Abstract
There is now good evidence in several taxa that animal coloration positively reflects an individual's antioxidant capacity. However, even though telomeres, a marker of ageing, are known to be vulnerable to reactive oxygen species (ROS) attacks, no studies have ever assessed whether colour fading reflects the rate of biological ageing in any taxa. Here, we measured colour fading, telomere erosion (a measure of biological ageing) and ROS levels in painted dragons. We show that individuals that were better at maintaining their coloration during the three months of the study suffered a higher cost in terms of telomere erosion, but overall ROS levels measured at the start of the study were not significantly related to colour maintenance and telomere shortening. We therefore suggest that colour maintenance is a costly phenomenon in terms of telomere erosion, and that overall ROS levels do not seem to be a crucial component linking ornamental coloration and telomere erosion in our study system.
Keywords: colour fading, telomere erosion, reactive oxygen species
1. Introduction
Colour fading in a given breeding year is a common phenomenon in the animal kingdom. Birds, for example, grow colourful plumage during periodic moults and then retain their feathers for extended periods of time, during which they are subject to abrasion, degradation and soiling by many biotic and abiotic factors, leading to a decrease in coloration [1]. Many species also display colours in fleshy structures where seasonal colour fading can even be faster and more pronounced, often reflecting the current physiological state and environmental conditions experienced by their bearers [2]. As colourful traits are used by animals to assess the quality of another individual as a mate or competitor, late season social interactions may be strongly affected by this colour fading.
The fact that colour signals fade during the breeding season or, even disappear when the reproductive season is over, strongly suggests that sexual coloration is a costly phenomenon. These costs have been extensively studied within the last two decades and there is now good evidence that coloration and individual antioxidant capacity are positively connected in several taxa, even if the causal mechanisms behind this relationship are not always fully understood [3]. This association has been primarily studied in species displaying carotenoid-based coloration, but recent studies suggest that melanin, structural or pteridine-based colorations might also signal the ability to keep oxidative stress to acceptable levels [4,5], highlighting the widespread nature of this association. For example, in Australian painted dragons (Ctenophorus pictus), male coloration is primarily generated by carotenoid and pteridine pigments, and male colour fading is predicted by increasing levels of the reactive oxygen species (ROS) superoxide (SO), an effect that is abolished by supplementation with a superoxide dismutase mimetic that eliminates SO [6].
Besides their association with colourful traits, ROS have also been shown to be related to telomere erosion and the rate of biological ageing. Telomeres are non-coding DNA sequences found at the end of chromosomes that protect genome integrity, and increased ROS levels accelerate telomere erosion [7]. When telomeres are shortened to a critical length, a permanent arrest in the cell cycle occurs through cellular senescence, increasing risk factors for a large number of diseases [7]. Thus, telomere erosion is considered an essential component of the ageing phenotype.
Despite the fact that ROS levels have been shown to be associated with telomere erosion and the maintenance of coloration, no studies have ever assessed whether colour fading reflects the rate of biological ageing. Here, we repeatedly measured male coloration and telomere length (TL) in Australian painted dragons during the second half of the breeding season. In addition, we measured ROS levels to assess how they affect the rate of colour fading and telomere erosion. In this species, males only live for a year, during which their head colour declines throughout the year from a peak just after hibernation (from red/orange to brown/yellow from the start to the end of the breeding season [6]). Based on our previous results in this species [6], we predicted that individuals better able to maintain their coloration during the breeding season would present lower levels of ROS and thus a slower rate of telomere shortening than individuals with a high rate of colour fading.
2. Material and methods
Lizards were caught (n = 53, October 2014) at Yathong Nature Reserve, New South Wales (145°35′; 32°35′) and were brought back at the University of Sydney. Individuals were kept in tanks (60 × 60 × 50 cm) in three different rooms on a 12 L : 12 D regime and fed crickets and mealworms to satiation. At the start of the study (16 December 2014), lizards were weighed, measured snout to vent, and body condition was calculated as residuals from a mass–snout-to-vent length regression. Blood samples were collected at the start and end of the study (26 March 2015). For TL measurements, cell pellets were resuspended after centrifugation (10 min at 1000g to remove plasma) in 200 µl of PBS, put in 1 ml of RNA later and kept at −80°C until further analyses. TL was measured by quantitative real-time PCR (see the electronic supplementary material). To quantify ROS levels, 10 µl of blood was added to 90 µl of PBS and, within 2 h, we used flow cytometry in combination with the dihydrorhodamine 123 (DHR) probe (Invitrogen) to measure various ROS species, including singlet oxygen, SO, H2O2 and peroxynitrite, hereafter referred to as unspecific ROS (see [6] for a detailed description of the methods used to collect blood and measure ROS levels).
Coloration was quantified at the start and end of the study using digital photography, following standard published methods [8]. Painted dragon skin does not reflect in the UV, thus techniques that rely on visible-light are sufficient to capture variation in coloration in this species. Using a Nikon D810, two separate photographs were taken of the left side of the head at each time point in standardized conditions and digital images were imported into Adobe Photoshop to extract hue, brightness and saturation values (in the HSB colour space). Because Photoshop assigns hue values around a 360° colour wheel, with red set at 0, higher hue scores denote yellower individuals. Values for the two images of each lizard/time point were averaged for statistical analyses (repeatability = 0.99 [9]).
(a). Statistics
We ran three generalized linear mixed models (GLMM) using SAS v. 9.4. The first model assessed, at the start of the study, whether TL and ROS levels influenced coloration (hue). The second model assessed whether telomere erosion (TL at the end of the study − TL at the start of the study), coloration at the start of the study and ROS levels at the start of the study influenced the change of coloration during the study (difference in hue between the end and the start of the study). We also ran the same model on the change of brightness and saturation (see the electronic supplementary material for results). In our study system, a high value of hue reflects a yellower coloration, while small values of hue reflect a red/orange coloration. Thus, positive values in the change of coloration during the experiment reflect a decrease in coloration while negative values reflect an increase in coloration during the experiment (figure 1). Finally, we performed a third model to assess whether TL and ROS levels at the start of the study influenced the change of TL during the study. We included the room where the lizards were housed as a random factor in each model. Body condition was first included in every model and then removed because it never reached significance. Telomere data were log-transformed to reach homoscedasticity and residuals normality assumptions.
Figure 1.
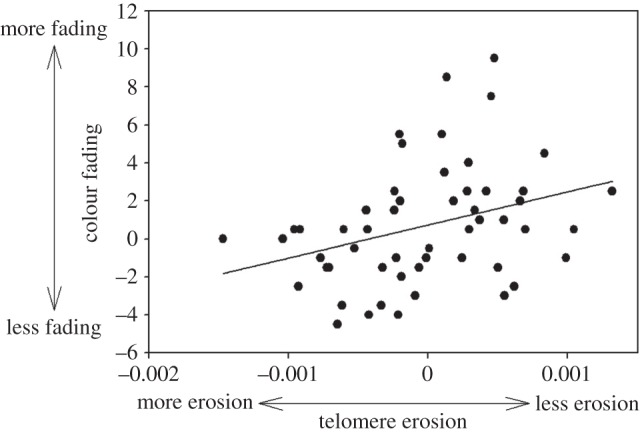
Relationships between telomere erosion (difference in TL between the end and the start of the study) and colour fading during the study (difference in hue between the end and the start of the study).
3. Results
At the start of the study, head coloration was not associated with TL, body condition or ROS levels (all p-values > 0.5). The change of coloration during the study was strongly positively predicted by coloration at the start of the study (F1,47 = 23.90, p < 10−4), telomere erosion (F1,47 = 5.64, p = 0.022) and tended to be positively predicted by ROS levels (F1,47 = 3.48, p = 0.068). Redder individuals at the start of the study suffered from a stronger colour loss and individuals better able to maintain their coloration suffered from a higher rate of telomere erosion (figure 1) and tended to have lower levels of ROS. Finally, the change of TL during the study was significantly and positively predicted by TL at the start of the study (individuals with longer telomeres had a higher telomere loss; F1,48 = 14.42, p < 10−3), but not by ROS levels (F1,48 = 0.71, p = 0.40).
4. Discussion
Contrary to our prediction, lizards that were better at maintaining their coloration, an important factor in social and sexually selected intraspecific interactions (coloration is associated with the probability of winning staged contests for resources (female or space) in painted dragons [10,11]), suffered increased telomere erosion. While trade-offs have been found between telomere maintenance and growth rates and longevity in other species [12,13], this is the first demonstration of a relationship consistent with a trade-off between telomere maintenance and a signalling trait. If telomere erosion is associated with early mortality in this species, then maintaining coloration with the associated benefits in terms of reproductive success but at the expense of longevity may be favoured if females store sperm [14]. In painted dragons, females store sperm across ovarian cycles and males may indeed sire offspring long after they have died [15]. Nonetheless, males whose colour has faded may live longer and experience fewer competitors later in the breeding season.
Coloration is primarily generated by carotenoid and pteridine pigments in Australian painted dragons and evidence suggests that the allocation of these two pigments might be connected with ROS biology and the antioxidant machinery [16,17]. Thus, in this study, individuals who constantly allocate substantial amounts of these pigments to maintain skin pigmentation might not be able to use them as antioxidants and would suffer from increased levels of oxidative stress and telomere shortening. However, the lack of relationships between ROS, telomere erosion and colour maintenance in our study is surprising, because telomere attrition and coloration are predicted to be mediated by ROS levels and ROS would represent a key link between ageing and condition-dependent signal expression [3]. In accordance with our result, Olsson et al. [6] have previously shown in this species that the rate of colour fading was not related to the overall ROS levels but was negatively correlated with SO levels and colour fading was prevented by supplementation with a superoxide dismutase mimetic. It thus seems that the balance between SO and superoxide dismutase might be a key underlying proximate mechanism controlling colour maintenance in our species. Unfortunately, SO levels were not specifically measured in this study and our overall measurement of ROS may not be accurate enough to detect potential associations between ROS production and telomere shortening and between ROS levels and colour maintenance.
In summary, we provide the first evidence of a trade-off between telomere erosion and a sexually dimorphic colour trait in an annual lizard. Future studies should now assess the cost of colour maintenance on telomere attrition in iteroparous species where an accelerated rate of ageing might be associated with earlier mortality and the loss of one or several breeding opportunities.
Supplementary Material
Ethics
This research was approved by the Animal Ethics Committee, Sydney University (#2013/6050) and animals were collected under a NSW National Parks & Wildlife Service scientific licence (SL100352).
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi:10.5061/dryad.11pp0 [18].
Authors' contributions
M.G., C.R.F., J.S., N.R. and M.O. collected the data; M.G., C.R.F. and M.O. designed the study; M.G. and M.O. analysed the data. C.M.W., N.R. and M.R.W. performed the laboratory analyses. M.G. wrote the manuscript. All authors agree to be held accountable for the content therein and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We thank the Australian Research Council for funding support to M.O.
References
- 1.McGraw KJ, Hill GE. 2004. Plumage color as a dynamic trait: carotenoid pigmentation of male house finches (Carpodacus mexicanus) fades during the breeding season. Can. J. Zool. 82, 734–738. ( 10.1139/z04-043) [DOI] [Google Scholar]
- 2.Velando A, Beamonte R, Torres R. 2006. Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149, 543–552. ( 10.1007/s00442-006-0457-5) [DOI] [PubMed] [Google Scholar]
- 3.von Schantz T, Bensch S, Grahn M, Wittzell H. 1999. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. Lond. B. 266, 1–12. ( 10.1098/rspb.1999.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvan I, Alonso-Alvarez C. 2009. The expression of melanin-based plumage is separately modulated by exogenous oxidative stress and a melanocortin. Proc. R. Soc. B 276, 3089–3097. ( 10.1098/rspb.2009.0774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopena R, Lopez P, Marin J. 2014. Relative contribution of dietary carotenoids and vitamin E to visual and chemical sexual signals of male Iberian green lizards: an experimental test. Behav. Ecol. Sociobiol. 68, 571–581. ( 10.1007/s00265-013-1672-9) [DOI] [Google Scholar]
- 6.Olsson M, Tobler M, Healey M, Perrin C, Wilson M. 2012. A significant component of ageing (DNA damage) is reflected in fading breeding colours: an experimental test using innate antioxidant mimetics in painted dragon lizards. Evolution 66, 2475–2483. ( 10.1111/j.1558-5646.2012.01617.x) [DOI] [PubMed] [Google Scholar]
- 7.Haussmann MF, Marchetto NM. 2010. Telomeres: linking stress and survival, ecology and evolution. Curr. Zool. 56, 714–727. [Google Scholar]
- 8.Lendvai ÁZ, Giraudeau M, Németh J, Bakó V, McGraw KJ. 2013. Carotenoid-based plumage coloration reflects feather corticosterone levels in male house finches. Behav. Ecol. Soc. 67, 1817–1824. ( 10.1007/s00265-013-1591-9) [DOI] [Google Scholar]
- 9.Lessells CM, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121. ( 10.2307/4087240) [DOI] [Google Scholar]
- 10.Healey M, Uller T, Olsson M. 2007. Seeing red: morph-specific contest success and survival rates in a colour-polymorphic agamid lizard. Anim. Behav. 74, 337–341. ( 10.1016/j.anbehav.2006.09.017) [DOI] [Google Scholar]
- 11.Healey M, Uller T, Olsson M. 2008. Variety is the spice of life: female lizards choose to associate with colour-polymorphic male dyads. Ethology 114, 231–237. ( 10.1111/j.1439-0310.2007.01469.x) [DOI] [Google Scholar]
- 12.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683. ( 10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 14.Uller T, Schwartz T, Koglin T, Olsson M. 2013. Sperm storage and sperm competition across ovarian cycles in the dragon lizard, Ctenophorus fordi. J. Exp. Zool. 319, 404–408. ( 10.1002/jez.1803) [DOI] [PubMed] [Google Scholar]
- 15.Olsson M, Schwartz T, Uller T, Healey M. 2009. Effects of sperm storage and male colour on probability of paternity in a polychromatic lizard. Anim. Behav. 77, 419–424. ( 10.1016/j.anbehav.2008.10.017) [DOI] [Google Scholar]
- 16.Tomášek O, Gabrielová B, Kačer P, Maršík P, Svobodová J, Syslová K, Vinkler M, Albrecht T. 2016. Opposing effects of oxidative challenge and carotenoids on antioxidant status and condition-dependent sexual signalling. Sci. Rep. 6, 23546 ( 10.1038/srep23546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oettl K, Reibnegger G. 2002. Pteridine derivatives as modulators of oxidative stress. Curr. Drug Metab. 3, 203–209. ( 10.2174/1389200024605127) [DOI] [PubMed] [Google Scholar]
- 18.Giraudeau M, Friesen C, Sudyka J, Rollings N, Whittington C, Wilson M, Olsson M. 2016. Data from Ageing and the cost of maintaining coloration in the Australian painted dragon. Dryad Digital Repository. ( 10.5061/dryad.11pp0) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi:10.5061/dryad.11pp0 [18].


