Abstract
Parasites are ubiquitous in wildlife populations, but physiological and behavioural responses of hosts to infection are difficult to measure. We experimentally treated semi-free-ranging red-capped mangabeys (Cercocebus torquatus) in Nigeria with antiparasitic drugs and examined subsequent changes in glucocorticoid production and individual behaviour. Because both parasites and stress impact energy balance and health, we measured (i) behavioural time re-allocation via activity budgets, (ii) social relationships (e.g. social connectivity and dominance hierarchy stability) and (iii) body condition. We collected triplicate faecal samples (n = 441) from 49 individuals prior to and following treatment. Cortisol levels fluctuated in parallel with parasite abundance. Elevations in cortisol, but not parasitism, were related to reduced body condition. Behaviour also shifted according to infection status, with uninfected individuals spending more time foraging and less time resting and vigilant compared with when they were infected. Time spent feeding, travelling or socializing did not differ between pre- and post-treatment time periods. Group cohesion, but not dominance stability, changed following treatment, suggesting parasite-induced social avoidance. Together, these findings show a coordinated response to infection that promotes host tolerance through stress and energy conservation, reduces transmission risk and increases protection when infected hosts are vulnerable.
Keywords: parasites, antiparasitic treatment, cortisol, sickness behaviour, social network, primates
1. Background
Infectious disease, like predation and resource limitation, can constrain population growth [1]. Pathogens with high case-fatality rates (e.g. epidemic viruses) can cause dramatic population declines and can even contribute to local and species extinctions [2–5]. Parasitic infections (e.g. protozoa and helminths), on the other hand, tend to cause only mild or subclinical effects, and their impact on populations is often less dramatic and more protracted [6,7]. As a result, we know less about the effects of ‘benign’ parasitism in wildlife populations than we do about the effects of more virulent pathogens.
Host behavioural and physiological responses to parasitic infection provide important clues into host defences as well as pathogenicity. Defence strategies against parasitic diseases include avoidance (behavioural mechanisms that reduce risk of exposure), resistance (immune response that reduces pathogen burden) and tolerance (multiple mechanisms that reduce susceptibility to fitness costs by limiting damage caused by the pathogen) [8]. Investment in avoidance and tolerance strategies, compared with resistance, has important implications for host infectiousness [9].
Tolerance-promoting behavioural and physiological responses can arise when immunoregulatory cytokines interact with the endocrine system, and cause downstream release of glucocorticoids and sickness behaviours [8,10–12]. Indeed, enhanced glucocorticoid production in response to experimental infection from parasites has been documented in domestic animals [13], laboratory amphibians [14] and fish [15,16]. Despite a growing understanding of such processes in controlled settings, little is known about host endocrine response to parasites in naturally infected populations, including the pathophysiological and behavioural consequences of parasite infection and stress.
Behavioural indicators that are expected to decline in frequency or magnitude with illness include social activity, exploratory behaviour and feeding [17–20]. Indeed, behaviours of red colobus monkeys (Procolobus rufomitratus tephrosceles) infected by whipworm (Trichuris spp.) included a shift to less energetically expensive behaviours [21]. In social animals, time constraints imposed by energetic responses to infection may also impair social relationships [22]. Parasitic infection may inhibit an individual's ability to engage in energetically costly behaviours, such as challenging dominant individuals [23,24]. Furthermore, social contact and proximity can facilitate parasite transmission [25–27], such that animals may modify their behaviour directly to avoid infected conspecifics and reduce infection risk [28].
Parasitism, hormonal changes and behavioural time reallocation can alter body condition and lead to reductions in fitness [29–32]. For example, low body condition in parasitized Iberian hares (Lepus granatensis) contributes to reduced antipredator defence and higher host mortality [33]. In male fence lizards (Sceloporus occidentalis), hormonal changes resulting from parasitism can reach levels capable of inhibiting reproduction [34]. In addition, time reallocation towards behaviours that conserve energy and facilitate recovery can impair survival and reproduction [22,35].
(a). Research aims
We investigated whether gastrointestinal and pulmonary parasites influenced the behaviour and physiology of hosts living in a complex social environment. We followed a group of semi-free-ranging red-capped mangabeys (Cercocebus torquatus) before and after chemotherapeutic treatment for protozoan and helminth parasites, and measured ensuing changes in (i) glucocorticoid production, (ii) activity budgets, (iii) social relationships (e.g. social connectivity and dominance hierarchy stability) and (iv) body condition. We tested the hypothesis that antiparasite treatment would reduce tolerance and avoidance strategies. Specifically, we predicted that a reduction in parasitic infections would result in a corresponding reduction in cortisol levels, time reallocation towards energetically expensive behaviours with potential positive fitness consequences (e.g. reduced stress, and enhanced resource acquisition and predator detection), increased social connectivity, decreased hierarchical stability, and a subsequent reversion in behavioural and hormonal changes as animals became reinfected.
2. Material and methods
(a). Study site and population
All study activities took place at Rhoko Research and Conservation Education Centre (41.21° N, 16.16° E), the forest site of the Centre for Education, Research and Conservation of Primates and Nature (CERCOPAN) in Nigeria. We studied 49 individually recognizable red-capped mangabeys living as a multi-male, multi-female social group in a 1 ha open-topped forest enclosure within the natural home range of the species. Animals were vulnerable to natural predators (e.g. snakes and birds of prey) and parasites. No immigration or emigration events took place, thereby limiting external changes in the social environment throughout the duration of the study. The population was provisioned three times daily, which lessened the effects of temporal variation in resource availability, but the animals still ate wild foods opportunistically and drank from a stream running through the enclosure [25]. Climate included a long wet season from April to November and a short dry season from November to March.
(b). Study design
Faecal sampling and behavioural and health data were collected during the rainy season (May–August 2012) to reduce effects of seasonality. In June, the population was treated for parasites using orally administered metronidazole for protozoans, mebendazole for nematodes and praziquantel for cestodes and trematodes [25]. Faecal and behavioural sampling was conducted over three sampling periods: 30 days prior to treatment (pre-treatment), and two 30-day post-treatment periods (post-treatment 1 and post-treatment 2). Triplicate faecal samples from each period were analysed to maximize detection of infections from parasites with variable shedding rates and generate mean glucocorticoid values for each individual. Pre-treatment sampling was conducted prior to any experimental procedures, and a 10-day gap without any behavioural observations was included between pre-treatment and post-treatment 1 periods to minimize influence of any behavioural changes that may have occurred during the drug administration process.
(c). Parasitological analyses
One gram of faeces was taken from formalin-preserved samples and concentrated via faecal sedimentation for assessment of gastrointestinal parasites [25,36]. The entirety of the sediment was systematically examined at 10× objective light magnification, and all helminth eggs and larvae and large protozoan trophozoites and cysts were counted. One drop of sediment from each sample was examined at 40× for identification of small protozoan cysts. Protozoan densities were scored as many (4), moderate (3), few (2), rare (1) or none (0) [37]. Population infection status at each time point was calculated by taking the average number of diagnostic stages (e.g. eggs, larvae, cysts or trophozoites; hereafter referred to as eggs per gram (epg) for simplicity) of triplicate samples. Mean abundance of infection (epg in any host) was calculated with bootstrap confidence limits using Quantitative Parasitology software [38,39].
(d). Faecal cortisol analyses
Faecal cortisol levels were measured via enzyme immunoassay [40]. Methodological details and assay validation are described in detail by Friant et al. [25]. Interassay variation for the high pool was 18.3% and for the low pool was 22.2%, whereas intra-assay variation was 3.8% for the high pool and 7.9% for the low pool. Individual average faecal cortisol levels (nanogram per gram) were calculated from triplicate samples collected during each time period.
(e). Behavioural observations
Behaviour was measured via 1 min focal observations with combined continuous and instantaneous point sampling, and structured ad libitum sampling methods [41,42]. The number of seconds an animal was vigilant (defined herein as any visual search or directed gaze beyond arm's reach) was recorded during 1 min continuous follows [42]. A single instantaneous point sample was taken at the end of each focal observation period to record activity (e.g. feed (bringing food to mouth, biting, or chewing), forage (actively searching for or externally processing foods, including nut cracking), travel, social or rest) and identifications of all the nearest neighbours within 2 m [42]. Focal individuals were selected opportunistically, and at least 30 s were allowed to elapse between observations of individuals to reduce interdependence of data [25]. Focal observations for each individual in the group were conducted three times daily (early morning, 7.00–10.00; mid-morning, 11.00–15.00; evening, 16.00–19.00), leaving a 15 min buffer period between observations and provisioning.
All observed agonistic interactions and directionality of submissions and supplants were recorded using structured ad libitum sampling as conducted for similar species [43]. All data were collected by three observers and inter-observer reliability was tested and accepted by calculating Fleiss's kappa test for categorical agreements between multiple observers (κ = 0.89, p < 0.001 for observers 1 and 2; κ = 0.92, p < 0.001 for observers 1 and 3), and Pearson's correlation coefficients for continuous measures of vigilance (r = 0.97, p < 0.001 for observers 1 and 2, and 1 and 3).
(f). Social variable construction
Dominance ranks at multiple time periods were calculated using the Elo rating procedure based on progressive evaluation of dyadic supplants, and aggressive and avoidance interactions between adults of the same sex throughout the study period. Elo ratings were used because they allow for rank assignment at multiple time points without constructing new matrices and are therefore preferred for comparisons across short time periods [44,45]. Hierarchical stability was calculated from Elo ratings over each sampling period, allowing a 10-day burn-in period during the pre-treatment period. The stability measure (S) represents the ratio of rank changes and ranges between 0 (unstable) and 1 (stable) [44,46]. Elo ratings and stability scores were calculated using the EloRating and zoo packages in R v. 3.2.2 [47].
Weighted and unweighted proximity networks were constructed based on observed pairwise associations between focal individuals and all their nearest neighbours within 2 m using SOCPROG v. 2.6 [48]. Weights were calculated from the total number of associations between dyads within each study period. Symmetric matrices with attribute information were imported into UCINET software for calculation of group cohesion during pre-treatment, post-treatment 1 and post-treatment 2 periods [49].
(g). Visual health assessments
Ordinal indices of health along five dimensions representing the major organ systems and clinical syndromes were recorded for each individual during a veterinary visual health assessment conducted between the pre- and post-treatment 1 period (modified from the electronic supplementary material, figure S1). Indices were scored as unaffected (0) to 100% affected (4), and included pelage condition (colour, sheen, roughness), body condition (prominence of ilium, scapula, ribs, vertebrate and cheek bones) and mobility (arms, legs, tail). Respiration was scored as the number of sneezes or coughs per min and faecal consistency as firm (0), soft (1), runny (2) or mucoid (3) averaged over three samples. Individual monkeys were scored independently by each of the three observers, then collectively to reach consensus where scores differed.
(h). Statistical analyses
The efficacy of chemotherapeutic treatment and occurrence of subsequent reinfection events were measured by comparing mean abundance between paired pre-treatment/post-treatment 1 under the directional hypothesis of reduced abundance following antiparasite treatment, and post-treatment 1/post-treatment 2 samples under the directional hypothesis of increased abundance following reinfection. Because of the skewed distributions characteristic of parasites, comparisons were made with permutation tests using the coin package in R v. 3.2.2 [39,47].
We incorporated mean cortisol levels, percentage of time allocated to different activities (i.e. feeding, foraging, socializing, travelling and resting), and average number of seconds per minute spent vigilant, into a series of mixed-effects linear models with an autocorrelation structure of 1. Response variables with non-normal distributions were transformed to meet assumptions of normality. We incorporated sample period, sex and age-class as main effects in each model, and included individual identification as a random effect. We set the first post-treatment period as the reference period to examine changes associated with removal of parasites (pre-treatment versus post-treatment 1) and subsequent reinfection (post-treatment 1 versus post-treatment 2). We initially included all variables in the models, and used backwards elimination and Akaike information criterion (AIC) to select the best models. Analysis of variance (ANOVA) was run on final models to test significance. We retained only significant variables and the first-order interactions (at the α < 0.05 level) in final models where AIC of the model was lower than the null (difference ≥ 2). Age and sex were only included as main effects in the final model if there was a significant interaction with sampling period, thus allowing us to test only for significant differences in physiological and behavioural responses to infection among individuals. We performed analyses with the nlme package in R v. 3.2.2 [47].
We quantified network density of weighted and unweighted networks as measures of group cohesion [50]. We compared network densities at different time points using a paired (same nodes) bootstrap technique in UCINET (analogous to the classical paired sample t-test) for comparing networks with the same actors [51]. Network diagrams were constructed using UCINET software's NetDraw program, with node size representing individual degree centrality (number of associates), weighted edges representing strength (number of associations between nodes) and without filters.
We used principal components analyses (PCA) to generate uncorrelated health indices from visual health assessments that retained much of the original variation. We compared principal components representing health indices with measures of parasitism and cortisol generated from samples collected prior to the date of the health assessment. The principal components representing at minimum 80% of the variance were plotted following standardization around zero on each axis. Measures of parasitism and cortisol were then colour coded and visualized using convex hulls. Hulls were constructed to represent cortisol, parasite richness (total number of species) and individual infection status from three parasites with high prevalence and intensities: a protozoan (Balantidium coli), a nematode (Abbreviata sp.) and a trematode (Paragonimus africanus). Individuals were assigned ‘high’ or ‘low’ richness and cortisol values by dichotomizing lognormal continuous variables at the median. Continuous measures were retained by scaling individuals according to parasite intensity, richness and cortisol level (nanogram per gram) within plots.
3. Results
(a). Treatment effect on parasites
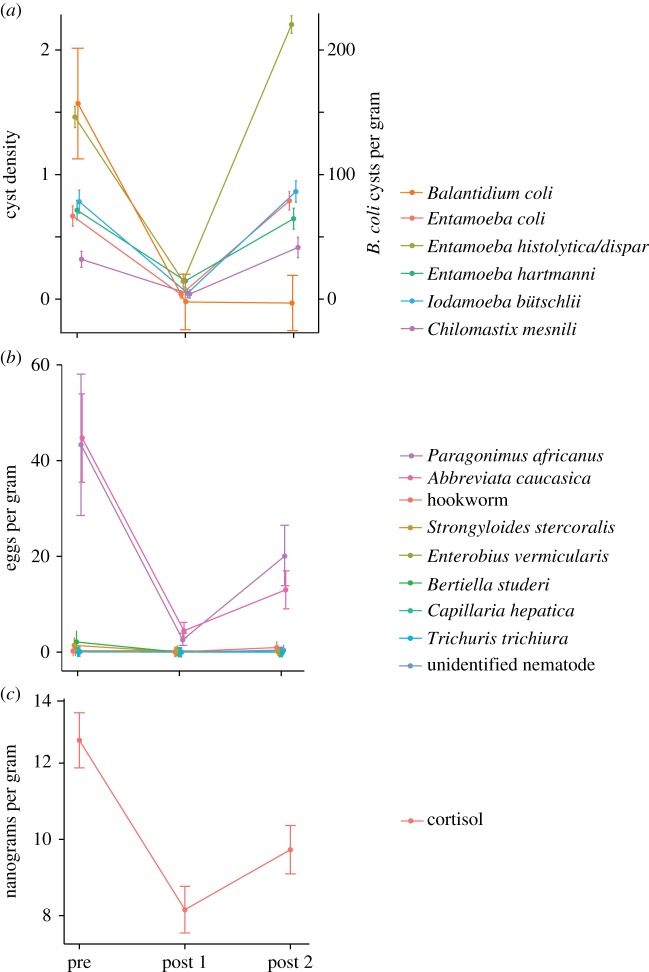
Parasites were recovered from triplicate faecal samples from every individual during each sampling period (n = 441). Mangabeys were infected with six protozoan and nine helminth taxa prior to treatment [25]. Chemotherapeutic treatment significantly reduced the abundance of protozoan (p < 0.001 (95% CI: 0.000–0.001)) and helminth (p < 0.001 (95% CI: 0.000–0.001)) infections from pre-treatment to post-treatment 1 samples. Mean parasite abundance significantly increased indicating reinfection between post-treatment 1 and 2 samples (protozoans (p < 0.001 (95% CI: 0.000–0.001)); helminths (p < 0.05 (95% CI: 0.008–0.013); electronic supplementary material, table S1; figure 1a,b).
Figure 1.
Parasite abundance and faecal cortisol relative to chemotherapeutic treatment for parasitic infections. Protozoan densities were scored as many (4), moderate (3), few (2), rare (1) or none (0). Balantidium coli cysts and all helminth eggs/larvae were counted as cysts or eggs per gram, respectively. Species identifications are putative based on size and morphological characteristics of eggs and cysts. (Online version in colour.)
(b). Treatment effect on faecal cortisol
Individual cortisol levels were calculated from triplicate faecal samples during three 30-day time periods (n = 441). Cortisol levels changed significantly over time (log transformed: F2,96 = 19.54, p < 0.0001; figure 1c). Mean cortisol decreased significantly following treatment (t96 = 6.23, p < 0.0001) and then increased in post-treatment 2 (t96 = 2.64, p < 0.01). Cortisol change did not differ significantly based on an individual's sex or age.
(c). Treatment effect on behavioural time allocation
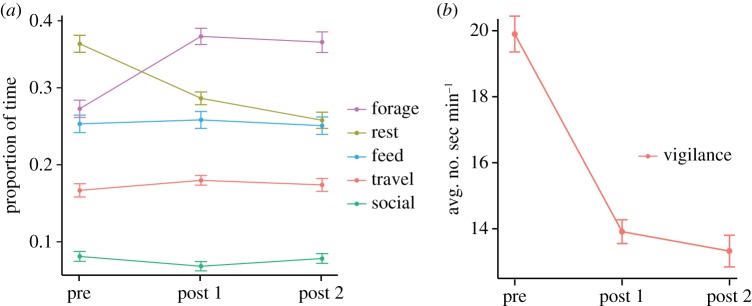
Activity budgets were calculated from 11 019 instantaneous point samples (M ± s.d. = 225 ± 6.66 per individual) over three 30-day time periods (pre-treatment n = 3661, post-treatment 1 n = 4063, post-treatment 2 n = 3295). Time spent foraging (F2,96 = 41.16, p < 0.0001) and resting (F2,96 = 44.51, p < 0.0001) changed significantly between treatment periods (figure 2a). Foraging behaviour increased significantly (9%) following treatment (t96 = 31.5, p < 0.0001), and corresponded to a significant decrease (7%) in resting behaviour (t96 = 6.52, p < 0.0001). Feeding, travelling and social behaviour did not change significantly between pre- and post-treatment 1 periods. Resting behaviour continued to decrease (3%) between post-treatment 1 and 2 periods (t96 = 2.64, p < 0.01). Other behaviours did not change significantly between post-treatments 1 and 2. Behavioural changes did not differ significantly between sex and age classes.
Figure 2.
Mean ± s.e. proportion of time that red-capped mangabeys spent (a) foraging, resting, feeding, travelling and socializing and (b) vigilant prior to and following chemotherapeutic treatment for parasites. (Online version in colour.)
Vigilance levels were calculated from 178 h (M ± s.d. = 3.63 h ± 14 min per individual) of focal observations over three 30-day time periods (pre-treatment n = 58 h, post-treatment 1 n = 66 h, post-treatment 2 n = 54 h). Vigilance levels changed significantly between time periods (F2,96 = 180.61, p < 0.0001; figure 2b). Specifically, vigilance reduced significantly following treatment (t96 = 15.80, p < 0.0001), but did not change significantly in the post-treatment 2 period. Change in vigilance did not differ significantly based on an individual's sex or age.
(d). Treatment effect on social relationships
Dominance ranks at multiple time periods were calculated from Elo ratings based on 888 dyadic supplants and aggressive and avoidance interactions between adults of the same sex (male n = 367 and female n = 521) throughout the study period. Rank changes occurred throughout the study, and dominance hierarchy stability varied by 1.38% in female hierarchies (pre-treatment S = 98.35, post-treatment 1 S = 98.39, post-treatment 2 S = 99.73) and 0.89% for male hierarchies (pre-treatment S = 98.32, post-treatment 1 S = 99.21, post-treatment 2 S = 98.85; electronic supplementary material, figure S2).
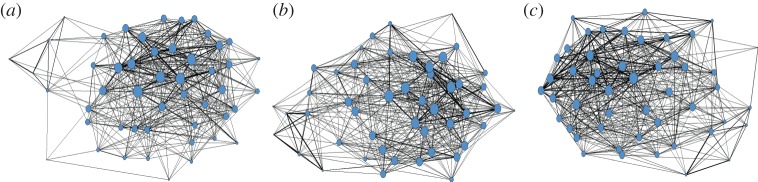
Proximity networks were constructed from 4042 observed pairwise associations (pre-treatment n = 970, post-treatment 1 n = 1112, post-treatment 2 n = 1117). The number of associates increased marginally between pre-treatment (binary density = 0.38) and post-treatment 1 sampling (binary density = 0.42; t48 = −1.92, p = 0.05, d = −0.39), and continued to increase between post-treatments 1 and 2 (binary density = 0.45; t48 = −1.98, p < 0.05, d = −0.40). Number of associations increased significantly between pre-treatment (valued density = 0.82) and post-treatment 1 sampling (valued density = 0.94; t48 = −2.30, p < 0.05, d = −0.46), but did not change between post-treatments 1 and 2 (valued density = 0.95).
(e). Relationship among body condition, infection and stress
Eighty-six per cent of the variation in visual health indices was explained by PC 1 (60%) and PC2 (26%) together. Principal component loadings were pelage colour (PC1: −0.42, PC2: −0.45), pelage roughness (PC1: −0.40, PC2: −0.22), pelage sheen (PC1: −0.48, PC2: −0.43), ilium prominence (PC1: −0.50, PC2: 0.53) and scapula prominence (PC1: −0.42, PC2: 0.53). Faecal consistency contributed only to PC4 and PC5, which together accounted for only 3% of the overall variance, and was therefore omitted from further analyses. Health measures with no observable variation were omitted from PCA. Individuals with higher cortisol levels occupied PCA values associated with more affected body condition (figures 3 and 4). Individuals infected with B. coli occupied PCA values associated with more variable body condition compared with uninfected individuals, which tended to be centred around the mean (electronic supplementary material, figure S2b). Body condition did not appear to be affected by Abbreviata sp. or P. africanus infection, nor overall parasite richness (electronic supplementary material, figure S2a,c,d).
Figure 3.
Social networks during (a) pre-treatment, (b) post-treatment 1 and (c) post-treatment 2 sampling periods. Nodes represent individuals and edges (lines connecting nodes) show interactions defined by proximity. Individuals with more associates (high degree centrality) are represented by larger nodes. Thickness of lines represents the number of associations between individuals (strength). (Online version in colour.)
Figure 4.
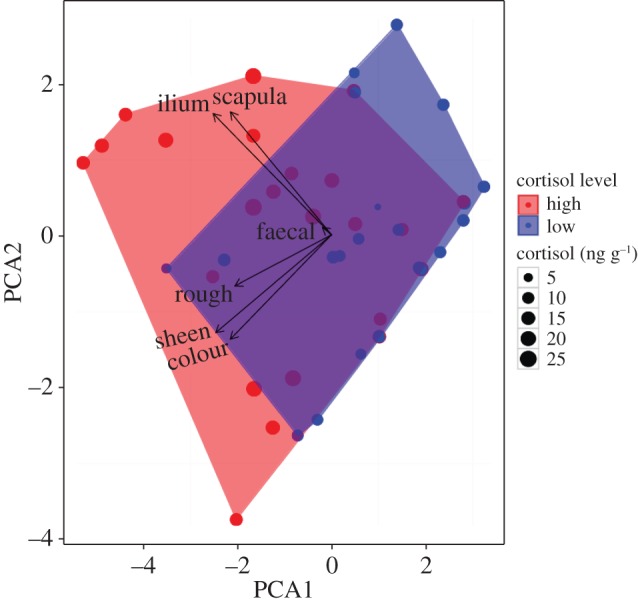
Visualization of principal component analysis (PCA) of five visual health indices (ilium and scapula prominence, faecal consistency, and fur roughness, sheen and colour), with first two components (PC1 and PC2) shown. Data points represent individuals, and are scaled by faecal cortisol level (nanogram per gram). Convex hulls are occupied by individuals with high (red) or low (blue) cortisol. The individual points projecting furthest in the direction of a given vector are the individuals with most affected body condition relevant to that variable, while those projecting opposite of variable vectors are least affected. (Online version in colour.)
4. Discussion
Our results show that reduced stress levels and altered behaviour accompanied treatment of parasitic infections in red-capped mangabeys. Average population cortisol levels covaried with parasite abundance, and high cortisol levels were associated with decreased body condition. When parasites were removed, individual activity patterns changed from resting and vigilance to active foraging. Although time spent engaging in social behaviour and hierarchical stability were not affected by parasite infection, the number and frequency of spatial associations increased following parasite treatment. Interestingly, behavioural changes did not revert to baseline levels by the end of the study, suggesting that parasite-induced behavioural change only occurs above a certain threshold of infection, or alternatively, that there is a delay in behavioural responses to immune defence and signalling. Behavioural and physiological responses to parasite treatment did not differ between sex and age classes, showing that the effects of parasitism were distributed equally across these subpopulations. Together, these results suggest that parasite-associated alterations in host physiology and behaviour have potential negative consequences for host fitness. Whether observed changes occurred as a coordinated host response to the effects of parasitism (e.g. tolerance) or as a result of parasite exploitation of the host [52,53] remains to be determined.
Parasite infections appear to have induced stress in naturally infected red-capped mangabeys. To date, it has remained unclear whether positive associations between cortisol and parasitism observed in wild primates was the result of increased susceptibility due to immunosuppressive effects, or if the parasites themselves induced a host stress response [54–56]. Indeed, explanations for patterns of parasite aggregation in certain hosts (e.g. males or dominant individuals) typically invoke hormonal regulation of the immune response as an important mechanism underlying susceptibility [57–60]. However, variation in cortisol levels did not explain time to reinfection [25]. Our results suggest that parasites themselves elicited a stress response, which to our knowledge had previously been demonstrated only in domestic and laboratory animals [13–16]. In addition, glucocorticoid production helped explain variation in physical estimators of health, suggesting that elevated stress, including contributions of parasitic infection to allostatic load, negatively influences body condition.
Experimental parasite reduction and associated reductions in glucocorticoid production corresponded with time reallocation away from resting and vigilance, and towards increased foraging activity. Movement around the enclosure was primarily foraging for food, as opposed to simply ‘travel’, which was observed far less frequently (figure 2). Energetic trade-offs between resting and foraging are consistent with sickness behaviour, in that parasitized animals favoured low-energy states when infected with parasites. Similarly, red colobus monkeys increased resting behaviour when infected with whipworm [21], and experimentally treated Grant's gazelles (Nanger granti) increased foraging behaviour and decreased vigilance compared with parasitized controls [61].
Interestingly, we found that parasite removal led to a significant increase in foraging but not feeding, despite feeding suppression being common during parasitic infections [17]. Our results lend support to the notion that parasite-induced feeding suppression results from a motivational state to conserve energy (i.e. reduced food consumption is a result of energetic trade-offs that decrease foraging) [62]. For example, early experiments in rodents found that operantly conditioned and experimentally infected rats stopped pressing a lever to receive water, but would drink it when readily available [63]. In this study, we may not have seen reductions in feeding because provisioned foods were readily available. Alternatively, infected individuals may have been less selective in their diets, thereby leading to reduced time spent foraging, yet equal time spent feeding. Further investigations combining information on food availability, feeding and foraging frequency, and dietary composition in naturally infected wild animals, will be useful in determining if food quality, as opposed to only food quantity, changes in infected versus uninfected individuals. In red colobus monkeys, for example, feeding frequency did not vary with infection status, but whipworm-infected animals shifted dietary composition to include more plants with medicinal properties [21].
Vigilance, which is protective against both predators and conspecific competition [64], decreased following treatment for parasites. Decreased vigilance (and increased foraging) was also observed in Grant's gazelles following experimental reduction in parasitism [61]. These findings suggest that parasite-infected individuals may allocate more time proportionally to vigilance to compensate for greater vulnerability [65]. Thus, reduced vigilance in our study population may indicate reduced vulnerability to predation and conspecific competition, resulting from parasite removal. Similar trade-offs between foraging and vigilance behaviour in inherently vulnerable animals were documented in pregnant European rabbits with poor physical condition (Oryctlagus cuniculus) [66], and vulnerable marmots (Marmota flaviventris) to enhance over-winter survival [67]. Whether these results indicate true re-allocation in response to a trade-off, or whether parasitized individuals spend time being vigilant because it is a low-energy activity linked predominately to resting, remains unclear.
Where social structure impacts transmission dynamics, social isolation of infected individuals should reduce transmission through avoidance [68]. For example, guppies (Poecilia reticulata) actively avoided experimentally infected individuals and reduced network clustering in the presence of infected conspecifics [28]. Indeed, risk of parasite transmission is increasingly attributed to social connectivity in primate populations [25–27]. In this study, individual connectivity through spatial associations, a known risk factor for acquisition of new infections in this population [25], increased when population levels of parasitism were reduced, despite no observed changes in time spent engaging in social behaviour. Determining whether reduced cohesion resulted from active avoidance of infected conspecifics or was a by-product of highly variable activity budgets will require further investigation.
5. Conclusion
In a population of semi-free-ranging red-capped mangabeys, parasitism was associated with avoidance and tolerance responses. Specifically, cortisol levels, activity budgets, vigilance and spatio-temporal associations covaried with levels of parasitism following treatment to remove parasites. This response to infection appears to maximize energy balance, reduce risk of transmission from infected conspecifics, and increase defence against competition and predation when animals are vulnerable. These findings suggest that fitness advantages of parasite-induced sickness behaviour may be mediated by neuroimmunoendocrine mechanisms that facilitate host tolerance.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Festus Onajde, Rosemary Gbegbaje and CERCOPAN staff for their assistance with data collection and support in the field, and Mason Saari, Kelsey Brown, Nicholas Segel and Julia Slezak for their assistance in the laboratory. We would also like to thank Dan Wittwer for his help with hormone assays, Dr Tim Yoshino for his assistance with parasite identifications, Dr Dorte Dopfer, Dr Ermias Emene, Dr Ria Ghai and Kelly Spoon for their input on statistical analyses, Dr Stephanie Salyer for design of the veterinary visual health assessment, and Dr Adrian Treves and Bill Rohde for their careful edits to this manuscript.
Ethics
The University of Wisconsin-Madison's Institutional Animal Care and Use committee (protocol # V1490) and CERCOPAN approved this research.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
S.F. designed the study, contributed to data collection in the field and laboratory, cleaned and analysed data, and drafted the manuscript. T.L.G. and T.E.Z. contributed to the study design, data analyses and manuscript writing. All authors approved the final version before publication.
Competing interests
We have no competing interests.
Funding
This research was funded by the Fulbright International Educational Exchange, the National Science Foundation's Doctoral Dissertation Improvement Grant (DDIG: 1403861), National Institutes of Health Parasitology and Vector Biology Training Program (T32AI007414); PI: T. Yoshino), Robert Wood Johnson Health Foundation Dissertation Grant, Graduate Women in Science Foundation, and John Ball Zoological Society Conservation Grant. Funding support was provided to the Assay Services Unit of the Wisconsin National Primate Research Centre by NIH: P51OD011106 to provide cost-efficient sample analyses.
References
- 1.Anderson RM, May RM. 1979. Population biology of infectious diseases: part I. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 2.Leroy EM. 2004. Multiple Ebola virus transmission events and rapid decline of Central African wildlife. Science 303, 387–390. ( 10.1126/science.1092528) [DOI] [PubMed] [Google Scholar]
- 3.Pounds AJ, et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. ( 10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 4.McCallum H, Jones M, Hawkins C, Hamede R, Lachish S, Sinn DL, Beeton N, Lazenby B. 2009. Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology 90, 3379–3392. ( 10.1890/08-1763.1) [DOI] [PubMed] [Google Scholar]
- 5.Paddle R. 2012. The thylacine's last straw: epidemic disease in a recent mammalian extinction. Aust. Zool. 36, 75 ( 10.7882/AZ.2012.008) [DOI] [Google Scholar]
- 6.Tompkins DM, Begon M. 1999. Parasites can regulate wildlife populations. Parasitol. Today 15, 311–313. ( 10.1016/S0169-4758(99)01484-2) [DOI] [PubMed] [Google Scholar]
- 7.Tompkins DM, Dunn AM, Smith MJ, Telfer S. 2011. Wildlife diseases: from individuals to ecosystems. J. Anim. Ecol. 80, 19–38. ( 10.1111/j.1365-2656.2010.01742.x) [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936–941. ( 10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanderWaal KL, Ezenwa VO. In press Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. ( 10.1111/1365-2435.12645) [DOI] [Google Scholar]
- 10.Dantzer R. 2001. Cytokine-induced sickness behavior: where do we stand? Brain. Behav. Immun. 15, 7–24. ( 10.1006/brbi.2000.0613) [DOI] [PubMed] [Google Scholar]
- 11.Kronfol Z, Remick DG. 2000. Cytokines and the brain: implications for clinical psychiatry. Am. J. Psychiatry 157, 683–694. ( 10.1176/appi.ajp.157.5.683) [DOI] [PubMed] [Google Scholar]
- 12.Besedovsky H, Rey A, del Sorkin E, Dinarello CA. 1986. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233, 652–654. ( 10.1126/science.3014662) [DOI] [PubMed] [Google Scholar]
- 13.Prichard RK, Hennessy DR, Griffiths DA. 1974. Endocrine responses of sheep to infection with Trichostrongylus colubriformis. Res. Vet. Sci. 17, 182–187. [PubMed] [Google Scholar]
- 14.Peterson JD, Steffen JE, Reinert LK, Cobine PA, Appel A, Rollins-Smith L, Mendonça MT. 2013. Host stress response is important for the pathogenesis of the deadly amphibian disease, Chytridiomycosis, in Litoria caerulea. PLoS ONE 8, e62146 ( 10.1371/journal.pone.0062146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sures B, Knopf K, Kloas W. 2001. Induction of stress by the swimbladder nematode Anguillicola crassus in European eels, Anguilla anguilla, after repeated experimental infection. Parasitology 123, 179–184. ( 10.1017/S003118200100823X) [DOI] [PubMed] [Google Scholar]
- 16.Tekmedash FS, Hemmatzadeh M, Khara H. In press Stress indices of Grass carp, Ceteopharyngodon idella, (Cuvier and Valenciennes, 1884) change in response to Monogenean parasites pollution, Gyrodactylus spp. and Dactylogyrus spp. J. Parasit. Dis. 1–3. ( 10.1007/s12639-014-0632-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crompton DW. 1984. Influence of parasitic infection on food intake. Fed. Proc. 43, 239–245. [PubMed] [Google Scholar]
- 18.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137. ( 10.1016/S0149-7634(88)80004-6) [DOI] [PubMed] [Google Scholar]
- 19.Hart BL. 1990. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294. ( 10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 20.Weary DM, Huzzey JM, von Keyserlingk MAG. 2009. Using behavior to predict and identify ill health in animals. J. Anim. Sci. 87, 770–777. ( 10.2527/jas.2008-1297) [DOI] [PubMed] [Google Scholar]
- 21.Ghai RR, Fugère V, Chapman CA, Goldberg TL, Davies TJ. 2015. Sickness behaviour associated with non-lethal infections in wild primates. Proc. R. Soc. B 282, 20151436 ( 10.1098/rspb.2015.1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunbar RIM, Korstjens AH, Lehmann J. 2009. Time as an ecological constraint. Biol. Rev. 84, 413–429. ( 10.1111/j.1469-185X.2009.00080.x) [DOI] [PubMed] [Google Scholar]
- 23.Rau ME. 1983. Establishment and maintenance of behavioural dominance in male mice infected with Trichinella spiralis. Parasitology 86, 319–322. ( 10.1017/S0031182000050484) [DOI] [PubMed] [Google Scholar]
- 24.Rau ME. 1984. Loss of behavioural dominance in male mice infected with Trichinella spiralis. Parasitology 88, 371–373. ( 10.1017/S0031182000054615) [DOI] [PubMed] [Google Scholar]
- 25.Friant S, Ziegler TE, Goldberg TL. 2016. Primate reinfection with gastrointestinal parasites: behavioural and physiological predictors of parasite acquisition. Anim. Behav. 117, 105–113. ( 10.1016/j.anbehav.2016.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIntosh AJJ, Jacobs A, Garcia C, Shimizu K, Mouri K, Huffman MA, Hernandez AD. 2012. Monkeys in the middle: parasite transmission through the social network of a wild primate. PLoS ONE 7, e51144 ( 10.1371/journal.pone.0051144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimbach R, Bisanzio D, Galvis N, Link A, Fiore AD, Gillespie TR. 2015. Brown spider monkeys (Ateles hybridus): a model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Phil. Trans. R. Soc. B 370, 20140110 ( 10.1098/rstb.2014.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croft DP, Edenbrow M, Darden SK, Ramnarine IW, van Oosterhout C, Cable J. 2011. Effect of gyrodactylid ectoparasites on host behaviour and social network structure in guppies Poecilia reticulata. Behav. Ecol. Sociobiol. 65, 2219–2227. ( 10.1007/s00265-011-1230-2) [DOI] [Google Scholar]
- 29.Irvine RJ, Corbishley H, Pilkington JG, Albon SD. 2006. Low-level parasitic worm burdens may reduce body condition in free-ranging red deer (Cervus elaphus). Parasitology 133, 465–475. ( 10.1017/S0031182006000606) [DOI] [PubMed] [Google Scholar]
- 30.Fairn ER, Schulte-Hostedde AI, Alarie Y. 2008. Water mite parasitism is associated with body condition and sex of the whirligig beetle Dineutus nigrior (Coleoptera: Gyrinidae). Ecoscience 15, 327–331. ( 10.2980/15-3-3134) [DOI] [Google Scholar]
- 31.Husak JF, Moore IT. 2008. Stress hormones and mate choice. Trends Ecol. Evol. 23, 532–534. ( 10.1016/j.tree.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 32.Pedersen AB, Fenton A. 2015. The role of antiparasite treatment experiments in assessing the impact of parasites on wildlife. Trends Parasitol. 31, 200–211. ( 10.1016/j.pt.2015.02.004) [DOI] [PubMed] [Google Scholar]
- 33.Alzaga V, Vicente J, Villanua D, Acevedo P, Casas F, Gortazar C. 2007. Body condition and parasite intensity correlates with escape capacity in Iberian hares (Lepus granatensis). Behav. Ecol. Sociobiol. 62, 769–775. ( 10.1007/s00265-007-0502-3) [DOI] [Google Scholar]
- 34.Dunlap KD, Schall JJ. 1995. Hormonal alterations and reproductive inhibition in male fence lizards (Sceloporus occidentalis) infected with the malarial parasite Plasmodium mexicanum. Physiol. Zool. 68, 608–621. ( 10.1086/physzool.68.4.30166347) [DOI] [Google Scholar]
- 35.Rauter CM, Moore AJ. 2004. Time constraints and trade-offs among parental care behaviours: effects of brood size, sex and loss of mate. Anim. Behav. 68, 695–702. ( 10.1016/j.anbehav.2003.09.018) [DOI] [Google Scholar]
- 36.Greiner EC, McIntosh A. 2009. Collection methods and diagnostic procedures for primate parasitology. In Primate parasite ecology: the dynamics and study of host-parasite relationships (eds Huffman MA, Chapman CA), pp. 3–27. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 37.Young KH, Bullock SL, Melvin DM, Spruill CL. 1979. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 10, 852–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efron B, Tibshirani R. 1993. An introduction to the bootstrap. London, UK: Chapman and Hall. [Google Scholar]
- 39.Rózsa L, Reiczigel J, Majoros G. 2000. Quantifying parasites in samples of hosts. J. Parasitol. 86, 228–232. ( 10.1645/0022-3395(2000)086%5B0228:QPISOH%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 40.Ziegler TE, Wittwer DJ. 2005. Fecal steroid research in the field and laboratory: improved methods for storage, transport, processing, and analysis. Am. J. Primatol. 67, 159–174. ( 10.1002/ajp.20175) [DOI] [PubMed] [Google Scholar]
- 41.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 42.Treves A. 1998. The influence of group size and neighbors on vigilance in two species of arboreal monkeys. Behaviour 135, 453–481. ( 10.1163/156853998793066168) [DOI] [Google Scholar]
- 43.Range F, Noe R. 2002. Familiarity and dominance relations among female sooty mangabeys in the Tai National Park. Am. J. Primatol. 56, 137–153. ( 10.1002/ajp.1070) [DOI] [PubMed] [Google Scholar]
- 44.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. ( 10.1016/j.anbehav.2011.07.016) [DOI] [Google Scholar]
- 45.Albers PCH, de Vries H. 2001. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim. Behav. 61, 489–495. ( 10.1006/anbe.2000.1571) [DOI] [Google Scholar]
- 46.McDonald DB, Shizuka D. 2012. Comparative transitive and temporal orderliness in dominance networks. Behav. Ecol. 24, 511–520. ( 10.1093/beheco/ars192) [DOI] [Google Scholar]
- 47.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 48.Whitehead H. 2009. SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. ( 10.1007/s00265-008-0697-y) [DOI] [Google Scholar]
- 49.Borgatti SP, Everett MG, Freeman LC. 2002. UCINET Software. Cambridge, MA: Analytic Technologies. [Google Scholar]
- 50.Borgatti SP, Everett MG, Johnson JC. 2013. Analyzing social networks, 1st edn Los Angeles, CA: Sage Publications. [Google Scholar]
- 51.Snijders TA, Borgatti SP. 1999. Non-parametric standard errors and tests for network statistics. Connections 22, 161–170. [Google Scholar]
- 52.Escobedo G, Roberts CW, Carrero JC, Morales-Montor J. 2005. Parasite regulation by host hormones: an old mechanism of host exploitation? Trends Parasitol. 21, 588–593. ( 10.1016/j.pt.2005.09.013) [DOI] [PubMed] [Google Scholar]
- 53.Perez AR, Bottasso O, Savino W. 2009. The impact of infectious diseases upon neuroendocrine circuits. Neuroimmunomodulation 16, 96–105. ( 10.1159/000180264) [DOI] [PubMed] [Google Scholar]
- 54.Foerster S, Kithome K, Cords M, Monfort SL. 2015. Social status and helminth infections in female forest guenons (Cercopithecus mitis). Am. J. Phys. Anthropol. 158, 55–66. ( 10.1002/ajpa.22764) [DOI] [PubMed] [Google Scholar]
- 55.Arlet ME, Chapman CA, Isbell LA, Molleman F, Mänd R, Hõrak P, Carey JR. 2015. Social and ecological correlates of parasitic infections in adult male gray-cheeked mangabeys (Lophocebus albigena). Int. J. Primatol. 36, 967–986. ( 10.1007/s10764-015-9866-9) [DOI] [Google Scholar]
- 56.Muehlenbein MP. 2006. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. Am. J. Phys. Anthropol. 130, 546–550. ( 10.1002/ajpa.20391) [DOI] [PubMed] [Google Scholar]
- 57.Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26, 1009–1024. ( 10.1016/S0020-7519(96)80001-4) [DOI] [PubMed] [Google Scholar]
- 58.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308, 648–652. ( 10.1126/science.1106477) [DOI] [PubMed] [Google Scholar]
- 59.Nunn CL, Altizer S. 2006. Infectious diseases in primates: behavior, ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 60.Cavigelli SA, Chaudhry HS. 2012. Social status, glucocorticoids, immune function, and health: can animal studies help us understand human socioeconomic-status-related health disparities? Horm. Behav. 62, 295–313. ( 10.1016/j.yhbeh.2012.07.006) [DOI] [PubMed] [Google Scholar]
- 61.Worsley-Tonks KEL, Ezenwa VO. 2015. Anthelmintic treatment affects behavioural time allocation in a free-ranging ungulate. Anim. Behav. 108, 47–54. ( 10.1016/j.anbehav.2015.07.018) [DOI] [Google Scholar]
- 62.Johnson RW. 2002. The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 87, 443–450. ( 10.1016/S0165-2427(02)00069-7) [DOI] [PubMed] [Google Scholar]
- 63.Miller NE. 1963. Some psychophysiological studies of motivation and of the behavioral-effects of illness. Bull. Brit. Psychol. Soc. 17, 1–21. [Google Scholar]
- 64.Treves A. 2000. Theory and method in studies of vigilance and aggregation. Anim. Behav. 60, 711–722. ( 10.1006/anbe.2000.1528) [DOI] [PubMed] [Google Scholar]
- 65.Elgar MA. 1989. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. 64, 13–33. ( 10.1111/j.1469-185X.1989.tb00636.x) [DOI] [PubMed] [Google Scholar]
- 66.Monclús R, Rödel HG. 2009. Influence of different individual traits on vigilance behaviour in European rabbits. Ethology 115, 758–766. ( 10.1111/j.1439-0310.2009.01661.x) [DOI] [Google Scholar]
- 67.Lea AJ, Blumstein DT. 2011. Age and sex influence marmot antipredator behavior during periods of heightened risk. Behav. Ecol. Sociobiol. 65, 1525–1533. ( 10.1007/s00265-011-1162-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gudelj I, White KAJ. 2004. Spatial heterogeneity, social structure and disease dynamics of animal populations. Theor. Popul. Biol. 66, 139–149. ( 10.1016/j.tpb.2004.04.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.