Abstract
Sexually antagonistic selection can drive both the evolution of sex chromosomes and speciation itself. The tropical butterfly the African Queen, Danaus chrysippus, shows two such sexually antagonistic phenotypes, the first being sex-linked colour pattern, the second, susceptibility to a male-killing, maternally inherited mollicute, Spiroplasma ixodeti, which causes approximately 100% mortality in male eggs and first instar larvae. Importantly, this mortality is not affected by the infection status of the male parent and the horizontal transmission of Spiroplasma is unknown. In East Africa, male-killing of the Queen is prevalent in a narrow hybrid zone centred on Nairobi. This hybrid zone separates otherwise allopatric subspecies with different colour patterns. Here we show that a neo-W chromosome, a fusion between the W (female) chromosome and an autosome that controls both colour pattern and male-killing, links the two phenotypes thereby driving speciation across the hybrid zone. Studies of the population genetics of the neo-W around Nairobi show that the interaction between colour pattern and male-killer susceptibility restricts gene flow between two subspecies of D. chrysippus. Our results demonstrate how a complex interplay between sex, colour pattern, male-killing, and a neo-W chromosome, has set up a genetic ‘sink' that keeps the two subspecies apart. The association between the neo-W and male-killing thus provides a ‘smoking gun' for an ongoing speciation process.
Keywords: Danaus chrysippus, male-killing, neo-W chromosome, colour pattern, speciation, Spiroplasma
1. Introduction
Many arthropod species are infected with maternally inherited endosymbionts that induce a shift in the sex ratio of their hosts by killing males, so-called ‘male-killing’. Male-killing can increase infected female fitness at the expense of non-transmitting infected males by increasing the resources available to females, either by reducing inter-sibling competition or via cannibalism [1]. Endosymbionts that have near-perfect transmission may reduce the effective population size (Ne) by a factor that approximates to the proportion of uninfected individuals in the population [2]. Theoretical studies of male-killing endosymbionts, where hybridizing subpopulations interbreed, have shown that local adaptation can be strongly impeded in the subpopulation with the more biased population sex ratio [3]. Further, it has been shown that both genetic drift and genetic influx are enhanced by male-killers; in cases where a nuclear gene is under selection (such as one which controls colour pattern), the two effects are unbalanced and the infection may have conspicuous effects [4].
One useful group of insects in which to examine the effects of male-killing are the butterflies. In East Africa, the African Queen butterfly, Danaus chrysippus (L.)—generation time one month, maximum adult lifespan around two months—is infected by a vertically transmitted, maternally inherited endosymbiotic bacterium, Spiroplasma ixodetis, that causes early mortality in males at the point of hatching from the egg, or occasionally in the first instar larva [5–10]. In the absence of suppression, vertical transmission of Spiroplasma in the Nairobi region of Kenya is 99.8% efficient (n = 1 240 offspring, n = 69 broods; electronic supplementary material, table S1). For Spiroplasma that are endosymbiotic, transmission is primarily vertical and there does not appear to be simultaneous reliance on horizontal and vertical transmission strategies. Spiroplasma-infected males which are assumed to carry a suppressor gene are normally rare and occur only seasonally. It is unknown if their sexual fitness is compromised.
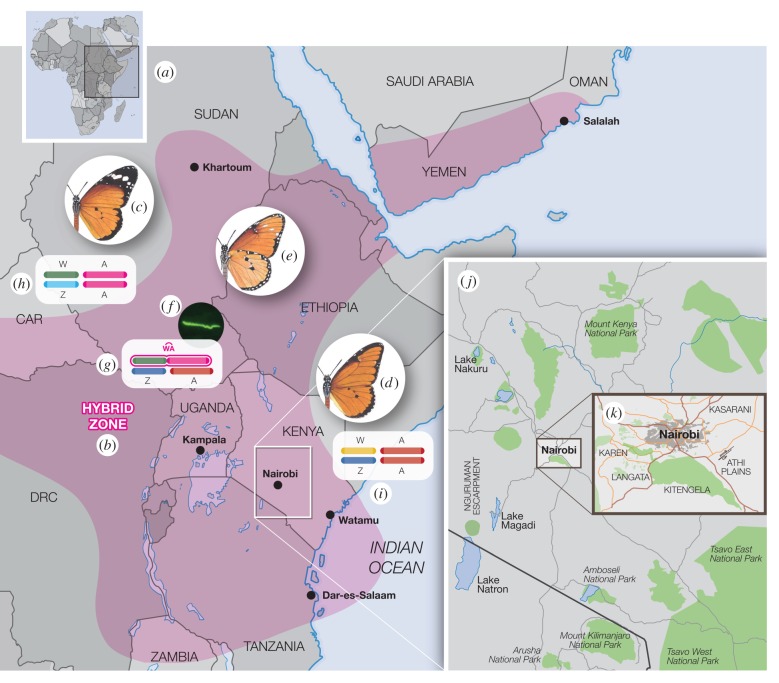
Alongside their differing susceptibility to male-killing, the African Queen also varies in colour pattern across its range. In fact, ever since Poulton [11] noted that two colour morphs could be bred from the same parents, D. chrysippus has been considered ‘polymorphic’. However, our long-term studies at three sites around Nairobi, Kenya (figure 1a,j and k), have established that in fact at this location the butterfly comprises two essentially parapatric (though migratory) subspecies. D. c. chrysippus and D. c. dorippus (here termed simply chrysippus and dorippus, figure 1c,d) each have an individual colour pattern controlled by a single autosomal locus C [12], dorippus being CC and chrysippus cc. The F1 hybrid transiens (Cc) closely resembles dorippus but is usually phenotypically detectable (figure 1e) [12]. The subspecies make frequent contact across East Africa creating colour polymorphisms within a mosaic of hybrid clines centred on Nairobi but reaching south to Tanzania [13], east into Uganda [5,8,10], and north to Sudan [10], Ethiopia [14], and Oman [15]. Outside the hybrid zone, both subspecies are monomorphic for the C locus through their separate ranges (figure 1, [16,17]). Previous investigations have explored relationships between colour pattern, male-killing, and sex-linkage. Throughout the hybrid zone female-biased sex ratios, driven by male-killing and marked by hybrid excess, predominate [16,17]. Two phenotypes are associated, the first being colour pattern, which may be sex-linked or independent of sex and the second is susceptibility to the male-killing Spiroplasma, or immunity to it [8–10].
Figure 1.
Map of the hybrid zone and the location of the different Danaus subspecies studied. (a) Map of East Africa showing the location of samples, the approximate position of the hybrid zone (b) and the distribution of Danaus chrysippus subspecies chrysippus (c) and dorippus (d). The hybrid form D. c. transiens (underside shown in e) is confined to the hybrid zone, as are the endosymbiont Spiroplasma ixodetis (f) and the ‘fused’ neo-W karyotype (g). Outside the hybrid zone the wild-type ‘unfused’ (Wu) karyotypes for chrysippus (h) and dorippus (i) are fixed. Sampling sites mentioned in the text are shown in the insets, southern Kenya (j) and the environs of Nairobi (k). The symbol A refers to the autosome carrying the C locus.
We have previously found that the c-autosome of chrysippus females strictly segregates with the W chromosome within the hybrid zone and have, therefore, postulated that a W-autosomal fusion (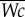 ) had occurred [18–20], physically linking the female determining W chromosome with the colour pattern locus C. Further, we have also found within the hybrid zone that chrysippus females and their transiens daughters are all infected with the male-killing Spiroplasma and thus produce all-female broods whereas, by contrast, dorippus females are largely uninfected by Spiroplasma and, therefore, continue to produce both males and females. The D. chrysippus hybrid zone is arguably an empirical example of the type of genetic ‘sink’ described in theoretical studies [3,4] which arises between hybridizing populations, one of which is infected and the other not. The aims of the present study were, therefore, to look at five specific factors likely to govern the population genetics of the hybrid zone. (i) To demonstrate, using cytogenetics, that an autosome has indeed become fused to the female-specific W-chromosome, as previously postulated. (ii) To document the frequency of Spiroplasma infection in the hybrid zone and to try to understand how this fusion maintains the highly female-biased sex ratios found. (iii) To investigate any possible relationship between population density and sex ratio. (iv) To document, via spermatophore count per female, potentially different rates of mating between hybrids and pure-bred individuals in the hybrid population. (v) Finally, to examine whether sexual selection or mate choice (assortative, random, or disassortative) within the hybrid zone affects sex ratio.
) had occurred [18–20], physically linking the female determining W chromosome with the colour pattern locus C. Further, we have also found within the hybrid zone that chrysippus females and their transiens daughters are all infected with the male-killing Spiroplasma and thus produce all-female broods whereas, by contrast, dorippus females are largely uninfected by Spiroplasma and, therefore, continue to produce both males and females. The D. chrysippus hybrid zone is arguably an empirical example of the type of genetic ‘sink’ described in theoretical studies [3,4] which arises between hybridizing populations, one of which is infected and the other not. The aims of the present study were, therefore, to look at five specific factors likely to govern the population genetics of the hybrid zone. (i) To demonstrate, using cytogenetics, that an autosome has indeed become fused to the female-specific W-chromosome, as previously postulated. (ii) To document the frequency of Spiroplasma infection in the hybrid zone and to try to understand how this fusion maintains the highly female-biased sex ratios found. (iii) To investigate any possible relationship between population density and sex ratio. (iv) To document, via spermatophore count per female, potentially different rates of mating between hybrids and pure-bred individuals in the hybrid population. (v) Finally, to examine whether sexual selection or mate choice (assortative, random, or disassortative) within the hybrid zone affects sex ratio.
Here, in a cytogenetic analysis using fluorescent in situ hybridization (FISH) to visualize telomeres, we show that in chrysippus females the W chromosome is indeed fused to an autosome, forming a trivalent with the Z chromosome and the autosome in meiosis, whereas in dorippus females W/Z and autosomes pair independently, as does the Z and all the autosomes in all males. We also show that in a female-dominated hybrid zone site at Kitengela, immigrant dorippus males are relatively immune to male-killing and that nearly all females are inseminated despite outnumbering males by around 5 : 1. Paradoxically, the female-biased hybrid population has a stable sex ratio which withstands perturbations induced by weather, population density, or immigration and reinforces a barrier to gene flow between two nascent species that has endured for at least 40 years around Nairobi [15–17]. The extraordinary effectiveness of this barrier is demonstrated by field data showing a rapid return to the prevailing female-biased sex ratios after a seasonal influx of Spiroplasma-infected and presumably male-killer-resistant males.
2. Material and methods
(a). Cytogenetics
Male metaphase I and II cells were obtained from testis cysts of pupae and last-instar larvae and female pachytene cells from the tips of ovarioles of adult females. Fixation in Carnoy's fluid (ethanol : chloroform : acetic acid, 6 : 3 : 1) was followed by spreading in 60% acetic acid and staining with 4′6-diamidino-2-phenylindole (DAPI). Female metaphase I was prepared from mature eggs by fixation in Carnoy's fluid, squashing in 60% acetic acid, freezing in liquid N2, flipping off the coverslip with a scalpel (dry ice method), post-fixation in ethanol, and staining with DAPI. Telomeres were visualized by FISH with a (TTAGG)n probe generated by substrate-free PCR (primers: TAGGTTAGGTTAGGTTAGGT and CTAACCTAACCTAACCTAAC) and labelled with Orange-dUTP.
(b). Spiroplasma screening
DNA from adult D. chrysippus was extracted from a 2 mm2 piece of thorax tissue using the Puragene DNA purification kit (Quiagen, Valencia, CA, USA). DNA was extracted using three times the suggested volume for a single Drosophila and then hydrated in 70 µl ddH2O. Previously described ixodetus-clade specific primers (SpixoF and SpixoR) and PCR cycling conditions [21] were used to amplify an 810 bp region of Spiroplasma 16S rDNA to confirm the presence of Spiroplasma. As a positive control for DNA quality, a region of host mitochondrial DNA (mtDNA) was amplified using primers LCO1490 and HCO2198 [22].
(c). Field sampling
The new field data from Kitengela comprise samples of flying adult butterflies collected by net in 19 2 h sessions from May 2013 to September 2015 (electronic supplementary material, table S2). As most butterflies seen were caught, the samples are ± random. All butterflies were date marked and released at the point of capture. Mated pairs were recorded; 260 wild females of random genotype were dissected to count spermatophores.
(d). A model for the relationship between sex ratio and the frequency of male-killing
A model for the relationship between the sex ratio of the population and the frequency of the male-killer or ‘MK’ (electronic supplementary material, figure S1) is derived from the following premises: (i) the sons of MK (neo-W) females all die, (ii) non-MK (Wu) females bear equal numbers of sons and daughters, (iii) MK and non-MK females produce equal numbers of surviving daughters, and (iv) the migration of females (though not of males) is effectively zero.
3. Results
(a). Cytogenetics
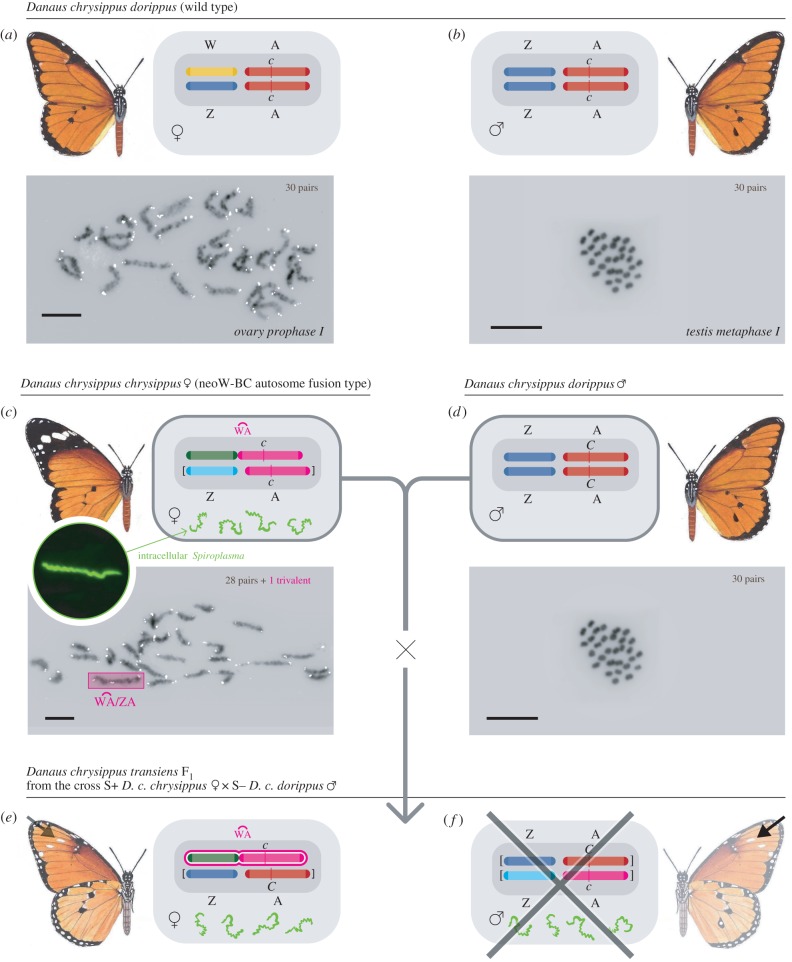
Previous cross-breeding has suggested that the autosome carrying the C locus may have become linked to the female-specific W (and not the Z) chromosome via a previously undocumented fusion ([18–20]; electronic supplementary material, table S1). To test this hypothesis, we examined the cytogenetics of butterflies of known descent for colour pattern from within the Nairobi hybrid zone (figure 2). We found that the diploid chromosome complement in F1 transiens (Cc) females from Nairobi contains a fusion chromosome (a neo-W): 28 bivalents and a trivalent are present in female meiosis (2n = 59), whereas in meiosis of females from outside the hybrid zone 30 bivalents (2n = 60) are present (figure 2b,d; electronic supplementary material, table S3). This confirms our earlier prediction and explains the strict sex-linkage found only in the hybrid zone (figure 2c,e). As the neo-W pairs in meiosis I of oogenesis with Z and the C-autosome, the former becomes Z1 and the latter Z2 in a W/Z1Z2 trivalent. All males from several sources had 30 bivalents (2n = 60), in agreement with older data [23,24]. The dataset, therefore, directly supports our earlier hypothesis that an autosome carrying the C locus has indeed become directly fused to the female-specific W-chromosome.
Figure 2.
Colour phenotypes, genotypes, and karyotypes of subspecies D. c. chrysippus (cc), D. c. dorippus (CC), and the hybrid form transiens (Cc) in the Nairobi region. (a–d) Meiosis prophase I pachytene chromosome pairs from female ovaries (a,c) and metaphase I pairs from male testes (b,d): Danaus chrysippus dorippus, a female, b male, 30 bivalents (2n = 60) in both sexes; D. c. chrysippus, c female, 28 bivalents, 1 trivalent (2n = 59); d male, (2n = 60) as b; the white dots in the female micrographs a and c are telomere signals. Scale bars represent 10 µm. (e,f) F1 progeny, female (e) and male (f) from a cross between a Spiroplasma-positive D. c. chrysippus female (c) and a Spiroplasma-negative D. c. dorippus male (d). The provenance of chromosomes is shown as red, dorippus C-autosome; pink, chrysippus c-autosome; dark blue, dorippus Z chromosome; light blue, chrysippus Z chromosome; yellow, W chromosome unfused; green, W chromosome fused. Wild-type bivalents (in a,b,d) comprise, respectively, two sex chromosomes (Z/Z or Z/W) with two autosomes carrying the C locus, whereas the 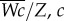 trivalent is found only in the mutant females c and e. Chromosomes in square brackets in females c, e, and male f are lost in successive generations of dead sons (marked with a cross in f). A few f males escape death by either immunity (MK suppression) or failed transmission of Spiroplasma [8]; most male survivors have the transiens (Cc) phenotype detectable by the white spots (arrowed in e,f) on the underside and/or scattered black scales on the upper side of the forewing apex.
trivalent is found only in the mutant females c and e. Chromosomes in square brackets in females c, e, and male f are lost in successive generations of dead sons (marked with a cross in f). A few f males escape death by either immunity (MK suppression) or failed transmission of Spiroplasma [8]; most male survivors have the transiens (Cc) phenotype detectable by the white spots (arrowed in e,f) on the underside and/or scattered black scales on the upper side of the forewing apex.
(b). Spiroplasma screening
In order to understand how infection with the male-killing Spiroplasma drives the female-biased sex ratios found within the hybrid zone, we typed 87 butterflies (72 females and 15 males) from the Kitengela field site for Spiroplasma (electronic supplementary material, table S4).
The high infection rate in female adults (87.9%, n = 72) at Kitengela, equally across all genotypes, is expected from the high frequency throughout the Nairobi region of the neo-W chromosome—78.7% of broods (n = 127) from wild females with sex-linkage for colour pattern and sex. The frequency of the male-killer is estimated from the proportion of wild females that produced all-female progenies in females as 71.7% (n = 127) ([18] and K Saitoti, IJ Gordon, and DAS Smith 2015, unpublished data) and is similar to that observed in 2009–2010 at Kasarani [20] (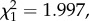 p = 0.158). However, Kitengela male samples had the highest ever recorded infection rates (66.7%) and significantly so (
p = 0.158). However, Kitengela male samples had the highest ever recorded infection rates (66.7%) and significantly so (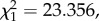 p < 0.0001) compared with the 2009–2010 Kasarani samples [20]. Comparing male infection rates for June (40.0%) and July (81.3%), the increase approaches significance (exact p (one-tailed) = 0.115). The increasing frequency of Spiroplasma-infected but male-killer-resistant males is reflected in a significant change in sex ratio from 86.6% female in May, through 82.1% in June, down to 72.4% in July (
p < 0.0001) compared with the 2009–2010 Kasarani samples [20]. Comparing male infection rates for June (40.0%) and July (81.3%), the increase approaches significance (exact p (one-tailed) = 0.115). The increasing frequency of Spiroplasma-infected but male-killer-resistant males is reflected in a significant change in sex ratio from 86.6% female in May, through 82.1% in June, down to 72.4% in July (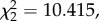 p = 0.005; electronic supplementary material, table S5). Each of these changes takes place within the period of a single generation, generation time being approximately one month. It should be noted that horizontal transmission of Spiroplasma has never been observed when vertical transmission has been demonstrated.
p = 0.005; electronic supplementary material, table S5). Each of these changes takes place within the period of a single generation, generation time being approximately one month. It should be noted that horizontal transmission of Spiroplasma has never been observed when vertical transmission has been demonstrated.
We paid particular attention to infection rates in the rarer males which appear after the rains (May–July 2015) and then appear to vanish over time. The high frequency of infected males (which can only exist if they are resistant) at this time was unusual. As invaders do not bring Spiroplasma from their population of origin, the infected males were probably first generation survivors of immigrants carrying suppressor genes. The fact that 85% of new males were of CC genotype, otherwise rare in the hybrid zone, supports this interpretation. However, factors other than migration may influence the frequency of male-killing. First, high population density may increase sibling competition, which is thought to be a main contributory factor promoting the spread of male-killers [25]. Although D. chrysippus females lay their eggs singly on the milkweed host plant, thus diminishing the risk from sibling competition, paradoxically they preferentially select isolated plants, which may then become overloaded at times of high population density [26]. Second, if Spiroplasma is to any extent horizontally transmitted, as may result from egg cannibalism [18], then high levels of infection may follow as a direct consequence of high population density [25]. As coincidental horizontal and vertical transmission of Spiroplasma has never been observed, however, we consider this alternative unlikely.
Prior to a brief period of high population density at Kitengela in May–July 2015, 90.8% (n = 76) of broods obtained from wild Cc males mated with cc females (electronic supplementary material, table S1) had produced all-female progeny. Two months after the period of high population density (in September) all males had vanished from the hybrid zone and the sample had returned to 100% female (electronic supplementary material, table S5). The all-female population was, therefore, restored and all males eliminated in as little as two butterfly generations. The summary statistics of the D. chrysippus populations which have been sampled for the physical presence of Spiroplasma and/or the male-killing phenotype, on the one hand, and hybridism on the other (electronic supplementary material, table S6) shows that the two phenomena are far from independent (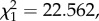 p < 0.0001). In conclusion, the high frequency of male-killing Spiroplasma and its association with the neo-W fusion within the hybrid zone rapidly cleanses the population of males. Following an unusual immigration of large numbers of males, they can be removed from the population within only a staggering two generations.
p < 0.0001). In conclusion, the high frequency of male-killing Spiroplasma and its association with the neo-W fusion within the hybrid zone rapidly cleanses the population of males. Following an unusual immigration of large numbers of males, they can be removed from the population within only a staggering two generations.
(c). Field data
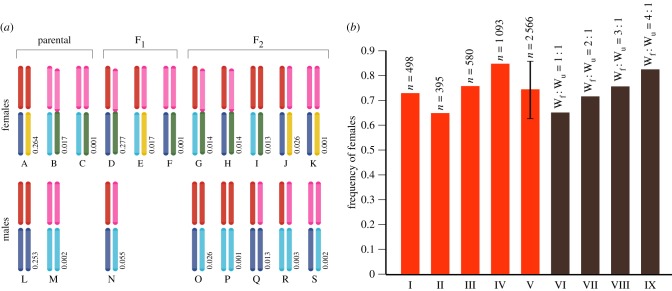
We estimated the density of the Kitengela population on several occasions. Bailey Index estimates for population size [27] at the 5 hectare (ha) site varied between extremes of 27 ± 16.7 (5.4 per ha, 100.0% female) in October 2014 and 344 ± 229.5 (68.8 per ha, 86.6% female) in May 2015, an approximately 13-fold difference in density which has no apparent effect on the sex ratio (Exact p = 0.063). However, as the small number of recaptures produced high standard errors to the population measures, this conclusion is, therefore, tentative. Even so, the Kitengela sex ratio data (electronic supplementary material, table S2) conform to a historical (1986–2010) norm for the region (figure 3b,I–III). The mean sex ratio of 84.1% female (figure 3b,IV), though the highest recorded in the Nairobi region, nonetheless lies within the C95 limits for the regional mean (figure 3b,V).
Figure 3.
(a) The array of karyotypes in the F2 from founder crosses between D. c. dorippus and D. c. chrysippus populations in the Nairobi hybrid zone, displayed by sex and the generation in which they first appear. F2 karyotype frequencies are calculated on the following assumptions: (1) the sex ratio in the founder crosses is 1 : 1 in dorippus and 90% female in chrysippus, (2) the numbers of dorippus and chrysippus females in the founder population is equal, (3) all females are mated, (4) all male offspring of neo-W females die, and (5) there is neutrality and no migration. The upper pair of chromosomes comprises the C-autosomes, red for dorippus and pink for chrysippus. The lower pair shows the Z chromosomes of dorippus (dark blue) and chrysippus (light blue); the yellow W chromosome is always unfused (Wu) in dorippus, whereas in chrysippus the W chromosome shown in green may be unfused or fused to the C-autosome (neo-W).
(d). Spermatophore counts
To test if females of different colour morphs experienced different rates of mating success, we counted the number of the male-transferred spermatophores found per female. Spermatophores per female at Kitengela (2013–2014, n = 260) averaged 1.73 ± 1.24 (range 0–6) with only 19 (7.3%) unmated; thus all females are expected eventually to mate. The sexual histories of Cc (n = 227) and cc females (n = 23) did not differ (t258 = 0.073, p > 0.99). The spermatophore count is significantly less than 3.50 ± 1.36 (n = 20) for a population in Ghana with a sex ratio of 1 : 1 (t278 = 6.126, p < 1.0 × 10−4). In conclusion, overall spermatophore counts per female do not differ between colour morphs within the hybrid zone.
(e). Sexual selection
Finally, we used a period of unusually high male frequency to study the potential effects of sexual selection and to test if mating between colour morphs was assortative, random, or disassortative. In May–July 2015, the Kitengela population irrupted during heavy rains after a long period of drought. For the first time males were relatively numerous, and male–female courtship and successful mating was observed in the field. We seized the corresponding opportunity to study sexual selection (electronic supplementary material, table S7). The frequency of males caught in copula was 63.4% compared with 13.4% for females. In other words, males were nearly five times more likely to mate. This remarkable statistic attests to the unrelenting pressure to mate on males. Sexual selection for colour genotype was not significant for either sex (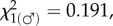 p = 0.662;
p = 0.662; 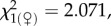 p = 0.150). However, with numbers corrected for penetrance (electronic supplementary material, table S7), the majority of males in copula (63.3%) were CC, whereas among females 84.2% were Cc, 15.8% cc, and 0% CC. Thus, the absence of CC females enforces males to pair disassortatively for genotype. This result is counterintuitive for two reasons; first, in Cc and cc females, all of which are neo-W and male-killer-susceptible, the genes contributed by the male partner will all subsequently be eliminated [2–4]; second, the absence of CC females suggests that migration into the hybrid zone is substantially male-biased [3].
p = 0.150). However, with numbers corrected for penetrance (electronic supplementary material, table S7), the majority of males in copula (63.3%) were CC, whereas among females 84.2% were Cc, 15.8% cc, and 0% CC. Thus, the absence of CC females enforces males to pair disassortatively for genotype. This result is counterintuitive for two reasons; first, in Cc and cc females, all of which are neo-W and male-killer-susceptible, the genes contributed by the male partner will all subsequently be eliminated [2–4]; second, the absence of CC females suggests that migration into the hybrid zone is substantially male-biased [3].
4. Discussion
This paper shows for the first time that a neo-W chromosome, a W-autosome ‘fusion’, is indeed responsible for the previously observed non-random segregation of colour pattern and sex in the African Queen butterfly and that the neo-W promotes the genetic separation of two incipient butterfly species across a hybrid zone. The data on morph frequencies and mated pairs also confirm for the first time that mating is disassortative within this hybrid zone despite being assortative outside it [7,28]. This paradoxical situation is entirely due to the restricted mate choice that results from male-killing and the neo-W fusion. Specifically, immigrant males are predominantly CC (dorippus), whereas all the infected females with which they mate are either Cc (transiens) or cc (chrysippus), and all their infected daughters carry the c allele. As mate choice in polymorphic populations is normally assortative for colour pattern [7,28], enforced disassortative mating potentially invokes sexual conflict [29–31]. Matrilineal descent of neo-W, combined with MK, would ensure that the progenies from such disparate pairings are overwhelmingly females of genotype 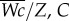 (electronic supplementary material, table S1) [17,18].
(electronic supplementary material, table S1) [17,18].
At this stage, we are uncertain as to exactly which autosome is fused to the female-specific W chromosome. However, what is clear is that this autosome carries loci (or indeed a single locus) controlling both colour pattern (the C locus) and susceptibility to the male-killing Spiroplasma. The high frequency of the neo-W fusion in the Kitengela population ensures that butterflies within the hybrid zone are predominantly female and that the sex ratio is stable despite seasonal influxes of male-killer-protected Spiroplasma-infected males. Hence, if these males carry a Z-linked suppressor gene, it would be lost in dead male offspring, thus perpetuating the female-biased sex ratio.
The African Queen has a generation time of approximately one month and a maximum adult lifespan of around two months. Remarkably, in this context, all females obtain at least one mating although their spermatophore count is significantly less than in a population with a 1 : 1 sex ratio. Thus, females in MK populations receive fewer (and possibly smaller) spermatophores from their exhausted male partners. As spermatophores contain nutrients and defensive pyrrolizidine alkaloids in addition to sperm [32], hybrid zone females are thereby disadvantaged. Moreover, as females outnumber males approximately 5 : 1, to achieve 1.7 pairings per female (as found at Kitengela) each male must mate an astonishing 8.5 times. Males will quickly be seduced by females, become entrapped and eventually exhausted [33]. Because within male-killer-susceptible females, all genes from the male partner are eliminated [2–4], strong pressure on the largely CC males to mate with unsusceptible CC females would be expected. However, as a result of male-biased migration the latter are absent. Indeed, if uninfected CC females were available this would quickly lead to restoration of a 1 : 1 sex ratio and this was never seen over 2 years of observation. Moreover, the highly unusual proportion of males caught in copula (63%, electronic supplementary material, table S7), all with male-killer-susceptible females, suggests that sexual selection is relaxed. As all males are in demand, all are expected to mate many times and their life expectancy must be curtailed. Furthermore, the paucity of males reduces the effective population size (Ne) by more than or equal to 90% [2] and severe inbreeding is the likely outcome from an average of 4.3 females sharing each male partner. Therefore, the prognosis for the male-killer-susceptible females is to be both short-changed for nuptial gifts and inbred.
In figure 3, we show the karyotypes that result after a founding cross between ‘unfused’ D. c. dorippus males with 30 bivalents and D. c. chrysippus females with 28 bivalents and a W/Z1Z2 trivalent. The array assumes all possible crosses within the hybrid population after the first two rounds of mating. Most of the rare recombinants are heterozygotes for either C-autosomes, sex chromosomes or both, and arguably unfit. However, karyotype J with an unfused W chromosome is probably the standard karyotype in chrysippus populations outside the hybrid zone, which, in the absence of the neo-W and male-killing, should be subject to Fisherian selection for a balanced sex ratio [34]. This suggests that once free from the hybrid zone, chrysippus populations should quickly revert to 1 : 1 sex ratios.
The neo-W fusion is apparently able to avoid suppression and ensures that male immigration does not reduce the high frequencies of females, except in the very short term; in effect the neo-W fusion imposes a female-biased sex ratio on the Kitengela population. The influx of dorippus males in July 2015 reduced the sex ratio only for the duration of their lifespan (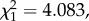 p = 0.043) and by September 2015, the female-biased sex ratio had been regained within just two generations (
p = 0.043) and by September 2015, the female-biased sex ratio had been regained within just two generations (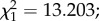 p = 0.0002; electronic supplementary material, table S5). The genetics of suppression are currently unclear, but, given the great selective advantage gained by male-killing suppressor genes [35], this extraordinary result is most easily explained if these genes are recessive and carried on the Z2 chromosome (approx. C-autosome) introduced by the immigrant males. This chromosome will be automatically eliminated in dead males within two generations, exactly as observed. Immigration by females from outside the hybrid zone does, however, have the potential to disrupt the sex ratio as it will always change the Wu : neo-W ratio (figure 3b). Ratios in the range 1 : 2 to 3 : 1 encompass a tipping point (electronic supplementary material, figure S1) between two adaptive peaks wherein the sex ratio switches rapidly from near normal to more than or equal to 80% female. When the neo-W is less common (less than or equal to 30%), the sex ratio remains relatively normal despite the presence of male-killing, and in practice (particularly in samples of active adults) will show no significant departures from 1 : 1. At Kitengela, the high frequency of the neo-W maintains sex ratios well above the tipping point and, even when males were most abundant in July 2015, adult female frequencies were never less than 70%. Moreover, all founding Wu : neo-W ratios in the range 1 : 1 to 1 : 4 fall inside C95 of the empirical mean for the Nairobi area (figure 3b,V), confirming the stability of the extreme sex ratios at Kitengela.
p = 0.0002; electronic supplementary material, table S5). The genetics of suppression are currently unclear, but, given the great selective advantage gained by male-killing suppressor genes [35], this extraordinary result is most easily explained if these genes are recessive and carried on the Z2 chromosome (approx. C-autosome) introduced by the immigrant males. This chromosome will be automatically eliminated in dead males within two generations, exactly as observed. Immigration by females from outside the hybrid zone does, however, have the potential to disrupt the sex ratio as it will always change the Wu : neo-W ratio (figure 3b). Ratios in the range 1 : 2 to 3 : 1 encompass a tipping point (electronic supplementary material, figure S1) between two adaptive peaks wherein the sex ratio switches rapidly from near normal to more than or equal to 80% female. When the neo-W is less common (less than or equal to 30%), the sex ratio remains relatively normal despite the presence of male-killing, and in practice (particularly in samples of active adults) will show no significant departures from 1 : 1. At Kitengela, the high frequency of the neo-W maintains sex ratios well above the tipping point and, even when males were most abundant in July 2015, adult female frequencies were never less than 70%. Moreover, all founding Wu : neo-W ratios in the range 1 : 1 to 1 : 4 fall inside C95 of the empirical mean for the Nairobi area (figure 3b,V), confirming the stability of the extreme sex ratios at Kitengela.
Within the hybrid mosaic the neo-W and male-killing are obligatorily linked as neither has ever been observed in the known absence of the other [18]. We speculate that the effect of the neo-W-male-killing package is to create a genetic ‘sink’ into which both chrysippus and dorippus male genomes are consigned, thus erasing their Z chromosomes and C-autosomes; these are precisely the expected locations of ‘speciation’ genes that would damage fitness in alien gene pools. The effectiveness of this genetic ‘sink’ is dramatically illustrated by the rapid return to all-female sex ratios observed at Kitengela in September after the incursion of male-killer suppressors in May–July 2015.
Our results are striking and unexpected for several reasons. First, they break Haldane's rule [36] which states: ‘when in the offspring of two animal races one sex is absent, rare, or sterile, that sex is the heterozygous [heterogametic] one’. Haldane's rule does not apply here because in D. chrysippus it is the homogametic males that die in the hybrid zone. Second, albeit with a different mechanism, the evolutionary outcome is similar to other animal hybrid zones between incipient species—stickleback fish [37], Drosophila or Anopheles [38], Podisma grasshoppers [39]—where gene flow is obstructed by hybrid male sterility, or in Heliconius butterflies by hybrid female sterility [40]. Third, unlike all the foregoing, the D. chrysippus genetic sink comprises a neo-W conserved by linkage in heterogametic females. It is, therefore, a reinforcement mechanism promoting speciation of a type that has not previously been described.
Finally, while we have yet to probe the molecular basis of this story, our results suggest two interesting possible alternatives for the future fate of the neo-W chromosome in the African Queen. The first alternative is suggested by the observation that Eurema butterflies carrying feminizing Wolbachia (wFem) lack W chromatin and, therefore, appear to have ‘lost’ their W chromosome [41]. This supports an overarching hypothesis whereby infection with a microorganism (albeit Wolbachia or Spiroplasma) can render the W chromosome somehow redundant and hence either gradually lost (as in Eurema) or, in our case, translocated to an autosome to form a neo-W. Alternatively, and in the specific case of the African Queen, the physical act of fusing the W chromosome to an autosome may also disrupt an autosomal locus controlling both colour pattern and sex. To this end, we suggest a simple and testable hypothesis that both male-killer susceptibility and colour pattern are controlled by the gene doublesex [42] which is autosomal but has become subsequently female-linked by the formation of the neo-W of the African Queen. We note that doublesex has recently been shown to control not only colour polymorphism in another butterfly, Papilio polytes [43], but can also change the penetrance of male-killing via manipulation of alternative splicing within the gene itself [44]. If doublesex is indeed disrupted in the formation of the neo-W this would help explain why we see changes in both colour pattern and susceptibility to male-killing within the hybrid zone, as both may actually be controlled by the same locus. Taken together, our data support a hypothesis that two sexually antagonistic traits have been translocated to the newly formed female-specific neo-W chromosome of this butterfly and are now driving its speciation around Nairobi. However, any local advantage enjoyed by the neo-W may in reality be transitory and rapidly erode because Muller's ratchet will be expected to ensure the pervasive loss of autosomal genes and subsequent rapid degeneration of the neo-W chromosome [45–47].
Supplementary Material
Acknowledgements
Matt McClements (Blink Studios Ltd) designed the figures, Bernard Rono assisted with fieldwork, and Samuel Katoi provided specimens from Watamu. Fieldwork at Silole Sanctuary (Kitengela) was sanctioned by Nani Croze, Eric Krystall, John Keen, and Mark van Rampelberg. Simon Martin scrutinized the first draft of the manuscript and made valuable suggestions for its improvement. Spiroplasma screening was carried out at icipe. D.A.S.S. thanks the Linnean Society of London and the Outreach Fund of the Royal Entomological Society of London for funding. I.J.G., D.A.S.S., W.T., and K.S. are Research Affiliates of the National Museums of Kenya.
Ethics
Research Clearance Permits NACOSTI/P15/3290/3607 to D.A.S.S. and NACOSTI/P15/2403/3602 to W.T. were issued by the National Commission for Science and Technology, Nairobi.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
D.A.S.S., I.J.G., and R.ff.-C. conceived the project and wrote the first and second drafts of the paper. I.J.G., S.C., and K.S. oversaw the breeding. I.J.G., D.A.S.S., K.S., and D.J.M. planned and supervised the fieldwork. W.T. performed the cytogenetic investigations, J.H. screened for Spiroplasma and P.I. counted spermatophores. All authors contributed to further revisions of the manuscript.
Competing interests
The authors confirm that they have no competing financial interests.
Funding
This work was supported by a grant (BB/H014268/1) from the Biotechnology and Biological Sciences Research Council of the UK to R.ff.-C.
References
- 1.Hurst GDD, Majerus MEN. 1993. Why do maternally inherited microorganisms kill males. Heredity 71, 81–95. ( 10.1038/hdy.1993.110) [DOI] [Google Scholar]
- 2.Engelstädter J. 2010. The effective size of populations infected with cytoplasmic sex-ratio distorters. Genetics 186, 309–320. ( 10.1534/genetics.110.120014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi Y, Achaz G, Telschow A. 2011. Effect of parasitic sex-ratio distorters on host gene frequencies in a mainland-island context. J. Evol. Biol. 24, 1695–1705. ( 10.1111/j.1420-9101.2011.02296.x) [DOI] [PubMed] [Google Scholar]
- 4.Telschow A, Engelstädter J, Yamamura N, Hammerstein P, Hurst GDD. 2006. Asymmetric gene flow and constraints on adaptation caused by sex ratio distorters. J. Evol. Biol. 19, 869–878. ( 10.1111/j.1420-9101.2005.01049.x) [DOI] [PubMed] [Google Scholar]
- 5.Owen DF, Chanter DO. 1968. Population of tropical African butterflies. 2. Sex ratio and polymorphism in Danaus chrysippus L. Rev. Zool. Bot. Afr. 78, 81–97. [Google Scholar]
- 6.Smith DAS. 1975. All-female broods in Danaus chrysippus L. and their ecological significance. Heredity 34, 363–371. ( 10.1038/hdy.1975.45) [DOI] [PubMed] [Google Scholar]
- 7.Gordon IJ. 1984. Polymorphism of the tropical butterfly Danaus chrysippus L. in Africa. Heredity 53, 583–593. ( 10.1038/hdy.1984.116) [DOI] [Google Scholar]
- 8.Jiggins FM, Hurst GDD, Jiggins CD, Schulenburg JHGvD, Majerus MEN. 2000. The butterfly Danaus chrysippus is infected by a male-killing bacterium. Parasitology 120, 439–446. ( 10.1017/S0031182099005867) [DOI] [PubMed] [Google Scholar]
- 9.Herren J, Gordon IJ, Holland PWH, Smith DAS. 2007. The butterfly Danaus chrysippus (L.) in Kenya is variably infected with respect to genotype and body size by a maternally transmitted male-killing symbiont (Spiroplasma). Insect. Sci. Appl. 27, 62–69. [Google Scholar]
- 10.Hassan SSM, Idris E, Majerus MEN. 2012. Male-killer dynamics in Danaus chrysippus (L.) (Lepidoptera: Nymphalidae) in East Africa. Afr. J. Ecol. 50, 489–499 ( 10.1111/j.1365-2028.2012.01347.x). [DOI] [Google Scholar]
- 11.Poulton EB. 1925. Danaida chrysippus L. and D. dorippus Klug, proved by breeding to be two forms of the same species. Proc. Entomol. Soc. Lond. 1924, cxix. [Google Scholar]
- 12.Smith DAS. 1975. Genetics of some polymorphic forms of the African butterfly Danaus chrysippus L. (Lepidoptera: Danaidae). Insect Syst. Evol. 6, 134–144. ( 10.1163/187631275X00235) [DOI] [Google Scholar]
- 13.Smith DAS. 1998. Non-Mendelian segregation and variable penetrance of colour genes in the polymorphic butterfly Danaus chrysippus (L.). Heredity 80, 474–480. ( 10.1046/j.1365-2540.1998.00314.x) [DOI] [Google Scholar]
- 14.Cross P. 2003. The butterflies of Wondo Genet: an introduction to the butterflies of Ethiopia. Addis Ababa, Ethiopia: Mega Printing Exercise. [Google Scholar]
- 15.Lushai G, Allen JA, Goulson D, Maclean N, Smith DAS. 2005. The butterfly Danaus chrysippus (L.) in East Africa comprises polyphyletic, sympatric lineages that are, despite behavioural isolation, driven to hybridization by female-biased sex ratios. Biol. J. Linn. Soc. 86, 117–131. ( 10.1111/j.1095-8312.2005.00526.x) [DOI] [Google Scholar]
- 16.Smith DAS, Owen DF, Gordon IJ, Lowis NK. 1997. The butterfly Danaus chrysippus (L.) in East Africa: polymorphism, and morph-ratio clines within a complex, extensive and dynamic hybrid zone. Zool. J. Linn. Soc. 120, 51–78. ( 10.1111/j.1096-3642.1997.tb01272.x) [DOI] [Google Scholar]
- 17.Smith DAS, Gordon IJ, Depew LA, Owen DF. 1998. Genetics of the butterfly Danaus chrysippus (L.) in a broad hybrid zone, with special reference to sex ratio, polymorphism and intragenomic conflict. Biol. J. Linn. Soc. 65, 1–40. ( 10.1111/j.1095-8312.1998.tb00349.x) [DOI] [Google Scholar]
- 18.Smith DAS. 2014. African queens and their kin. Taunton, UK: Brambleby Books. [Google Scholar]
- 19.Smith DAS, Gordon IJ, Allen JA. 2010. Reinforcement in hybrids among once isolated semispecies of Danaus chrysippus (L.) and evidence for chromosome evolution. Ecol. Ent. 35, 77–89. ( 10.1111/j.1365-2311.2009.01143.x) [DOI] [Google Scholar]
- 20.Gordon IJ, Ireri P, Smith DAS. 2014. Hologenomic speciation: synergy between a male-killing bacterium and sex-linkage creates a ‘magic trait’ in a butterfly hybrid zone. Biol. J. Linn. Soc. 110, 92–109. ( 10.1111/bij.12185) [DOI] [Google Scholar]
- 21.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstädter J, Hurst GDD. 2008. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 6, 27 ( 10.1186/1741-7007-6-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers of mitochondrial cytochrome c oxidase subunit I from diverse invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299. [PubMed] [Google Scholar]
- 23.de Lesse H, Condamin M. 1962. Formules chromosomiques de quelques Lépidoptères Rhopalocères du Sénégal. Bull. Inst. Fond. Afr. Noire 24, 464–473. [Google Scholar]
- 24.Gupta Y. 1964. Chromosome studies of some Indian Lepidoptera. Chromosoma 15, 540–561. ( 10.1007/BF00319989) [DOI] [Google Scholar]
- 25.Hurst GDD, Hurst LD, Majerus M. 1997. Cytoplasmic sex ratio distorters. In Influential passengers (eds O'Neill SL, Hoffman A, Werren JH), pp. 125–154. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Gordon IJ, Ireri P, Smith DAS. 2014. Preference for isolated hosts facilitates invasion of Danaus chrysippus (Linnaeus, 1758) (Lepidoptera; Nymphalidae) by a bacterial male-killer Spiroplasma. Austral Entomol. 54, 210–216. ( 10.1111/aen.12113) [DOI] [Google Scholar]
- 27.Bailey NTJ. 1951. On estimating the size of mobile populations from recapture data. Biometrica 38, 293–306. ( 10.1093/biomet/38.3-4.293) [DOI] [Google Scholar]
- 28.Smith DAS. 1984. Mate selection in butterflies: competition, coyness, choice and chauvinism. In The biology of butterflies (eds Vane-Wright RI, Ackery PR), pp. 225–244. London, UK: Academic Press. [Google Scholar]
- 29.Rice WR. 1987. The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced combination between primitive sex chromosomes. Evolution 41, 911–914. ( 10.2307/2408899) [DOI] [PubMed] [Google Scholar]
- 30.Gavrilets S. 2000. Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889. ( 10.1038/35002564) [DOI] [PubMed] [Google Scholar]
- 31.Perry JC, Rowe L. 2014. The evolution of sexually antagonistic phenotypes. Cold Spring Harb. Perspect. Biol. 7, a017558 ( 10.1101/cshperspect.a017558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dussourd DE, Harvis CA, Meinwald J, Eisner T. 1989. Paternal allocation of sequestered plant pyrrolizidine alkaloid to eggs in the danaine butterfly Danaus gilippus. Cell. Mol. Life Sci. 45, 896–898. ( 10.1007/BF01954068) [DOI] [PubMed] [Google Scholar]
- 33.Charlat S, Reuter M, Dyson EA, Hornett EA, Duplouy A, Davies N, Roderick GK, Wedell N, Hurst GDD. 2007. Male-killing bacteria trigger a cycle of increasing male fatigue and female promiscuity. Curr. Biol. 17, 273–277. ( 10.1016/j.cub.2006.11.068) [DOI] [PubMed] [Google Scholar]
- 34.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 35.Hornett EA, Moran B, Reynolds LA, Charlat S, Tazzyman S, Wedell N, Jiggins CD, Hurst GDD. 2014. The evolution of sex ratio distorter suppression affects a 25cM genomic region in the butterfly Hypolimnas bolina. PLoS Genet. 10, e1004822 ( 10.1371/journal.pgen.1004822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldane JBS. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 37.Kitano J, et al. 2009. A role for a neo-sex chromosome in stickleback speciation. Nature 461, 1079–1083. ( 10.1038/nature08441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalak P. 2013. Speciation: natural processes, genetics and biodiversity. New York, NY: Nova Biomedical. [Google Scholar]
- 39.Veltsos P, Keller I, Nichols RA. 2008. The inexorable spread of a newly arisen neo-Y chromosome. PLoS Genet. 4, e1000082 ( 10.137/journal.pgen.1000082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naisbit RE, Jiggins CD, Linares M, Salazar C, Mallet J. 2002. Hybrid sterility, Haldane's rule and speciation in Heliconius cydno and H. melpomene. Genetics 161, 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kern P, Cook JM, Kageyama D, Riegler M. 2015. Double trouble: combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biol. Lett. 11, 20150095 ( 10.1098/rsbl.2015.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunte K, Zhang W, Tenger-Trolander A, Palmer DH, Martin A, Reed RD, Mullen SP, Kronforst MR. 2014. doublesex is a mimicry supergene. Nature 507, 229–232. ( 10.1038/nature13112) [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa H, et al. 2015. A genetic mechanism for female-limited Batesian mimicry in a Papilio butterfly. Nat. Genet. 47, 404–409. ( 10.1038/ng.3241) [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto TN, Ishikawa Y. 2012. A male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sex-determining system of its host. Biol. Lett. 8, 412–415. ( 10.1098/rsbl.2011.1114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlesworth B, Charlesworth D. 2000. The degeneration of Y chromosomes. Phil. Trans. R. Soc. Lond. B 355, 1563–1572. ( 10.1098/rstb.2000.0717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traut W, Sahara K, Marec F. 2007. Sex chromosomes and sex determination in Lepidoptera. Sex. Dev. 1, 332–346. ( 10.1159/000111765) [DOI] [PubMed] [Google Scholar]
- 47.Sahara K, Yoshido A, Traut W. 2012. Sex chromosome evolution in moths and butterflies. Chromosome Res. 20, 83–94. ( 10.1007/s10577-011-9262-z) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.