Abstract
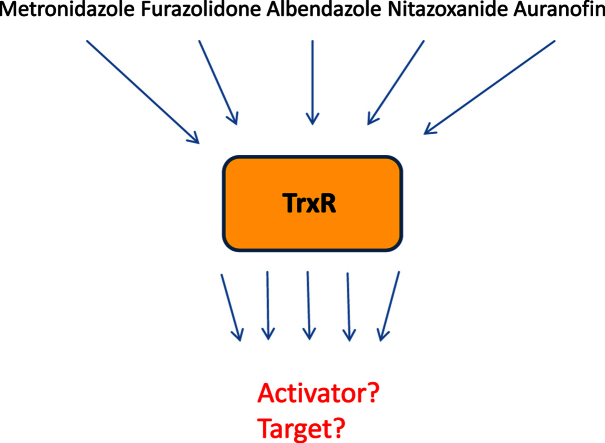
The antioxidative enzyme thioredoxin reductase (TrxR) has been suggested to be a drug target in several pathogens, including the protist parasite Giardia lamblia. TrxR is also believed to catalyse the reduction of nitro drugs, e.g. metronidazole and furazolidone, a reaction required to render these compounds toxic to G. lamblia and other microaerophiles/anaerobes. It was the objective of this study to assess the potential of TrxR as a drug target in G. lamblia and to find direct evidence for the role of this enzyme in the activation of metronidazole and other nitro drugs.
TrxR was overexpressed approximately 10-fold in G. lamblia WB C6 cells by placing the trxR gene behind the arginine deiminase (ADI) promoter on a plasmid. Likewise, a mutant TrxR with a defective disulphide reductase catalytic site was strongly expressed in another G. lamblia WB C6 cell line. Susceptibilities to five antigiardial drugs, i.e. metronidazole, furazolidone, nitazoxanide, albendazole and auranofin were determined in both transfectant cell lines and compared to wildtype. Further, the impact of all five drugs on TrxR activity in vivo was measured.
Overexpression of TrxR rendered G. lamblia WB C6 more susceptible to metronidazole and furazolidone but not to nitazoxanide, albendazole, and auranofin. Of all five drugs tested, only auranofin had an appreciably negative effect on TrxR activity in vivo, albeit to a much smaller extent than expected. Overexpression of TrxR and mutant TrxR had hardly any impact on growth of G. lamblia WB C6, although the enzyme also exerts a strong NADPH oxidase activity which is a source of oxidative stress.
Our results constitute first direct evidence for the notion that TrxR is an activator of metronidazole and furazolidone but rather question that it is a relevant drug target of presently used antigiardial drugs.
Keywords: Giardia lamblia, Thioredoxin reductase, Antigiardial drugs
Graphical abstract
Highlights
-
•
Thioredoxin reductase can be overexpressed in Giardia lamblia more than tenfold.
-
•
Thioredoxin reductase is an activator of metronidazole and furazolidone.
-
•
Thioredoxin is not an important target of presently used antigiardial drugs.
1. Introduction
Giardia lamblia (syn duodenalis, intestinalis) is a microaerophilic protist parasite that occurs in all parts of the world and infects hundreds of millions of people every year (Centers for Disease Control and Prevention, CDC). It colonizes the small intestine and causes gastrointestinal symptoms like nausea, diarrhea, bloating and malabsorption of nutrients. Although not life-threatening in most cases, Giardia infections can be persistent and cause growth retardation in children (Buret, 2008). Treatment mainly relies on 5-nitroimidazoles, such as metronidazole and tinidazole, or albendazole, a benzimidazole drug (Leitsch, 2015). 5-nitroimidazoles have been in use against practically all microaerophilic or anaerobic pathogens for more than 50 years due to the comparably low rate of resistance (Leitsch, 2015). However, metronidazole-resistant microaerophiles and anaerobes, including isolates of G. lamblia, do occur. Due to the importance of 5-nitroimidazoles, especially metronidazole which is listed among the “essential medicines” by the WHO (World Health Organisation, 2015), a large number of studies on 5-nitroimidazole action and resistance have been conducted throughout the last 30 years.
5-Nitroimidazoles are essentially prodrugs and not very reactive. Reduction at the nitro group, however, activates nitroimidazoles which react with numerous cell constituents - in G. lamblia e.g. DNA (Uzlikova and Nohynkova, 2014), proteins (Leitsch et al., 2012), and thiols (Leitsch et al., 2012). Due to the extremely low reduction potential of 5-nitroimidazoles reduction at the nitro group occurs quantitatively only in microaerophilic and anaerobic organisms (Müller, 1983). In the protist parasites G. lamblia, Entamoeba histolytica and Trichomonas vaginalis, several enzymatic pathways were identified that are likely to play a role in 5-nitroimidazole reduction, including the central metabolic enzyme pyruvate:ferredoxin oxidoreductase (PFOR) together with ferredoxin (Townson et al., 1996, Rasoloson et al., 2002, Leitsch et al., 2011) and thioredoxin reductase (TrxR) (Leitsch et al., 2007, Leitsch et al., 2009, Leitsch et al., 2011), a central redox regulator. Further, another 5-nitroimidazole reducing enzyme, nitroreductase 1 (NR1), was identified in G. lamblia (Müller et al., 2007). A correlation between expression levels of nitroreductase 1 and PFOR/ferredoxin and metronidazole sensitivity in G. lamblia is well documented, as PFOR and nitroreductase 1 are less strongly expressed in many metronidazole-resistant cell lines (Müller et al., 2008, Leitsch et al., 2011). Moreover, overexpression of NR1 from a plasmid renders G. lamblia more sensitive to metronidazole (Nillius et al., 2011). Direct evidence for a role of TrxR in 5-nitroimidazole reduction in vivo, however has been missing so far.
Importantly, TrxR was not only found to reduce 5-nitroimidazoles but also to be targeted by reduced nitroimidazole intermediates (Leitsch et al., 2007, Leitsch et al., 2009, Leitsch et al., 2011), resulting, at least in E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009), in a diminished thioredoxin reducing activity of the enzyme (Leitsch et al., 2007, Leitsch et al., 2009). Thus, TrxR has an intriguing double role as an activator and target of 5-nitroimidazoles. It was hypothesised that inhibition of TrxR could be one of the major toxic effects brought about by 5-nitroimidazoles (Leitsch et al., 2007, Leitsch et al., 2009). The TrxR/thioredoxin (Trx) redox system has multiple roles in most organisms, including reduction of peroxiredoxins and maintaining the activity of enzymes like ribonucleotide reductase and methionine sulfoxide reductase. In G. lamblia, however, the role of the TrxR/Trx system is still poorly understood. Although G. lamblia TrxR displays marked disulphide reduction and NADPH oxidase activities (Tejman-Yarden et al., 2013), a functional thioredoxin has not be identified so far (Leitsch et al., 2011; manuscript in preparation). Further, several enzymes known to be dependent on thioredoxin-mediated reduction, such as ribonucleotide reductase, are absent from the parasite. However, despite the current lack of knowledge about the physiological role of TrxR it is generally believed to be an important target not only of metronidazole (Leitsch et al., 2012) but also of auranofin, an antirheumatic drug that has been reprofiled for off-label use against G. lamblia (Tejman-Yarden et al., 2013) and E. histolytica (Debnath et al., 2012).
In order to evaluate the role of TrxR as an activator of antigiardial drugs and as a drug target, expression levels of the enzyme were strongly increased in G. lamblia trophopzoites by transfection of an episomal copy of the TrxR gene under control of the arginine deiminase (ADI) promoter. Likewise, a dominant negative mutant of the TrxR gene under control of the ADI promoter was introduced. Transfectants were assayed for altered drug susceptibilities and used for enzyme inhibition assays.
2. Materials and methods
2.1. Chemicals
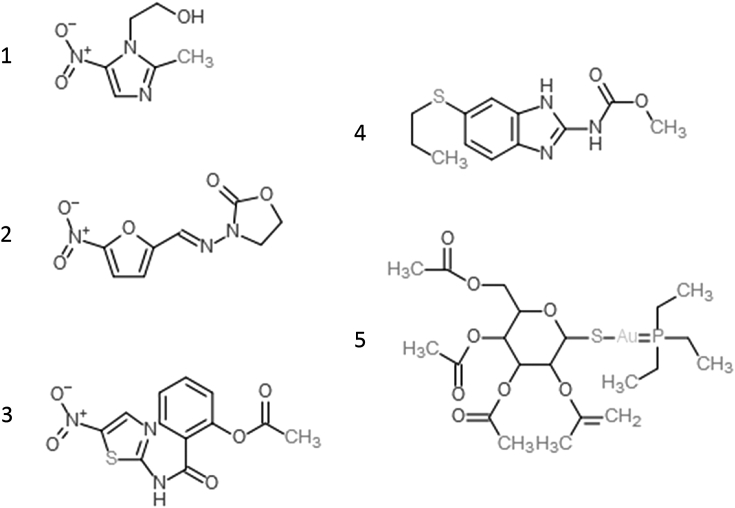
Metronidazole, furazolidone, nitazoxanide, auranofin and albendazole were purchased from Sigma (St. Louis, Mo, USA). All drugs tested are depicted in Fig. 1. Growth medium constituents were purchased from Merck (peptone from casein, yeast extract, sodium chloride, glucose, ammonium iron (III) citrate). Fetal calf serum was purchased from Biochrom (Bioswisstec AG, Schaffhausen, Switzerland).
Fig. 1.
The five antigiardial drugs tested in this study. 1, metronidazole; 2, furazolidone; 3, nitazoxanide; 4, albendazole, and 5, auranofin.
2.2. Cell culture
G. lamblia WB C6 (ATCC 50803) trophozoites were axenically cultivated in Keister's modified Diamond's medium. Media were sterile-filtered. Subcultures were performed every third day.
2.3. Construction of a TrxR overexpressing transfectant
The TrxR gene (GL50803_9827; XM_001707116) was amplified from genomic DNA isolated from WB C6 (ATCC 50803) with primers bearing 50 bp of the upstream region and 50 bp of the downstream region, respectively, of the arginine deiminase gene (GL50803_112103; XM_001705703), and PacI and XbaI restriction sites for cloning into the pPac-VInteg vector (Štefanić et al., 2009). The primer sequences were as follows: (forward) CATCTAGAAACGTCTACACGTGAGGTG TGTAAACTTCCGGAGAAAAAAATCCTAGTACATGTCTGCTCAAGCATTCGA, (reverse) CATTAATTAAC TGGATATGAACATGTCAATTATTTGATATCTGAATTACAATTCACTGTTTTAGTGATGGTGATGGTGAT. Transfections of the new plasmid pTrxR into WB C6 trophozoites were performed in a BTX Electro cell manipulator 600 (Harvard Apparatus) with the settings 500 V, 800 μF, and 720 Ω. Transfectant WB C6 cells were selected via the plasmid-encoded puromycin N-acetyl-transferase (pac) gene by adding puromycin to the growth medium (100 mg/l). The plasmid constructs are schematically depicted in Supplementary Fig. 1.
2.4. Construction of an episomal mutagenized TrxR gene
The second cysteine of the active site of TrxR on pTrxR was mutated to serine using the QuikChange II XL Site-Directed Mutagenesis kit (Agilent) according to the manufacturer's instructions. The mutagenesis primers introduced one single nucleotide exchange in order to alter a cysteine codon (TGC) to a serine codon (AGC). The sequences of the primers were as follows: (forward) GTCGGCCTGCGCTGTCAGCGATTCTGC, (reverse) GCAGAATCGCTGACAGCGCAGGCCGAC. The resulting plasmid pTrxR-mut was transfected into WB C6 as described above.
2.5. Two-dimensional gel electrophoresis of G. lamblia protein extracts (2DE)
Two-dimensional gel electrophoresis (2DE) with G. lamblia cell extracts was performed as described previously (Leitsch et al., 2011, Leitsch et al., 2012). Gels were stained with Coomassie Blue R-250 and evaluated using Melanie™ 4 software (Genebio).
2.6. mRNA quantification of expression by real-time RT–PCR
For quantification of TrxR mRNA expression, cells were harvested as described above and RNA was extracted using a Qiagen RNeasy Kit (Qiagen, Hilden, Germany). Synthesis of first-strand cDNA was performed using a Qiagen OmniscriptRT Kit (Qiagen). The primers used for the amplification of a 189 bp TrxR gene fragment: (forward) CGTTGGCCACGATCCCC, (reverse) GGGGATCGTGGCCAACG. TrxR mRNA levels were calculated using actin mRNA as internal standard (primers: ACTquantF, ACATATGAGCTGCCAGATGG; ACTquantR TCGGGGAGGCCTGCAAAC).
Quantitative RT–PCR was performed on a LightCyclerTM Instrument (Roche Diagnostics, Rotkreuz, Switzerland) as described previously (Nillius et al., 2011). Expression levels of TrxR mRNA were calculated as arbitrary units in relation to the quantity of actin mRNA. PCRs were performed in triplicate in three independent experiments.
2.7. Drug susceptibility and growth assays
Culture medium (10 ml) was inoculated with 10,000 trophozoites/ml and drugs were added in appropriate amounts. After incubation (37 °C, 48 h), cell numbers were determined in a Bürker-Türk chamber and IC50 values were calculated using Grafit 7 software (Erithacus software). The generation times of the cell lines were determined in duplicates in three independent biological replicates applying identical inoculates and incubation conditions.
2.8. Nitroreductase assay
5 μg/mL recombinant G. lamblia TrxR and 100 μM of either furazolidone or nitazoxanide were added to Tris pH 7.5 buffer, containing 1 mM EDTA, 0.5 mM NADPH and 50 μM cytochrome c (Leitsch et al., 2011). Reduction of cytochrome c (Sigma, St. Louis, Mo, USA) was measured in a Lambda 25 UV/Vis spectrometer (Perkin Elmer) at λ = 550 (Δε550 = 20 mM−1 cm−1). Recombinant G. lamblia TrxR was expressed and purified as described (Leitsch et al., 2011).
2.9. Disulphide reductase and NADPH oxidase assays with G. lamblia cell extracts
Disulphide reductase and NADPH oxidase activities of TrxR were measured photometrically using cell extracts of the respective G. lamblia cell lines. Cells were harvested and lysed by resuspension of the pellet in 10 times the volume of ultrapure water. Cell debris was removed (20,000 × g for 10 min) and protein concentrations of the supernatants determined by Bradford assay. 50 μg protein/mL were used in all measurements. Disulphide reductase activity in G. lamblia extracts was measured at 37 °C by quantifying reduction of DTNB (5,5'-dithiobis-(2-nitrobenzoic acid)) to TNB (2-nitro-5-thiobenzoate) at λ = 412 (Δε412 = 13.6 mM−1 cm−1) in 100 mM Tris pH 7.5 buffer containing 0.5 mM NADPH, 1 mM EDTA and 1 mM DTNB (Merck). Net TrxR activity without background reduction of DTNB by thiols and proteins was calculated by adding 10 μM of the non-competitive flavin inhibitor diphenyleneiodonium (DPI). NADPH oxidase activity was measured in 100 mM Tris pH 7.5 buffer containing 0.2 mM NADPH and 1 mM EDTA by determining oxidation of NADPH at λ = 340 (Δε340 = 6.2 mM−1 cm−1). Again, 10 μM DPI were added in order to determine the net NADPH oxidase activity of TrxR.
2.10. Western blotting
Polyclonal antibodies were raised in rabbit against G. lamblia TrxR (GenicBio Biotech, Shanghai, China). Sera were diluted 1:1000 for the detection of TrxR on western blots of G. lamblia cell extracts using anti-rabbit IgG (Sigma-Aldrich; 1:5000). Western blotting was performed according to standard protocols.
3. Results
3.1. Generation of TrxR overexpressing transfectant WB C6 lines
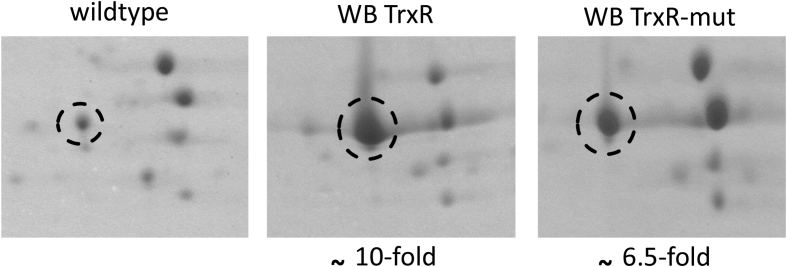
In order to assess the role of TrxR in the reduction of metronidazole and other drugs containing a nitro group, an expression construct based on pPAV-VInteg (Štefanić et al., 2009) was devised for the manipulation of TrxR expression levels in G. lamblia. Preliminary experiments harnessing 2DE and RT-qPCR had shown that episomal expression of the TrxR gene under its own promoter did not result in recognisable higher copy numbers of TrxR protein and mRNA, respectively (data not shown). The position of TrxR in 2DE gels had been identified earlier (Leitsch et al., 2011) and was hence known to us at the start of the study. Several promoters of very highly expressed proteins were tested as replacement of the trxR promoter on the plasmid, including 50 bp of the upstream and downstream regions, respectively, of the arginine deiminase (ADI) gene, one of the most strongly expressed proteins in G. lamblia as recognisable on 2DE gels (Supplementary Fig. 2). The resulting vector was named pTrxR and transfected into G. lamblia WB C6 trophozoites. Transfectants bearing pTrxR (WB TrxR) had an approximately 10-fold higher expression level of TrxR (Fig. 2) than wildtype WB C6, rendering TrxR one of the most prominent proteins in 2DE gels. The elevated expression level of TrxR in WB pTrxR was further confirmed in a western blot assay using an anti-TrxR antibody (Supplementary Fig. 3). The increase in protein levels was mirrored by strong increases of TrxR mRNA levels (Table 1). In order to check whether TrxR was functional in WB TrxR, disulphide reductase and NADPH oxidase activities of TrxR were measured in cell extracts. Indeed, WB TrxR displayed approximately tenfold disulphide reductase and NADPH oxidase activities as compared to wildtype (Table 1). It is important to note that disulphide reductase activity in wildtype cells was low and difficult to measure because background reduction of DTNB by cell constituents of the added extract was several times higher. Another expression construct was designed that placed a mutated TrxR gene behind the ADI promoter. To this end the second cysteine of the enzyme's active site was mutated to serine which nullifies disulphide reductase activity because thioredoxin reductases require two cysteines in their active site to reduce disulfide bonds in the substrate (Schlosser et al., 2013). Mutant TrxR could be overexpressed from pTrxR-mut in WB C6 trophozoites (WB TrxR-mut) to levels comparable to those in WB TrxR but disulphide reductase activity was practically identical to wildtype (Fig. 2, Table 1). NADPH oxidase activity, however, was even slightly higher (p < 0,05) in WB TrxR-mut than in WB TrxR (Table 1). When 10 μM of the flavin inhibitor diphenylene iodonium (DPI) were added to reactions, both disulphide reductase and NADPH oxidase activities were completely inhibited, showing that NADPH oxidase activity is independent of the catalytic cysteines but requires the cofactors NADPH and FAD. Importantly, the very high expression levels of native TrxR and mutant TrxR did hardly, if at all, affect growth of the transfected cell lines in G. lamblia growth medium although the at least tenfold NADPH activity would be expected to cause considerable oxidative stress through generation of superoxide radicals. Still, wildtype WB C6 displayed practically the same generation time (410 ± 10 min) as WB C6 pTrxR (435 ± 15 min) and WB C6 pTrxR-mut (430 ± 10 min). Further, no effect of altered TrxR levels on motility or shape could be observed under the light microscope. WB TrxR-mut was used as a control in the ensuing drug susceptibility assays in order to demonstrate that increased nitroreductase activity of the enzyme and not overexpression of the enzyme by itself is responsible for altered drug susceptibilities.
Fig. 2.
Sections of 2D-gels from cell extracts of G. lamblia WB C6 without plasmid (wildtype), with a plasmid harbouring the trxR gene behind the ADI promoter (WB TrxR), and with a plasmid harbouring a mutated trxR gene behind the ADI promoter (WB TrxR-mut). TrxR is encircled. The degree of overexpression of TrxR (functional or mutated) in relation to wildtype is indicated beneath the gel sections. The gel shown is representative for three biological replicates in total.
Table 1.
Expression of TrxR at the mRNA and protein levels in WB TrxR and WB TrxR-mut as compared to wildtype WB C6, and resulting changes in disulphide reductase and NADPH oxidase activities in these cell lines.
| TrxR expression | Relative expression level (protein) | Relative expression level (mRNA) | Disulphide reductase activity in cell extract (nmol min−1 mg−1) | NADPH oxidase activity in cell extract (nmol min−1 mg−1) |
|---|---|---|---|---|
| WB C6 (wildtype) | ×1 | ×1 | 10.2 ± 1.5 | 27 ± 7 |
| WB TrxR | ×10 | ×25 | 105 ± 14 | 213 ± 17 |
| WB TrxR-mut | ×6.5 | ×11 | 12.1 ± 2.3 | 278 ± 27 |
3.2. Evaluation of G. lamblia TrxR as an activator of and target of antigiardial drugs
Five antigiardial drugs (Fig. 1) were selected for susceptibility testing in the transfected cell lines: the 5-nitroimidazole metronidazole, the 5-nitrofuran furazolidone, the nitrothiazolide nitazoxanide, the benzimidazole albendazole, and auranofin. The first three drugs have nitro groups which need to be reduced for activity (Müller et al., 2007, Leitsch, 2015) and are thus potential substrates for TrxR. Albendazole, however, lacks a nitro group and does not require reduction for toxicity. Also Auranofin, a novel antigiardial drug known to semicompetitively inhibit TrxR in vitro (Tejman-Yarden et al., 2013), lacks a nitro group. Thus, prior to the susceptibility assays we hypothesised that WB TrxR would be more susceptible to the three nitro drugs but less susceptible to auranofin, due to much higher concentrations of the target molecule, i.e. TrxR. Susceptibility to albendazole was expected to be unchanged in WB TrxR.
Indeed, overexpression of TrxR had no influence on the susceptibility to albendazole (Table 2). In contrast to our prediction, however, overexpression of TrxR did not confer more tolerance to auranofin but had no effect at all (Table 2). In accordance with TrxR's capacity to function as a nitroreductase, however, overexpression of functional TrxR rendered WB TrxR more sensitive to metronidazole and furazolidone (Table 2) than wildtype or WB TrxR-mut. Surprisingly, the effect of TrxR overexpression was not significant in case of nitazoxanide although this drug has a nitro group which was shown to be essential for toxicity (Müller et al., 2007). In order to check whether nitazoxanide is a potential substrate for G. lamblia TrxR, we used purified recombinant G. lamblia TrxR for measuring reduction of nitazoxanide along the lines described for metronidazole and other 5-nitroimidazoles in an earlier study (Leitsch et al., 2011). Indeed, no nitroreductase activity of recombinant TrxR activity could be observed with nitazoxanide as substrate (Table 3). In contrast, furazolidone, was strongly reduced by recombinant G. lamblia TrxR (Table 3). This suggests that the elevated expression levels of TrxR in WB TrxR are responsible for a higher susceptibility to metronidazole and furazolidone, whereas the susceptibility to nitazoxanide remains unchanged because it is not a substrate of TrxR.
Table 2.
IC50 values determined for wildtype WB C6, WB TrxR, and WB TrxR-mut for all five drugs. Asterisks indicate statistically significant differences to wildtype (unpaired t-test, two sided; *P < 0.01; **P < 0.05). ND … not determined.
| Drug | IC50 |
||
|---|---|---|---|
| Wild type | WB TrxR | WB TrxR-mut | |
| Metronidazole (μM) | 3.44 ± 0.06 | 2.33 ± 0.03* | 3.71 ± 0.36 |
| Furazolidone (μM) | 0.73 ± 0.04 | 0.52 ± 0.03** | 0.79 ± 0.02 |
| Nitazoxanide (μM) | 1.86 ± 0.05 | 1.63 ± 0.04 | 1.76 ± 0.03 |
| Albendazole (nM) | 59.3 ± 0.4 | 60.8 ± 0.4 | 59.9 ± 1.4 |
| Auranofin (μM) | 16.5 ± 1 | 16.8 ± 0.1 | ND |
Table 3.
Recombinant G. lamblia TrxR as a nitroreductase with the substrates metronidazole, furazolidone, and nitazoxanide. *already published in Leitsch et al., 2011.
| Drug | Reduction by G. lamblia TrxR |
|---|---|
| Metronidazole (1 mM) | 81 ± 30 nmol min−1 mg−1* |
| Furazolidone (100 μM) | 1094 ± 25 nmol min−1 mg−1 |
| Nitazoxanide | 0 |
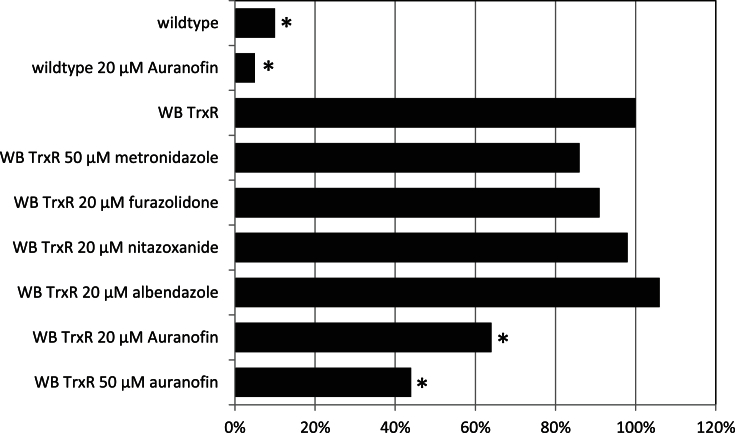
As a second step we delineated the role of TrxR as a drug target and assessed the effect of the five drugs on disulphide reductase activity of TrxR in WB TrxR (Fig. 3). Inhibition of TrxR by metronidazole and furazolidone was not significant, whereas nitazoxanide and albendazole did not inhibit TrxR at all (Fig. 3). Auranofin was the only drug that substantially reduced disulphide reductase activity of TrxR in WB TrxR (Fig. 3). When applied at 20 μM auranofin reduced TrxR activity to 60% as compared to control but even at a concentration of 50 μM, which is about the 300-fold dose of the IC50 as determined with recombinant G. lamblia TrxR in vitro (Tejman-Yarden et al., 2013), residual activity of TrxR in WB TrxR did not drop below 40% as compared to untreated WB TrxR. This indicates that the dose-response curve is rather flat and that even much higher concentrations of auranofin would not reduce TrxR activity in WB TrxR below the rate as observed in wildtype (Fig. 3). Indeed, TrxR activity in WB TrxR treated with 50 μM auranofin, was still 4–5-fold higher than in untreated wildtype (Fig. 3), at least after 2 h of incubation. Our results demonstrate that TrxR is a target of auranofin in the living parasite. It is not likely, however, that TrxR is a critical target of auranofin in G. lamblia since the susceptibility of WB TrxR to auranofin is equal to that of wildtype (Table 2). In fact, the results of this experiment suggest that G. lamblia TrxR is not a relevant target of any drug presently used for the treatment of giardiasis.
Fig. 3.
Impact of the drugs tested on disulphide reductase activity of TrxR in cell extracts of WB TrxR after 2 h of treatment. Untreated WB TrxR represents 100% activity. TrxR activities from untreated wildtype and wildtype treated with auranofin are given for comparison. Asterisks indicate statistically significant differences to TrxR activity in untreated WB TrxR (paired t-test, two sided; *P < 0.05).
4. Discussion
We devised a plasmid-based expression construct that coupled the trxR gene to the ADI promoter, resulting in approximately tenfold higher intracellular concentrations of the enzyme as compared to wildtype. Overexpression of TrxR rendered G. lamblia more susceptible to metronidazole and furazolidone (Table 2), two drugs which are reduced by recombinant TrxR in vitro (Leitsch et al., 2011) (Table 3). Although indirect evidence for the reduction of metronidazole in vivo by TrxR had already been presented for E. histolytica (Leitsch et al., 2007), T. vaginalis (Leitsch et al., 2009), and G. lamblia (Leitsch et al., 2007), this study constitutes first direct evidence that TrxR is an activator of nitro drugs in the living parasite. It can be ruled out that the higher susceptibility of WB TrxR to metronidazole and furazolidone was due to a decreased fitness caused by overexpression of TrxR as such because the susceptibilities to the other drugs tested were unaltered. Further, TrxR had to be functional in order to render WB C6 more susceptible to metronidazole and furazolidone (Table 2), as overexpression of mutated TrxR in WB TrxR-mut did not result in increased sensitivity to metronidazole and furazolidone (Table 2). Finally, since NADPH oxidase activity in WB TrxR-mut was even higher than in WB TrxR, it can be also ruled out that TrxR indirectly renders G. lamblia more susceptible to metronidazole and furazolidone by causing oxidative stress through generation of superoxide radicals. It is evident, however, that the effect of TrxR overexpression on metronidazole and furazolidone susceptibility was only moderate. This finding is in line with the notion that several factors contribute to nitro drug reduction in G. lamblia and other microaerophilic parasites. One well documented example is nitroreductase 1 from G. lamblia which, when overexpressed, increases susceptibility to metronidazole quite to the same extent as shown here for TrxR (Nillius et al., 2011; reviewed in Leitsch, 2015).
Surprisingly, the direct effect of four of the five drugs on TrxR activity was minute, if at all measurable. Only auranofin, a compound known to inhibit G. lamblia TrxR effectively in vitro also inhibited TrxR in vivo to a relevant extent (Fig. 3). Still, even if auranofin was applied in very high concentrations (50 μM) the residual TrxR activity was still 4 to 5-times higher than in untreated wildtype (Fig. 3). Since wildtype and WB TrxR were equally susceptible to auranofin (Table 2), we conclude that TrxR, quite in contrast to the commonly held belief (Tejman-Yarden et al., 2013, Watkins and Eckmann, 2016), is not the primary target of this drug in G. lamblia.
Acknowledgements
David Leitsch was supported by grant J3492 of the Austrian Science Fund (FWF). Norbert Müller [NM] and Joachim Müller [JM] were supported by the Swiss National Science Foundation (grants SNF 31003A_138353 [NM and JM] and 31003A_163230 [NM]).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2016.07.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Buret A.G. Pathophysiology of enteric infections with Giardia duodenalis. Parasite. 2008;15:261–265. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2016. Parasites—Giardia.https://www.cdc.gov/parasites/giardia/ Last time accessed: June 27th. [Google Scholar]
- Debnath A., Parsonage D., Andrade R.M., He C., Cobo E.R., Hirata K., Chen S., García-Rivera G., Orozco E., Martínez M.B., Gunatilleke S.S., Barrios A.M., Arkin M.R., Poole L.B., McKerrow J.H., Reed S.L. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012;18:956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D. Drug Resistance in the microaerophilic parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015;2015(2):128–135. doi: 10.1007/s40475-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D., Burgess A.G., Dunn L.A., Krauer K.G., Tan K., Duchêne M., Upcroft P., Eckmann L., Upcroft J. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5- nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 2011;66:1756–1765. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D., Kolarich D., Wilson I.B.H., Altmann F., Duchêne M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. Plos. Biol. 2007;5:1820–1834. doi: 10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D., Kolarich D., Binder M., Stadlmann J., Altmann F., Duchêne M. Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol. Microbiol. 2009;72:518–536. doi: 10.1111/j.1365-2958.2009.06675.x. [DOI] [PubMed] [Google Scholar]
- Leitsch D., Schlosser S., Burgess A., Duchêne M. Nitroimidazole drugs vary in their mode of action in the human parasite Giardia lamblia. Intern. J. Parasitol. Drugs Drug Resis. 2012;2:166–170. doi: 10.1016/j.ijpddr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Ley S., Felger I., Hemphill A., Müller N. Identification of differentially expressed genes in a Giardia lamblia WB C6 clone resistant to nitazoxanide and metronidazole. J. Antimicrob. Chemother. 2008;2008(62):72–82. doi: 10.1093/jac/dkn142. [DOI] [PubMed] [Google Scholar]
- Müller J., Wastling J., Sanderson S., Müller N., Hemphill A. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 2007;51:1979–1986. doi: 10.1128/AAC.01548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–171. [PubMed] [Google Scholar]
- Nillius D., Müller J., Müller N. Nitroreductase (GlNR1) increasessusceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J. Antimicrob. Chemother. 2011;66:1029–1035. doi: 10.1093/jac/dkr029. [DOI] [PubMed] [Google Scholar]
- Rasoloson D., Vanacová S., Tomková E., Rázga J., Hrdý I., Tachezý J., Kulda J. Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis. Microbiology. 2002;48:2467–2477. doi: 10.1099/00221287-148-8-2467. [DOI] [PubMed] [Google Scholar]
- Schlosser S., Leitsch D., Duchêne M. Entamoeba histolytica: identification of thioredoxin- targeted proteins and analysis of serine acetyltransferase-1 as a prototype example. Biochem. J. 2013;451:277–288. doi: 10.1042/BJ20121798. [DOI] [PubMed] [Google Scholar]
- Štefanić S., Morf L., Kulangara C., Regös A., Sonda S., Schraner E., Spycher C., Wild P., Hehl A.B. Neogenesis and maturation of transient Golgi-like cisternae in a simple eukaryote. J. Cell Sci. 2009;122:2846–2856. doi: 10.1242/jcs.049411. [DOI] [PubMed] [Google Scholar]
- Tejman-Yarden N., Miyamoto Y., Leitsch D., Santini J., Debnath A., Gut J., McKerrow J.H., Reed S.L., Eckmann L. Auranofin, a reprofiled drug, is effective against metronidazole-resistant Giardia lamblia. Antimicrob. Agents Chemother. 2013;57:2029–2035. doi: 10.1128/AAC.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson S.M., Upcroft J.A., Upcroft P. Characterisation and purification of pyruvate: ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 1996;79:183–189. doi: 10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- Uzlikova M., Nohynkova E. The effect of metronidazole on the cell cycle and DNA in metronidazole-susceptible and -resistant Giardia cell lines. Mol. Biochem. Parasitol. 2014;198:75–81. doi: 10.1016/j.molbiopara.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Watkins R.R., Eckmann L. Treatment of giardiasis: current status and further directions. Curr. Infect. Dis. Rep. 2016;16:396. doi: 10.1007/s11908-014-0396-y. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO), model list of essential medicines, 19th list (April) 2015, http://www.who.int/selection_medicines/committees/expert/20/EML_2015_FINAL_amended_AUG2015.pdf?ua=1, Last time accessed: June 27th, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.