Abstract
We studied the evolution of developmental plasticity in populations of Drosophila melanogaster that evolved at either constant or fluctuating temperatures. Consistent with theory, genotypes that evolved at a constant 16°C or 25°C performed best when raised and tested at that temperature. Genotypes that evolved at fluctuating temperatures performed well at either temperature, but only when raised and tested at the same temperature. Our results confirm evolutionary patterns predicted by theory, including a loss of plasticity and a benefit of specialization in constant environments.
Keywords: experimental evolution, Drosophila, performance, plasticity, temperature, selection
1. Background
Optimality models predict that species evolve to perform best in the range of conditions they experience most frequently [1,2]. A generalist, which performs well under many conditions, would only outperform a specialist when conditions fluctuate from generation to generation [3]. However, generalists must spend more energy to function at different extremes [4], usually by making isoforms of proteins and stabilizing the cellular environment [5]. This energetic cost favours a genotype that anticipates future conditions and develops the appropriate phenotype [6], a process called developmental plasticity. The cost of sensing environmental change and the risk of misinterpreting this change offset the benefit of developmental plasticity [7]. Thus, developmental plasticity should evolve when environments fluctuate slowly and reliably.
Ideally, hypotheses about the evolution of developmental plasticity would be tested by manipulating environmental fluctuations and observing genetic changes in plasticity [8,9]. However, experimental studies of evolution in fluctuating environments have focused on unicellular organisms ([10–13], but see [14]), which develop far less than plants and animals do. Thus, we lack sufficient evidence to infer whether the plasticity of multicellular organisms evolves according to hypothetical costs and benefits.
Here, we evaluated the evolutionary theory of developmental plasticity with experimental populations of Drosophila melanogaster that evolved at either constant or fluctuating temperatures [15]. During experimental evolution, fluctuations in temperature occurred between generations, analogous to the seasonal variation in temperature that occurs in temperate environments. Following evolution, we compared the thermal sensitivity of flight performance [16] between genotypes from fluctuating environments and genotypes from constant environments. Our experimental design enabled us to tease apart the genetic, developmental and acute effects of temperature on performance.
2. Material and methods
We studied populations of D. melanogaster that evolved at either a constant 16°C (C populations; n = 5), a constant 25°C (H populations; n = 5), or temporal fluctuations between 16°C and 25°C (T populations; n = 5). Temporal fluctuations were achieved by moving populations between 16°C and 25°C every four weeks. These populations were sampled after 32 and 64 generations at 16°C and 25°C, respectively; populations at fluctuating temperatures experienced an intermediate number of generations. Yeaman et al. [15] described the origin and maintenance of the experimental populations. Isofemale lines were founded by pairing virgin flies from each population [17]. These lines were subsequently transferred to vials of fresh medium every three weeks.
Our experiment included 6–8 isofemale lines from each population (31, 31 and 38 lines from T, C and H populations, respectively). We controlled the density of each line for two generations by mating two pairs of flies in a new vial. These adults were removed after 48 h to limit the density of offspring. Seven days after offspring emerged from pupae, females from each line were transferred to fresh vials kept at 20.5°C—a temperature that lies between those experienced during evolution. After two generations, flies were divided randomly between an incubator set at 16°C and another set at 25°C. About 7–10 days after adults emerged, we measured performances of two females of each isofemale line from each developmental temperature.
We quantified flight performances at 16°C and 25°C in a custom chamber located in a temperature-controlled room. Twenty-four hours before testing, each fly was anaesthetized with CO2, transferred to a new vial of medium and placed in an environmental room set at the test temperature (±0.3°C). The next day, each fly was transferred without anaesthesia to an empty vial just prior to testing performance. The flight chamber (25.4 × 25.4 × 25.4 cm) was constructed from clear acrylic, with a circular opening of 2.5 cm in diameter at the top. This opening was temporarily sealed by a movable plate. A vial with a fly was inverted on top of this plate. When the fly was on the wall of the vial, the plate was slid aside and the fly was tapped into the chamber. Flies either fell uncontrollably or flew to a surface. To ensure objectivity, a fall was scored when a fly landed on the floor within 10 cm of the point below the vial. The order of testing at each temperature was randomized among selective treatments, experimental populations, and isofemale lines.
To model the probability of flight, we fit a generalized linear mixed model using the nlme library of the R Statistical Package [18]. Selective treatment, developmental temperature, and test temperature were fixed factors. Isofemale line was a random factor, nested within the random factor of experimental population. Following Burnham & Anderson [19], we used multimodel averaging to estimate the most likely values of means. First, we estimated the most likely random effects according to Zuur et al. [20]. Then, we used the MuMIn library [21] to fit all possible sets of fixed effects to the data. Finally, we calculated the Akaike information criterion and Akaike weight of each model (table 1), the latter being the probability that the model best describes the data. The weighted average of each parameter, including estimates from all models, was used to calculate the mean for each group. This approach eliminates the need to interpret p-values, because all models (including the null model) contributed to the most likely value of each mean.
Table 1.
All likely models included an effect of selective treatment on flight performance. Likely models are ranked according to their Akaike information criterion (AICc). For each model, we provide the Akaike weight, which equals the probability that the model describes the data better than other models. All models contained an intercept and error terms associated with experimental population and isofemale line.
| model | parameters | log likelihood | AICc | ΔAICc | Akaike weight |
|---|---|---|---|---|---|
| (1) dev temp + test temp + selection + (dev temp × test temp) + (dev temp × selection) + (test temp × selection) | 12 | −340.3 | 705.1 | 0 | 0.62 |
| (2) dev temp + test temp + selection + (dev temp × test temp) + (dev temp × selection) + (test temp × selection) + (dev temp × test temp × selection) | 14 | −339.6 | 707.9 | 2.76 | 0.16 |
| (3) dev temp + test temp + selection + (dev temp × test temp) + (test temp × selection) | 10 | −344.5 | 709.4 | 4.28 | 0.07 |
| (4) dev temp + test temp + selection + (dev temp × test temp) + (dev temp × selection) | 10 | −344.7 | 709.8 | 4.68 | 0.06 |
| (5) dev temp + test temp + (dev temp × test temp) | 6 | −348.9 | 709.8 | 4.74 | 0.06 |
| (6) dev temp + test temp + selection + (dev temp × test temp) | 8 | −347.8 | 711.8 | 6.69 | 0.02 |
3. Results and discussion
Our results confirm major patterns of evolution predicted by theory, including a loss of developmental plasticity and a benefit of thermal specialization in constant environments (figure 1). Regardless of their evolutionary background, flies performed worse when raised at one temperature and subsequently tested at another temperature, suggesting that flies benefitted from developmental responses to temperature [22]. Adaptive plasticity was most pronounced in genotypes from T populations, which evolved at fluctuating temperatures. When raised and tested at 16°C, these genotypes performed almost as well as genotypes from C populations. Moreover, when raised and tested at 25°C, genotypes from T populations performed as well as genotypes from H populations. However, this adaptive plasticity was associated with a loss of performance at other test temperatures. When raised at 16°C, flies from T populations performed better at 16°C but worse at 25°C than did flies raised at 25°C. Conversely, when raised at 25°C, flies from T populations performed better at 25°C but worse at 16°C than did flies raised at 16°C. The best performance was observed in flies that evolved, developed and were tested at 25°C, supporting the view that ‘hotter is better’ [23].
Figure 1.
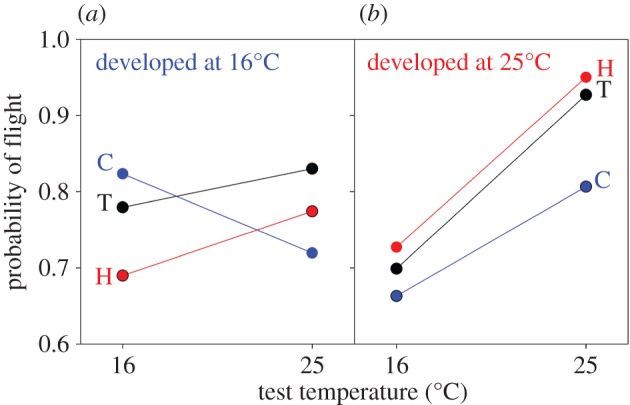
Flies performed best when they were raised and tested at the temperature experienced by their ancestors. Data are mean probabilities of flight estimated from multimodel averaging. Data for flies that developed at (a) 16°C and (b) 25°C. The text next to each datum denotes the selective environment (H, C or T).
Constant environments should favour specialists over generalists when a cost of generalization exists [1,3]. Accordingly, genotypes from C populations or H populations performed best when raised and tested at 16°C or 25°C, respectively. In fact, flies from the C populations were more likely to perform successfully when raised and tested at 16°C than when raised and tested at any other combination of temperatures. No other group of flies performed better at 16°C than at 25°C, suggesting that the optimal temperature for performance decreased during the evolution of C populations. Furthermore, genotypes from either C or H populations performed poorly at either temperature when raised at a temperature that differed from their selective environment. Thus, selection for specialization resulted in genotypes that produced poor phenotypes, in general, when developing at a novel temperature, a pattern referred to as detrimental acclimation [24]. Such clear costs of thermal adaptation, though commonly assumed [25,26], have rarely been documented through comparative or experimental studies of closely related populations (e.g. see [27,28]). More often, adaptation of performance at an extreme temperature occurs without a large loss of performance at other temperatures [29,30].
The evolution of developmental plasticity likely involves mechanisms for expressing genes that promote physiological functions in extreme conditions. Several biochemical mechanisms could account for plastic responses to temperature, such as the ability to express protein isoforms [31], adjust membrane fluidity [32] or regulate mitochondrial density [33]. In a previous experiment, flies from our T populations adjusted membrane fluidity to developmental temperature more readily than did flies from our C or H populations [34]. However, this effect resulted primarily from differences in fluidity after developing at 25°C, with all populations having similar fluidity when developing at 16°C. Thus, this biochemical change can only partially explain our results. Additional studies of biochemical mechanisms are needed to understand the mutations required for developmental plasticity to evolve.
Acknowledgements
We thank Sam Yeaman and Michael Whitlock for providing the selection lines. Brandon Cooper and Catrina Condon helped to maintain isofemale lines. Arianne Cease granted access to her environmental chamber.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.6rk67 [35].
Authors' contributions
All authors designed the study, interpreted results, revised the manuscript, and approved the final version. Data were collected by J.L.V.T. and analysed by M.J.A. Both J.L.V.T. and M.J.A. wrote early drafts. All authors approved the final version of the manuscript and agreed to be accountable for its content.
Competing interests
We have no competing interests.
Funding
Research was supported by Barrett Honors College and Midwestern University.
References
- 1.Lynch MJ, Gabriel W. 1987. Environmental tolerance. Am. Nat. 129, 283–303. ( 10.1086/284635) [DOI] [Google Scholar]
- 2.Levins R. 1968. Evolution in changing environments: some theoretical explorations. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Gilchrist GW. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270. ( 10.1086/285797) [DOI] [Google Scholar]
- 4.Angilletta MJ, Wilson RS, Navas CA, James RS. 2003. Tradeoffs and the evolution of thermal reaction norms. Trends. Ecol. Evol. 18, 234–240. ( 10.1016/S0169-5347(03)00087-9) [DOI] [Google Scholar]
- 5.Somero GN. 1995. Proteins and temperature. Annu. Rev. Physiol. 57, 43–68. ( 10.1146/annurev.ph.57.030195.000355) [DOI] [PubMed] [Google Scholar]
- 6.Gabriel W. 2005. How stress selects for reversible phenotypic plasticity. J. Evol. Biol. 18, 873–883. ( 10.1111/j.1420-9101.2005.00959.x) [DOI] [PubMed] [Google Scholar]
- 7.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends. Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 8.Kassen R. 2002. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 15, 173–190. ( 10.1046/j.1420-9101.2002.00377.x) [DOI] [Google Scholar]
- 9.Murren CJ, et al. 2015. Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115, 293–301. ( 10.1038/hdy.2015.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan AB, Fellous S, Quillery E, Kaltz O. 2011. Adaptation of Paramecium caudatum to variable conditions of temperature stress. Res Microbiol. 162, 939–944. ( 10.1016/j.resmic.2011.04.012) [DOI] [PubMed] [Google Scholar]
- 11.Kassen R, Bell G. 1998. Experimental evolution in Chlamydomonas. IV. Selection in environments that vary through time at different scales. Heredity 80, 732–741. ( 10.1046/j.1365-2540.1998.00329.x) [DOI] [Google Scholar]
- 12.Maughan H, Masel J, Birky CW, Nicholson WL. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177, 937–948. ( 10.1534/genetics.107.075663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes BS, Cullum AJ, Bennett AF. 2007. An experimental evolutionary study on adaptation to temporally fluctuating pH in Escherichia coli. Physiol. Biochem. Zool. 80, 406–421. ( 10.1086/518353) [DOI] [PubMed] [Google Scholar]
- 14.Scheiner SM, Yampolsky LY. 1998. The evolution of Daphnia pulex in a temporally varying environment. Genet. Res. 72, 25–37. ( 10.1017/S0016672398003322) [DOI] [Google Scholar]
- 15.Yeaman S, Chen Y, Whitlock MC. 2010. No effect of environmental heterogeneity on the maintenance of genetic variation in wing shape in Drosophila melanogaster. Evolution 64, 3398–3408. ( 10.1111/j.1558-5646.2010.01075.x) [DOI] [PubMed] [Google Scholar]
- 16.Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G. 2004. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Dev. Biol. 131, 2183–2194. ( 10.1242/dev.01095) [DOI] [PubMed] [Google Scholar]
- 17.Condon C, Cooper BS, Yeaman S, Angilletta MJ. 2014. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution 68, 720–728. ( 10.1111/evo.12296) [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 19.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 20.Zuur AF, Leno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 21.Bartoń K. 2013. MuMIn: multi-model inference, R package version 1.9.13.
- 22.Huey RB, Berrigan D. 1996. Testing evolutionary hypotheses of acclimation. In Animals and temperature: phenotypic and evolutionary adaptation (eds Johnston IA, Bennett AF), pp. 205–237. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Angilletta MJ, Huey RB, Frazier MR. 2010. Thermodynamic effects on organismal performance: is hotter better? Physiol Biochem Zool. 83, 197–206. ( 10.1086/648567) [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Franklin CE. 2002. Testing the beneficial acclimation hypothesis. Trends Ecol Evol. 17, 66–70. ( 10.1016/S0169-5347(01)02384-9) [DOI] [Google Scholar]
- 25.Huey RB, Kingsolver JG. 1989. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol. 4, 131–135. ( 10.1016/0169-5347(89)90211-5) [DOI] [PubMed] [Google Scholar]
- 26.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Sun HJ, Friedmann EI. 2005. Communities adjust their temperature optima by shifting producer-to-consumer ratio, shown in lichens as models: II. Experimental verification. Microb. Ecol. 49, 528–535. ( 10.1007/s00248-005-3679-x) [DOI] [PubMed] [Google Scholar]
- 28.Knies JL, Izem R, Supler KL, Kingsolver JG, Burch CL. 2006. The genetic basis of thermal reaction norm evolution in lab and natural phage populations. PLoS Biol. 4, 1–8. ( 10.1371/journal.pbio.0040201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett AF, Lenski RE, Mittler JE. 1992. Evolutionary adaptation to temperature. I. Fitness responses of Escherichia coli to changes in its thermal environment. Evolution 46, 16–30. ( 10.2307/2409801) [DOI] [PubMed] [Google Scholar]
- 30.Knies JL, Kingsolver JG, Burch CL. 2009. Hotter is better and broader: thermal sensitivity of fitness in a population of bacteriophages. Am. Nat. 173, 419–430. ( 10.1086/597224) [DOI] [PubMed] [Google Scholar]
- 31.Hochachka PW, Somero GN. 2002. Biochemical adaptation. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Hazel JR. 1995. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19–42. ( 10.1146/annurev.ph.57.030195.000315) [DOI] [PubMed] [Google Scholar]
- 33.Guderley H. 2004. Metabolic responses to low temperature in fish muscle. Biol. Rev. 79, 409–427. ( 10.1017/S1464793103006328) [DOI] [PubMed] [Google Scholar]
- 34.Cooper BS, Hammad LA, Fisher NP, Karty JA, Montooth KL. 2012. In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution 66, 1976–1984. ( 10.1111/j.1558-5646.2011.01566.x) [DOI] [PubMed] [Google Scholar]
- 35.Le Vinh Thuy J, Vandenbrooks JM, Angilletta MJ. 2016. Data from: Developmental plasticity evolved according to specialist–generalist tradeoffs in experimental populations of Drosophila melanogaster. Dryad Digital Repository. ( 10.5061/dryad.6rk67) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.6rk67 [35].


