Abstract
The initial stages of a disease outbreak can determine the magnitude of the ensuing epidemic. Though rarely tested in unison, two factors with important consequences for the transmission dynamics of infectious agents are the collective traits of the susceptible population and the individual traits of the index case (i.e. ‘patient zero’). Here, we test whether the personality composition of a social group can explain horizontal transmission dynamics of cuticular bacteria using the social spider Stegodyphus dumicola. We exposed focal spiders of known behavioural phenotypes with a GFP-transformed cuticular bacterium (Pantoea sp.) and placed them in groups of 10 susceptible individuals (i.e. those with no experience with this bacterium). We measured bacterial transmission to groups composed of either all shy spiders, 10% bold spiders or 40% bold spiders. We found that colonies with 40% bold spiders experienced over twice the incidence of transmission compared to colonies with just 10% bold individuals after only 24 h of interaction. Colonies of all shy spiders experienced an intermediate degree of transmission. Interestingly, we did not detect an effect of the traits of the index case on transmission. These data suggest that the phenotypic composition of the susceptible population can have a greater influence on the degree of early transmission events than the traits of the index case.
Keywords: group composition, horizontal transmission, index case, patient zero, social spider
1. Introduction
The transmission of microbial agents from exposed to susceptible hosts represents one of the central forces regulating infectious disease dynamics. It has long been recognized that hosts vary in their likelihood of acquiring and transmitting infectious agents in both human and wildlife diseases [1,2]. Recently, variation among hosts in their behavioural traits and social tendencies have emerged as potential explanations for heterogeneity in disease susceptibility and transmission potential [3,4]. Few studies, though, link the behavioural traits of infected and susceptible individuals to identify combinations that may facilitate the transmission of microbes.
A posteriori studies using molecular tools or theoretical modelling have identified that the actions of one or a few infected individuals are often important in the early stages of epidemics [5–7]. Likewise, populations harbouring a greater diversity of genotypes [8], behavioural phenotypes [9] and patterns of social interactions [10,11] can reduce the spread of infectious agents. However, few studies have compared the relative influence of the individual traits of the index case versus the collective traits of the susceptible population (i.e. its phenotypic composition) in determining the degree of transmission.
Here, we use the social spider Stegodyphus dumicola as a model to test how the behavioural composition of a social group affects the transmission of a fluorescent cuticular bacterium from the first exposed individual. Previous work demonstrated that cuticular bacterial transmission between two individuals is more likely from bolder to shyer spiders and to individuals in better body condition [12]. Thus, we hypothesize that (i) groups composed of a mixture of personality types and body conditions will exhibit a greater incidence of bacterial transmission and (ii) that bacterial transmission will be greater when a bolder spider is the index case.
2. Material and methods
Stegodyphus dumicola is a southwestern African social spider that lives in colonies of several hundred individuals that cooperate in collective foraging, web-building, and reproduction [13]. We collected 11 colonies along roadside Acacia trees in the Northern Cape of South Africa in October 2015. Colonies were transported to the University of Pittsburgh and fed domestic crickets ad libitum. Individual adult S. dumicola females were isolated into 30 ml plastic housing cups before the onset of behavioural assays.
‘Boldness’ is a repeatable behavioural trait (i.e. ‘personality type’, repeatability ≈0.63; [14]) which impacts colonies' ability to attack prey effectively [15]. We measured boldness by placing a spider in a clear plastic arena and administering two rapid puffs of air atop the spider using an infant nose-cleaning bulb, after a 30 s acclimation period. Spiders then halt movement and their latency to resume activity estimates their boldness. Here, we identify bold individuals as those that resume movement within 1–200 s and shy individuals as those that take 600 s or more to resume activity (similar to [15]). We measured spiders' mass and prosoma width to estimate their body conditions using the residuals of a linear regression of body mass and body size [16].
To examine bacterial transmission dynamics, we constructed 53 colonies of 10 spiders each in one of three personality compositions: all shy (n = 18), 10% bold (n = 18) or 40% bold (n = 17). Spiders originated from seven different source colonies, and we did not mix spiders from different source colonies. Colonies were housed in plastic containers with a wire substrate to facilitate web-building. After 24 h, we added to each colony a spider to serve as the ‘index case’, i.e. a spider of known behavioural type (n = 26 bold; n = 27 shy) which had been topically exposed to a cuticular bacterium, Pantoea sp., that had been transformed via electroporation with a plasmid containing the reporter genes for green fluorescent protein and ampicillin resistance (methods described in [12,17]). We exposed the index case to the bacterium by submerging it in a liquid bacterial solution (approx. 109 CFU ml−1 in phosphate-buffered saline) for 3 s and leaving it to dry for 24 h before transferring it to a colony. After 20 h, we noted whether or not the index case was in body contact with any susceptible individuals in the social group. After a further 4 h (24 h total interaction time), we tested for the presence of the transformed Pantoea on each of the susceptible spiders' cuticles. We vortexed each spider separately in 1 ml of sterile-selective LB broth (100 µg ml−1 ampicillin, 20% arabinose) for 10 s and incubated the solution for 20 h at 30°C. Green fluorescence in this solution indicates the presence of the transformed Pantoea on the spider's cuticle. Group-wide bacterial transmission was quantified as the proportion of individuals on which we identified the transformed bacteria, not including the index case.
We used a generalized linear mixed model (GLMM) to determine how group-wide bacterial transmission was affected by group composition, boldness of the index case, the interaction term between them, body condition of the index case and mean colony body condition. The proportions of individuals exposed to bacteria were arcsine square root transformed to meet model assumptions. We analysed the number of instances where susceptible spiders were observed in contact with the index case using a nominal logistic regression with the same independent variables listed above. Experimental group ID nested in source colony ID was included as a random effect in each model. The experiment was conducted in two consecutive trials, so we also included trial number as a random effect. Data used for all analyses can be found in the Dryad Digital Repository [18].
3. Results and discussion
Groups' behavioural compositions significantly influenced bacterial transmission (GLMM: F2,41 = 6.93, p = 0.003; table 1). Colonies of 40% bold spiders experienced twice the incidence of bacterial transmission compared to groups with only 10% bold individuals, while colonies with all shy spiders experienced an intermediate level (Tukey's post-hoc test: Q = 2.43, p < 0.05; figure 1a). However, neither the boldness nor the body condition of the index case significantly influenced bacterial transmission (figure 1a and table 1a). The index case was more likely to rest in contact with susceptible spiders in colonies of all shy individuals than in the other two group compositions (nominal logistic regression: χ2 = 7.65, d.f. = 2, p = 0.02; figure 1b), regardless of whether the index case was bold or shy (table 1b).
Table 1.
Summary for statistical models predicting (a) colony-wide bacterial transmission and (b) contacts with the index case while resting. Significant effects in italic.
| effect | F-value | d.f. | p-value |
|---|---|---|---|
| (a) colony-wide bacterial transmission | |||
| boldness of index case | 0.60 | 1, 41 | 0.44 |
| colony composition | 6.93 | 2, 41 | 0.003 |
| colony composition × index boldness | 0.85 | 2, 41 | 0.44 |
| body condition of index case | 2.97 | 1, 41 | 0.09 |
| colony mean body condition | 2.66 | 1, 41 | 0.11 |
| effect | χ2 | d.f. | p-value |
|---|---|---|---|
| (b) contact with index case | |||
| boldness of index case | 0.31 | 1 | 0.58 |
| colony composition | 7.65 | 2 | 0.02 |
| colony composition × index boldness | 2.48 | 2 | 0.29 |
| body condition of index case | 0.09 | 1 | 0.77 |
| colony mean body condition | 2.87 | 1 | 0.09 |
Figure 1.
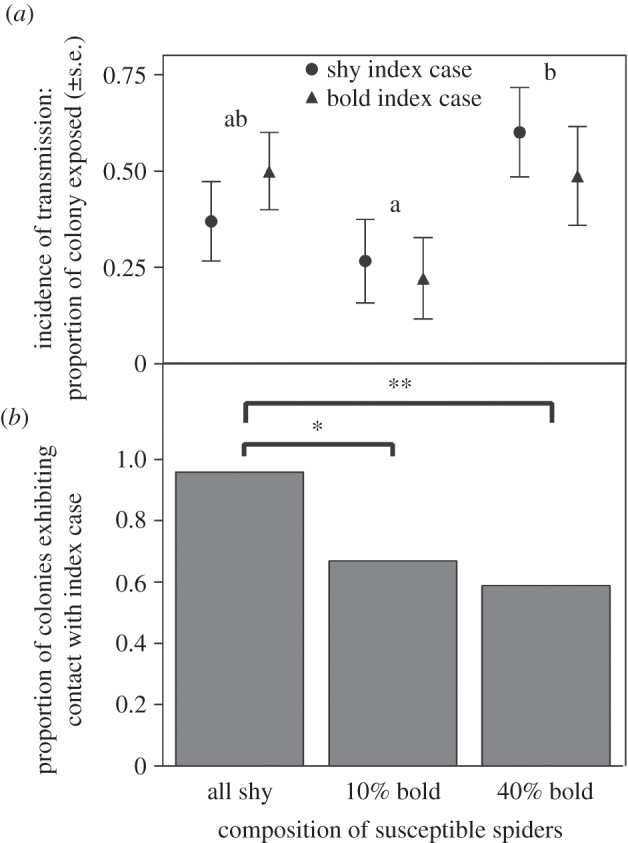
(a) Colonies containing 40% bold spiders experienced the greatest incidence of colony-wide bacterial transmission. That is, the proportion of spiders from which fluorescent Pantoea was collected was greatest in colonies of 40% bold spiders, regardless of the behavioural type of the index case. Colony compositions with different letter headings denote statistically different values. (b) Colonies containing all shy spiders were more likely to be found in physical contact with the index case, *p < 0.05, **p < 0.01.
The actions of just a few key individuals during early stages of an epidemic may have a large influence over its trajectory and magnitude [19]. Here, it appears that group personality composition is more important than the personality of the index case in predicting bacterial transmission. Modelling approaches have demonstrated that the traits of the index case can influence their infectiousness in a heterogeneous population [20], and that transmission can depend on their social connectedness [21]. We were unable to differentiate transmission events that arose from the index case versus those that arose from secondary or tertiary events from newly exposed spiders. However, it appears that bodily contact with the index case is not the only likely explanation for colony-wide transmission (figure 1). Further, some of the transmission events may have occurred indirectly via the environment (contaminated silk) or via social contacts that we failed to observe [12].
Interestingly, group personality composition may drive trade-offs among ecological challenges. In S. dumicola, a greater proportion of bold spiders in the colony facilitates more effective collective foraging [15,22]. Here, a greater number of bold spiders in the group facilitated more widescale transmission of bacteria. Thus, the group compositions that promote success in the context of collective foraging also experience the greatest incidence of bacterial transmission. Such transmission is potentially costly because increased bacterial load on key group members can hinder a group's ability to execute important collective behaviours like foraging and web-building [23]. Natural colonies of S. dumicola harbour a small proportion of bold individuals [24]. We reason that having a small proportion of bold individuals, as compared with a large proportion of bold individuals or none at all, may provide benefits to colonies in the context of foraging while imposing the smallest costs with respect to bacterial transmission. Taken together, indirect evidence suggests that group traits like phenotypic composition may drive trade-offs in group performance, ensuring that no group composition experiences superior performance in all contexts/environments [25].
Acknowledgements
We thank the South Africa Department of Tourism, Environment, and Conservation for providing permits for animal collection (FAUNA 1690/2014). We thank Sara Geary, Michael Ziemba, and Jacob Kaminski for laboratory assistance.
Ethics
All activities were conducted in compliance with all relevant guidelines for the care and use of invertebrate study species.
Data accessibility
The data associated with this manuscript are deposited in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.g56g1).
Authors' contributions
C.N.K. and K.A.H. carried out the experiment; C.N.K. transformed the bacteria and performed statistical analyses; N.P.W. and J.N.P. assisted in experimental design and manuscript preparation. All authors gave final approval for publication and agreed to be accountable for all aspects of the work.
Competing interests
We declare that we have no competing interests.
Funding
Funding for this research was provided by the University of Pittsburgh and the National Science Foundation IOS grant nos. 1352705 and 1455895 to J.N.P. and 1456010 to N.P.W.
References
- 1.Woolhouse ME, et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342. ( 10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White LA, Forester JD, Craft ME. 2015. Using contact networks to explore mechanisms of parasite transmission in wildlife. Biol. Rev. ( 10.1111/brv.12236) [DOI] [PubMed] [Google Scholar]
- 3.Kortet R, Hedrick AV, Vainikka A. 2010. Parasitism, predation and the evolution of animal personalities. Ecol. Lett. 13, 1449–1458. ( 10.1111/j.1461-0248.2010.01536.x) [DOI] [PubMed] [Google Scholar]
- 4.Barron D, Gervasi S, Pruitt J, Martin L. 2015. Behavioral competence: how host behaviors can interact to influence parasite transmission risk. Curr. Opin. Behav. Sci. 6, 35–40. ( 10.1016/j.cobeha.2015.08.002) [DOI] [Google Scholar]
- 5.Faria NR, et al. 2016. Zika virus in the Americas: early epidemiological and genetic findings. Science 352, 345–349. ( 10.1126/science.aaf5036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley S, et al. 2003. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science 300, 1961–1966. ( 10.1126/science.1086478) [DOI] [PubMed] [Google Scholar]
- 7.Adelman JS, Moyers SC, Farine DR, Hawley DM. 2015. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. R. Soc. B 282, 20151429 ( 10.1098/rspb.2015.1429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Power AG. 1991. Virus spread and vector dynamics in genetically diverse plant populations. Ecology 72, 232–241. ( 10.2307/1938917) [DOI] [Google Scholar]
- 9.Dizney L, Dearing MD. 2013. The role of behavioural heterogeneity on infection patterns: implications for pathogen transmission. Anim. Behav. 86, 911–916. ( 10.1016/j.anbehav.2013.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins SE, Cagnacci F, Stradiotto A, Arnoldi D, Hudson PJ. 2009. Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. J. Anim. Ecol. 78, 1015–1022. ( 10.1111/j.1365-2656.2009.01557.x) [DOI] [PubMed] [Google Scholar]
- 11.Bansal S, Grenfell BT, Meyers LA. 2007. When individual behaviour matters: homogeneous and network models in epidemiology. J. R. Soc. Interface 4, 879–891. ( 10.1098/rsif.2007.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiser CN, Pinter-Wollman N, Augustine DA, Ziemba MA, Lawrence JG, Pruitt JN. 2016. Individual differences in boldness influence patterns of social interactions and the transmission of cuticular bacteria among group-mates. Proc. R. Soc. B 283, 20160457 ( 10.1098/rspb.2016.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilde T, Coates K, Birkhofer K, Bird T, Maklakov A, Lubin Y, Aviles L. 2007. Survival benefits select for group living in a social spider despite reproductive costs. J. Evol. Biol. 20, 2412–2426. ( 10.1111/j.1420-9101.2007.01407.x) [DOI] [PubMed] [Google Scholar]
- 14.Keiser CN, Modlmeier AP, Singh N, Jones DK, Pruitt JN. 2014. Exploring how a shift in the physical environment shapes individual and group behavior across two social contexts. Ethology 120, 825–833. ( 10.1111/eth.12256) [DOI] [Google Scholar]
- 15.Keiser CN, Pruitt JN. 2014. Personality composition is more important than group size in determining collective foraging behaviour in the wild. Proc. R. Soc. B 281, 20141424 ( 10.1098/rspb.2014.1424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakob EM, Marshall SD, Uetz GW. 1996. Estimating fitness: a comparison of body condition indices. Oikos 77, 61–67. ( 10.2307/3545585) [DOI] [Google Scholar]
- 17.Keiser CN, Shearer TA, DeMarco AE, Brittingham HA, Knutson KA, Kuo C, Zhao K, Pruitt JN. 2016. Cuticular bacteria appear detrimental to social spiders in mixed but not monoculture exposure. Curr. Zool. ( 10.1093/cz/zow015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keiser CN, Howell KA, Pinter-Wollman N, Pruitt JN. 2016. Data from: Personality composition alters the transmission of cuticular bacteria in social groups. Dryad Digital Repository. ( 10.5061/dryad.g56g1) [DOI] [PMC free article] [PubMed]
- 19.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz W. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diekmann O, Heesterbeek J, Metz JA. 1990. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 28, 365–382. ( 10.1007/BF00178324) [DOI] [PubMed] [Google Scholar]
- 21.Ciccolini M, Dahl J, Chase-Topping ME, Woolhouse MEJ. 2012. Disease transmission on fragmented contact networks: livestock-associated methicillin-resistant Staphylococcus aureus in the Danish pig-industry. Epidemics 4, 171–178. ( 10.1016/j.epidem.2012.09.001) [DOI] [PubMed] [Google Scholar]
- 22.Wright CM, Keiser CN, Pruitt JN. 2015. Personality and morphology shape task participation, collective foraging and escape behaviour in the social spider Stegodyphus dumicola. Anim. Behav. 105, 47–54. ( 10.1016/j.anbehav.2015.04.001) [DOI] [Google Scholar]
- 23.Keiser CN, Wright CM, Pruitt JN. 2016. Increased bacterial load can reduce or negate the effects of keystone individuals on group collective behaviour. Anim. Behav. 114, 211–218. ( 10.1016/j.anbehav.2016.02.010) [DOI] [Google Scholar]
- 24.Pinter-Wollman N, Keiser CN, Wollman R, Pruitt JN. 2016. The effect of keystone individuals on tradeoffs between collective outcomes can be mediated through interaction rules or behavioral persistence. Am. Nat. ( 10.1086/687235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrish JK, Edelstein-Keshet L. 1999. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101. ( 10.1126/science.284.5411.99) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with this manuscript are deposited in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.g56g1).


