Abstract
Large consumers have ecological influence disproportionate to their abundance, although this influence in food webs depends directly on productivity. Evolutionary patterns at geologic timescales inform expectations about the relationship between consumers and productivity, but it is very difficult to track productivity through time with direct, quantitative measures. Based on previous work that used the maximum body size of Cenozoic marine invertebrate assemblages as a proxy for benthic productivity, we investigated how the maximum body size of Cenozoic marine mammals, in two feeding guilds, evolved over comparable temporal and geographical scales. First, maximal size in marine herbivores remains mostly stable and occupied by two different groups (desmostylians and sirenians) over separate timeframes in the North Pacific Ocean, while sirenians exclusively dominated this ecological mode in the North Atlantic. Second, mysticete whales, which are the largest Cenozoic consumers in the filter-feeding guild, remained in the same size range until a Mio-Pliocene onset of cetacean gigantism. Both vertebrate guilds achieved very large size only recently, suggesting that different trophic mechanisms promoting gigantism in the oceans have operated in the Cenozoic than in previous eras.
Keywords: gigantism, marine mammals, predators, escalation, fossil record
1. Introduction
Tracking primary productivity in the oceans over time is one of the major goals of palaeoceanography and palaeoecology because the rate of carbon fixation by marine primary producers depends on global patterns of ocean circulation, climate and ecosystem structure that have changed markedly over geologic time [1,2]. Despite the lack of direct metrics for primary productivity in past oceans, one promising indirect avenue is to measure the maximum body sizes of consumers (herbivores and planktivores) that benthic seaweeds and benthic photosymbiotic animals can sustain [3,4]. In this regard, consumer body size is a direct consequence of the available primary productivity within the sphere of influence of an individual consumer [3]. We argue that, over geologic time, patterns in the maximum body size of consumers indicate temporal changes in the enabling factor of primary productivity on the seafloor and in the pelagic zone.
Here, we evaluated the maximum sizes of two ecological groups of large, metabolically active marine consumers (bottom-feeding herbivorous and pelagic filter-feeding mammals) in the North Pacific and Atlantic oceans, two basins whose marine vertebrate record is adequate and well documented [5]. Vermeij [3] indicated that the history of gigantism in bottom-feeding molluscs, among several ecological guilds and trophic groups, differed between these two basins, and that maximum size became greater in the temperate North Pacific during or shortly before the Pliocene (5.3–2.6 Ma). Because marine vertebrates occupy broad geographical ranges, inferences of past primary productivity based on their maximum body sizes probably reflect global rather than more regional patterns chronicled by molluscs. Furthermore, temporal patterns in filter-feeding vertebrates should reflect productivity in the open ocean, whereas those of herbivores should reflect coastal productivity. Our findings imply a sharp rise in oceanic productivity and coastal North Pacific productivity during the Mio-Pliocene boundary, raising questions about the possible mechanisms underlying this surprisingly late increase.
2. Material and methods
We compiled maximal body size for two guilds of marine mammals in both the North Pacific and North Atlantic Ocean basins during the Cenozoic. We restricted our search to fossil-bearing rock units of the western and eastern coasts of these basins, while excluding fossils from the Mediterranean region and elsewhere (see electronic supplementary materials). We identified the largest single individual specimen known, either published or in a museum collection, binned by sub-epoch. Although these bins are unequal lengths of geologic time, this coarse scale permitted inter-basinal and cross-taxonomic comparisons. Known biasing factors in the fossil record (e.g. rock area, collecting efforts) do not appear to distort the diversity records of these groups, nor do they display strong ‘pull of the Recent’ signals (see electronic supplementary material, figure S1).
First, we examined large marine herbivorous mammals, comprising Sirenia and Desmostylia [6,7]. Comparability among fossil taxa in this guild is challenging because intact, associated skeletons are relatively rare and body plans differ sufficiently to prevent using traditional comparative proxies (i.e. post-Eocene sirenians lack weight-bearing hind limbs). We used cranial length as a proxy for body size, based on the strong allometric correlations known for sirenians [6]; we presume that similar allometries constrain desmostylian feeding ecology [7], given the ecomorphologic similarities in the rostrum and dentition of desmostylians and aquatic sloths (Thalassocnus spp.), and to a lesser extent, sirenians [8]. Although desmostylians probably retained some degree of terrestrial locomotion, multiple lines of evidence place their feeding palaeoecology firmly in aquatic environments [7], and thus in direct competition with sirenians, until the Late Neogene. Second, we collected skull width on mysticete cetaceans, which is a reliable size proxy [9] for the largest and exclusive members of the mammalian filter-feeding guild. We did not include so-called ‘toothed’ stem mysticetes in our dataset because it remains unclear whether they belonged to the same filter-feeding guild as baleen-bearing mysticetes (Eomysticetidae and crown-ward mysticetes).
3. Results and discussion
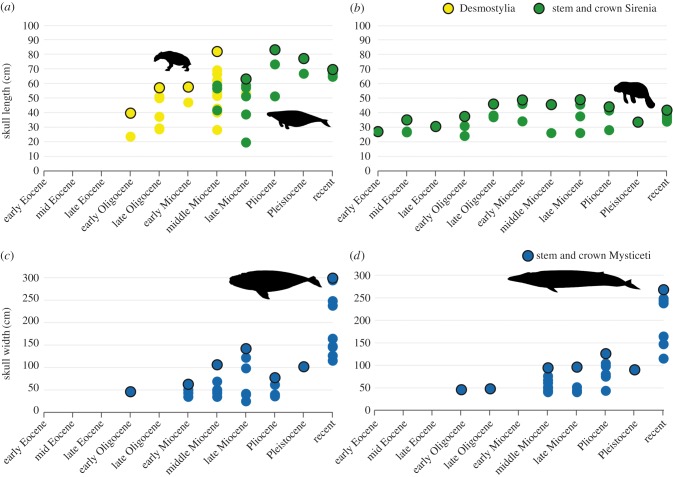
While marine mammal herbivores quickly attained maximum size in both ocean basins, this guild never achieved the large body sizes of mysticetes (figure 1 and table 1). In the North Pacific, the late and slight rise in body size in the Plio-Pleistocene is entirely attributable to Steller's sea cow (Hydrodamalis gigas) and fossil hydrodamalines [10], which postdate the extinction of desmostylians. While this rise may reflect an adaptive response to colder conditions at high latitude, desmostylians did attain similar sizes at temperate latitudes in the Mid Miocene (figure 1). North Atlantic sirenians never attained the maxima thresholds in the North Pacific. There are strong geographical differences in the maxima of this guild because fossil sirenians in the North Atlantic are primarily subtropical and tropical and associated with seagrasses [11], especially in the western Atlantic and Caribbean region, whereas herbivores in the North Pacific span temperate to sub-polar latitudes. By contrast, mysticetes remained within a narrow body size range until the Plio-Pleistocene, with marked size increases driven by the lineage leading to blue whales (Balaenoptera musculus), which lack a fossil record, and right whales (Eubalaena spp.), including a smaller Late Miocene relative, Eubalaena shinshuensis, from Japan (see electronic supplementary material, table S1). While not all mysticetes feed at the same trophic level, we argue that the high variability in their diet [12], over geologic time and geography, minimizes these differences and essentially averages them across the entire guild.
Figure 1.
(a,b) Maximal body size in North Pacific and North Atlantic marine mammal herbivores, and (c,d) similarly for mammalian filter-feeders, during the Cenozoic. PhyloPics of herbivores, except Hydrodamalis, by Steven Traver.
Table 1.
Maximal body size in North Pacific and North Atlantic marine mammal herbivores and mammalian filter-feeders during the Cenozoic. Measurements show cranial dimensions in centimetres; see the text and electronic supplementary material for details and data.
| North Pacific |
North Atlantic |
|||||||
|---|---|---|---|---|---|---|---|---|
| marine herbivore guild |
filter-feeding guild |
marine herbivore guild |
filter-feeding guild |
|||||
| recent | 69.5 | Sirenia | 299 | Mysticeti | 41.7 | Sirenia | 268 | Mysticeti |
| Pleistocene | 77 | Sirenia | 101.6 | Mysticeti | 33.5 | Sirenia | 90.2 | Mysticeti |
| Pliocene | 83 | Sirenia | 85 | Mysticeti | 44 | Sirenia | 126 | Mysticeti |
| late Miocene | 63 | Sirenia | 160 | Mysticeti | 48.9 | Sirenia | 96.2 | Mysticeti |
| mid Miocene | 81.8 | Desmostylia | 106 | Mysticeti | 45.5 | Sirenia | 94.5 | Mysticeti |
| early Miocene | 57.5 | Desmostylia | 62.5 | Mysticeti | 48.7 | Sirenia | 0 | none |
| late Oligocene | 57 | Desmostylia | 0 | none | 45.9 | Sirenia | 48 | Mysticeti |
| early Oligocene | 39.6 | Desmostylia | 46 | Mysticeti | 37.4 | Sirenia | 46 | Mysticeti |
| late Eocene | 0 | none | 0 | none | 30.5 | Sirenia | 0 | none |
| mid Eocene | 0 | none | 0 | none | 35 | Sirenia | 0 | none |
| early Eocene | 0 | none | 0 | none | 27 | Sirenia | 0 | none |
Generally, our findings fit with previous work showing a dramatic rise in maximal body size of vertebrate filter-feeders and other marine mammals, especially after the Mio-Pliocene boundary [9,13,14]. Data on plankton-feeding molluscs do not indicate a sharp increase in maximum size from the Pliocene to the Pleistocene as the mysticete data do, but instead show an increase in the later Miocene and Pliocene, especially in the North Pacific, North Atlantic and tropics [3]. The maximum sizes of suspension-feeding molluscs reflect primary productivity of coastal plankton, whereas those for mammals integrate productivities on an oceanic scale, from near- to offshore. Based on modern stranding records [15], there is no reason to expect biases against pelagic fossil marine mammals or oversampling of near-shore ones (i.e. habitats of sirenians and desmostylians [7]). Inter-oceanic differences in maximum size, and therefore by inference in pelagic productivity, are less severe than differences among basins in coastal benthic size and productivity.
Our results are consistent with the hypothesis that benthic primary production was higher in the temperate North Pacific than in the subtropical Atlantic from at least Early Oligocene time, a pattern inferred for temperate molluscs [4,16]. The North Pacific is the basin of origin for kelps (Laminariales), which include the largest marine plants (Nereocystis and Macrocystis). Kelps, especially the two large-bodied genera, grow rapidly, transport nutrients through phloem-like medullary tissues and are adapted to intense herbivory [4]. The coincident rise of kelps and large marine herbivorous mammals indicates a positive escalation, culminating in the relatively recent origin of Nereocystis and Macrocystis, probably in response to intense herbivory by desmostylians and sirenians [10]. The seagrasses on which Atlantic sirenians feed are also productive, but the plants are smaller and rates of production are lower than that for kelps. No marine mammalian herbivores have evolved in the temperate North Atlantic or in most of the Southern Hemisphere, despite the spread of Laminariales to these basins and of Macrocystis to Australasia and western South America [17]. Mio-Pliocene-age marine sloths (Thalassocnus spp.) that evolved in Peru and Chile never attained the large body sizes of North Pacific sirenians and probably fed on seagrasses [8]. While our data do not focus on the Southern Hemisphere, the improving fossil record of South American fossil marine vertebrates (e.g. [18,19]) will soon provide sufficient basis for such inter-hemispheric comparisons.
The general increase in maximum body size to a broad Neogene peak in herbivorous mammals appears to coincide with a rise in near-shore primary benthic productivity, especially in the North Pacific [3,4], where exceptionally large and productive seaweeds arose and diversified. Primary production near-shore was stimulated by increased runoff from the tectonically highly active continents during the Neogene [16]. The all-time maximum size of filter-feeding mammals coincides with the onset of large-scale glaciation in the Pleistocene, which together with continuing intense erosion and chemical weathering invigorated ocean circulation and productivity worldwide [20]. Intriguingly, mammalian body size patterns at sea are far delayed from those on land, where maxima were achieved relatively quickly following the end of the Cretaceous, across different lineages and continents [13].
We argue that maximal body size increases among marine mammals were enabled by increasing marine productivity in benthic and pelagic ecosystems during the Neogene. The largest herbivorous and filter-feeding marine animals are mammals, whose metabolic rates are higher than those of functionally equivalent ectothermic fishes [21], which mysticetes replaced as dominant guild members at least by the Neogene [22]. Cenozoic body size patterns may not be comparable with those in Mesozoic oceans, where the largest suspension feeders were smaller than their Cenozoic counterparts [22], and where marine herbivores did not evolve until the Cretaceous [23]. Although the largest guild members studied here outstripped Mesozoic body size maxima for marine reptiles, widespread modes of hypercarnivory within Mesozoic feeding guilds point to major differences in community size structuring, probably underpinned by differences in trophic structuring [24]. Such patterns may be broader in the fossil record than previously recognized [25].
Supplementary Material
Supplementary Material
Acknowledgements
We thank J. Vélez-Juarbe for help with data collection and four anonymous reviewers along with A. H. Fleming, J. A. Goldbogen, A. O'Dea, J. F. Parham, C. M. Peredo and J. Vélez-Juarbe for helpful comments.
Data accessibility
All additional data are in the electronic supplementary material file.
Authors' contributions
N.D.P collected the data. N.D.P. and G.J.V. analysed the data, wrote the manuscript, approved the final draft of the manuscript and agree to be held accountable for the content herein.
Competing interests
We have no competing interests.
Funding
N.D.P. is supported by the Smithsonian Institution, its Remington Kellogg Fund, and the Basis Foundation. All figures are our own.
References
- 1.Falkowski P, Knoll AH. 2011. Evolution of primary producers in the sea. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 2.Norris RD, Kirtland Turner S, Hull PM, Ridgwell A. 2013. Marine ecosystem responses to Cenozoic global change. Science 341, 492–498. ( 10.1126/science.1240543) [DOI] [PubMed] [Google Scholar]
- 3.Vermeij GJ. 2011. Shifting sources of productivity in the coastal marine tropics during the Cenozoic era. Proc. R. Soc. B 278, 2362–2368. ( 10.1098/rspb.2010.2362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeij GJ. 2012. The evolution of gigantism on temperate seashores. Biol. J. Linn. Soc. 106, 776–793. ( 10.1111/j.1095-8312.2012.01897.x) [DOI] [Google Scholar]
- 5.Uhen MD, Pyenson ND. 2007. Diversity estimates, biases, and historiographic effects: resolving cetacean diversity in the Tertiary. Palaeo. Electron. 10, 11–22. [Google Scholar]
- 6.Sarko DK, Domning DP, Marino L, Reep RL. 2010. Estimating body size of fossil sirenians. Mar. Mamm. Sci. 26, 937–959. ( 10.1111/j.1748-7692.2010.00384.x) [DOI] [Google Scholar]
- 7.Clementz MT, Hoppe KA, Koch PL. 2003. A paleoecological paradox: the habitat and dietary preferences of the extinct tethythere Desmostylus, inferred from stable isotope analysis. Paleobiology 29, 506–519. () [DOI] [Google Scholar]
- 8.de Muizon C, McDonald HG, Salas R, Urbina M. 2004. The evolution of feeding adaptations of the aquatic sloth Thalassocnus. J. Vert. Paleo. 24, 398–410. ( 10.1671/2429b) [DOI] [Google Scholar]
- 9.Pyenson ND, Sponberg SN. 2011. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. J. Mamm. Evol. 18, 269–288. ( 10.1007/s10914-011-9170-1) [DOI] [Google Scholar]
- 10.Domning DP. 1978. Sirenian evolution in the North Pacific Ocean. Univ. Calif. Pub. Geol. Sci. 118, 1–176. [Google Scholar]
- 11.Vélez-Juarbe J. 2014. Ghost of seagrasses past: using sirenians as a proxy for historical distribution of seagrasses. Palaeogeogr. Palaeoclimatol. Palaeoecol. 400, 41–49. ( 10.1016/j.palaeo.2013.05.012) [DOI] [Google Scholar]
- 12.Fleming AH, Clark CT, Calambokidis J, Barlow J. 2016. Humpback whale diets respond to variance in ocean climate and ecosystem conditions in the California Current. Glob. Change Biol. 22, 1214–1224. ( 10.1111/gcb.13171) [DOI] [PubMed] [Google Scholar]
- 13.Evans AR, et al. 2012. The maximum rate of mammal evolution. Proc. Natl Acad. Sci. USA 109, 4187–4190. ( 10.1073/pnas.1120774109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churchill M, Clementz MT, Kohno N. 2015. Cope's rule and the evolution of body size in Pinnipedimorpha (Mammalia: Carnivora). Evolution 69, 201–215. ( 10.1111/evo.12560) [DOI] [PubMed] [Google Scholar]
- 15.Pyenson ND. 2011. The high fidelity of the cetacean stranding record: insights into measuring diversity by integrating taphonomy and macroecology. Proc. R. Soc. B 278, 3608–3616. ( 10.1098/rspb.2011.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs DK, Haney TA, Louie KD. 2004. Genes, diversity, and geologic process on the Pacific coast. Annu. Rev. Earth Planet Sci. 32, 601–652. ( 10.1146/annurev.earth.32.092203.122436) [DOI] [Google Scholar]
- 17.Bolton JJ. 2010. The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular phylogenetics. Helgoland Mar. Res. 64, 263–279. ( 10.1007/s10152-010-0211-6) [DOI] [Google Scholar]
- 18.Villafaña JA, Rivadeneira MM. 2014. Rise and fall in diversity of Neogene marine vertebrates on the temperate Pacific coast of South America. Paleobiology 40, 659–674. ( 10.1666/13069) [DOI] [Google Scholar]
- 19.Pyenson ND, et al. 2014. Repeated mass strandings of Miocene marine mammals from Atacama Region of Chile point to sudden death at sea. Proc. R. Soc. B 281, 20133316 ( 10.1098/rspb.2013.3316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman F, Seward D, Valla PG, Carter A, Kohn B, Willett SD, Ehlers TA. 2013. Worldwide acceleration of mountain erosion under a cooling climate. Nature 504, 423–426. ( 10.1038/nature12877) [DOI] [PubMed] [Google Scholar]
- 21.Vermeij GJ. 2016. Gigantism and its implications for the history of life. PLoS ONE 11, e0146092 ( 10.1371/journal.pone.0146092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman M, Shimada K, Martin LD, Everhart MJ, Liston J, Maltese A, Triebold M. 2010. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 327, 990–993. ( 10.1126/science.1184743) [DOI] [PubMed] [Google Scholar]
- 23.Parham JF, Pyenson ND. 2010. New sea turtle from the Miocene of Peru and the iterative evolution of feeding ecomorphologies since the Cretaceous. J Paleo. 84, 231–247. ( 10.1666/09-077R.1) [DOI] [Google Scholar]
- 24.Kelley NP, Pyenson ND. 2015. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science 348, aaa3716. ( 10.1126/science.aaa3716) [DOI] [PubMed] [Google Scholar]
- 25.Klug C, de Baets K, Kröger B, Bell M, Korn D, Payne J. 2014. Normal giants? Temporal and latitudinal shifts of Palaeozoic marine invertebrate gigantism and global change. Lethaia 48, 267–288. ( 10.1111/let.12104) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All additional data are in the electronic supplementary material file.