of the foundation for understanding the mechanisms underlying disease transmission dynamics, virulence, and the design of effective integrated control strategies.
Here we review the state of knowledge regarding the biology of Asian liver Fluke-host relationship, parasitology, phylodynamics, drug-based interventions and liver Fluke-related cancer etiology from an evolutionary biology perspective. We consider how evolutionary principles, mechanisms and research methods could help refine our understanding of clinical disease associated with infection by Liver Flukes as well as their transmission dynamics.
We identify a series of questions for an evolutionary biology research agenda for the liver Fluke that should contribute to an increased understanding of liver Fluke-associated diseases.
Finally, we describe an integrative evolutionary medicine approach to liver Fluke prevention and control highlighting the need to better contextualize interventions within a broader human health and sustainable development framework.
Keywords: Opisthorchis, Epidemiology, Transmission, Infectious Disease, Host-Parasite, Evolution
1. Introduction
Pointing out that specialization and reductionism in scientific research represents a major challenge to infectious disease control, Restif (2009) argued that integrative approaches are needed in which evolutionary biology has a central role. This involves the ‘evolutionary epidemiology’ perspective first advocated by Ewald (1988) that integrates public health, ecology and evolutionary biology. The perspectives offered by evolutionary biology and evolutionary epidemiology have increasingly permeated microparasite research (Levin, 1996; Read et al., 1999; Galvani, 2003). For instance, significant insights have been gained in our understanding of influenza antigenic reassortments and geographic strain distribution (Grenfell, 2004; Koelle et al., 2006), bacterial resistance to antibiotics (Palumbi, 2001), and vaccine strategies in relation to virulence evolution (Gandon et al., 2001). Aside from applications to malaria (Michalakis and Renaud, 2009) and schistosomiasis (Webster et al., 2008), the integration of evolutionary principles to parasitic diseases in general remains relatively limited (but see Schmid-Hempel, 2011).
Liver Flukes are food borne trematode parasites with which, as with helminths in general, humans are believed to share a deep co-evolutionary history (Brinkworth and Pechenkina, 2013). Food borne trematodes have been the subject of increasing attention as a result of their association with persistent neglected tropical diseases whose public health impacts are suggested to have been vastly underestimated (Keiser and Utzinger 2009). Prominent among these are the Asian Liver Flukes that have been the focus of increasing research and disease control efforts for several decades in Southeast Asia (Sripa et al., 2015). In spite of these efforts a number of key aspects of their biology remain obscure and they reportedly continue to contribute significantly to the disease burden in the region (Sripa et al., 2015).
The need to fill research gaps in host-Fluke biology and develop integrated interventions has recently been described (Lustigman et al., 2012; Sripa et al., 2015). Related and perhaps more fundamentally important is a need for evolutionary thinking in framing research questions and interpretation of existing research results as well as public health interventions (Nesse and Stearns, 2008). In this paper we review research and intervention campaigns focused on the liver Flukes in Southeast Asia that have been largely uninformed by evolutionary biology. We also identify new research questions and describe alternative explanations based on evolutionary thinking to what is generally found in the biomedical literature about the role of liver Fluke in disease. We also report how with an evolutionary framework these explanations offer new insights with regard to research and public health intervention priorities.
2. Medical and Public Health Significance
Chlornorchis sinensis (Cs), Opisthorchis viverini (Ov) and Opisthorchis felineus (Of) are important food–borne trematodes that have been argued as having a high impact on human health and a wide distribution, covering most of Eastern Europe and Eastern and Southeast Asia (Keiser and Utzinger 2009; Petney et al. 2013; Sithithaworn et al. 2014). Approximately 700 million people are thought to be at risk of infection by these liver Flukes through the consumption of raw or partially cooked freshwater cyprinid fish (Keiser and Utzinger, 2005; 2009). An estimated 35 million people are infected with Cs, while 600 million people may be at risk of infection (Lun et al., 2005). In China, where clonorchiasis is considered to be one of the fastest growing food-borne parasitic infections, Cs infections have been reported in 27 of the 34 provinces (Li et al., 2010). In the case of Of, an estimated 1.6 million people are infected while 12.5 million people are thought to be at risk in Russia, Ukraine, Kazakstan and Siberia (Keiser and Utzinger, 2009; Petney et al., 2013). In Southeast Asia, more than 90 million people are at risk of infection, and at least 10 million people are estimated to be infected with Ov (Andrews et al., 2008; Sripa et al., 2010; Sithithaworn et al., 2012a). In Lao PDR, high endemicity and prevalence rates to up to 90% in certain communities have been observed (Rim et al., 2003; Sayasone et al., 2009; Ayé Soukhathammavong et al., 2015). Reliable national data from Cambodia and southern Vietnam are lacking but local prevalence estimates can be high in each of these countries (Sithithaworn et al. 2012a). In Thailand, for which reasonably reliable data are available, in spite of decades of campaigns targeting uncooked fish consumption, at least 8 million people are estimated to be infected, most of whom live in the northern and northeastern regions of the country (Sithithaworn et al., 2012a; Sripa et al., 2011; 2015; Table 1 and 2).
Table 1.
Historical O. viverrini prevalence in the four geographical regions of Thailand. The reported prevalences are based on Sithitaworn et al. 2012a and Jongsuksuntigul and Imsomboon, 2003 reviews of Thai National Surveys.
| Region | Prevalence (%) | |||
|---|---|---|---|---|
| 1981 | 1991 | 2001 | 2009 | |
| North | 5.6 | 22.9 | 29.7 | 10 |
| North East | 34.6 | 24.1 | 15.7 | 21.3 |
| Central | 6.3 | 7.3 | 3.8 | 1.3 |
| South | 0.11 | 0.3 | 0.1 | 0.1 |
|
| ||||
| Overall | 11.6 | 13.6 | 12.3 | 8.2 |
Table 2.
Provincial O. viverrini prevalence in the Northeastern region of Thailand. The reported prevalences are from the 2009 Thai national survey reviewed in Sithitaworn et al. 2012a.
| Province | Prevalence (%) |
|---|---|
| AmnatCharoen | 32.6 |
| Buriram | 20 |
| Chayaphum | 17.6 |
| Kalasin | 27.4 |
| Khon Kaen | 14.2 |
| Loei | 5.2 |
| Mahasarakam | 11.6 |
| Mukdahan | 29.5 |
| Nakhon Phanom | 60.8 |
| Nakhon Ratchasima | 4.6 |
| Nongbua Lampoo | 24.7 |
| NongKhai | 8.8 |
| Roiet | 16.8 |
| SakolNakhon | 20 |
| Sisaket | 38.6 |
| Surin | 16.1 |
| Ubon Ratchathani | 20.2 |
| Udon Thani | 13.8 |
| Yasothorn | 22.5 |
|
| |
| Overall | 21.3 |
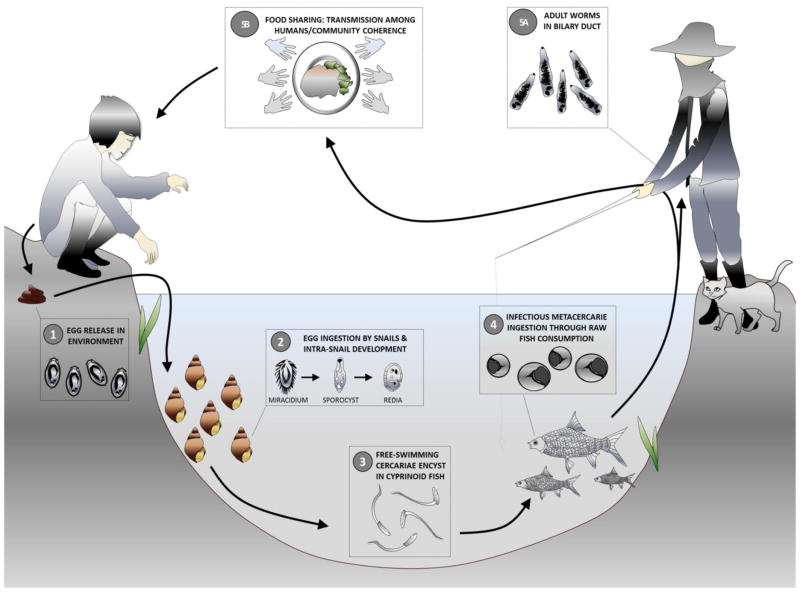
All three liver Fluke species exhibit two free living parasitic stages and require three host species, a freshwater snail, a fish and a mammal (e.g. humans) to complete their life cycle (Fig. 1). Considerable detailed knowledge describing Ov and Cs, and to a lesser extent Of, pathology and infection prevalence in humans has been aquired over several decades of investigation and reviewed (Sripa, 2003; Sripa et al., 2010; Sithithaworn et al., 2012a; Petney et al., 2013). While the vast majority of liver Fluke infections are light and often asymptomatic, it is reported that chronically infected individuals are more likely to develop advanced hepatobiliary conditions (Haswell-Elkins et al. 1991; Sithithaworn et al. 1991; Mairiang and Mairiang 2003; reviewed in Sithithaworn et al. 2014). Especially when the worm burden is high, liver Fluke infection can induce serious hepatobiliary pathology such as fibrosis, hepatomegaly, cholangitis and gallstone formation. In combination with other risk factors (reviewed in Sithithaworn et al. 2014), opisthorchiasis can lead to cholangiocarcinoma a malignant tumor of the biliary tract associated with a poor prognosis upon diagnosis and high mortality rates (Smout et al. 2011; Sripa et al. 2012; Sithithaworn et al. 2014). Cholangiocarcinoma incidence in Northeast Thailand is the world’s highest, ranging from 93.8 to 317.6/100,000 (Sithithaworn et al. 2014). Although there is strong evidence indicating that Ov infection is a primary cause of CCA carcinogenesis (IARC, 1994, 2011, Miwa et al. 2014, Sithithaworn et al. 2014) other factors, including dietary, life style and genetic components (Miwa et al. 2014, Sithithaworn et al. 2014), are also likely to play a role calling for more integrative investigations (Ziegler et al. 2016).
Fig. 1. O. viverrini transmission cycle.
Human infection occurs when cyprinid fish bearing metacercariae in their tissues and organs are consumed raw or partially cooked (including smoked, pickled and salted ; Grundy-Warr et al., 2012). Metacercariae in the fish excyst in the duodenum and enter the bile ducts, where they mature sexually. The adult worms produce eggs which are passed out in faeces to the environment. When freshwater Bithynia snails ingest the eggs, miracidium hatch and develop into sporocysts which undergo asexual multiplication, and develop into rediae and cercariae. Upon release in the environment, free-swimming cercariae actively search for a fish host, most often from the cyprinidae family, to penetrate the tissues and skin and develop into metacercariae infective to humans and other fish-eating mammals (Kaewkes, 2003; Wykoff et al., 1965).
3. Host-Parasite Evolutionary Biology and its Clinical and Implications
In contrast with micro-parasites and characteristic of helminths in general, adult liver Flukes do not multiply in their human host and are long-lived organisms, with individuals presumably surviving more than ten years in their host (Harinasuta and Harinasuta, 1984; Kaewpitoon et al., 2008). Therefore, liver Fluke infections tend to be of a persistent nature. This implies an evolutionary strategy which includes adult worms’ contribution to the capacity of their host’s sustained provision of the resources needed by the worms. The selection pressures associated with these long-lasting intimate interactions and dictated by the complex nature of helminths’ life cycles and transmission mode, generate co-evolutionary trajectories that are different from microparasite-hosts interactions. This has important consequences for virulence evolution, thus disease manifestation, as well as transmission dynamics. Their understanding, and especially the lack of appreciation of the implications to disease control, has long been problematic in the case of helminthes in general (Warren, 1981).
3.1. Infection along a cost-benefit continuum
Asian liver Flukes have fully zoonotic cycles (with no human involvement) in addition to that with humans as final hosts. This implies that any co-evolutionary patterns will necessarily reflect adaptation to multiple host species, resulting in less host specificity (see section 5 for more details). In the case of Ov, the situation is more complex as there is good evidence that this is a species complex with limited gene flow between certain watersheds, which is suspected to be associated with potential local adaptation and co-evolution, particularly with its snail hosts (Andrews et al., 2008; Saijuntha et al., 2007; Sithithaworn et al., 2012b) and possibly with its human host (Andrews et al. 2008). For instance, evolution of « disease tolerance » by the host in the presence of these parasites as a consequence of the close relationship between humans and helminths, over history is widely documented (Little et al., 2010; Allen and Wynn, 2011; Medzhitov et al., 2012). The immuno-modulatory capacity of the worms result in most infections being asymptomatic (McSorley and Maizels, 2012) and the tempering of responses to non-helminth antigens, like allergens and self-antigens, leading to a reduced incidence of allergies and autoimmune diseases in chronically infected individuals (Yazdanbakhsh et al., 2001). These observations have been argued to warrant a refined interpretation of the nature of the interaction between Flukes and humans. This may range from parasitic to mutualistic, the latter particularly in endemic areas where people have been persistently exposed to helminths and other infectious agents. An evolutionary perspective (i.e. dynamic view of host-parasite interactions cf. Renaud and de Meeüs, 1991; Horwitz and Wilcox, 2005; Fellous and Salvaudon, 2009) and a precise understanding of the immune regulatory mechanisms underlying helminth infection is key to better identify the thresholds beyond which helminth infections are prejudiciable or beneficial and better assess how the local context modulate these thresholds (Fig. 2). Host’s helminth tolerance mechanisms limit disease severity by preventing tissue damage or ameliorating tissue function without interfering with parasite load (Soares et al., 2014). A key component of the immune system that has evolved to foster host tolerance and minimize the virulence of helminths is a more pronounced “non-harmful” type 2 (or Th2) response (Medzhitov et al., 2012; Mishra et al., 2014). The type 2 response likely evolved both to provide resistance by limiting the number of helminths that can live in our intestinal tract (Artis and Grencis, 2008; Maizels et al., 2012) and to repair the tissue damage that is caused by the helminths that have colonized our tissues (Allen and Wynn, 2011), while inhibiting a more inflammatory and tissue damaging Th1 pathway (Bashyam, 2007; Ludwig-Portugall and Layland, 2012). The Th2 response is characterized by the production of cytokines such as interleukin-4 (IL-4), IL-5, IL-9, and IL-13 as well as the increased secretion of Immunoglobulin E (IgE) and IL 10 by regulatory B cells (Hussaarts et al., 2011). Similarly, Treg cells generally also expand during helminths infections which promote the survival of the parasites, limit protective immunity upon reinfection (Maizels et al., 2012) as well as inadvertantly limit autoimune diseases (Taylor et al., 2012) through, among other factors, TGF-b-like and IL 10 increased product expression (Wilson and Maizels 2004; Ben-Ami Shor et al. 2013 for a review). For instance, helminth infection and the Th2 based-immunomodulation that it induces has been associated with improvement of inflammatory bowel disease, multiple sclerosis, type 1 diabetes, and autoimmune liver disease (reviewed in Ben-Ami Shor et al. 2013). Increasing evidence also suggests that the intensity of metabolic syndrome (MetS), a disorder of energy utilization and storage leading to hypertension, abdominal obesity and altered glucose metabolism (related to Type 2 diabetes and cardiovascular diseases), is minimized in case of reduced inflammation /helminths infection (Wiria et al., 2012; 2014).
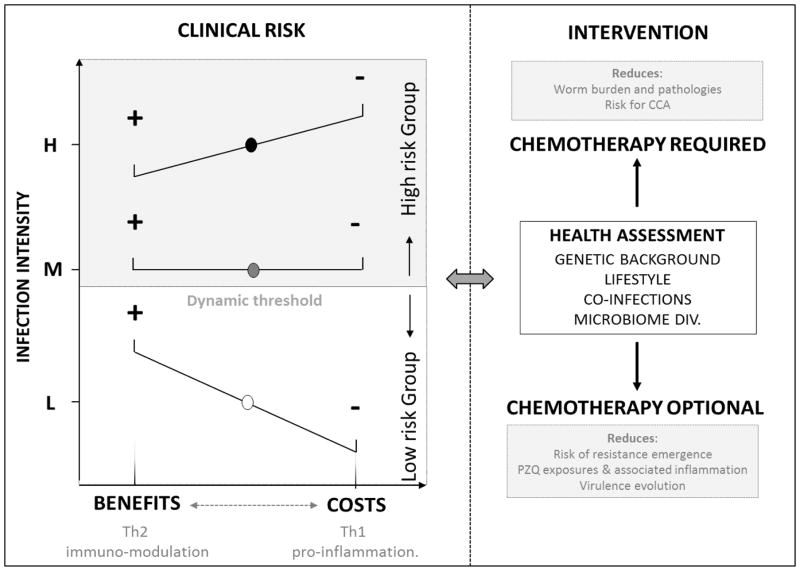
Fig. 2. A dynamic view of O. viverrini infection and associated clinical risks.
A) Liver fuke infection may vary from beneficial to costly depending on the infection intensity which for sake of simplicity in the figure we categorize as Low (L), Moderate (M) or High (H). The infection intensity will influence the relative dominance of anti-inflammatory and beneficial TH2 responses vs pro-infammatory harmful/costly TH1 responses. In case of chronic immuno-modulation by the Fluke and associated reduction of inflammation, the benefits can be a reduced risk of autoimmune and allergic diseases as well as metabolic disorders including obesity, cardiovascular and type 2 diabetes (in the figure lowest panel (L) the benefits (+) outweight the costs (−)). In case of chronic inflammation when Th1 response dominates and impart costs to the host (i.e. in the upper panel of the figure, costs (−) are higher than benefits (+)). Costs of infection range from anemia, hepathobiliary abnormalities to more severe pathologies such as fibrosis, cholangitis, hepatoegaly and in combination with other risk factors such as alcohol, Cholangiocarcinoma. The thresholds (the line represented in the middle of the figure) - beyond which infection intensity triggered hyper-inflammation and where the costs outwheight the benefits - are dynamic and change in relation to the host genetic background, diet and lifestyle as well as microbiome. B) Health assessments incoprorating knowledge about infection intensity, genetic predisposition to inflammation as well as lifestyle is necessary to refine interventions and avoid disrupting possible beneficial settings. When costs of infection are found to outwheight potential benefits, intervention, likely drug administration (chemotherapy) is warranted. When the benefits are thought to outwheight the costs (in case of low intensity infection) intervention and drug administration may be nuanced as possibly administrering drug in this case can lead to unwanted clinical conditions that are more detrimental than having a low worm burden. This « trade-off » rationale should help design epidemiological investigation where risk groups (risk of developping severe hepathobiliary conditions) are defined based on infection intensity and if possible other biological and socio-economic attributes as advocated by the WHO (Montresor et al. 1998).
In the case of Asian liver Flukes, information is limited on the timing and extent of their immuno-modulatory capacity in human hosts. Most studies have focused on identifying immune-markers in individuals with advanced clinical conditions and declared hyper-inflammation resulting from immune environments skewed towards pro-inflammatory pathways (e.g IL6; Sripa, 2003; Sripa et al., 2009). However, evidence of immuno-suppression has been provided in animal models where chronic Ov infection was associated with a predominance of Th2 responses and high level of TGF-β and IL10 which was believed to inhibit the immune functions and allowed the parasites to evade the host immune response (Wongratanacheewin et al., 1987; Sripa and Kaewkes, 2000; Jittimanee et al., 2012). The immuno-modulatory capacity of the liver Flukes and their potential to down regulate overall inflammation is also indirectly supported by results from recent human cohort studies. For instance, chronic Of infection in Siberia has been shown to be associated with lower serum total cholesterol and significant attenuation of atherosclerosis (Magen et al., 2005; 2013) a condition typically associated with Th1 polarized immune response (Castrillo and Tontonoz, 2004). In the case of Ov in Southeast Asia data suggests increased cholesterol levels and fatty livers in patients following deworming (from 10% to 15% of fatty livers; Mairiang et al., 2012, Sripa unpublished data) which corroborate the observation that liver Flukes cannot produce cholesterol by themselves and need to obtain it from their host (Young et al., 2014). Similarly, preliminary observations of nation-wide cardiovascular diseases (CVD) distribution in Thailand indicate a negative relationship with liver Fluke infection, corroborating the observation of cholesterol protection afforded by liver Fluke infection as hypercholesterolemia is known to be strongly associated with CVD (Magen et al. 2013). Regarding Type 2 diabetes, while low levels of cholesterol and fatty livers usually predicts low occurrence of type 2 diabetes (Sung and Kim, 2011), nation-wide diabetes distribution mapping in Thailand intriguingly matches liver Fluke (and low CVD prevalence) distribution and suggest a positive relationship that warrants further investigations.
3.2. Context-dependent outcomes
Several factors, including Fluke infection intensity, host genetic polymorphisms and diet may influence the immuno-modulatory capacity of liver Flukes, its timing and the balance between indirect benefits (i.e. reduced inflammation) and direct costs (i.e. hyper-inflammation and pathology). For instance, serious hepatobiliary diseases associated with liver Fluke infection have only been observed in cases of heavy infections, while the intensity of the majority (>80%) of infections lies far below (Sithithaworn et al. 1991; Mairiang and Mairiang 2003; Sripa et al. 2011; Sithithaworn et al. 2014). Asymptomatic hepatobiliary abnormalities may be present in apparently healthy infected subjects with low worm burden (Elkins et al., 1996) but the high proportion of asymptomatic carriers still suggests that the immuno-modulatory mechanisms allowing persistent Fluke infestation and low pathology are in place in most individuals with low infection intensity in endemic communities (McSorley and Maizels, 2012). This observation indicates that Fluke intensity of infection may be a key factor contributing to the cross-regulation of Th1 vs Th2 pathways in infected individuals and that in cases of low infection (more efficient immune-modulation) the indirect benefits of being infected (i.e. reduced risk of MetS and allergic diseases and an overall more balanced metabolism) may outweigh the costs of being infected as long as the worm burden remains low (Fig. 2). When the worm burden increases and a more inflammatory immune environment sets in, possible mechanisms influencing the switch from a dominant Th2 response to a dominant Th1 response may include parasite overcrowding and/or increased strain diversity, that lead to more sustained intra-host competition (Frank, 1996; Read and Taylor, 2001). Intra-host competition is known to involve induction of increased parasite feeding activity or more aggressive behaviors. Although more damaging to the host this form of phenotypic plasticity has an obvious fitness advantage when parasite density becomes hight enough (Antia et al., 1994; May and Nowak, 1994; Davies et al., 2002). This is likely to be associated with a higher parasite death rate and possibly increased detectability of parasite antigens (that were otherwise hidden under the worm’s tegument). This would in turn elicit more inflammation through Th1 pro-inflammatory networks and increased pathologies (Woolhouse and Hagan, 1999).
The infection intensity thresholds beyond which hyper-inflammatory rather than anti-inflammatory environments are observed (Fig. 2) are known to be strongly affected by host’s genetic background and involve polymorphic loci presumably maintained in part by selection pressure on humans populations exerted by the Flukes in endemic areas (Fumagalli et al., 2009). For instance, the 48892 A/C (Asp358Ala) of exon 9 of the interleukin 6 receptor (IL-6R) gene is polymorphic, with one of the two known alleles associated with higher risk of hepathobiliary complication associated with Ov (Prayong et al., 2014). This suggests the possibility of balanced polymorphisms much like sickle cell gene in regions with endemic malaria. Similarly but more indirectly, genetic factors along with host diet may dictate the microbial community structure in hosts which are increasingly recognized as playing an important role in establishing a balanced immune response (Cho and Blaser, 2012).
3.3. Research gaps and disease control implications
A view of human-Fluke interactions informed by evolutionary biology emphasizes the dynamic and context-dependent nature of liver Fluke infection outcomes ranging along a cost-benefit continuum. This is in stark contrast with the more common, simplistic view held in tropical medicine that equates disease with infection. The reality, particularly in the case of liver Flukes, is the relationship between infection and disease is highly complex. There is a need to clarify the mechanisms underlying pro-inflammatory/anti-inflammatory cross-regulation (Nair et al., 2011) and the potentially protective effects of liver Fluke infection against MetS, allergic and auto-immune diseases in hosts living in areas where Flukes are highly endemic and where co-evolution is suspected to have occurred. At the cross-roads of this research, the mediating role of infection intensity needs particular attention and should be used as an additional parameter to characterize at-risk populations. The consideration of infection intensity rather than infection prevalence in epidemiological investigations and disease risk mapping as well as in public health intervention appears to be the most critical practical application emerging from the cost-benefit framework we described. This view echoes Anderson and May and others (Anderson and May, 1982; 1991; Montresor et al., 1998; Andrews et al., 2008) who demonstrate that targeting intensely infected individuals is more effective from a transmission dynamics and disesase prevention standpoints. Additionally, considering that liver Fluke infection may be beneficial depending on infection intensity and an individual’s genetic background raises questions about the broader public health implications of mass drug administration. This connection is particularly relevant in the current context of the epidemiological transition these rural communities currently are undergoing (Rigg, 2006) and the increasing incidence of MetS associated with livelihood and diet shifts (Wiria et al., 2012). Defining risk groups based on infection intensity and genetic polymorphism (i.e. proneness to inflammation) and adjusting drug administration strategies accordingly could be part of evidence based, integrated intervention protocols that are more likely to prove sustainable. Similarly revised intervention protocols with adjusted drug administration may reduce the risk for drug resistance emergence (Read et al., 2011) and may limit repeated use of praziquantel thought to be associated with elevated risk of cancer after the first administration (see section 6).
4. Parasite life history adaptations and consequences on transmission dynamics
Life history traits (longevity, replication mode, timing of reproduction, etc.) are particularly critical components of fitness. Even in the absence of comprehensive data, partial information on a species life history characteristics considered in light of the theory of life history evolution (e.g. Strearns 1992; Roff 1993) can provide important insights. For example, liver Fluke’s iteroparity along with extremely large number of eggs produced in an individual worm’s lifetime indicates the enormous odds against an individual egg or offspring in surviving to complete the reproductive cycle. This has clear implications to the possibilities for interrupting the transmission cycle as part of a disease control strategy.
Understanding of the schedule of liver Fluke egg production and release in humans and alternate hosts is one stage of the Ov life cycle that has particularly significant implications for transmission dynamics and thus disease control. In many complex life cycle parasites that produce eggs in their definitive hosts, the timing of egg production is adjusted to match the chances of encounter with the down-stream host (Poulin, 2011). Given the highly seasonal environment in which liver Flukes are generally found it follows there is strong selection pressure to allocate resources to egg production in periods corresponding to the availability of Bithynia snails, the first intermediate host. Highest snail abundance is usually observed during the rainy season from July to October in northeastern Thailand (Kim et al. pers. comm.), although dams and associated irrigation systems undoubtedly have altered the natural cycles of water availability.
The capacity of parasite eggs to remain viable in the environment for extended periods of time is another adaptation for coping with the uncertainty of down-stream host availability and therefore a strategy to maximize transmission success in variable environments (Poulin, 2011). While extensive data have been collected for Ascaris and other nematodes (Smith, 1999), relatively few studies have examined liver Fluke egg survival in the environment. A series of field investigation from Russia from the 1980’s found Of eggs are able to survive several months in water and soils (Krivenko, 1979; Krivenko et al., 1981). Ongoing work by the authors of the present article suggests similar survival trends for Ov, but infectiousness, measured as a combination of egg hatching rate and infection success in snails, appear to be limited to only two to three weeks in moderately warm conditions. This suggests that high risk of transmission to snail hosts may be restricted to certain periods of the year mostly dictated by seasonality (Echaubard et al. pers. comm.). Robust manipulative field studies in different environmental settings are needed to confirm and generalize the trends observed in the laboratory.
In digeneans, such as liver Flukes, the life cycle also includes the release of cercariae from the mollusk host and the subsequent infection in cyprinid fish. Parasite genotypes that allow a good match between the timing of cercarial release and the presence as well as susceptibility of the target host should be favored. In the case of liver Fluke both the size of the snails and environmental factors are known to influence cercarial emergence patterns (Kaewkes et al., 2012; Kiatsopit et al., 2014). In a recent study, cercarial output was highest during the hot-dry season and during day time suggesting that peak cercarial release may be driven by temperature and light intensity (Kiatsopit et al., 2014). While the adaptive significance of diurnal emergence, which may increase the cercariae chances to encounter fish mostly active during the day, appears plausible, the adaptive significance of peak cercariae release during the hot-dry season is more difficult to understand as fish abundance may be reduced during this drought-prone period. Contrary to this possibility, fish may be forced into greater concentrations in shallower depth as water bodies evaporate and shrink in size. Several other mechanisms, adaptive or not, may thus contribute to the patterns observed. For example, higher temperature and brighter conditions contribute to enhance snail metabolism, which may provide more energy resources to the parasites (Kiatsopit et al., 2014). In some digenean species cercarial production declines with the age of infection, a strategy assumed to maintain the host alive longer which inadvertently prolongs the period of cercarial production. This bet hedging strategy would optimize transmission success in situations where the contact rate with the next host is variable (Karvonen et al., 2004). Additionally, the occurrence of different Ov strains or cryptic species have been recently documented with discussions of potential local adaptations and concomitant virulence differences among genotypes cross-infecting sympatric/allopatric hosts (see section 5) possibly blurring the relationship between cercariae release and fish abundance.
Understanding the mechanisms responsible for liver Fluke egg production, survival in the environment as well as cercariae release, among other factors using an evolutionary rationale can help refine our comprehension of parasite transmission dynamics and identify critical temporal windows of transmission risks in the parasite’s life cycle.
5. Genetic polymorphism geographic variation and phylodynamics
5.1. Review of current knowledge and research gaps
Recent population genetic studies using multilocus enzyme electrophoresis (MEE) on Ov, and hosts Bithynia snails and cyprinid fish have demonstrated high genetic variability and population structure associated with river basins and wetland zones (Andrews et al. 2008; Sithithaworn et al. 2012b). More particularly, two major lineages of Ov, further subdivide into 6 distinct genetic groups, correlated with 5 different wetland systems, including the Chi, Mun, Songkram and Wang River wetlands in Thailand, and the Nam Ngum River wetland in Lao PDR. Subsequent DNA-based studies confirmed the occurrence of genetically divergent Ov lineages, although the association remains to be fully elucidated, especially in relation to river basin and wetland water flow connectivity as well as intermediate host populations genetics and demographic dynamics (Saijuntha et al., 2007; Laoprom et al., 2009; 2010; Thaenkham et al., 2010). The level of genetic variability within and between species and subspecies of freshwater Bithynia snails has also been examined by MEE analyses and has provided preliminary evidence of genetic variation within B. s. goniomphalos in northeast Thailand. Results suggest an association with river wetlands and possible co-evolution between Ov and snail hosts (Saijuntha et al., 2007; Kiatsopit et al., 2013). This suggests genotype by genotype-driven interaction outcomes between snails and parasite may have occured (Sithithaworn et al., 2012b).
In the case of cyprinid fish, the second intermediate host, allozyme genotyping failed to detect genetic structure variation in Ov retrieved from four cyprinid fish host species in one endemic area indicating a high rate of gene flow and relatively limited potential for local co-adaptation on the scale of a single wetland (Saijuntha et al., 2009). However without a better understanding of host fish demographic structure and wider spatial scale gene flow patterns, little can be said about host fish and Ov co-evolution. Given the diversity of cyprinid fish species in the region, differences in the intensity of infection and differences in the biology and ecology of these intermediate hosts, definitive conclusions are limited at present. A further complicating matter is the increasing use of cyprinid fish species in aquaculture (Naylor et al., 2000), which provides opportunities for host species switching and concomitant host and parasite genetic structure modification (reviewed in Sitithaworn et al. 2012b).
In the specific case of humans, evidence suggests that Asian liver Flukes are ancient human parasites. C. sinensis eggs, for instance, have been detected in the fecal remains from the Warring States Period (475–221 BC) in Hubei, China (Thaenkham et al., 2010). The invention of wet rice cultivation and its spread throughout East Asia 7,000 to 10,000 years bp (MacNeish, 1992) would have provided an extraordinary ecological and evolutionary opportunity for liver Flukes. The transmission cycle—which may have originally involved only two hosts (snails and fish) with fish-eating mammals subsequently becoming the definitive hosts (Petney et al. 2013)—evolved as human population density increased as a result of wetlands rice cultivation. This would have imposed selective pressures on the parasite to survive in this increasingly frequent human host (Parker et al., 2003; Poulin 2011). As a consequence, humans and Flukes have likely co-evolved for millennia and developed traits and mechanisms that maximize each other’s fitness, including the immune immuno-modulation mechanisms characteristic of helminthes infections described in section 3. Genetic polymorphism underlying immune response modulation processes and tolerance has been documented in northeastern Thailand and specific alleles or gene expression profiles providing advantages to infected humans are known (see section 3). Similarly, records indicate that high incidences of hepathobiliary diseases, including cholangiocarcinoma, are aggregated in certain communities within Khon Kaen Province in northeastern Thailand, where locally specific allozyme and mitochondrial DNA (mtDNA) haplotypes have been found (Vatanasapt et al., 1990; Sriamporn et al., 2004; Andrews et al., 2008).
The research initiatives and results described above have set the basis for an improved understanding of co-evolutionary and phylodynamic relationships between the Flukes and their hosts, but certain limitations remain. In particular, the taxonomy and phylodynamics of the Flukes needs to be refined including acknowledging the potential cryptic species and related possible virulence phenotypes that this could foster. For instance Opisthorchis lobatus was recently described as a distinct member of the Opisthorchis genus (Thaenkham et al., 2011) and this recent discovery confirm the suspiscion and substantial evidence that liver Flukes are not one homogeneous genetic clade (Sithithaworn et al. 2012). Additionally, although recent investigations suggest that cats and dogs may serve as alternate hosts (Aunpromma et al., 2012), very little is known about the role of wildlife as reservoir (but see Wenz-Mücke et al., 2013 for a case study on crab-eating macaques in Khon Kaen province) and possibly source of genetic differentiation.
Finally, a refined understanding of gene flow patterns across the host spectrum as well as of host-parasite demographic processes in a metapopulation context is needed (Thrall and Burdon, 2002; Gandon and Nuismer, 2009; Laine et al., 2014). Greater understanding of gene flow in association with landscape connectivity assessment at different spatial scales can help (1) better characterizing host-parasite local adaptations and geographic co-evolutionary trends, ultimately improving our understanding of virulence, and disease hot spots and (2) infer past and present host and parasite population demographics, including host population expansions and bottlenecks in relation to major landscape/environmental changes, which could in turn help identify which human activities are most likely to foster host population spread or constriction.
5.2. Local adaptations and gene flow across scales: a framework to guide future research
Recent theoretical developments can be used to guide and reinforce current research initiatives in their ability to assess gene flow and its implications. For instance the recent review by Auld and Tinsley (2014) provide a framework to understand how the complex life cycles of parasites (i.e. up to three taxonomically different hosts with markedly different ecologies and metapopulation dynamics) influence local adaptation patterns across spatial scales and through the host taxonomic range. The host taxonomic diversity generally characteristic of complex life-cycle parasites (CLPs) implies that the parasites have evolved different mechanisms and gene expression profiles to infect each host type effectively (Auld and Tinsley 2014). It also suggests non-trivial consequences in terms of gene flow, and local adaptations to hosts (Fig. 3). For instance, a recent meta-analysis of trematode species showed that parasites bound to complete their life cycle within water (i.e. with aquatic intermediate and definitive hosts) had significantly more pronounced genetic structuring than those leaving water through a bird or mammal host (Blasco-Costa and Poulin, 2013).
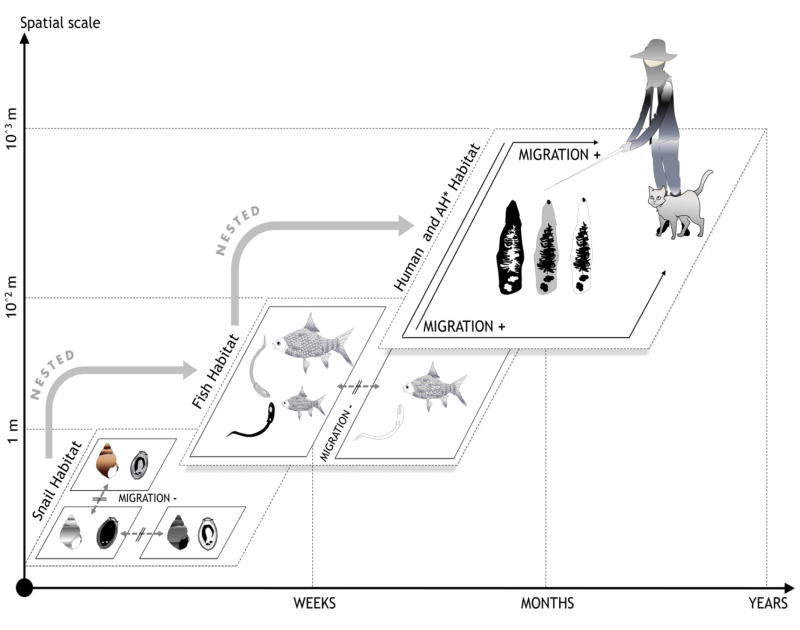
Fig. 3. The evolutionary consequences of hosts migrations for Complex Life Cycle parasites (CLP) local adaptations.
Migration rates often vary among the different hosts of a CLP. The migration rate of a CLP is expected to be most similar to that of its most mobile host—in this case the definitive host. Immigration of mobile hosts into a population can supply novel parasite genotypes, promoting parasite LA to the less-mobile host (which experience movement restriction at smaller scales. In snail populations, finer grain movement restriction may foster tighter genetic local adaptations as one snail subpopulation is likely to be exposed to a unique Ov local strain (in the figure, eggs from one particular color only encounter snails from one particular color). Fish are likely to be exposed to several Ov strains as they can navigate entire wetlands or watersheds (fish from one colour can encounter a limited number of cercariae of different colors). Human hosts are sharing fish and parasite from different wetlands, watersheds and even regions which contributes to parasite genotype mixing and fosters exposure to a greater diversity of parasite lineages hence minimizing tight local co-adaptations. *AH = Alternate Hosts.
This suggests that genetic differentiation of parasites reflects the mobility of their hosts and that parasite dispersal depends on host traits (Blasco-Costa and Poulin, 2013). In the case of liver Flukes, while freshwater snails, the first intermediate host, demonstrate relatively small-scale migration patterns, the second intermediate host, cyprinid fish, may exhibit larger movement and migration potential in particular in relation to breeding activities which entail extended migration up and downstream or laterally from rivers or lakes to floodplains (Rainboth, 1991). The definitive host, humans, at least in the case of Ov, has the potential to carry and transmit parasites over very large distances. Consequently, parasite gene flow spatial extent and significance are likely to differ from host to host, creating a situation where the parasite has a limited ability to adapt to one specific host species because patterns of allelic movement at different scales may disrupt specific directional selection at the host level (Slatkin, 1987; Lenormand, 2002; Prugnolle et al., 2005, Fig. 3). However, this prediction is only an approximation of what is actually seen in nature and there are many examples of accurate host specialization among CLPs, suggesting that genotypic specificity and local adaptation between host types and parasites and thus patterns of selection depend on a combination of mechanisms, including genetic drift and demographic events in relation to spatial scales (Lenormand, 2002; Prugnolle et al., 2005).
Beyond the emerging evidence of possible Ov and snail host local co-adaptations described earlier, host-parasite genetic specificity has been found in Schistosoma mansoni (Mitta et al., 2012; Webster and Woolhouse, 1998) and trematodes of the genus Microphallus (Dybdahl et al., 2008; Lively and Dybdahl, 2000), as well as in Plasmodium falciparum (Lambrechts et al., 2005). Following this line of thought, it appears that CLPs can show local adaptation (LA) to at least one host species (that is, achieve greater infection success in sympatric hosts than allopatric hosts), provided parasite migration rate exceeds host migration rate in the metapopulation (Gandon et al., 1996; Gandon and Michalakis, 2002). Auld and Tinsley (2014) suggested the following predictions in this regard: (1) more mobile hosts promote CLP dispersal, and thus increase the supply of novel genotypes for infection of less mobile hosts and (2) CLPs have higher likelihood of exhibiting LA to their least mobile host species. Designing research projects to test these predictions and to assess how landscape structure and its modification can impact host and Fluke movements as well as subsequent gene flow (i.e. landscape genetics; Manel et al., 2003; Manel and Holderegger, 2013) appears critically important, particularly in the current context of the extensive landscape modification in Southeast Asia, which lead to natural metapopulation dynamics disruption, livelihood changes and labor migrations.
Furthermore, complementary laboratory or mesocosm experiments that specifically address host genotype by parasite genotype interactions in variable environmental contexts (i.e. water quality, temperature, density, etc.; Wolinska and King, 2009; Echaubard et al., 2014) are needed to refine our understanding of the biological significance of the genotypic structuring documented in landscape genetics studies (i.e. are hosts better able to tolerate infection by sympatric parasites? What are the consequences for transmission?) and help better understand the geographic mosaic of host-Fluke co-adaptations (Thompson, 2005).
6. Evolutionary concepts in predicting and assessing the impact of chemical controls on liver Flukes and their hosts
There is currently no vaccine against liver Fluke infection and while the molluscicide Bayluscide has been proven efficient in the laboratory against B.s. goniomphalos (Tesana et al., 2012), its field effectiveness remains limited due to the behavior of the snail, which can live in deep layers of the muddy shore during the dry season. Consequently molluscicide has not been used for the snail first intermediate host control (Petney et al., 2012). Chemotherapy to treat human infections remains the principal tool for reducing transmission and disease. As such Praziquantel (PZQ) has been the drug of choice in the treatment of most of the food-borne trematode infections in SEA, including Ov infections (Montresor et al., 2008). Community-based treatment after active screening, through indiscriminate mass treatment, or in specific target groups is now the major control tool in Thailand and LaoPDR for Ov infections (Montresor et al., 2008; Sripa et al., 2015).
Despite the relative success of chemotherapy-based interventions in reduction of infection prevalence (Sithithaworn et al., 2012a; Sripa et al., 2015), reduction in parasite transmission may be limited and quite local. Drug-administration campaigns are voluntary and the targeted population may not be responsible for most of transmission in a community. Moreover, reinfection of those treated may not be uncommon due to the deeply entrenched habit of eating uncooked fish dishes (Grundy-Warr et al., 2012). Some individuals reportedly even take PZQ repeatedly (Saengsawang et al., 2013) as a preventive measure despite possible long-term increased risk of developing hepathobiliary conditions through increased inflammation (Pinlaor et al., 2004; 2008). Finally, legitimate concerns are growing regarding the possibility for drug resistance emergence (Webster et al., 2008; Read et al., 2011) and Fluke virulence evolution (Ewald, 1983; Paterson and Barber, 2007; Webster et al., 2008) in response to mass liver Fluke chemotherapy is a possibility.
6.1. Chemotherapy influence on human hosts inflammation
Besides the potential for increased risk of metabolic disorders, autoimmune and allergic diseases indirectly associated with deworming that we described in section 3, repeated administration of PZQ may indirectly foster a more persistent inflammatory state through an increased risk of reinfection. In spite of helminths’ immuno-modulatory capacity, the onset of infection is often accompanied by a period of acute inflammation (Th1 response dominance) potentially damaging to the host (Mishra et al., 2014). After a period of time ranging from few weeks to months, a chronic down-regulated immune environment (Th2 dominated) sets in (Mishra et al., 2014). It is noteworthy that subsequent infections are associated with faster and stronger immune response and consequently higher temporary inflammation. Repeated reinfection may thus create a hyper-inflammatory immune environment prone to pathologies. For instance, lab experiments using hamsters show that more frequent Ov infection can induce the expression of nitric oxyde both in inflammatory cells and in the epithelium of bile ducts and subsequently cause nitrosative and oxidative damage to nucleic acids, which may participate in the initiation and/or promotion steps of cholangiocarcinoma development (Pinlaor et al., 2004, 2008). The cost associated with chemotherapy-induced inflammation may be balanced if chemotherapy would provide protection against reinfection and therefore reduce infection events. This argument is currently under intense scrutiny in the community studying PZQ chemotherapy and schistosomiasis. Recent evidence suggests that repeated treatments with PZQ do not procure effective protection against possible reinfection by schistosomes as high levels of parasite-specific IL-10 were found to be a risk factor for reinfection while high levels of IL-5 were associated with hematuria development (van den Biggelaar et al., 2002 but see Bourke et al., 2013). While the risk of increasing inflammation-driven pathologies following PZQ treatment remain to be fully elucidated in humans (Kamsa-Ard et al., 2013), preliminary evidence in animal models indicates that repeated PZQ treatment may favor hyper-inflammatory environments most likely indirectly through increased reinfection risk. Further research is needed to clarify the relationship between increased PZQ administration, Ov reinfection and the cross-regulation of anti vs pro-inflammatory immune responses.
6.2. Drug resistance emergence
The state of our understanding of drug resistance is much less advanced in the case of helminths than for other macroparasites (e.g., Plasmodium spp.) and microparasites despite an increasing recognition of the problem by biomedical researchers (Read et al. 2011). The general opinion has been that helminths are less likely to develop resistance, or that doing so would occur relatively slowly compared to microparasites because of their longer generation time (Cerami and Warren, 1994). Moreover, treatment failures would often go unnoticed since most helminths infections are asymptomatic. Thus, the problem of drug resistance is not well acknowledged and the understanding of the mechanisms underpinning resistance emergence has been slow to grow in the research communities studying helminths infections (Waller, 1985). More particularly, the understanding of resistance from an evolutionary perspective where resistance emerges and establishes when the selective advantage of the drug resistant phenotype exceeds that of the wild type at the population level is not well appreciated despite its clear epidemiological significance (Antonovics et al., 2007; Webster et al., 2008).
While no studies to our knowledge have formally investigated resistance to PZQ, or reported evidence for its occurrence in the case of Ov (but see Ayé Soukhathammavong et al. 2015), resistance to PZQ in schistosomes infection treatment has been relatively thoroughly investigated. To date, there is no evidence to suggest that schistosomes have already developed and/or established resistance to PZQ under field conditions, even within China where praziquantel-based chemotherapy has reached more than 50 million people since 1992 (Doenhoff et al., 2002; Liang et al., 2011; Seto et al., 2011; Wang et al., 2010; 2012). However, clinical investigations carried out, primarily in Egypt and Senegal on S. mansoni (Ismail et al., 1999; Gryseels et al., 2001), report occasionally individual failures of PZQ in treatment of travellers with schistosomiasis (Alonso et al., 2006). Furthermore, the results from a range of laboratory studies and successful artificial selection of PZQ resistance lines as well as previous evidence of the ability of the schistosome parasites to evolve resistance to oxaminiquine which was in use prior the development of PZQ (Bonesso-Sabadini and de Souza Dias, 2002), continue to raise concerns about the existence or development of potential PZQ resistance in schistosomes in the future (Webster et al., 2008). Considering the relative epidemiological similarity between schistosomes and Ov as well as the convergent intervention mindset targeting them with high frequency and coverage of indiscriminate MDA campaigns, it follows that resistance to PZQ could become an issue in Ov endemic areas. Further research and improved monitoring of PZQ administration is needed to detect subtle changes in drug efficacy and to assess the promises of dual therapies combining praziquantel and Tribendimidine (Keiser et al., 2013) as a mean to reduce the risks for drug resistance emergence.
6.3. Evolution of parasite life histories in response to chemotherapy
Like all living organisms, parasites acquire resources from their environment, which happens to be in their case the bodies of other living organisms (i.e. their hosts), then allocate them to different functions, including development, survival and reproduction (Stearns 1992; Roff 1993; Michalakis 2009). Given that resources are most often limited, the resource allocation patterns observed in organisms (i.e. amount and timing of energy differentially allocated to the above functions) involve trade-offs (i.e. resources allocated to growth and survival will not be available for reproduction) that are constrained by physiological, involving phenotypic plasticity, or genetic (micro-evolutionary) adaptations. While a discussion of the mechanisms underlying these trade-off goes beyond the scope of this article, it is important to highlight the potential for strong selective agents, such as PZQ, to affect significantly the patterns of resource allocation underlying parasite biological functioning and consequently to influence the parasite’s survivorship, reproductive schedule and virulence (Paterson and Barber, 2007; Webster et al., 2008). To our knowledge these evolutionary considerations have not been applied to liver Flukes. Yet a strong case can be made as follows for research to assess the Flukes’ life history plasticity and their underlying trade-offs to better understand their epidemiology and virulence.
Chemotherapeutic agents against human helminthes which reduce adult worms’ life expectancy have been suggested to select for parasites that mature earlier (Medley, 1994; Poulin, 1998) allowing parasites to produce eggs before the host is given anthelminthics. However, reproducing earlier means that the worms will be smaller and overall less fecund as a result of a resource allocation trade-off between development and reproduction schedule. Conversely, other authors argue that parasitic nematodes with larvae in host tissues (poorly susceptible to treatments) and adults in the gastrointestinal tract (highly susceptible to treatment) should postpone maturity, become larger and more fecund (Skorping and Read, 1998). Support for this hypothesis has been found in Teladorsagia cicumcinta worms infecting sheep, where adult worms became larger when subjected to massive chemotherapy (Skorping and Read 1998). Similarly, differences in schistosome susceptibility to PZQ possibly associated with delayed reproduction have been documented (Fallon et al., 1997).
Perhaps more importantly, massive use of chemotherapeutic agents can affect parasite virulence through the modification of the trade-offs between traits and optimum virulence levels that maximize parasite fitness (Bull, 1994; Ewald, 1994; Dieckmann et al., 2002). For example, Porco et al. (2005) considered that treatment exerts selective pressure by reducing the duration of an infection and hence opportunities for continued reproduction, thereby reducing the value of duration-increasing strategies to the pathogen (i.e. immuno-modulation) and favoring the parasite’s strategies that maximize the rate of transmission (egg production), which inadvertently may harm the host. In other scenarios, such as if there is a trade-off between virulence and host activity levels (as in the case of liver Flukes which require their host to be present and active in the wetlands), which may determine opportunities for contacts with intermediate hosts, they concluded that treatment decreases the virulence. Theoretical studies based on parasite adaptive evolution have also proposed multiple or mixed genotype infections as a further mechanism to promote the maintenance and evolution of virulence in parasite populations, as intra-host competition can favor high virulence parasite strains of lower potential fitness than less virulent strains wherever they have a local growth or transmission advantage (Antia et al., 1994; May and Nowak, 1994; Frank, 1996; Read and Taylor, 2001). This is because competition between strains may select for genotypes that exploit host resources sooner, regardless of whether they would produce fewer new infections in the single genotype situation (reviewed in Webster et al., 2008) and may be directly observed as an increase in the virulence.
6.4. Significance
The projected intensification of interventions, including mass and indiscriminate use of PZQ, in endemic communities in northeastern Thailand (Sripa et al. unpublished) underline the timeliness and importance of addressing the questions highlighted above. The risk for drug resistance is real and the overlooked consequences of chemotherapy on both hosts and parasite together with the potential increased risk of metabolic disorders indirectly associated with deworming (see section 3), strongly argues for the need to consider Ov interventions based on a more moderate or refined drug strategy.
7. Liver Fluke, cancer and evolutionary thinking
While cholangiocarcinoma (CCA) etiology in particular and cancer biology in general have been mainly the purview of the clinical biomedical sciences, a new understanding of cancer is emerging from a broader research community informed by evolutionary biology (Aktipis and Nesse, 2013). Recent progress in genetics and cell biology has revealed details about cancer that question conceptual frames historically prevalent in biomedicine lacking an evolutionary biology perspective. The findings suggest cancer can be better understood, prevented and treated when considered within a larger framework of the six types of evolutionary explanations for traits that leave organisms vulnerable to disease. These are (1) mismatches with novel environments, (2) co-evolution with fast-evolving pathogens, (3) constraints on what selection can do, (4) trade-offs, (5) reproductive success (RS) at the expense of health, and (6) defenses with costs as well as benefits (Aktipis and Nesse, 2013; Nesse and Stearns, 2008). Importantly, these evolutionary explanations not only can improve our understanding of cell proliferation patterns and cancer progression but also help to anticipate cancer initiation and promotion (Lochhead et al., 2015), which may be particularly valuable in the context of CCA to clarify the extent to which Ov infection promotes cancer and to subsequently adjust Ov/CCA-focused interventions.
The “mismatch with novel environments” in terms of “exposure to aspects of modern environments for which our bodies are ill prepared” (Aktipis and Nesse, 2013) may be particularly applicable to Ov and CCA. Rural communities in SEA are experiencing fundamental changes in their social, physical and biological environments largely associated with ‘deagrarianization’ and associated patterns of wealth and poverty (Rigg, 2006). This transition including increased economic disparity generally is known to affect environmental, physiological and psychosocial health with mutually reinforcing influences (Bircher and Kuruvilla, 2014). Chronic psychosocial stress in particular is known to initiate a cascade of information processing in both the peripheral nervous system and the central nervous system (Sapolsky, 2004) which induces the mis-regulation of neuroendocrine hormones, particularly catecholamines and cortisol (Seeman and McEwen, 1996). In other words, under both chronic and acute stress conditions, the body remains in a constant state of ‘overdrive’, with deleterious downstream effects on regulation of stress response systems, as well as many organ systems which may increase the risk for cancer initiation as well as the progression of existing malignancies (Seeman and McEwen, 1996; IOM, 2001; Reiche et al., 2004; Glaser and Kiecolt-Glaser, 2005; Moreno-Smith et al., 2010). Chronic psychosocial stress also is associated with behavorial changes that predispose to cancer such as increasing alcohol consumption (Harling et al., 2009; José et al., 2000) which has reportedly grown several fold in Thailand since the 1960’s (WHO, 2014), with the northeast having the highest rates of consumption and abuse (Moolasart and Chirawatkul, 2012).
Pesticide use and exposure have also increased dramatically in rural Thailand in recent decades (Panuwet et al., 2012) with 73% of the agrochemical imports into Thailand classified as extremely hazardous by the WHO. Indeed, severe health complications are increasingly reported in central and northeastern regions (Tawatsin, 2015). In fact, cancer risk of greater than one in a million was estimated in fishermen throwing and/or placing a fish net in the water in central Thailand because of the presence of and exposure to dieldrin, DDT, β-HCH, heptachlor, and heptachlor epoxide in the canals (Panuwet et al., 2012). Similarly drinking water or eating food contaminated with nitrates (from fertilizers) has a potential role in developing cancers of the digestive tract, and has also been associated with other types of cancer (Townsend et al., 2003; Ward et al., 2005).
This growing literature provides evidence that the current de-agrarianization of rural northeastern Thailand and the “mismatch with novel environment” that it creates—including diet shifts, social reorganization of rural communities, agriculture intensification and new economic pressure for productivity as well as the inherent psychosocial stress associated with these changes—represents an important combination of determinants possibly affecting CCA etiology. An evolutionary perspective helps contextualize current Ov/CCA research within a broader socio-ecological framework, identify distal influences and generate interventions more considerate of global health and governance issues as well as more aligned with sustainable development goals.
8. Evolutionary medicine and liver Fluke disease prevention and control
In the now classic “Plagues and Peoples” (McNeill, 1976), McNeill identified “the ecological impact of medical science as the most recent (at the time of the book’s publication) of seven major historical eras of infectious disease social-ecology prior to the present era of globalization-driven disease emergence. The emergence of modern medicine beginning in the 16th and 17th centuries, including Koch’s and Pasteur’s discoveries in the 19th century and the formulation of the ‘germ theory’, led to interventions such as hygiene and quarantine, pesticides, anti-microbial drugs and vaccines which together with improvements in living conditions led to the control of major diseases in many parts the developed world.
By the 1960’s and 70’s the belief that infectious diseases would soon be conquered led to complacency and overconfidence–soon to be shattered by the appearance of HIV and looming anti-biotic resistant drugs (IOM, 2006). A litany of new and re-emerging microbial threats followed, leading the new phenomena of ‘emerging and re-emerging infectious diseases. A founding father of microbial genetics and the prime mover in the scientific campaign drawing attention to this new phenomenon, Joshua Lederberg (Lederberg, 2000) emphasized that our misplaced optimism stemmed (and still does) in large part from a lack of evolutionary thinking. Specifically, a failure to recognize the microbial evolutionary consequences of our medical interventions as well as other human-microbe “ecological imbalances” being anthropogenically created. He went on to stress the irrevocable interdependence of humans and microbes or co-evolution and the need to shift away from the simplistic notion: “We good, they evil.”
Evolutionary medicine represents a complementary alternative to this narrow view—nonetheless appropriate for treatement of infected individuals in the clinical setting—by offering sustainable, population-level solutions: If we cannot eradicate infections by frontal assault, we may be able to keep them at bay durably provided we can understand the fundamental nature of host-parasite relationships (Nesse and Stearns, 2008). Evolutionary medicine also helps foster a mindset where health is seen as a functional and context-dependent state, with adaptability and resilience as key attributes. The evolutionary perspective asks ultimate questions, which are about why mechanisms or features are the way they are (i.e. How does this mechanism give a selective advantage? What is the phylogeny of this mechanism?). Most medical research and perspectives focus on proximal questions, those about the mechanisms themselves (i.e. How does the mechanism work and what is the ontogeny of the mechanisms?). The distinction between proximate or mechanistic and ultimate or evolutionary explanations was emphasized by Tinbergen (1963) and Mayr (1982) but remain unfamiliar to the medical sciences despite its epistemological importance. Indeed, the medical literature systematically avoids use of the term evolution even in instances where it is apparently understood (Antonovics et al 2007). Both proximate and ultimate types of explanations are necessary, neither substitutes for the other, and they inform each other (Nesse and Stearns, 2008; Nesse, 2013). The biomedical model, the most reductionisty of health models (Tamm, 1993), has dominated liver Fluke research until now.
Evolutionary thinking complements the biomedical proximate perspective with ultimate/evolutionary causation toward improving our understanding of liver Flukes transmission dynamics, infection patterns and disease manifestation. Evolutionary biology can help identify new, relevant questions such as: what are the direct cost and indirect benefits of liver Fluke infections? What is the reproductive schedule of liver Fluke adult worms and how does it affect transmission dynamics? How does migration of hosts influence gene flow, local adaptations and ultimately disease manifestation? How do drug administration strategies select for drug resistance? How do psychosocial stress, diet shifts and pesticide use, associated with transitioning rural landscapes, influence CCA etiology and how this helps to clarify the CCA-Ov relationship and refine intervention (Table 3)?
Table 3.
Summary of an evolutionary research agenda for liver Flukes in Southeastern Asia.
| Questions | Data availability/novelty | Research design suggested | Significance | |
|---|---|---|---|---|
| BIOLOGY OF H-P INTERACTIONS | Q1. What are the mechanisms underlying pro/anti-inflammatory cross-regulation and the potentially protective effects of liver Fluke infection? | Moderate/High. While studies have not looked at these questions specifically, the data collected for the purpose of addressing other clinical or epidemiological questions may be sorted out and analyzed to detect trends confirming (or not) the relevance of addressing the proposed question. | Cohort studies, with dewormed and control groups screened for MetS, autoimmune diseases, parasite effects and controlled for ethnicity/genetic background | Clinical and Public Health (PH): help refine interventions and contextualize liver Fluke research and control initiatives within a broader health map. Suggests caution about systematic deworming. |
| Q2. How do infection intensity, genetic polymorphisms and diet interact to modulate thresholds of beneficial vs costly outcomes of liver Fluke infection? | Moderate/High. Infection intensity is known to be associated with increased expression of pro-inflammatory cytokines but limited information is available in case of low infection intensity. Genetic polymorphisms are known but limited information is available regarding the influence of diet in influencing Th1/Th2 polarization. | Cohort studies involving variable worm burden, known genetic polymorphisms and contrasting livelihoods and diets | Clinical and PH: underline the importance of the context in disease manifestation and emphasize the need to refine intervention based on infection intensity | |
|
| ||||
| PARASITE ADAPTATIONS | Q3. What is the reproductive schedule of liver Fluke adult worms? | Low/High. Ov eggs per gram of faeces (EPG) data are usually collected prior deworming and subsequently during follow up. No egg count data are usually available for the same individual over a long period of time. | Eco-epidemiology designs and cohort studies incorporating information on snail seasonal abundance and seasonal EPG counts in humans in different endemic areas. | Epidemiology. Refine our understanding of transmission dynamics. Help identify temporal windows of transmission risks and schedule for intervention. For example, with a refined understanding of the seasonal presence of eggs in local wetlands, or risk of infection in fish, snail removal or health education campaigns can be adjusted during particular time of the year. |
| Q4. How long do Ov eggs remain infective in the environment? | Moderate/High. Preliminary experimental data is available for Ov while field data is available for Of. There is a need to develop field manipulative experiments to assess the influence of environmental variability on egg survival and infectious trends. | Manipulative field experiments were Ov egg are distributed in porous containers and placed in local wetlands with regular survival and infectiousness assessments. | Epidemiology/Transmission dynamics | |
| Q5. Is cercarial release adjusted to maximize fish encounter? Is there a genetic basis underlying cercarial release? | Moderate/High. Preliminary field data is available for Ov cercaria release seasonal trends. No formal assessment of the relationship between cercarial release and fish abundance. No information regarding cercarial release variation in relation to Ov genotypes. | Field epidemiological studies assessing local snail cercarial release and fish abundance patterns coupled with factorial lab infection experiments with snails and Ov geographic strains cross-exposed. | Epidemiology/Transmission dynamics | |
|
| ||||
| LANDSCAPE GENETICS / METAPOPULATION DYNAMICS | Q6. How does migration of hosts and landscape modification influence gene flow, local adaptations and ultimately disease manifestation? | Low/High. Historical texts on Northeastern Thailand, Laos, Cambodia and Vietnam are difficult to access to but provide evidence and traceability of human migrations during the last centuries. Little information is available regarding human ethnic groups differential exposure to liver Flukes or genetic make-up specifically tailored to cope with liver Flukes. More recently, seasonal and semi-permanent labor migrations are important elements of the livelihood transition occurring in rural SEA. Limited demographic information is available. Information regarding gene flow in human populations in this context is inexistent. More information is available for snails and to a lesser extent fish but investigations of the role of landscape structure on gene flow is lacking. |
Cohort studies, paeloparasitological studies and human population genetics studies accounting for ethnicity and assessing past and present population migration. Landscape genetics studies assessing landscape fragmentation/functional connectivity on genetic diversity and structuration and host and parasite genotypes geographic co-occurrence. |
Evolutionary Epidemiology. Refine our understanding of co-evolutionary and disease manifestation hotspots. |
| Q7. Are there host and parasite genotypes more compatible? | Low/Moderate. Geographic genotypes have been identified but cross infection experiments are lacking. | Host genotype x Parasite genotype factorial lab experiments assessing frequency-dependent selection and the potential for local adaptations. | Epidemiology | |
|
| ||||
| CHEMOTHERAPY | Q8. How does PZQ influence TH1/Th2 polarization | Moderate/High. Understanding PZQ influence on pro/anti-inflammatory cross regulation is limited though indirect evidence suggest risk of inflammation after repeated exposure. | Prospective cohort studies involving immunodiagnostic of infected patient treated vs non-treated with PZQ | Epidemiology/Cinical. Help refine Mass Drug Administration campaigns and overall treatment strategy. |
| Q9. How does chemotherapy select for drug resistance in liver fukes? | Low/High. Mechanisms of resistance emergence are unknown and data documenting resistance emergence (or non-emergence) to praziquantel are inexistent in the context of liver Flukes. Results from schistosomes resistance to praziquantel do not provide evidence of exacerbated risk for resistance emergence. | Cohort studies incorporating groups of patients having received none, one or multiple praziquantel doses over a selected period of time screened for treatment efficiency through faeces examination. | ||
| Q10. How does chemotherapy affects parasite virulence and reproductive schedule? | Low/High. No data available. | Experimental factorial designs involving lines of worms exposed to PZQ in vitro. | ||
|
| ||||
| CANCER PREVENTION | Q.11 How do psychosocial stress, diet shifts and pesticide use associated with transitioning rural landscape influence CCA etiology and how this helps to clarify the CCA-Ov relationship and refine interventions? | Low/High. Livelihood transition in rural SEA has immense implications on all dimensions of health including psychosocial and physiological health. From a medical perspective, this transition is not recognized as a strong determinant that has the potential to promote cancer through increased emotional and physiological stress. Accompanying the livelihood transition, traditional subsistence agricultural practices are transformed into profit-making activities requiring the heavy use of pesticide implying high rate of blood poisoning. Little information is available in the context of liver Fluke and CCA in this regard. | Social-epidemiological cohort studies incorporating groups of different socio-economic conditions and belonging to various social-ecological systems (traditional, peri-urban, urban) assessing psychosocial stress, clinical status and propensity for cancer development, accounting for Ov infection. | Epidemiology/Clinical/Governance. Help refine prevention campaigns for CCA as well as for liver Flukes. Stimulate the need to reconsider government priorities accounting for the health consequences of economic decisions and subsequent local landscape management as well as agrarian politics. |
Moreover, evolutionary biology cultivates a holistic mindset sensitive to context-dependency including the role of environmental and socio-cultural factors. Hence, it contributes to an understanding of host-parasite dynamics as emedded in, and disease as an emergent property of, complex social-ecological systems (Horwitz and Wilcox, 2005; Wilcox and Colwell, 2005). Evolutionary principles help us understand the trade-offs that individuals face within this environment; embedded within these dynamics, hierarchical systems across temporal and spatial scales. This invokes an appreciation of the role of the interaction of social, cultural and biophysical environmental factors in disease transmission.
Highlights.
Liver flukes in Southeast Asia remain an important public health concern
Liver fluke research prevention and control is uninformed by evolutionary biology
We review host-liver fluke biology, epidemiology, parasitology and phylodynamics
We identify a series of questions for an evolutionary biology research agenda
We describe an integrative evolutionary medicine approach to liver fluke control
Acknowledgments
We would like to thank John F. Smith, Carsten Richter, Radhakrishnan Muthukumar, Kanin Salao and Isabelle Jala and two anonymous reviewers for comments and suggestions during the preparation of the manuscript, as well as the organizers and participants of the Molecular Epidemiology and Evolutionary Genetic of Infectious Diseases (MEEGID) conference held in Bangkok, Thailand in December 2014 for fruitful discussions. We gratefully acknowledge support from the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University, from the Thailand Research Fund (TRF) grant number RTA 5680006 and from the National Institute of Allergy and Infectious Diseases (NIAID), NIH, grant number P50AI098639. PE is a post-doctoral research scholar of Khon Kaen University. BS is a TRF Senior Research Scholar. Other financial support was provided by the Department of Biology, Laurentian University, Sudbury, Ontario, Canada. The content is solely the responsibility of the authors and does not necessarily represent the official views of the TRF, NIAID or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aktipis CA, Nesse RM. Evolutionary foundations for cancer biology. Evol Appl. 2013;6:144–159. doi: 10.1111/eva.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JE, Wynn TA. Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso D, Muñoz J, Gascón J, Valls ME, Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am J Trop Med Hyg. 2006;74:342–344. [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; 1991. Reprint edition. ed. [Google Scholar]
- Anderson RM, May RM. Co-evolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/S0031182000055360. [DOI] [PubMed] [Google Scholar]
- Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antia R, Levin BR, May RM. Within-Host Population Dynamics and the Evolution and Maintenance of Microparasite Virulence. Am Nat. 1994;144:457–472. [Google Scholar]
- Antonovics J, Abbate JL, Baker CH, Daley D, Hood ME, Jenkins CE, Johnson LJ, Murray JJ, Panjeti V, Rudolf VH, et al. Evolution by any other name: antibiotic resistance and avoidance of the E-word. PLoS Biol. 2007;5:e30. doi: 10.1371/journal.pbio.0050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1:252–264. doi: 10.1038/mi.2008.21. [DOI] [PubMed] [Google Scholar]
- Auld SK, Tinsley MC. The evolutionary ecology of complex lifecycle parasites: linking phenomena with mechanisms. Heredity. 2015:125–132. doi: 10.1038/hdy.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunpromma S, Tangkawattana P, Papirom P, Kanjampa P, Tesana S, Sripa B, Tangkawattana S. High prevalence of Opisthorchis viverrini infection in reservoir hosts in four districts of Khon Kaen Province, an opisthorchiasis endemic area of Thailand. Parasitol Int. 2012;61:60–64. doi: 10.1016/j.parint.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Ayé Soukhathammavong P, Rajpho V, Phongluxa K, Vonghachack Y, Hattendorf J, Hongvanthong B, Rasaphon O, Sripa B, Akkhavong K, Hatz C, Odermatt P. Subtle to severe hepatobiliary morbidity in Opisthorchis viverrini endemic settings in southern Laos. Acta Trop. 2015;141:303–309. doi: 10.1016/j.actatropica.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Bashyam H. Th1/Th2 cross-regulation and the discovery of IL-10. J Exp Med. 2007;204:237. doi: 10.1084/jem.2042fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Shor D, Harel M, Eliakim R, Shoenfeld Y. The Hygiene Theory Harnessing Helminths and Their Ova to Treat Autoimmunity. Clin Rev Allergy Immunol. 2013;45:211–216. doi: 10.1007/s12016-012-8352-9. [DOI] [PubMed] [Google Scholar]
- Bircher J, Kuruvilla S. Defining health by addressing individual, social, and environmental determinants: new opportunities for health care and public health. J Public Health Policy. 2014;35:363–386. doi: 10.1057/jphp.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Costa I, Poulin R. Host traits explain the genetic structure of parasites: a meta-analysis. Parasitology. 2013;140:1316–1322. doi: 10.1017/S0031182013000784. [DOI] [PubMed] [Google Scholar]
- Bonesso-Sabadini PIP, de Souza Dias LC. Altered response of strain of Schistosoma mansoni to oxamniquine and praziquantel. Mem Inst Oswaldo Cruz. 2002;97:381–385. doi: 10.1590/s0074-02762002000300019. [DOI] [PubMed] [Google Scholar]
- Bourke CD, Nausch N, Rujeni N, Appleby LJ, Mitchell KM, Midzi N, Mduluza T, Mutapi F. Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis. J Infect Dis. 2013;208:159–169. doi: 10.1093/infdis/jis524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkworth JF, Pechenkina K. Primates, Pathogens, and Evolution. Springer Science & Business Media; Berlin: 2013. [Google Scholar]
- Bull JJ. Perspective: virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Castrillo A, Tontonoz P. Nuclear Receptors in Macrophage Biology: At the Crossroads of Lipid Metabolism and Inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- Cerami A, Warren KS. Drugs. Parasitol Today. 1994;10:404–406. doi: 10.1016/0169-4758(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Cho I, Blaser MJ. The Human Microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CM, Fairbrother E, Webster JP. Mixed strain schistosome infections of snails and the evolution of parasite virulence. Parasitology. 2002;124:31–38. doi: 10.1017/s0031182001008873. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, editor. International Institute for Applied Systems Analysis, editor. Adaptive dynamics of infectious diseases: in pursuit of virulence management, Cambridge studies in adaptive dynamics. IIASA : Cambridge University Press, [Laxenburg]; Cambridge; New York: 2002. [Google Scholar]
- Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- Dybdahl MF, Jokela J, Delph LF, Koskella B, Lively CM. Hybrid fitness in a locally adapted parasite. Am Nat. 2008;172:772–782. doi: 10.1086/592866. [DOI] [PubMed] [Google Scholar]
- Echaubard P, Leduc J, Pauli B, Chinchar VG, Robert J, Lesbarrères D. Environmental dependency of amphibian-ranavirus genotypic interactions: evolutionary perspectives on infectious diseases. Evol Appl. 2014;7:723–733. doi: 10.1111/eva.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins DB, Mairiang E, Sithithaworn P, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, Haswell-Elkins MR. Cross-sectional patterns of hepatobiliary abnormalities and possible precursor conditions of cholangiocarcinoma associated with Opisthorchis viverrini infection in humans. Am J Trop Med Hyg. 1996;55:295–301. doi: 10.4269/ajtmh.1996.55.295. [DOI] [PubMed] [Google Scholar]
- Ewald PW. The Evolution of Infectious Diseases. Oxford University Press; Oxford: 1994. [Google Scholar]
- Ewald PW. Oxford Surveys in Evolutionary Biology. Oxford University Press; 1988. Cultural vectors, virulence and the emergence of evolutionary epidemiology. [Google Scholar]
- Ewald PW. Host-Parasite Relations, Vectors, and the Evolution of Disease Severity. Annu Rev Ecol Syst. 1983;14:465–485. doi: 10.1146/annurev.es.14.110183.002341. [DOI] [Google Scholar]
- Fallon PG, Mubarak JS, Fookes RE, Niang M, Butterworth AE, Sturrock RF, Doenhoff MJ. Schistosoma mansoni: maturation rate and drug susceptibility of different geographic isolates. Exp Parasitol. 1997;86:29–36. doi: 10.1006/expr.1997.4149. [DOI] [PubMed] [Google Scholar]
- Fellous S, Salvaudon L. How can your parasites become your allies? Trends Parasitol. 2009;25:62–66. doi: 10.1016/j.pt.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, Bresolin N, Sironi M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani AP. Epidemiology meets evolutionary ecology. Trends Ecol Evol. 2003;18:132–139. [Google Scholar]
- Gandon S, Capowiez Y, Dubois Y, Michalakis Y, Olivieri I. Local Adaptation and Gene-For-Gene Co-evolution in a Metapopulation Model. Proc Biol Sci. 1996;263:1003–1009. [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- Gandon S, Michalakis Y. Local adaptation, evolutionary potential and host–parasite co-evolution: interactions between migration, mutation, population size and generation time. J Evol Biol. 2002;15:451–462. [Google Scholar]
- Gandon S, Nuismer SL. Interactions between Genetic Drift, Gene Flow, and Selection Mosaics Drive Parasite Local Adaptation. Am Nat. 2009;173:212–224. doi: 10.1086/593706. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Grenfell BT. Unifying the Epidemiological and Evolutionary Dynamics of Pathogens. Science. 2004;303:327–332. doi: 10.1126/science.1090727. [DOI] [PubMed] [Google Scholar]
- Grundy-Warr C, Andrews RH, Sithithaworn P, Petney TN, Sripa B, Laithavewat L, Ziegler AD. Raw attitudes, wetland cultures, life-cycles: socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol Int. 2012;61:65–70. doi: 10.1016/j.parint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Mbaye A, De Vlas SJ, Stelma FF, Guissé F, Van Lieshout L, Faye D, Diop M, Ly A, Tchuem-Tchuenté LA, Engels D, Polman K. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop Med Int Health. 2001;6:864–873. doi: 10.1046/j.1365-3156.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- Harinasuta C, Harinasuta T. Opisthorchis viverrini: life cycle, intermediate hosts, transmission to man and geographical distribution in Thailand. Arzneimittelforschung. 1984;34:1164–1167. [PubMed] [Google Scholar]
- Harling M, Strehmel P, Schablon A, Nienhaus A. Psychosocial stress, demoralization and the consumption of tobacco, alcohol and medical drugs by veterinarians. J Occup Med Toxicol. 2009;4:4. doi: 10.1186/1745-6673-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz P, Wilcox BA. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Int J Parasitol. 2005;35:725–732. doi: 10.1016/j.ijpara.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hussaarts L, van der Vlugt LEPM, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128:733–739. doi: 10.1016/j.jaci.2011.05.012. [DOI] [PubMed] [Google Scholar]
- IARC. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis) World Health Organization, International Agency for Research on Cancer; Lyon: 2011. [Google Scholar]
- IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- IOM, (Institue of Medicine) Ending the War Metaphor: The Changing Agenda for Unraveling the Host-Microbe Relationship. National Academies Press; Washington DC: 2006. [PubMed] [Google Scholar]
- IOM, (Institue of Medicine) New Horizons in Health: An Integrative Approach. National Academies Press; Washington DC: 2001. [PubMed] [Google Scholar]
- Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- Jittimanee J, Sermswan RW, Kaewraemruaen C, Barta JR, Macinnes JI, Maleewong W, Wongratanacheewin S. Protective immunization of hamsters against Opisthorchis viverrini infection is associated with the reduction of TGF-β expression. Acta Trop. 2012;122:189–195. doi: 10.1016/j.actatropica.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- José BS, Oers HaMV, Mheen HDVD, Garretsen HFL, Mackenbach JP. Stressors and Alcohol Consumption. Alcohol Alcohol. 2000;35:307–312. doi: 10.1093/alcalc/35.3.307. [DOI] [PubMed] [Google Scholar]
- Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Kaewkes S, Kaewkes W, Boonmars T, Sripa B. Effect of light intensity on Opisthorchis viverrini cercarial shedding levels from Bithynia snails—A preliminary study. Parasitol Int. 2012;61:46–48. doi: 10.1016/j.parint.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: The carcinogenic human liver fluke. World J Gastroenterol WJG. 2008;14:666–674. doi: 10.3748/wjg.14.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsa-Ard S, Laopaiboon M, Luvira V, Bhudhisawasdi V. Association between Praziquantel and Cholangiocarcinoma in Patients Infected with Opisthorchis viverrini: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2013;14:7011–7016. doi: 10.7314/APJCP.2013.14.11.7011. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Kirsi S, Hudson PJ, Valtonen ET. Patterns of cercarial production from Diplostomum spathaceum: terminal investment or bet hedging? Parasitology. 2004;129:87–92. doi: 10.1017/s0031182004005281. [DOI] [PubMed] [Google Scholar]
- Keiser J, Adelfio R, Vargas M, Odermatt P, Tesana S. Activity of tribendimidine and praziquantel combination therapy against the liver fluke Opisthorchis viverrini in vitro and in vivo. J Helminthol. 2013;87:252–256. doi: 10.1017/S0022149X12000387. [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507–1514. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatsopit N, Sithithaworn P, Kopolrat K, Andrews RH, Petney TN. Seasonal cercarial emergence patterns of Opisthorchis viverrini infecting Bithynia siamensis goniomphalos from Vientiane Province, Lao PDR. Parasit Vectors. 2014;7:551. doi: 10.1186/s13071-014-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatsopit N, Sithithaworn P, Saijuntha W, Petney TN, Andrews RH. Opisthorchis viverrini: Implications of the systematics of first intermediate hosts, Bithynia snail species in Thailand and Lao PDR. Infect Genet Evol. 2013;14:313–319. doi: 10.1016/j.meegid.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Koelle K, Cobey S, Grenfell B, Pascual M. Epochal evolution shapes the phylodynamics of interpandemic influenza A (H3N2) in humans. Science. 2006;314:1898–1903. doi: 10.1126/science.1132745. [DOI] [PubMed] [Google Scholar]
- Krivenko VV. Action of environmental factors on the survivability of Opisthorchis eggs in reservoirs and in soil. Gig Sanit. 1979:80–81. [PubMed] [Google Scholar]
- Krivenko VV, Filatov VG, Kuzovlev AP, Glazkov GA. Significance of Opisthorchis egg survival in the environment in the transmission cycle in opisthorchiasis. Gig Sanit. 1981:62–64. [PubMed] [Google Scholar]
- Laine AL, Burdon JJ, Nemri A, Thrall PH. Host ecotype generates evolutionary and epidemiological divergence across a pathogen metapopulation. Proc R Soc B Biol Sci. 2014;281:20140522–20140522. doi: 10.1098/rspb.2014.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar J. 2005;4:3. doi: 10.1186/1475-2875-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoprom N, Saijuntha W, Sithithaworn P, Wongkham S, Laha T, Ando K, Andrews RH, Petney TN. Biological variation within Opisthorchis viverrini sensu lato in Thailand and Lao PDR. J Parasitol. 2009;95:1307–1313. doi: 10.1645/GE-2116.1. [DOI] [PubMed] [Google Scholar]
- Laoprom N, Sithithaworn P, Ando K, Sithithaworn J, Wongkham S, Laha T, Klinbunga S, Webster JP, Andrews RH. Microsatellite loci in the carcinogenic liver fluke, Opisthorchis viverrini and their application as population genetic markers. Infect Genet Evol. 2010;10:146–153. doi: 10.1016/j.meegid.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Lederberg J. Infectious History. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17:183–189. doi: 10.1016/S0169-5347(02)02497-7. [DOI] [Google Scholar]
- Levin BR. The evolution and maintenance of virulence in microparasites. Emerg Infect Dis. 1996;2:93–102. doi: 10.3201/eid0202.960203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YS, Li HJ, Dai JR, Wang W, Qu GL, Tao YH, Xing YT, Li YZ, Qian K, Wei JY. [Studies on resistance of Schistosoma to praziquantel XIII resistance of Schistosoma japonicum to praziquantel is experimentally induced in laboratory]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi Chin. J Schistosomiasis Control. 2011;23:605–610. [PubMed] [Google Scholar]
- Li T, He S, Zhao H, Zhao G, Zhu XQ. Major trends in human parasitic diseases in China. Trends Parasitol. 2010;26:264–270. doi: 10.1016/j.pt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Little TJ, Shuker DM, Colegrave N, Day T, Graham AL. The Co-evolution of Virulence: Tolerance in Perspective. PLoS Pathog. 2010;6:e1001006. doi: 10.1371/journal.ppat.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively CM, Dybdahl MF. Parasite adaptation to locally common host genotypes. Nature. 2000;405:679–681. doi: 10.1038/35015069. [DOI] [PubMed] [Google Scholar]
- Lochhead P, Chan AT, Nishihara R, Fuchs CS, Beck AH, Giovannucci E, Ogino S. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol. 2015;28:14–29. doi: 10.1038/modpathol.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Portugall I, Layland LE. TLRs, Treg, and B Cells, an Interplay of Regulation during Helminth Infection. Front Immunol. 2012;3:1–7. doi: 10.3389/fimmu.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- Lustigman S, Geldhof P, Grant WN, Osei-Atweneboana MY, Sripa B, Basáñez MG. A Research Agenda for Helminth Diseases of Humans: Basic Research and Enabling Technologies to Support Control and Elimination of Helminthiases. PLoS Negl Trop Dis. 2012;6:e1445. doi: 10.1371/journal.pntd.0001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeish RS. The Origins of Agriculture and Settled Life. University of Oklahoma Press; 1992. [Google Scholar]
- Magen E, Borkow G, Bentwich Z, Mishal J, Scharf S. Can worms defend our hearts? Chronic helminthic infections may attenuate the development of cardiovascular diseases. Med Hypotheses. 2005;64:904–909. doi: 10.1016/j.mehy.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Magen E, Bychkov V, Ginovker A, Kashuba E. Chronic Opisthorchis felineus infection attenuates atherosclerosis–An autopsy study. Int J Parasitol. 2013;43:819–824. doi: 10.1016/j.ijpara.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Mairiang E, Laha T, Bethony JM, Thinkhamrop B, Kaewkes S, Sithithaworn P, Tesana S, Loukas A, Brindley PJ, Sripa B. Ultrasonography assessment of hepatobiliary abnormalities in 3,359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61:208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop, Opisthorchis viverrini and Opisthorchiasis: The 21st Century Review. 2003;88:221–227. doi: 10.1016/j.actatropica.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol, Host pathogens / Immune senescence. 2012;24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel S, Holderegger R. Ten years of landscape genetics. Trends Ecol Evol. 2013;28:614–621. doi: 10.1016/j.tree.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Manel S, Schwartz MK, Luikart G, Taberlet P. Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol. 2003;18:189–197. doi: 10.1016/S0169-5347(03)00008-9. [DOI] [Google Scholar]
- Mayr E. The Growth of Biological Thought: Diversity, Evolution, and Inheritance. Harvard University Press; Cambridge, MA: 1982. [Google Scholar]
- May RM, Nowak MA. Superinfection, metapopulation dynamics, and the evolution of diversity. J Theor Biol. 1994;170:95–114. doi: 10.1006/jtbi.1994.1171. [DOI] [PubMed] [Google Scholar]
- McNeill WH. Plagues and peoples. Anchor Press; New York: 1976. [Google Scholar]
- McSorley HJ, Maizels RM. Helminth Infections and Host Immune Regulation. Clin Microbiol Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley GF. Parasitic and Infectious Diseases: Epidemiology and Ecology. Academic Press; San Diego, CA: 1994. Chemotherapy; pp. 141–157. [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease Tolerance as a Defense Strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis Y. Ecology and Evolution of Parasitism. Oxford University Press; Oxford: 2009. Parasitism and the evolution of life-history traits; pp. 19–30. [Google Scholar]
- Michalakis Y, Renaud F. Malaria: Evolution in vector control. Nature. 2009;462:298–300. doi: 10.1038/462298a. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014;7:753–762. doi: 10.1038/mi.2014.23. [DOI] [PubMed] [Google Scholar]
- Mitta G, Adema CM, Gourbal B, Loker ES, Theron A. Compatibility polymorphism in snail/schistosome interactions: From field to theory to molecular mechanisms. Dev Comp Immunol. 2012;37:1–8. doi: 10.1016/j.dci.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa M, Honjo S, You G, Tanaka M, Uchida K, Srivatanakul P, Khuhaprema T, Loilome W, Techasen A, Wongkham C, Limpaiboon T, Yongvanit P, Wongkham S. Genetic and environmental determinants of risk for cholangiocarcinoma in Thailand. World J Gastrointest Pathophysiol. 2014;5:570–578. doi: 10.4291/wjgp.v5.i4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor A, Cong DT, Sinuon M, Tsuyuoka R, Chanthavisouk C, Strandgaard H, Velayudhan R, Capuano CM, Le Anh T, Tee Dató AS. Large-scale preventive chemotherapy for the control of helminth infection in Western Pacific countries: six years later. PLoS Negl Trop Dis. 2008;2:e278. doi: 10.1371/journal.pntd.0000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva World Health Organ. 1998:1–49. [Google Scholar]
- Moolasart J, Chirawatkul S. Drinking culture in the Thai-Isaan context of northeast Thailand. Southeast Asian J Trop Med Public Health. 2012;43:795–807. [PubMed] [Google Scholar]
- Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–1881. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SS, Bommana A, Pakala SB, Ohshiro K, Lyon AJ, Suttiprapa S, Periago MV, Laha T, Hotez PJ, Bethony JM, Sripa B, Brindley PJ, Kumar R. Inflammatory response to liver fluke Opisthorchis viverrini depends on host master coregulator, MTA1, a marker for parasite induced cholangiocarcinoma. Hepatol Baltim Md. 2011;54:1388–1397. doi: 10.1002/hep.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. Effect of aquaculture on world fish supplies. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Tinbergen’s four questions, organized: a response to Bateson and Laland. Trends Ecol Evol. 2013;28:681–682. doi: 10.1016/j.tree.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Stearns SC. The great opportunity: Evolutionary applications to medicine and public health: Evolutionary applications to medicine and public health. Evol Appl. 2008;1:28–48. doi: 10.1111/j.1752-4571.2007.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- Panuwet P, Siriwong W, Prapamontol T, Ryan PB, Fiedler N, Robson MG, Barr DB. Agricultural pesticide management in Thailand: status and population health risk. Environ Sci Policy. 2012;17:72–81. doi: 10.1016/j.envsci.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA, Chubb JC, Ball MA, Roberts GN. Evolution of complex life cycles in helminth parasites. Nature. 2003;425:480–484. doi: 10.1038/nature02012. [DOI] [PubMed] [Google Scholar]
- Paterson S, Barber R. Experimental evolution of parasite life-history traits in Strongyloides ratti (Nematoda) Proc R Soc B Biol Sci. 2007;274:1467–1474. doi: 10.1098/rspb.2006.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petney TN, Andrews RH, Saijuntha W, Wenz-Mücke A, Sithithaworn P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol. 2013;43:1031–1046. doi: 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Petney T, Sithithaworn P, Andrews R, Kiatsopit N, Tesana S, Grundy-Warr C, Ziegler A. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol Int. 2012;61:38–45. doi: 10.1016/j.parint.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25:1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- Pinlaor S, Prakobwong S, Hiraku Y, Kaewsamut B, Dechakhamphu S, Boonmars T, Sithithaworn P, Pinlaor P, Ma N, Yongvanit P, Kawanishi S. Oxidative and nitrative stress in Opisthorchis viverrini-infected hamsters: an indirect effect after praziquantel treatment. Am J Trop Med Hyg. 2008;78:564–573. [PubMed] [Google Scholar]
- Porco TC, Lloyd-Smith JO, Gross KL, Galvani AP. The effect of treatment on pathogen virulence. J Theor Biol. 2005;233:91–102. doi: 10.1016/j.jtbi.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. Evolutionary Ecology of Parasites: (Second Edition) Princeton University Press; Princeton: 2011. [Google Scholar]
- Poulin R. Evolutionary Ecology of Parasites: From Individuals to Communities. Chapman & Hall; London: 1998. [Google Scholar]
- Prayong P, Mairiang E, Pairojkul C, Chamgramol Y, Mairiang P, Bhudisawasdi V, Sripa B. An interleukin-6 receptor polymorphism is associated with opisthorchiasis-linked cholangiocarcinoma risk in Thailand. Asian Pac J Cancer Prev APJCP. 2014;15:5443–5447. doi: 10.7314/apjcp.2014.15.13.5443. [DOI] [PubMed] [Google Scholar]
- Prugnolle F, Théron A, Pointier JP, Jabbour-Zahab R, Jarne P, Durand P, de Meeûs T. Dispersal in a Parasitic Worm and Its Two Hosts: Consequence for Local Adaptation. Evolution. 2005;59:296–303. doi: 10.1111/j.0014-3820.2005.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Rainboth WJ. Cyprinids of South East Asia, in: Cyprinid Fishes: Systematics, Biology and Exploitation. Chapman & Hall; 1991. pp. 156–210. [Google Scholar]
- Read AF, Aaby P, Antia R, Ebert D, Ewald PW, Gupta S, Holmes EC, Sasaki A, Shields DC, Taddei F, Moxon RE. Evolution in Health and Disease. Oxford University Press; 1999. What can Evolutionary Biology can contribute to understand virulence? pp. 205–218. [Google Scholar]
- Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc Natl Acad Sci. 2011;108:10871–10877. doi: 10.1073/pnas.1100299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Taylor LH. The Ecology of Genetically Diverse Infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Renaud F, de Meeüs T. A simple model of host-parasite evolutionary relationships. Parasitism: Compromise or conflict? J Theor Biol. 1991;152:319–327. doi: 10.1016/S0022-5193(05)80197-3. [DOI] [PubMed] [Google Scholar]
- Restif O. Evolutionary epidemiology 20 years on: Challenges and prospects. Infect Genet Evol. 2009;9:108–123. doi: 10.1016/j.meegid.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Rigg J. Land, farming, livelihoods, and poverty: Rethinking the links in the Rural South. World Dev. 2006;34:180–202. doi: 10.1016/j.worlddev.2005.07.015. [DOI] [Google Scholar]
- Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CH, Hoang EH. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- Roff D. Evolution Of Life Histories: Theory and Analysis. Springer Science & Business Media; 1993. [Google Scholar]
- Saengsawang P, Promthet S, Bradshaw P. Infection with Opisthorchis viverrini and Use of Praziquantel among a Working-age Population in Northeast Thailand. Asian Pac J Cancer Prev. 2013;14:2963–2966. doi: 10.7314/APJCP.2013.14.5.2963. [DOI] [PubMed] [Google Scholar]
- Saijuntha W, Sithithaworn P, Chilton NB, Petney TN, Klinbunga S, Satrawaha R, Webster JP, Andrews RH. Impact of temporal changes and host factors on the genetic structure of a population of Opisthorchis viverrini sensu lato in Khon Kaen Province (Thailand) Parasitology. 2009;136:1057. doi: 10.1017/S0031182009006441. [DOI] [PubMed] [Google Scholar]
- Saijuntha W, Sithithaworn P, Wongkham S, Laha T, Pipitgool V, Tesana S, Chilton NB, Petney TN, Andrews RH. Evidence of a species complex within the food-borne trematode Opisthorchis viverrini and possible co-evolution with their first intermediate hosts. Int J Parasitol. 2007;37:695–703. doi: 10.1016/j.ijpara.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Why Zebras Don’t Get Ulcers: The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping - Now Revised and Updated. Macmillan; Toronto: 2004. [Google Scholar]
- Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, Akkhavong K, Odermatt P. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:247–254. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. OUP Oxford; 2011. [Google Scholar]
- Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine regulation. Psychosom Med. 1996;58:459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Seto EYW, Wong BK, Lu D, Zhong B. Human schistosomiasis resistance to praziquantel in China: should we be worried? Am J Trop Med Hyg. 2011;85:74–82. doi: 10.4269/ajtmh.2011.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Petney TN, Saijuntha W, Laoprom N. The systematics and population genetics of Opisthorchis viverrini sensu lato: Implications in parasite epidemiology and bile duct cancer. Parasitol Int. 2012a;61:32–37. doi: 10.1016/j.parint.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Van De N, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012b;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Tesana S, Pipitgool V, Kaewkes S, Pairojkul C, Sripa B, Paupairoj A, Thaiklar K. Relationship between faecal egg count and worm burden of Opisthorchis viverrini in human autopsy cases. Parasitology. 1991;102:277–281. doi: 10.1017/S0031182000062594. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepato-Biliary-Pancreat Sci. 2014;21:301–308. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- Skorping A, Read AF. Drugs and parasites: global experiments in life history evolution? Ecol Lett. 1998;1:10–12. doi: 10.1046/j.1461-0248.1998.0007d.x. [DOI] [Google Scholar]
- Slatkin M. Gene flow and the geographic structure of natural populations. Science. 1987;236:787–792. doi: 10.1126/science.3576198. [DOI] [PubMed] [Google Scholar]
- Smith HV. Detection of parasites in the environment. Parasitology. 1999;117:113–141. [PubMed] [Google Scholar]
- Smout MJ, Sripa B, Laha T, Mulvenna J, Gasser RB, Young ND, Bethony JM, Brindley PJ, Loukas A. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biosyst. 2011;7:1367. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MP, Gozzelino R, Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35:483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health TM IH. 2004;9:588–594. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Acta Tropica Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120:S158–S168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini–multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, Tessana S, Loukas A, Brindley PJ, Bethony JM. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of IL-6. Hepatol Baltim Md. 2009;50:1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Tangkawattana S, Laha T, Kaewkes S, Mallory FF, Smith JF, Wilcox BA. Toward integrated opisthorchiasis control in northeast Thailand: The Lawa project. Acta Trop. 2015;141:361–367. doi: 10.1016/j.actatropica.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press; 1992. [Google Scholar]
- Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1093–1097. doi: 10.1210/jc.2010-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ME. Models of health and disease. Br J Med Psychol. 1993;66:213–228. doi: 10.1111/j.2044-8341.1993.tb01745.x. [DOI] [PubMed] [Google Scholar]
- Tawatsin A. Pesticides used in thailand and toxic effects to human health. Med Res Arch 0 2015 [Google Scholar]
- Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Tesana S, Thapsripair P, Thammasiri C, Prasopdee S, Suwannatrai A, Harauy S, Piratae S, Khampoosa P, Kulsantiwong J, Donthaisong C, Chalokepanrat P, Viyanant V, Malone JB. Effects of Bayluscide on Bithynia siamensis goniomphalos, the first intermediate host of the human liver fluke, Opisthorchis viverrini, in laboratory and field trials. Parasitol Int. 2012;61:52–55. doi: 10.1016/j.parint.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Thaenkham U, Nuamtanong S, Sa-nguankiat S, Yoonuan T, Touch S, Manivong K, Vonghachack Y, Sato M, Waikagul J. Monophyly of Opisthorchis viverrini populations in the lower Mekong Basin, using mitochondrial DNA nad1 gene as the marker. Parasitol Int. 2010;59:242–247. doi: 10.1016/j.parint.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Thaenkham U, Nuamtanong S, Vonghachack Y, Yoonuan T, Sanguankiat S, Dekumyoy P, Prommasack B, Kobayashi J, Waikagul J. Discovery of Opisthorchis lobatus (Trematoda: Opisthorchiidae): a new record of small liver flukes in the Greater Mekong Sub-region. J Parasitol. 2011;97:1152–1158. doi: 10.1645/GE-2764.1. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The Geographic Mosaic of Co-evolution. University of Chicago Press; 2005. [Google Scholar]
- Thrall PH, Burdon JJ. Evolution of gene-for-gene systems in metapopulations: the effect of spatial scale of host and pathogen dispersal. Plant Pathol. 2002;51:169–184. doi: 10.1046/j.1365-3059.2002.00683.x. [DOI] [Google Scholar]
- Tinbergen N. On aims and methods of Ethology. Z Für Tierpsychol. 1963;20:410–433. doi: 10.1111/j.1439-0310.1963.tb01161.x. [DOI] [Google Scholar]
- Townsend AR, Howarth RW, Bazzaz FA, Booth MS, Cleveland CC, Collinge SK, Dobson AP, Epstein PR, Holland EA, Keeney DR, et al. Human health effects of a changing global nitrogen cycle. Front Ecol Environ. 2003;1:240–246. [Google Scholar]
- van den Biggelaar AH, Borrmann S, Kremsner P, Yazdanbakhsh M. Immune responses induced by repeated treatment do not result in protective immunity to Schistosoma haematobium: interleukin (IL)–5 and IL-10 responses. J Infect Dis. 2002;186:1474–1482. doi: 10.1086/344352. [DOI] [PubMed] [Google Scholar]
- Vatanasapt V, Uttaravichien T, Mairiang EO, Pairojkul C, Chartbanchachai W, Haswell-Elkins M. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- Waller PJ. Resistance in Nematodes to Anthelmintic Drugs. CSIRO Division of Animal Health/Australian Wool Corporation; Glebe, NSW: 1985. Resistance to anthelminthics and the implication for animal production; pp. 1–11. [Google Scholar]
- Wang W, Dai JR, Li HJ, Shen XH, Liang YS. Is there reduced susceptibility to praziquantel in Schistosoma japonicum? Evidence from China. Parasitology. 2010;137:1905–1912. doi: 10.1017/S0031182010001204. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, VanDerslice J. Workgroup Report: Drinking-Water Nitrate and Health—Recent Findings and Research Needs. Environ Health Perspect. 2005;113:1607–1614. doi: 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KS. The control of helminths: nonreplicating infectious agents of man. Annu Rev Public Health. 1981;2:101–115. doi: 10.1146/annurev.pu.02.050181.000533. [DOI] [PubMed] [Google Scholar]
- Webster JP, Gower CM, Norton AJ. Evolutionary concepts in predicting and evaluating the impact of mass chemotherapy schistosomiasis control programmes on parasites and their hosts: Applying evolutionary principles to schistosome control. Evol Appl. 2008;1:66–83. doi: 10.1111/j.1752-4571.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Woolhouse MEJ. Selection and Strain Specificity of Compatibility between Snail Intermediate Hosts and Their Parasitic Schistosomes. Evolution. 1998;52:1627–1634. doi: 10.2307/2411336. [DOI] [PubMed] [Google Scholar]
- Wenz-Mücke A, Sithithaworn P, Petney TN, Taraschewski H. Human contact influences the foraging behaviour and parasite community in long-tailed macaques. Parasitology. 2013;140:709–718. doi: 10.1017/S003118201200203X. [DOI] [PubMed] [Google Scholar]
- WHO. Global status report on alcohol and health. Geneva: 2014. [Google Scholar]
- Wilcox BA, Colwell RR. Emerging and Reemerging Infectious Diseases: Biocomplexity as an Interdisciplinary Paradigm. EcoHealth. 2005;2:244–257. doi: 10.1007/s10393-005-8961-3. [DOI] [Google Scholar]
- Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26:35–50. doi: 10.1385/CRIAI:26:1:35. [DOI] [PubMed] [Google Scholar]
- Wiria AE, Djuardi Y, Supali T, Sartono E, Yazdanbakhsh M. Helminth infection in populations undergoing epidemiological transition: a friend or foe? Semin Immunopathol. 2012;34:889–901. doi: 10.1007/s00281-012-0358-0. [DOI] [PubMed] [Google Scholar]
- Wiria AE, Sartono E, Supali T, Yazdanbakhsh M. Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome. PLoS Pathog. 2014;10:e1004140. doi: 10.1371/journal.ppat.1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinska J, King KC. Environment can alter selection in host–parasite interactions. Trends Parasitol. 2009;25:236–244. doi: 10.1016/j.pt.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Wongratanacheewin S, Rattanasiriwilai W, Priwan R, Sirisinha S. Immunodepression in hamsters experimentally infected with Opisthorchis viverrini. J Helminthol. 1987;61:151–156. doi: 10.1017/s0022149x00009913. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Hagan P. Seeking the ghost of worms past. Nat Med. 1999;5:1225–1227. doi: 10.1038/15169. [DOI] [PubMed] [Google Scholar]
- Wykoff DE, Harinasuta C, Juttijudata P, Winn MM. Opisthorchis viverrini in Thailand: The Life Cycle and Comparison with O. felineus. J Parasitol. 1965;51:207–214. doi: 10.2307/3276083. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22:372–377. doi: 10.1016/s1471-4906(01)01958-5. [DOI] [PubMed] [Google Scholar]
- Young ND, Nagarajan N, Lin SJ, Korhonen PK, Jex AR, Hall RS, Safavi-Hemami H, Kaewkong W, Bertrand D, Gao S, Seet Q, Wongkham S, Teh BT, Wongkham C, Intapan PM, Maleewong W, Yang X, Hu M, Wang Z, Hofmann A, Sternberg PW, Tan P, Wang J, Gasser RB. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat Commun. 2014;5 doi: 10.1038/ncomms5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler AD, Echaubard P, Lee YT, Chuah CJ, Wilcox BA, Grundy-Warr C, Sithithaworn P, Petney TN, Laithevewat L, Ong X, Andrews RH, Ismail T, Sripa B, Khuntikeo N, Poonpon K, Tungtang P, Tuamsuk K. Untangling the Complexity of Liver Fluke Infection and Cholangiocarcinoma in NE Thailand Through Transdisciplinary Learning. EcoHealth. 2016 doi: 10.1007/s10393-015-1087-3. [DOI] [PubMed] [Google Scholar]